Abstract
Background
Diabetes mellitus is generally accompanied by dyslipidaemia, but inconsistent relationships between lipid profiles and diabetes are noted. Moreover, genetic variations in insertion/deletion (I/D) polymorphisms at angiotensin-converting enzyme gene (ACE) and T/C polymorphisms in the angiotensin type 1 receptor gene (AGTR1) are related to diabetes and lipid levels, but the associations are controversial. Thus, the current research aimed to explore the effects of ACE I/D, AGTR1 rs5182 and diabetes mellitus on serum lipid profiles in 385 Chinese participants with an average age of 75.01 years.
Methods
The ACE I/D variant was identified using the polymerase chain reaction (PCR) method, whereas the AGTR1 rs5182 polymorphism was identified using the PCR-based restriction fragment length polymorphism (PCR-RFLP) method and verified with DNA sequencing. Total cholesterol (TC), triglyceride (TG), apolipoprotein A (ApoA), apolipoprotein B (ApoB), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) levels were measured using routine methods, and the lipid ratios were calculated.
Results
ACE I/D, but not AGTR1 rs5182, was a predictor of TG/HDL-C for the whole study population. Both ACE I/D and AGTR1 rs5182 were predictors of HDL-C and LDL-C levels in females but not in males. Moreover, in females, diabetes mellitus and ACE I/D were identified as predictors of TG and TG/HDL-C, whereas AGTR1 rs5182 and diabetes mellitus were predictors of TG/HDL-C. Moreover, diabetes mellitus and the combination of ACE I/D and AGTR1 rs5182 variations were predictors of TG and TG/HDL-C exclusively in females.
Conclusions
The results demonstrated the potential for gender-dependent interactions of ACE I/D, AGTR1 rs5182, and diabetes on lipid profiles. These findings may serve as an additional explanation for the inconsistent changes of blood lipids in individuals with diabetes mellitus, thereby offering a novel perspective for the clinical management of blood lipid levels in diabetic patients.
Similar content being viewed by others

Background
Diabetes mellitus has become an expanding public health concern globally, affecting more than 37% of individuals aged 65 years and older [1]. Moreover, diabetes mellitus is generally accompanied by dyslipidaemia [2], the typical features of which include elevated triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) levels as well as decreased high-density lipoprotein cholesterol (HDL-C) levels [3]. Elevated TG levels and reduced HDL-C levels are often accompanied by diabetes mellitus [4]. In addition, as a sensitive parameter reflecting blood lipid profiles, the TG-to-HDL-C ratio (TG/HDL-C) is significantly positively associated with diabetes mellitus [5]. However, inconsistent relationships between serum lipids and diabetes mellitus have also been reported. For example, TG is associated with a reduced risk of diabetes mellitus in patients who exhibit genetic susceptibility to elevated TG levels [6]. Similarly, the protective effect of HDL for diabetes was found only in Iranian women but not in Iranian men [7]. Thus, the critical effects of genetic background should be considered in the context of the complex relationship between lipid fractions and diabetes mellitus.
The renin‒angiotensin system (RAS), a complex hormonal regulatory system, is associated with not only blood pressure but also dyslipidaemia [8]. Interfering with the RAS using drugs has become a type of diabetes treatment [9]. In the RAS, angiotensin-converting enzyme (ACE) can convert angiotensin I to angiotensin II through the removal of carboxy-terminal dipeptides [8]. The significant effects of ACE on glycaemic disturbances suggest a correlation between ACE and diabetes mellitus [10], which was further confirmed by the utilization of ACE inhibitors to prevent diabetes mellitus [11]. The ACE gene (ACE), which is located on the long arm of chromosome 17 (17q23), is 21 kb long and consists of 26 exons and 25 introns [12]. The ACE I/D polymorphism is distinguished by a distinctive 287-base pair repetitive element located within intron 16, which gives rise to the variation of either an insertion or a deletion (I/D) within the genetic sequence [13]. This variation leads to 3 possible genotypes, including II, ID and DD [13], as well as a change in ACE concentration [14]. Previous studies have shown that ACE I/D is associated with diabetes mellitus [15], and II homozygotes had higher HDL-C levels than did subjects with the D allele [13, 16]. On the other hand, other studies noted that the association between the ACE I/D polymorphism and dyslipidaemia was insignificant in Chinese diabetic patients [17, 18]. Obviously, other factors should be considered to explain the conflicting findings regarding the influence of ACE I/D on lipid profiles in diabetic patients.
