Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) represents a significant proportion of heart failure cases. Accurate diagnosis is challenging due to the heterogeneous nature of the disease and limitations in traditional echocardiographic parameters.
Main body
This review appraises the application of Global Longitudinal Strain (GLS) and Left Atrial Strain (LAS) as echocardiographic biomarkers in the diagnosis and phenotyping of HFpEF. Strain imaging, particularly Speckle Tracking Echocardiography, offers a superior assessment of myocardial deformation, providing a more detailed insight into left heart function than traditional metrics. Normal ranges for GLS and LAS are considered, acknowledging the impact of demographic and technical factors on these values. Clinical studies have demonstrated the prognostic value of GLS and LAS in HFpEF, especially in predicting cardiovascular outcomes and distinguishing HFpEF from other causes of dyspnea. Nevertheless, the variability of strain measurements and the potential for false-negative results underline the need for careful clinical interpretation. The HFA-PEFF scoring system's integration of these biomarkers, although systematic, reveals gaps in addressing the full spectrum of HFpEF pathology. The combined use of GLS and LAS has been suggested to define HFpEF phenogroups, which could lead to more personalized treatment plans.
Conclusion
GLS and LAS have emerged as pivotal tools in the non-invasive diagnosis and stratification of HFpEF, offering a promise for tailored therapeutic strategies. Despite their potential, a structured approach to incorporating these biomarkers into standard diagnostic workflows is essential. Future clinical guidelines should include clear directives for the combined utilization of GLS and LAS, accentuating their role in the multidimensional assessment of HFpEF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Heart failure with preserved ejection fraction (HFpEF) accounts for over one-half of all Heart Failure(HF) cases [1]. It is the most common form of HF, with its incidence and prevalence are growing as risk factors, including obesity, diabetes, and hypertension increase [2].
HFpEF is defined as the inability of the heart to pump blood properly at normal filling pressures. From a hemodynamic point of view, subjects with pulmonary arterial wedge pressure (PAWP) > 15 mmHg in rest conditions or > 25 mmHg during exercise have HFpEF [1]. However, the clinical definition of HFpEF varies substantially among studies, resulting in inconsistencies in non-invasive diagnosis [3,4,5].
The echocardiogram is a main non-invasive diagnostic tool that can provide several parameters to assess patients with suspected HFpEF. Even though the echocardiographic evaluation of diastolic function can provide parameters such as myocardial relaxation velocities and estimate the filling pressure non-invasively, those echocardiographic features present limited predictive capacities to diagnose HFpEF [2, 5].
The evaluation of left ventricular myocardial strain assessed by the Global Longitudinal Strain of the left ventricle (GLS) and Left Atrial Strain (LAS) have been proposed as robust and sensitive markers of left heart function with promising results to determine diagnosis and prognosis in HFpEF [6, 7]. Notwithstanding, there is a lack of data addressing the additive value of those echocardiographic features in the context of the recent recommendations to standardize the diagnostic criteria.
This review discusses whether the impressive evidence base for GLS and LAS justifies their use as biomarkers of HFpEF.
Technical considerations of strain imaging
Definition of strain
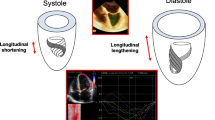
Strain corresponds to the amount of deformation of an object in relation to its original form. In Cardiology, this concept is represented as the percentage (%) of shortening/lengthening of the heart in relation to its initial measurement [7, 8]. Mathematically, the Lagrangian strain is computed by the algorithm as strain(t) = 100 [L(t) – L(ED)/L(ED)], where L(t) is the longitudinal length at time t, and L(ED) is the end-diastolic length [9] (Fig. 1). Significant differences exist between software regarding the L(ED) length used: an entire line of the ROI x average of a certain number of ROI points x average of the values in each segment of the same frame [9].
The concept of Left Ventricular Strain (LV Strain) depicted on the left side and Left Atrial Strain (LA Strain) depicted on the right side represent the relative changes in chamber length over a specified time interval -L(t) (depicted by red full circle), in comparison to the chamber length in end-diastole, denoted as L(ED) (depicted by blue full circle). The L(ED) is often defined as the time at which the mitral valve closes or the automatic detection of peak QRS occurs. For LV Strain, the time ‘t’ corresponds to end-systole (ES), which is precisely defined as aortic valve closure (AVC). On the other hand, for LA strain, the reservoir phase corresponds to the zenith of the strain curve
The Speckle Tracking technique has become the most recommended technique to estimate cardiac strain due to its better reproducibility and lower sensitivity to the ultrasound insonation angle, despite having lower temporal resolution when compared to Tissue Doppler Imaging [9].