As the major biologically active hormone generated by the RAS system, angiotensin II regulates blood pressure via angiotensin type 1 receptor (AGTR1) [8, 19]. The human AGTR1 gene (AGTR1), which contains 5 exons and 4 introns, is located on the long arm of chromosome 3 (3q21-25) [20]. The rs5182 polymorphism (C573T) of AGTR1 alters AGTR1 expression [21] and is related to the presence of diabetes combined with hypertension in the Han population of Inner Mongolia [20]. The AGTR1 rs5182 variant is related to nonalcoholic fatty liver disease, which is often characterized by dyslipidaemia [22, 23]. However, a study conducted in another Chinese population failed to observe a significant association between AGTR1 rs5182 and dyslipidaemia [21]. Thus, the effects of ACE I/D and AGTR1 rs5182 on lipid profiles, as well as their interaction with diabetes mellitus, should be studied to obtain a better understanding about the influence of diabetes and genetic polymorphisms on lipids.
This study seeks to explore possible explanations for the controversial findings concerning the relationship between diabetes and dyslipidaemia reported in previous studies and to evaluate the cumulative effect of ACE I/D and AGTR1 rs5182 on lipid levels, which has not yet been reported. It is hypothesized that interactions potentially occur among ACE I/D variation, the AGTR1 rs5182 polymorphism and diabetes mellitus to impact serum lipid profiles in the current study. Therefore, ACE I/D variation, the AGTR1 rs5182 polymorphism and serum lipid levels were measured in 385 Chinese subjects with an average age of 75.01 years. The interactions between genetic backgrounds and diabetes mellitus, as well as their contributions to lipid profiles, were analysed. The investigation of the combined effects of ACE I/D and AGTR1 rs5182 on lipid profiles, as well as their interactions with diabetes mellitus in the current study, may contribute to preventing and managing dyslipidaemia in individuals with diabetes mellitus.
Methods
Study population
One thousand one hundred nineteen volunteers were enrolled, and the inclusion criteria for the participants were as follows: (1) understood the procedures involved and provided written consent; (2) had a history of diabetes; (3) provided current medication use status for antidiabetic and/or lipid-lowering drugs; (4) provided complete serum lipid and glucose measurements; (5) provided blood samples; and (6) were aged ≥ 50 years. In total, 385 of the participants (average age = 75.01 ± 24.90 years) who met the above criteria were involved in the present study. The study was approved by the Human Research Ethics Committee of Chongqing Orthopedic Hospital of Traditional Chinese Medicine.
Biochemical measurements
Venous blood samples were collected from the participants in the morning after a 12-hour fast. Serum was isolated via centrifugation (3000 rpm, 20 min) at 4 °C and stored at -80 °C for further analyses. Glucose levels were measured using the glucose oxidase‒peroxidase (GOD‒POD) method [24]. Serum TG levels were determined using the glycerol phosphate oxidase-p-aminophenazone (GPO-PAP) method [25]. TC, HDL-C and LDL-C levels were measured using the cholesterol oxidase-peroxidase and 4-aminoantipyrine phenol (CHOD-PAP) method [25]. Apolipoprotein A (ApoA) and apolipoprotein B (ApoB) levels were determined via immunoturbidimetry [26]. Ratios of TG/HDL-C, TC/HDL-C and LDL-C/HDL-C were calculated.
DNA extraction and genotyping
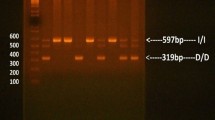
Genomic DNA was extracted using a DNA extraction kit per the manufacturer’s instructions (Kuang Yuan, Suzhou, China). The ACE I/D genotype was detected via the polymerase chain reaction (PCR) method, and the AGTR1 rs5182 variant was identified via the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method followed by confirmation using DNA sequencing. Briefly, for ACE I/D genotype detection, the target DNA fragments were amplified with the primers 5’-CTGGAGACCACTCCCATCCTTTCT-3’ (forward) and 5’-GATGTGGCCATCACATTCGTCAGA T-3’ (reverse) [13]. The samples were denatured at 94 °C for 4 min, followed by 32 cycles, which consisted of denaturation at 94 °C for 1 min, annealing at 56 °C for 1 min and extension at 72 °C for 90 s, with a final extension at 72 °C for 5 min. A 190-bp PCR fragment was produced in the absence of the insertion (D), and a 490-bp fragment was produced in the presence of the insertion (I). In the absence of the insertion (D), a PCR product of 190 bp was generated, whereas the presence of the insertion (I) resulted in the amplification of a 490-bp fragment. Moreover, for AGTR1 rs5182 genotype identification, two oligonucleotide primers, 5’-GGCTTTGCTTTGTCTTGTTG-3’ (forward) and 5’-AATGCTTGTAGCCAAAGTCACCT-3’ (reverse), were used for amplification [27]. The PCR procedure consisted of 3 min at 94 °C for denaturation; 40 cycles of 30 s at 94 °C, 30 s at 60 °C, and 90 s at 72 °C; and a final elongation step of 5 min at 72 °C [27]. The PCR-amplified products were digested overnight with the restriction endonuclease MnII, which cuts at position 580, when the C allele is present instead of the T allele at 573, and at positions 905, 1032, 1062, and 1147. The PCR-amplified products of ACE I/D and the restriction endonuclease MnII-digested products of AGTR1 rs5182 were identified using 1.5% agarose gel electrophoresis and verified with DNA sequencing.
Dummy variable coding
Owing to the limited number, the minor allele homozygotes were combined with their heterozygotes and defined as D allele carriers of ACE I/D and C allele carriers of AGTR1 rs5182 for further analysis. To further clarify the cumulative effects of ACE I/D and AGTR1 rs5182 on lipid levels, the combined genotypes of ACE I/D and AGTR1 rs5182 were used as dummy variables:
Statistical analyses
The data are expressed as the means ± standard deviations (SDs) unless otherwise specified. The sample size calculation was conducted using the G*Power software program (version 3.1.9.7, Germany) [28], and the current sample size was sufficient for a minimum power of 80%. The deviation from Hardy‒Weinberg equilibrium [29], which can estimate the number of homozygous and heterozygous variant vectors in an unevolved population [30], was analysed with the χ2 goodness-of-fit test. The chi-square test was used to determine the distribution of genotypes and alleles, the prevalence of diabetes and the percentage of drug use between subjects of different genders. The normal distribution of each variable was initially analysed using the Kolmogorov-Smirnov test. Because of the abnormal distribution, logarithmic transformations were applied to TG and the TG/HDL-C ratio to reduce skewness before performing the statistical analyses. Independent sample t tests were conducted to compare the differences in blood lipid levels between the males and the females. Potential factors associated with blood lipid and blood glucose levels were analysed using the stepwise multiple linear regression analysis. Statistical significance was defined as P < 0.05.
Results
Characteristics of the study population
Table 1 shows the characteristics of the metabolic variables, prevalence of diabetes, and drug usage in the current study population. As displayed in Table 1, TG, TC, HDL-C, ApoA, LDL-C, and ApoB levels as well as the prevalence of diabetes were significantly greater in females than in males (P = 0.017, p = 0.016, P = 0.049, P = 0.002, P = 0.021, P = 0.022, and P = 0.045, respectively). However, no statistically significant differences in age or glucose were noted between the genders. When considering the use of lipid-lowering drugs and treatment with antidiabetic drugs, no significant difference was found between male and female subjects.
Genotypes and alleles of ACE I/D and AGTR1 rs5182 in the participants
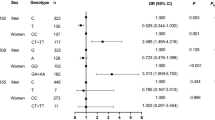
The identification of ACE I/D and AGTR1 rs5182 using gel electrophoresis, followed by confirmation via DNA sequencing, is shown in Fig. 1. The frequencies of genotypes and alleles are presented in Fig. 2. The genotype frequencies of either ACE I/D or AGTR1 rs5182 were in Hardy‒Weinberg equilibrium in the current study (P = 0.820 and P = 0.480, respectively). No statistically significant differences in the genotype frequencies of the ACE I/D genotype and AGTR1 rs5182 genotype were noted between male and female subjects. However, the D allele of ACE I/D and C allele of AGTR1 rs5182 exhibited significantly greater frequencies in males compared with females. Owing to the limited numbers of samples, the minor allele homozygotes were combined with their heterozygotes and defined as D allele carriers of ACE I/D and C allele carriers of AGTR1 rs5182, respectively, for further analysis.
Gel images of ACE I/D and AGTR1 rs5182 genotyping and sequencing results. (A) Gel images of ACE I/D and rs5182 genotyping: (1) gel images of ACE I/D genotyping, M: DNA ladder, 1: blank, 2/3: DD, 4/5: II, 6/7: ID; (2) gel images of AGTR1 rs5182 genotyping, M: DNA ladder, 1: blank, 2/3: TT, 4/5: CC, 6/7: TC. (B) Sequencing results of the ACE I/D (1) DD genotype, (2) ID genotype, and (3) II genotype. (C) Sequencing results for AGTR1 rs5182: (1) TT genotype, (2) TC genotype, (3) CC genotype
Predictors of lipid levels in the subjects
To further explore the predictors of lipid profiles in the current study population, stepwise multiple linear regression analyses were performed. The ACE I/D genotype (Model A) or AGTR1 rs5182 genotype (Model B) was used as an independent variable separately or in combination with age, gender, diabetes mellitus, and the use of antidiabetic drugs or lipid-lowering drugs as other independent variables.