The use of the concept of strain to assess myocardial function has been validated experimentally in vivo using sonomicrometry and cardiac magnetic resonance [10].
Left ventricular strain: definition and normal range
The GLS reflects the relative longitudinal contraction (%) of the myocardium that occurs from the period of isovolumetric contraction until the end of the ejection period [9, 11].
The GLS normality value is approximately 20%. There is evidence of variations in normality values according to sex and age. Absolute values of GLS ranging from 16–18% are frequently stated as borderline, while an absolute below 16% indicates systolic dysfunction.
Farsalinos et al. [12] demonstrated that the greatest absolute difference between manufacturers for GLS values was 3.7 percentage units of strain, with a significant and strong correlation between the measurements of the different manufacturers and also with the average measurement of all the manufacturers. Furthermore, caution is required regarding software updates, which can also impact GLS calculations.
Despite these findings, GLS is an echocardiographic feature that is less susceptible to technical factors than ejection fraction assessments by conventional 2D echocardiography, and it can be applied in daily practice [7, 13].
Left atrial strain: definition and normal range
Analogous to the left ventricle, LAS is a metric that quantifies the dynamic changes in the length of the atrial myocardium, indicating both shortening and lengthening, across the cardiac cycle. It serves as a valuable indicator of LA deformation. LAS proves to be an important tool with prognostic value for a broad spectrum of cardiovascular diseases [14]. Imaging in the far ultrasound field and a thinner left atrial (LA) myocardium may pose additional challenges to LAS analysis. For the same reason, assessment of radial strain or a subdivision of the LA wall into segments is not recommended [15]. Several studies have demonstrated the use of echocardiographic LAS to evaluate LA mechanical function [16,17,18].
The LAS comprises three distinct phases: reservoir, conduit, and contractile. The reservoir phase signifies the stretching of the LA wall resulting from the filling of the left atrium through pulmonary veins while the mitral valve is closed. The conduit phase takes place in the early diastole when the mitral valve opens, and the LA discharges into the left ventricle, corresponding to the E wave (early transmitral flow). The contractile phase corresponds to the A wave (late transmitral flow) and denotes the contraction of the left atrium. Each component of the LA is represented by a LAS element: LAS reservoir (LASr), LAS conduit (LAS cd), and LAS contractile (LASct). In the LAS curve, each phase can be represented by a measure obtained through the difference in values at two points [15]. Based on actual evidence, LASr has the most important clinical utility due to its prognostic and morbimortality prediction.
Reference values for LA function parameters using strain have been investigated in various publications. A meta-analysis [19] comprising 40 studies and involving 2542 healthy patients defined the following normal values: 39.4% (95% CI: 38.0–40.8%) for LASr, 23% (95% CI: 20.7–25.2%) for LAScd, and 17.4% (95% CI: 16.0–19.0%) for LASct. Heterogeneity was attributed to sample size, heart rate, and body surface area. Age, gender, racial groups, reference zero for strain, and differences between software types were not identified as contributors to the variation.
Due to the wide range of reference values for LAS compared to LV strain, the lower limit of normality demonstrated has been considerably lower than the average values described.
Results from the EACVI NORRE study [20], which included 371 healthy patients, indicated the following absolute normal values and lower expected values (indicated in parentheses) for LA strain: 42.5% (26.1%) for reservoir function, 25.7% (12.0%) for conduit function, and 16.3% (7.7%) for contractile function.
Nyberg et al. [21], based on 1329 healthy individuals, described the values of 17%, 3%, and 7% as the lower limits of normality for LASr, LAScd, and LASct, respectively.
Given the variability in reference ranges for LAS, caution should be exercised when incorporating it into clinical decision-making in various contexts.
Evidence of left ventricular and atrial myocardial strain in HFpEF
The left ventricular global longitudinal strain in HFpEF
The role of GLS in HFpEF has been assessed in animal models and clinical studies. Despite all the challenges related to producing an animal model that recapitulates all major features of HFpEF syndrome, recent data has provided new insightful findings. Using a model of chronic hypertension in rats, Shah et al. [22] shows that GLS could be a biomarker in transitioning from adaptive cardiac hypertrophy to dysfunction toward cardiac failure. The abnormalities in strain imaging seem to happen when there are changes in transverse (T)-tubule organization. This leads to altered intracellular Ca2 + cycling, abnormal excitation–contraction coupling, and the development of abnormal myocardial mechanics. These changes seem to manifest prior to the development of significant cardiac fibrosis and precede the development of overt cardiac dysfunction and HF, indicating that impairments in GLS may have a role in pre-clinical HF (pre-HFpEF, previously called stage B). These findings are in agreement with clinical studies suggesting that impairments in GLS may occur in early-stage HFpEF. For example, Kosmala et al. [23] demonstrated that in asymptomatic patients with type 2 diabetes mellitus, impaired GLS is independent and incremental to left ventricular hypertrophy in predicting incident HF.