As shown in Table 2, gender was the only predictor of TG, TC, and ApoB levels, explaining 1.2%, 1.5%, and 1.4% of the total variance, respectively. Gender, antidiabetic drug use, and lipid-lowering drug use were predictors of HDL-C levels, accounting for 2.7%, 1.1% and 0.8% of the total variance, respectively. In addition, gender, the use of antidiabetic drugs, lipid-lowering drugs and diabetes mellitus were predictors of ApoA levels, accounting for 2.3%, 3.2%, 2.3%, and 0.9% of the total variance, respectively. Moreover, gender, the use of antidiabetic drugs, and diabetes mellitus were predictors of LDL-C levels, accounting for 0.9%, 1.3% and 1.2% of the total variance, respectively. The use of antidiabetic drugs, diabetes mellitus status and age were predictors of glucose levels, accounting for 12.3%, 2.8% and 2.3% of the total variance, respectively. Notably, ACE I/D and the use of antidiabetic drugs were predictors of the TG/HDL-C ratio, accounting for 1.0% and 1.6% of the total variance, respectively. However, AGTR1 rs5182 was not a predictor of TG/HDL-C.
When gender was taken into consideration in the ACE I/D analysis, as displayed in Table 3, the use of antidiabetic drugs, the use of lipid-lowering drugs and diabetes mellitus were predictors of HDL-C (accounting for 2.4%, 2% and 2.8% of the total variance, respectively) and ApoA (accounting for 4.3%, 3.4%, and 3.6% of the total variance, respectively) levels in males. The use of antidiabetic drugs and diabetes mellitus were predictors of LDL-C levels, contributing 2.7% and 1.6% of the total variance, respectively, whereas the use of antidiabetic drug was a predictor of ApoB levels in males, contributing 2.4% of the total variance. Furthermore, the use of antidiabetic drugs, diabetes mellitus, and age were predictors of glucose levels in males, contributing to 12.3%, 2.1% and 2.3% of the total variance, respectively. Although ACE I/D was not a predictor of any lipid profile in males, notably, in female subjects, ACE I/D and diabetes mellitus were predictors of TG (accounting for 3.7% and 3.5% of the total variance, respectively) and TG/HDL-C (accounting for 4.3% and 3.9% of the total variance, respectively). Moreover, age was the only predictor of ApoA levels, and ACE I/D was the only predictor of LDL-C levels in females, accounting for 3.6% and 4.4% of the total variance, respectively. In terms of HDL-C levels in females, ACE I/D, age and the use of antidiabetic drugs were predictors, accounting for 2.9%, 2.7% and 2.3% of the total variance, respectively. The predictors of glucose in females were the same as those in identified males, including the use of antidiabetic drugs, diabetes mellitus, and age, contributing to 2.8%, 14% and 1.9% of the total variance, respectively.
The results of the analysis of the effects of AGTR1 rs5182 in males and females are shown in Table 4. The predictors of HDL-C, ApoA, LDL-C, ApoB, and glucose levels, as well as the contribution of each factor to the total variance in males, were the same as those identified in the ACE I/D analysis (Table 3). Similarly, the predictors of glucose levels and their contributions to the total variance in females were the same as those identified in the ACE I/D analysis (Table 3). Nevertheless, in female subjects, diabetes mellitus was the only predictor of TG levels, whereas age was the only predictor of ApoA levels, accounting for 3.5% and 3.6%, respectively, of the total variance. Interestingly, AGTR1 rs5182, age and the use of antidiabetic drugs were predictors of HDL-C levels in females, accounting for 2.9%, 2.3%, and 2.2%, of the total variance, respectively. The AGTR1 rs5182 was the only predictor of LDL-C levels in females, accounting for 2.0% of the total variance. Furthermore, AGTR1 rs5182 and diabetes mellitus were predictors of TG/HDL-C, accounting for 2.4% and 3.9% of the total variance, respectively.
ACE I/D and AGTR1 rs5182 combination analysis in the subjects
To further investigate the combined influence of the ACE I/D polymorphism and the AGTR1 rs5182 variant on lipid profiles, stepwise multiple linear regression analyses were performed. Dummy variables 1 (ACE II + AGTR1 rs5182 TT), 2 (ACE II + AGTR1 rs5182 C allele), and 4 (ACE D allele + AGTR1 rs5182 C allele) were incorporated as independent variables, whereas dummy variable 3 (ACE D allele + AGTR1 rs5182 TT) served as the reference category. Additionally, age, gender, the prevalence of diabetes mellitus, and the use of antidiabetic drugs or lipid-lowering drugs were included as other independent variables to estimate their individual predictive value for lipid levels, lipid ratios and glucose levels.
In the whole study population, as shown in Table 5, although the predictors of TG, TC, HDL-C, ApoA, ApoB, LDL-C, and glucose levels, as well as the contribution of each factor to the total variance, were the same as those in Table 2, it is interesting to note that dummy variable 1 and the use of antidiabetic drugs, accounting for 0.8% and 1.6% of the total variance, respectively, were predictors of TG/HDL-C.
When gender was taken into consideration, as displayed in Table 6, dummy variable 4 was the only predictor of TG levels in males, accounting for 3.8% of the total variance. Dummy variable 4 and diabetes mellitus were identified as predictors of TG levels in females, accounting for 3.8% and 3.7% of the total variance, respectively. None of the dummy variables were predictors of TC, HDL-C, ApoA, LDL-C, ApoB, TG/HDL-C, TC/HDL-C, LDL-C/HDL-C or glucose levels in males. In contrast, dummy variable 1 was the only predictor of TC (accounting for 5.8%), ApoB (accounting for 2.4%) and LDL-C (accounting for 7.7%) levels, whereas dummy variable 1 and age were predictors of ApoA (accounting for 3.3% and 3.6%, respectively) in females. Moreover, the predictors of HDL-C in females included dummy variable 1, age, and the use of antidiabetic drugs, accounting for 8.4%, 3.2%, and 2.4% of the total variance, respectively. Dummy variable 1, dummy variable 4, and diabetes mellitus were predictors of TG/HDL-C in female subjects and accounted for 5%, 1.9%, and 4% of the total variance, respectively.
Discussion
Diabetes mellitus is often accompanied by dyslipidaemia [5]. However, inconsistencies in the relationship between diabetes mellitus and dyslipidaemia have been reported [6, 31], and the mechanism of the observed discrepancy remains unclear. Moreover, the RAS has been reported as a critical system in controlling blood pressure. For example, ACE activity has been reported to be involved in changes in the retinoic acid receptor (RAR)/retinoid X receptor (RXR)-peroxisome proliferator-activated receptor (PPAR) signalling pathway and the suppression of cellular retinol-binding protein 1 (CRBP1), ultimately affecting adipocyte homeostasis and blood lipids [32, 33]. Moreover, AGTR1 activation is related to lipid accumulation in both the livers of C57BL/6 mice and in HepG2 cells [34, 35]. Thus, ACE and AGTR1 could be involved in diabetes and dyslipidaemia. The ACE I/D polymorphism and AGTR1 rs5182 polymorphism are associated with lipid levels, but the findings are also contradictory [21, 22]. Therefore, investigating the effects of the interactions of the ACE I/D and AGTR1 rs5182 polymorphisms with diabetes and the subsequent effects on lipid profiles has the potential to elucidate the possible mechanism underlying the inconsistency among genetic variations, diabetes and lipid levels.
In the present study, diabetes mellitus was a predictor of ApoA and LDL-C levels in the whole study population but not a predictor of other lipid profiles or ratios. Moreover, although diabetes mellitus was identified as a predictor of HDL-C, ApoA, LDL-C and glucose, neither ACE I/D nor AGTR1 rs5182 contributed to changes in lipid and lipid ratios in males (Tables 2, 3 and 4). In contrast, both ACE I/D and AGTR1 rs5182 were identified as predictors of HDL-C and LDL-C exclusively in females (Tables 3 and 4). Furthermore, in the present study, ACE I/D, not AGTR1 rs5182, was identified as a predictor of the TG/HDL-C ratio (Table 2), which might be attributable to the increased expression levels and increased activity of ACE in DD homozygotes [36, 37]. In female subjects, diabetes mellitus and ACE I/D were predictors of TG and TG/HDL-C levels (Table 3), whereas diabetes mellitus and AGTR1 rs5182 were predictors of TG/HDL-C ratios (Table 4). Thus, the interactions among ACE I/D variation or the AGTR1 rs5182 polymorphism with gender and diabetes mellitus are likely involved in the heterogeneous relationships between TG metabolism and diabetes.