Clinical studies also have evaluated GLS in patients with overt HFpEF syndrome. A large cohort study [24] demonstrated that an abnormal GLS was strongly associated with a more than twofold increase in the composite endpoint of cardiovascular (CV) death, hospitalization due to heart failure, or aborted cardiac arrest. This association remained significant even after adjusting for risk factors and other echocardiographic features.
Sakaguchi et al. [23] demonstrated the enduring prognostic significance of GLS changes in HFpEF patients. Assessing GLS during acute HF hospitalization and in a stable phase, they observed that patients with major cardiovascular events (MACE) had a significantly lower GLS change rate than those without MACE (10,6% vs. 26%; p < 0.001), highlighting impaired GLS as a robust prognostic indicator for a more adverse disease course.
In the context of recent efforts to standardize diagnostic criteria such as the H2FPEF and HFA-PEFF scores, there is a notable gap in data addressing the additional value of Global Longitudinal Strain (GLS). The H2FPEF score [25] does not incorporate GLS, making it challenging to assess its contributory significance based on existing evidence.
The HFA-PEFF score, introduced by European guidelines in 2019 [26] and posteriorly validated in two large cohorts [24], presents a systematic framework for diagnosing patients suspected of HFpEF. While this score provides a valuable evaluative metric, physicians should be aware of the potential for false negatives, which could account for up to 25% [27], necessitating a careful application of the score alongside comprehensive clinical assessments to ensure diagnostic accuracy.
The acronym “PEFF” represents the following sequential steps: Pre-test assessment (P), Echocardiography (E), Functional evaluation (F1), and Final etiology determination (F2). During Step E, the echocardiographic parameters are assessed alongside plasmatic biomarkers (Fig. 2), where, GLS is categorized as a minor parameter within the functional domain. Thus, not all patients with abnormal GLS will have HFpEF.
Echocardiographic and natriuretic peptide heart failure with preserved ejection fraction workup and scoring system. TR: Tricuspid Regurgitation. LAVI: Left Atrial Volume Index. LVMI: left ventricular mass index. RWT: Relative Wall Thickness. M: Men. W: Women. SR: Sinus Rhythm.AF: atrial fibrillation. Adapted from: Pieske, B et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association of the European Society of Cardiology
The abnormal GLS has been associated with conditions that are known to affect the myocardium, such as hypertension, diabetes mellitus, subclinical atherosclerosis, aortic stenosis and amyloidosis, These conditions can be considered as risk factors or even as HFpEF mimics [7, 9].
This abnormal GLS without others pathophysiological impairments may not be sufficient to lead to an increase in LV filling pressures. Indeed, there is evidence pointing that GLS have only a moderate correlation with the time constant of LV pressure decay, tau [28], and with resting pre-A pressure [29].
Since patients with HFpEF may exhibit increased filling pressures exclusively during exercise, studies addressing the behavior of GLS during hemodynamic stress such as exercise, preload challenge and afterload challenge can potentially have relevant information. Recent data reported the myocardial dysfunction mediated by increased afterload in patients with HFpEF with preserved resting GLS [30]. Those subjects displayed an accentuated decrease in the Longitudinal Strain during provoked pressure overload.
Furthermore, other aspects of LV mechanics beyond the GLS, such as the degree of temporal heterogeneity of contraction (mechanical dyssynchrony), are more associated to exercise capacity in HFpEF patients and may provide new insights on how the heart could be more prone to the steep increase filling pressures during exercise that characterized HFpEF [31].
These data support the notion that overt HFpEF can occur without impaired resting GLS, indicating that the isolated use of GLS may have sub-optimal sensitivity in the HFpEF population. Indeed several studies have substantial proportion of patients with fully developed HFpEF with preserved GLS [32] as shown in Table 1.
The left atrial strain in HFpEF
The LA strain has emerged as a valuable echocardiographic parameter in the assessment of HFpEF, offering insights into the pathophysiology and prognostication of this complex syndrome [39,40,41,42,43,44]. The role of LA strain in HFpEF is multifaceted, primarily focusing on its correlation with LV filling pressures, major adverse cardiovascular events (MACE), and the risk of atrial fibrillation (Table 2).