There are limited data from previous studies concerning the interplay of ACE I/D and AGTR1 rs5182, as well as their associations with diabetes mellitus in terms of lipid profiles in Chinese subjects. These results revealed that the combination of ACE I/D and AGTR1 rs5182 contributed to the TG/HDL-C ratio in the whole study population; TG levels in males; and TC, ApoB, LDL-C, ApoA and HDL-C levels in females (Tables 5 and 6). The association of ACE I/D with lipid levels has been inconsistently reported [38, 39]. In addition, a significant correlation was observed between the AGTR1 rs5186 variant and TG levels, but the mechanism has not yet been fully elucidated [40, 41]. Therefore, the combined effect of ACE I/D and AGTR1 rs5182 on lipid levels in the current study provides valuable insights into the intricate relationships between the RAS and dyslipidaemia.
Previous studies have shown an association between gender and blood lipids. For example, adult females had lower LDL-C levels and higher HDL-C levels compared with adult males [42]. Moreover, the prevalence of TC, TG, and LDL-C at borderline high or greater levels increased with age in females, but it remained stable or even decreased in males [42]. Although diabetes is often accompanied by hyperlipidaemia [2], TG is associated with a decreased risk of diabetes when increased genetic susceptibility is considered [6]. As a potential predictive marker of insulin resistance (IR), the TG/HDL-C ratio is related to diabetes mellitus [5]. However, a previous study reported that no differences in TG/HDL-C were found between obese normal glucose-tolerant individuals and patients with type 2 diabetes [43]. Interestingly, the combination of genetic variations and diabetes mellitus was demonstrated to be a predictor of TG and TG/HDL-C only in the female subjects (Table 6). Thus, the gender-dependent associations with the combination of genetic variations found in the present study potentially provide an additional explanation for the inconsistent changes in TG and other indicators related to diabetes mellitus and provide new ideas to target blood lipids clinically.
Strengths and limitations
The current study is the first to assess the correlations between the ACE I/D variant and the AGTR1 rs5182 polymorphism both independently and synergistically with diabetes mellitus in a Chinese elderly population. A potential limitation of the current study was that serum ACE levels were not detected. However, the combination of different genetic variations was taken into consideration. Moreover, analysis of the younger Chinese population with diabetes is highly recommended in future studies because of the elevated prevalence of diabetes in elderly subjects [44]. Additionally, given the diverse effects of different antidiabetic and lipid-lowering drugs on metabolism [45], the identification of the associations among specific medications, diabetes and genetic backgrounds in China will be valuable.
Conclusions
The findings of the present study suggest potential interactions among gender, ACE I/D, AGTR1 rs5182 and diabetes mellitus in terms of lipid and lipid ratios, especially in terms of TG levels and the TG/HDL-C ratio. This information provides possible explanations for the contradictory associations between diabetes mellitus and lipid metabolism. Furthermore, in elderly Chinese females, TG and TG/HDL-C levels might be more susceptible to the cumulative effect of ACE I/D and AGTR1 rs5182 as well as their combined effect with diabetes. Such an understanding may suggest the development of personalized treatments based on ACE and AGTR1 genetic polymorphisms to lower elevated TG levels in elderly diabetic female patients that have the potential to normalize the dyslipidaemia induced by diabetes mellitus.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- AGTR1:
-
Angiotensin type 1 receptor
- ACE:
-
Angiotensin converting enzyme gene
- AGTR1:
-
Angiotensin type 1 receptor gene
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- ApoA:
-
Apolipoprotein A
- ApoB:
-
Apolipoprotein B
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- RAS:
-
Renin-Angiotensin System
- SDs:
-
Standard deviations
- RAR:
-
Retinoic acid receptor
- RXR:
-
Retinoid X receptor
- PPAR:
-
Peroxisome proliferator-activated receptor
- CRBP1:
-
Cellular retinol-binding protein 1
References
Ning G, Bloomgarden Z. Diabetes in China: prevalence, diagnosis, and control. J Diabetes. 2013;5(4):372. https://doi.org/10.1111/1753-0407.12088.
Kaze AD, Santhanam P, Musani SK, Ahima R, Echouffo-Tcheugui JB. Metabolic Dyslipidemia and Cardiovascular outcomes in type 2 diabetes Mellitus: findings from the look AHEAD study. J Am Heart Assoc. 2021;10(7):e016947. https://doi.org/10.1161/jaha.120.016947.
Berberich AJ, Hegele RA. A Modern Approach to Dyslipidemia. Endocr Rev. 2022;43(4):611–53. https://doi.org/10.1210/endrev/bnab037.
Huang JX, Liao YF, Li YM. Clinical features and microvascular complications risk factors of early-onset type 2 diabetes Mellitus. Curr Med Sci. 2019;39(5):754–8. https://doi.org/10.1007/s11596-019-2102-7.
Yang T, Liu Y, Li L, Zheng Y, Wang Y, Su J, et al. Correlation between the triglyceride-to-high-density lipoprotein cholesterol ratio and other unconventional lipid parameters with the risk of prediabetes and type 2 diabetes in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):93. https://doi.org/10.1186/s12933-022-01531-7.
Ahmad S, Mora S, Ridker PM, Hu FB, Chasman DI. Gene-based elevated triglycerides and type 2 diabetes Mellitus Risk in the women’s Genome Health Study. Arterioscler Thromb Vasc Biol. 2019;39(1):97–106. https://doi.org/10.1161/atvbaha.118.311562.
Sun T, Chen J, Yang F, Zhang G, Chen J, Wang X, et al. Lipidomics reveals new lipid-based lung adenocarcinoma early diagnosis model. EMBO Mol Med. 2024;16(4):854–69. https://doi.org/10.1038/s44321-024-00052-y.
Vargas Vargas RA, Varela Millán JM, Fajardo Bonilla E. Renin-angiotensin system: Basic and clinical aspects-A general perspective. Endocrinol Diabetes Nutr (Engl Ed). 2022;69(1):52–62. https://doi.org/10.1016/j.endien.2022.01.005.
Rein J, Bader M. Renin-angiotensin system in diabetes. Protein Pept Lett. 2017;24(9):833–40. https://doi.org/10.2174/0929866524666170728144357.
Jhawat V, Gupta S, Agarwal BK, Roy P, Saini V. Angiotensin converting enzyme gene Insertion/Deletion polymorphism is not responsible for antihypertensive therapy Induced New Onset of type 2 diabetes in essential hypertension. Clin Med Insights Endocrinol Diabetes. 2019;12:1179551418825037. https://doi.org/10.1177/1179551418825037.
McCall KL, Craddock D, Edwards K. Effect of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers on the rate of new-onset diabetes mellitus: a review and pooled analysis. Pharmacotherapy. 2006;26(9):1297–306. https://doi.org/10.1592/phco.26.9.1297.
Król-Kulikowska M, Abramenko N, Jakubek M, Banasik M, Kepinska M. The role of angiotensin-converting enzyme (ACE) polymorphisms in the risk of Development and Treatment of Diabetic Nephropathy. J Clin Med. 2024;13(4):995. https://doi.org/10.3390/jcm13040995.
Kong LN, Shen YL, Chen YL, Chen X, Su GM, Wang JH, et al. Insertion/deletion polymorphism at angiotensin-converting enzyme gene in PTSD individuals and their reciprocal effects on blood pressure. Clin Exp Hypertens. 2022;44(3):208–14. https://doi.org/10.1080/10641963.2021.2018598.
Hu X, Zou L, Wang S, Zeng T, Li P, Shen Y, et al. Performance of serum angiotensin-converting enzyme in diagnosing Sarcoidosis and Predicting the active status of sarcoidosis: a Meta-analysis. Biomolecules. 2022;12(10):1400. https://doi.org/10.3390/biom12101400.
Hemeed RN, Al-Tu’ma FJ, Al-Koofee DAF, Al-Mayali AH. Relationship of angiotensin converting enzyme (I/D) polymorphism (rs4646994) and coronary heart disease among a male Iraqi population with type 2 diabetes mellitus. J Diabetes Metab Disord. 2020;19(2):1227–32. https://doi.org/10.1007/s40200-020-00632-y.
Erbas T, Cinar N, Dagdelen S, Gedik A, Yorgun H, Canpolat U, et al. Association between ACE and AGT polymorphism and cardiovascular risk in acromegalic patients. Pituitary. 2017;20(5):569–77. https://doi.org/10.1007/s11102-017-0819-5.
Pan YH, Wang M, Huang YM, Wang YH, Chen YL, Geng LJ, et al. ACE gene I/D polymorphism and obesity in 1,574 patients with type 2 diabetes Mellitus. Dis Markers. 2016;2016:7420540. https://doi.org/10.1155/2016/7420540.