Correlation with LV Filling Pressure (PAWP)
LA strain serves as a non-invasive marker for assessing LV filling pressures, often reflected by pulmonary artery wedge pressure (PAWP). Abnormalities in LA strain, especially in reservoir phase, have shown consistent correlation with elevated LV filling pressures, aiding in the diagnosis and monitoring of HFpEF patients. A decrease in LV filling pressures is associated with a reduction in LA volumes, although normalization is infrequent. Notably, a robust correlation exists between the reduction in LV filling pressure and the enhancement of LA function, as evidenced by improvements in LA strain [43]. This correlation is particularly crucial as elevated filling pressures are a hallmark of HFpEF and contribute to its clinical manifestations.
Association with major adverse cardiovascular events (MACE)
LA strain has demonstrated predictive value for MACE in HFpEF patients. Impaired LA strain is associated with adverse cardiovascular outcomes, including all-cause mortality, cardiovascular mortality, and HF hospitalizations. The compromised LA function, as reflected by reduced strain, highlights the intricate interplay between LA dysfunction and the progression of HFpEF. As a result, LA strain becomes a valuable prognostic tool, aiding clinicians in risk stratification and identifying patients at a higher risk of cardiovascular events.
Risk of Atrial Fibrillation (AF)
HFpEF patients frequently exhibit a high prevalence of atrial fibrillation, a condition associated with increased morbidity and mortality. LA strain has shown promise in predicting the development of AF in HFpEF cohorts. Impaired LA function, as assessed by reduced strain values, is associated with atrial remodeling and electrical disturbances, contributing to the initiation and perpetuation of AF. Therefore, LA strain not only aids in assessing the current state of HFpEF but also serves as a potential marker for identifying individuals at an increased risk of developing AF.
In this context, LA strain plays a pivotal role in HFpEF management by offering valuable insights into LV filling pressures, predicting major adverse cardiovascular events, and identifying patients at risk of atrial fibrillation. As an integral component of advanced echocardiographic assessment, the LAS has been shown to be the most robust imaging marker distinguishing HFpEF from noncardiac causes of dyspnea, and abnormalities in LAS are more associated with adverse outcomes than those of LV [39, 40].
Both H2FPEF and HFA-PEFF scoring systems do not incorporate the potential insights provided by LAS. The LASr improves the feasibility and diagnostic accuracy of the 2016 ASE/EACVI diastolic algorithm in patients with HFpEF and, so, can furnish additional information that aids in the diagnosis of HFpEF. Furthermore, the LAS reservoir has proven aid to identify those patients with normal resting filling pressure who subsequently developed elevated LVFP during exercise [40].
Proposal for the combined use of GLS and LAS to Phenotype HFpEF patients
Recently it was proposed the potential Phenotyping of HFpEF syndrome according to the abnormalities in LV strain [32, 65]. Abnormalities in left ventricular longitudinal strain have been identified as a valuable marker for distinguishing a specific phenogroup within the diverse spectrum of HFpEF syndrome. This phenogroup consists of individuals with reduced GLS (HFpEF-rLS), indicating the presence of contractile dysfunction, myocardial fibrosis, maladaptive myocardial hypertrophy, and other myocardial diseases [7, 13, 66]. These underlying mechanisms contribute to the alteration of GLS in HFpEF.
Conversely, a notable proportion of HFpEF patients (ranging from 18 to 48%) exhibit preserved LV longitudinal systolic function (HFpEF-pLS) [32, 67]. This subgroup may represent a distinct phenogroup that has progressed toward other major pathophysiological abnormalities such as atrial dysfunction, chronotropic incompetence, pulmonary vascular disease, unbalanced blood volume distribution, and low peripheral oxygen extraction, which can combine in different permutations, ultimately contributing to the development of heart failure syndrome.
In this context, given the growing body of research providing high-quality, evidence-based support for LAS as a robust biomarker in Heart Failure with Preserved Ejection Fraction (HFpEF) and Left Atrial (LA) myopathy, the integration of LAS into the assessment of patients with HFpEF-pLS appears promising and holds an excellent pathophysiological rationale. Furthermore, this additional value seems to manifest independently of the presence of persistent or paroxysmal atrial fibrillation, thereby amplifying both diagnostic and prognostic insights.
In patients with HFpEF-pLS and reduced LAS, there is a disproportionate impairment within the left atrium chamber due to atrial cardiomyopathy [65], establishing the left atrium as a pivotal mechanical epicentrum in the pathophysiology mechanism of the disease. The evidence supporting this rationale is relatively new in the field of Cardiology, as most classical knowledge about diastology assumes LA disease as a direct consequence of advanced LV diastolic dysfunction with chronic increase in LV end-diastolic pressure [68].
Recent data suggests that despite having better LV diastolic function, these patients exhibit a worse hemodynamic profile characterized by higher pulmonary artery pressure and pulmonary vascular resistance, coupled with a lower stroke volume [65]. Additionally, they are more prone to displaying abnormalities in LV strain during exercise, further supporting the evidence of an association with microvascular dysfunction.
With a more pronounced degree of impairments in LA function, these patients may demonstrate reduced LV filling, leading to a decreased LV end-diastolic volume. Consequently, they may exhibit heart failure with a supranormal LVEF profile [69, 70].
The concurrent application of both GLS and LAS for phenotyping subjects with HFpEF is depicted in Fig. 3. This proposal strategy has the potential to provide a more comprehensive insight into the specific cardiac impairments of HFpEF patients. Moreover, it holds promise for the development of innovative randomized controlled trial designs such as enrichment trials [3].
Proposed methodology for phenotyping Heart Failure with Preserved Ejection Fraction (HFpEF) through parameters derived from left heart myocardial mechanics. There are two mains distinct HFpEF phenotypes based on left ventricular strain: HFpEF with reduced longitudinal systolic function (HFpEF-rLS) and HFpEF with preserved longitudinal systolic function (HFpEF-pLS). Additionally, the integration of the left atrium strain reservoir (LASr) is suggested to introduce a comprehensive dimension addressing the impairments in both the left ventricle and left atrium. This proposed approach offers a nuanced perspective for refining HFpEF phenotyping and may contribute to targeted therapeutic insight
This type of trial design can test interventions to specific sub-phenogroups. This type of study represents one of the main tools to deal with HFpEF heterogeneity since the traditional randomized controlled trial design may have significant limitations in this scenario [3].
Conclusions
The current evidence substantiates the utility of GLS and LAS for diagnostic of HFpEF. Their combined use offers a promising approach to phenotyping patients with HFpEF, potentially guiding more personalized therapeutic interventions. While compelling, the evidence calls for a carefully structured integration of GLS and LAS into standard diagnostic protocols to fully leverage their potential in clinical practice.
Availability of data and materials
None.
Abbreviations
- ACC:
-
American College of Cardiology
- AF:
-
Atrial Fibrillation
- AHA:
-
American Heart Association
- CV:
-
Cardiovascular
- EF:
-
Ejection Fraction
- GLS:
-
Global Longitudinal Strain
- HF:
-
Heart Failure
- HFA:
-
Heart Failure Association
- HFpEF:
-
Heart Failure with Preserved Ejection Fraction
- HFpEF-pLS:
-
Heart Failure with preserved Ejection Fraction and preserved Longitudinal Systolic function
- HFpEF-rLS:
-
Heart Failure with preserved Ejection Fraction and reduced Longitudinal Systolic function
- LAS:
-
Left atrial strain
- LASr:
-
Left atrial strain reservoir
- LAScd:
-
Left atrial strain conduit
- LASct:
-
Left atrial strain contractile
- LAVI:
-
Left Atrial Volume Index
- L(ED):
-
End-Diastolic Length.
- LV:
-
Left Ventricular
- LVH:
-
Left Ventricular Hypertrophy
- LVMI:
-
Left Ventricular Mass Index
- MACE:
-
Major Cardiovascular Events
- NP:
-
Natriuretic Peptides
- PAWP:
-
Pulmonary Wedge Pressure
- RWT:
-
Relative Wall Thickness
- SP:
-
Speckle Tracking
- SR:
-
Sinus Rhythm
- TR:
-
Tricuspid Regurgitation
References
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):1757–80.
Kittleson MM, Panjrath GS, Amancherla K, Davis LL, Deswal A, Dixon DL, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835–78.
Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R, et al. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 2020;141(12):1001–26.
Ho JE, Zern EK, Wooster L, Bailey CS, Cunningham T, Eisman AS, et al. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated With Existing HFpEF Definitions. Circulation. 2019;140(5):353–65.
Hortegal RA. Decodificando os Dilemas Diagnósticos da Insuficiência Cardíaca com Fração de Ejeção Preservada: Aplicando a Definição Universal para uma Melhor Padronização Diagnóstica. 2023;36(3):-.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876–94.
Almeida ALC, Melo MDTd, Bihan DCdSL, Vieira MLC, Pena JLB, Castillo JMD, et al. Posicionamento do Departamento de Imagem Cardiovascular da Sociedade Brasileira de Cardiologia sobre o Uso do Strain Miocárdico na Rotina do Cardiologista – 2023. Arquivos Brasileiros de Cardiologia. 2023;120(9):00-.
Sutherland GR, Di Salvo G, Claus P, D’Hooge J, Bijnens B. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr. 2004;17(7):788–802.
Loizou CP PC, D'Hooge J. Handbook of speckle filtering and tracking in cardiovascular ultrasound imaging and video2018.
MacGowan GA, Shapiro EP, Azhari H, Siu CO, Hees PS, Hutchins GM, et al. Noninvasive measurement of shortening in the fiber and cross-fiber directions in the normal human left ventricle and in idiopathic dilated cardiomyopathy. Circulation. 1997;96(2):535–41.
Sousa RD, Regis CDM, Silva IDS, Szewierenko P, Hortegal RA, Abensur H. Software for Post-Processing Analysis of Strain Curves: The D-Station. Arq Bras Cardiol. 2020;114(3):496–506.
Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr. 2015;28(10):1171-81e2.
Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28(2):183–93.
Thomas L, Muraru D, Popescu BA, Sitges M, Rosca M, Pedrizzetti G, et al. Evaluation of Left Atrial Size and Function: Relevance for Clinical Practice. J Am Soc Echocardiogr. 2020;33(8):934–52.
Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600.
Sirbu C, Herbots L, D’Hooge J, Claus P, Marciniak A, Langeland T, et al. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects. Eur J Echocardiogr. 2006;7(3):199–208.
Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. 2009;7:6.
Liu L, Zhang B, Yang Y, Qi L, Wang S, Meng L, et al. Reduced left atrial contractile strain with speckle tracking analysis predicts abnormal plasma NTproBNP in an asymptomatic community population. Cardiovasc Ultrasound. 2022;20(1):27.
Pathan F, D’Elia N, Nolan MT, Marwick TH, Negishi K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr. 2017;30(1):59-70e8.
Sugimoto T, Robinet S, Dulgheru R, Bernard A, Ilardi F, Contu L, et al. Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2018;19(6):630–8.
Nyberg J, Jakobsen EO, Ostvik A, Holte E, Stolen S, Lovstakken L, et al. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. JACC Cardiovasc Imaging. 2023;16(12):1516–31.
Shah SJ, Aistrup GL, Gupta DK, O’Toole MJ, Nahhas AF, Schuster D, et al. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol. 2014;306(1):H88-100.
Sakaguchi E, Yamada A, Naruse H, Hattori H, Nishimura H, Kawai H, et al. Long-term prognostic value of changes in left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction. Heart Vessels. 2023;38(5):645–52.
BarandiaranAizpurua A, Sanders-van Wijk S, Brunner-La Rocca HP, Henkens M, Heymans S, Beussink-Nelson L, et al. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(3):413–21.
Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation. 2018;138(9):861–70.
Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297–317.
Churchill TW, Li SX, Curreri L, Zern EK, Lau ES, Liu EE, et al. Evaluation of 2 Existing Diagnostic Scores for Heart Failure With Preserved Ejection Fraction Against a Comprehensively Phenotyped Cohort. Circulation. 2021;143(3):289–91.
Hayashi T, Yamada S, Iwano H, Nakabachi M, Sakakibara M, Okada K, et al. Left Ventricular Global Strain for Estimating Relaxation and Filling Pressure - A Multicenter Study. Circ J. 2016;80(5):1163–70.
Tan TS, TuranSerifler N, Demirtola AI, Akbulut IM, Ozyuncu N, Vurgun VK, et al. Invasive validation of the left ventricular global longitudinal strain for estimating left ventricular filling pressure. Echocardiography. 2021;38(7):1133–40.
Hortegal RA, Valeri R, Grizante M, Cancellier R, Uemoto V, Gun C, et al. Afterload increase challenge unmasks systolic abnormalities in heart failure with preserved ejection fraction. Int J Cardiol. 2023;380:20–7.
Hortegal RA, Hossri C, Giolo L, Cancellier R, Gun C, Assef J, et al. Mechanical dispersion is a superior echocardiographic feature to predict exercise capacity in preclinical and overt heart failure with preserved ejection fraction. Int J Cardiovasc Imaging. 2023.
Argulian E, Chandrashekhar Y, Shah SJ, Huttin O, Pitt B, Zannad F, et al. Teasing Apart Heart Failure With Preserved Ejection Fraction Phenotypes With Echocardiographic Imaging: Potential Approach to Research and Clinical Practice. Circ Res. 2018;122(1):23–5.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92.
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–77.
Donal E, Lund LH, Oger E, Bosseau C, Reynaud A, Hage C, et al. Importance of combined left atrial size and estimated pulmonary pressure for clinical outcome in patients presenting with heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2017;18(6):629–35.
Buggey J, Alenezi F, Yoon HJ, Phelan M, DeVore AD, Khouri MG, et al. Left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: outcomes following an acute heart failure hospitalization. ESC Heart Fail. 2017;4(4):432–9.
Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–95.
Huang W, Chai SC, Lee SGS, MacDonald MR, Leong KTG. Prognostic Factors After Index Hospitalization for Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2017;119(12):2017–20.
Morris DA, Gailani M, Vaz Perez A, Blaschke F, Dietz R, Haverkamp W, et al. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24(6):651–62.
Ye Z, Miranda WR, Yeung DF, Kane GC, Oh JK. Left Atrial Strain in Evaluation of Heart Failure with Preserved Ejection Fraction. J Am Soc Echocardiogr. 2020;33(12):1490–9.
Nishida G, Calvilho Junior AA, Assef JE, Dos Santos NSS, de Andrade VA, Braga SLN. Left atrial strain as a predictor of left ventricular filling pressures in coronary artery disease with preserved ejection fraction: a comprehensive study with left ventricular end-diastolic and pre-atrial contraction pressures. Int J Cardiovasc Imaging. 2023;39(11):2193–204.
Inoue K, Khan FH, Remme EW, Ohte N, Garcia-Izquierdo E, Chetrit M, et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging. 2021;23(1):61–70.
Donal E, Galli E, Schnell F. Left Atrial Strain: A Must or a Plus for Routine Clinical Practice? Circ Cardiovasc Imaging. 2017;10(10).
Venkateshvaran A, Tureli HO, Faxen UL, Lund LH, Tossavainen E, Lindqvist P. Left atrial reservoir strain improves diagnostic accuracy of the 2016 ASE/EACVI diastolic algorithm in patients with preserved left ventricular ejection fraction: insights from the KARUM haemodynamic database. Eur Heart J Cardiovasc Imaging. 2022;23(9):1157–68.
Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, et al. Correlation of Left Atrial Strain and Doppler Measurements with Invasive Measurement of Left Ventricular End-Diastolic Pressure in Patients Stratified for Different Values of Ejection Fraction. Echocardiography. 2016;33(3):398–405.
Lin J, Ma H, Gao L, Wang Y, Wang J, Zhu Z, et al. Left atrial reservoir strain combined with E/E’ as a better single measure to predict elevated LV filling pressures in patients with coronary artery disease. Cardiovasc Ultrasound. 2020;18(1):11.
Ma C-S, Liao Y-P, Fan J-L, Zhao X, Su B, Zhou B-Y. The novel left atrial strain parameters in diagnosing of heart failure with preserved ejection fraction. Echocardiography. 2022;39(3):416–25.
Zhou Y, Zhao C-M, Shen Z-Y, Zhao X, Zhou B-Y. Mitral early-diastolic inflow peak velocity (E)-to-left atrial strain ratio as a novel index for predicting elevated left ventricular filling pressures in patients with preserved left ventricular ejection fraction. Cardiovasc Ultrasound. 2021;19(1):17.
Reddy YNV, Obokata M, Egbe A, Yang JH, Pislaru S, Lin G, et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21(7):891–900.
Lundberg A, Johnson J, Hage C, Bäck M, Merkely B, Venkateshvaran A, et al. Left atrial strain improves estimation of filling pressures in heart failure: a simultaneous echocardiographic and invasive haemodynamic study. Clin Res Cardiol. 2019;108(6):703–15.
Telles F, Nanayakkara S, Evans S, Patel HC, Mariani JA, Vizi D, et al. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21(4):495–505.
Singh A, Medvedofsky D, Mediratta A, Balaney B, Kruse E, Ciszek B, et al. Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures. Int J Cardiovasc Imaging. 2019;35(1):23–32.
Inoue K, Khan FH, Remme EW, Ohte N, García-Izquierdo E, Chetrit M, et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging. 2021;23(1):61–70.
Hummel YM, Liu LCY, Lam CSP, Fonseca-Munoz DF, Damman K, Rienstra M, et al. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail. 2017;19(12):1651–60.
Park J-H, Hwang I-C, Park JJ, Park J-B, Cho G-Y. Prognostic power of left atrial strain in patients with acute heart failure. Eur Heart J Cardiovasc Imaging. 2020;22(2):210–9.
Bouwmeester S, van der Stam JA, van Loon SLM, van Riel NAW, Boer A-K, Dekker LR, et al. Left atrial reservoir strain as a predictor of cardiac outcome in patients with heart failure: the HaFaC cohort study. BMC Cardiovasc Disord. 2022;22(1):104.
Ersbøll M, Andersen MJ, Valeur N, Mogensen UM, Waziri H, Møller JE, et al. The Prognostic Value of Left Atrial Peak Reservoir Strain in Acute Myocardial Infarction Is Dependent on Left Ventricular Longitudinal Function and Left Atrial Size. Circulation. Circ Cardiovasc Imaging. 2013;6(1):26–33.
Kim J, Yum B, Palumbo MC, Sultana R, Wright N, Das M, et al. Left Atrial Strain Impairment Precedes Geometric Remodeling as a Marker of Post-Myocardial Infarction Diastolic Dysfunction. JACC Cardiovasc Imaging. 2020;13(10):2099–113.
Inciardi RM, Claggett B, Minamisawa M, Shin S-H, Selvaraj S, Gonçalves A, et al. Association of Left Atrial Structure and Function With Heart Failure in Older Adults. J Am Coll Cardiol. 2022;79(16):1549–61.
Shin SH, Claggett B, Inciardi RM, Santos ABS, Shah SJ, Zile MR, et al. Prognostic Value of Minimal Left Atrial Volume in Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2021;10(15):e019545.
Oike F, Usuku H, Yamamoto E, Yamada T, Egashira K, Morioka M, et al. Prognostic value of left atrial strain in patients with wild-type transthyretin amyloid cardiomyopathy. ESC Heart Failure. 2021;8(6):5316–26.
Jasic-Szpak E, Marwick TH, Donal E, Przewlocka-Kosmala M, Huynh Q, Gozdzik A, et al. Prediction of AF in Heart Failure With Preserved Ejection Fraction: Incremental Value of Left Atrial Strain. JACC Cardiovasc Imaging. 2021;14(1):131–44.
Weber J, Bond K, Flanagan J, Passick M, Petillo F, Pollack S, et al. The Prognostic Value of Left Atrial Global Longitudinal Strain and Left Atrial Phasic Volumes in Patients Undergoing Transcatheter Valve Implantation for Severe Aortic Stenosis. Cardiology. 2021;146(4):489–500.
Nagueh SF, Khan SU. Left Atrial Strain for Assessment of Left Ventricular Diastolic Function: Focus on Populations With Normal LVEF. JACC Cardiovasc Imaging. 2023;16(5):691–707.
Patel RB, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, et al. Disproportionate left atrial myopathy in heart failure with preserved ejection fraction among participants of the PROMIS-HFpEF study. Sci Rep. 2021;11(1):4885.
Barberato SH, Romano MMD, Beck ALS, Rodrigues ACT, Almeida ALC, Assuncao B, et al. Position Statement on Indications of Echocardiography in Adults - 2019. Arq Bras Cardiol. 2019;113(1):135–81.
DeVore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail. 2017;19(7):893–900.
Appleton CP, Hatle LK. The Natural History of Left Ventricular Filling Abnormalities: Assessment by Two-Dimensional and Doppler Echocardiography. Echocardiography. 1992;9(4):437–57.
Fauchier L, Bisson A, Bodin A. Heart failure with preserved ejection fraction and atrial fibrillation: recent advances and open questions. BMC Med. 2023;21(1):54.
Shah S, Segar MW, Kondamudi N, Ayers C, Chandra A, Matulevicius S, et al. Supranormal Left Ventricular Ejection Fraction, Stroke Volume, and Cardiovascular Risk: Findings From Population-Based Cohort Studies. JACC Heart Fail. 2022;10(8):583–94.
Acknowledgements
None.
Funding
No.
Author information
Authors and Affiliations
Contributions
MS, GN, and RH wrote the main manuscript text. MS, NS prepared all figures and tables. HM, JA, FF. RH overall supervision All authors revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree with the publication of this article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shinzato, M.H., Santos, N., Nishida, G. et al. Left ventricular and atrial myocardial strain in heart failure with preserved ejection fraction: the evidence so far and prospects for phenotyping strategy. Cardiovasc Ultrasound 22, 4 (2024). https://doi.org/10.1186/s12947-024-00323-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12947-024-00323-1