Khamlaoui W, Mehri S, Hammami S, Elosua R, Hammami M. Association of angiotensin-converting enzyme insertion/deletion (ACE I/D) and angiotensinogen (AGT M235T) polymorphisms with the risk of obesity in a Tunisian population. J Renin Angiotensin Aldosterone Syst. 2020;21(2):1470320320907820. https://doi.org/10.1177/1470320320907820.
Paz Ocaranza M, Riquelme JA, García L, Jalil JE, Chiong M, Santos RAS, et al. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17(2):116–29. https://doi.org/10.1038/s41569-019-0244-8.
Hou L, Quan X, Li X, Su X. Correlation between gene polymorphism in angiotensin II type 1 receptor and type 2 diabetes mellitus complicated by hypertension in a population of Inner Mongolia. BMC Med Genet. 2020;21(1):83. https://doi.org/10.1186/s12881-020-01021-1.
Zuo S, Xiong J, Wei Y, Chen D, Chen F, Liu K, et al. Potential interactions between genetic polymorphisms of the transforming growth Factor-β pathway and environmental factors in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;50(1):71–7. https://doi.org/10.1016/j.ejvs.2015.04.010.
Zhu P, Lu H, Jing Y, Zhou H, Ding Y, Wang J, et al. Interaction between AGTR1 and PPARγ gene polymorphisms on the risk of nonalcoholic fatty liver disease. Genet Test Mol Biomarkers. 2019;23(3):166–75. https://doi.org/10.1089/gtmb.2018.0203.
Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75(18):3313–27. https://doi.org/10.1007/s00018-018-2860-6.
Ichimura S, Kakita H, Asai S, Mori M, Takeshita S, Ueda H, et al. Acetaminophen elevates unbound bilirubin levels by the glucose oxidase-peroxidase method. Pediatr Int. 2021;63(9):1069–74. https://doi.org/10.1111/ped.14608.
Li J, Yuan H, Zhao Z, Li L, Li X, Zhu L, et al. The mitigative effect of isorhamnetin against type 2 diabetes via gut microbiota regulation in mice. Front Nutr. 2022;9:1070908. https://doi.org/10.3389/fnut.2022.1070908.
Zeng M, Liu Y, Xie Y. Association between ALDH2 polymorphisms and the risk of diabetes Mellitus in Hypertensive patients. Int J Gen Med. 2023;16:5719–27. https://doi.org/10.2147/ijgm.S435598.
Chaves FJ, Pascual JM, Rovira E, Armengod ME, Redon J. Angiotensin II AT1 receptor gene polymorphism and microalbuminuria in essential hypertension. Am J Hypertens. 2001;14(4 Pt 1):364–70. https://doi.org/10.1016/s0895-7061(00)01284-x.
Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. https://doi.org/10.3352/jeehp.2021.18.17.
Kässens JC, Wienbrandt L, Ellinghaus D, BIGwas. Single-command quality control and association testing for multi-cohort and biobank-scale GWAS/PheWAS data. Gigascience. 2021;10(6). https://doi.org/10.1093/gigascience/giab047.
Abramovs N, Brass A, Tassabehji M. Hardy-Weinberg equilibrium in the large scale genomic sequencing era. Front Genet. 2020;11:210. https://doi.org/10.3389/fgene.2020.00210.
Yuge H, Okada H, Hamaguchi M, Kurogi K, Murata H, Ito M, et al. Triglycerides/HDL cholesterol ratio and type 2 diabetes incidence: Panasonic Cohort Study 10. Cardiovasc Diabetol. 2023;22(1):308. https://doi.org/10.1186/s12933-023-02046-5.
Kim JY, Barua S, Jeong YJ, Lee JE, Adiponectin. The potential Regulator and therapeutic target of obesity and Alzheimer’s Disease. Int J Mol Sci. 2020;21(17):6419. https://doi.org/10.3390/ijms21176419.
Klobučar I, Habisch H, Klobučar L, Trbušić M, Pregartner G, Berghold A, et al. Serum levels of Adiponectin are strongly Associated with Lipoprotein subclasses in healthy volunteers but not in patients with metabolic syndrome. Int J Mol Sci. 2024;25(9):5050. https://doi.org/10.3390/ijms25095050.
Wu Y, Ma KL, Zhang Y, Wen Y, Wang GH, Hu ZB, et al. Lipid disorder and intrahepatic renin-angiotensin system activation synergistically contribute to non-alcoholic fatty liver disease. Liver Int. 2016;36(10):1525–34. https://doi.org/10.1111/liv.13131.
Trejo-Moreno C, Alvarado-Ojeda ZA, Méndez-Martínez M, Cruz-Muñoz ME, Castro-Martínez G, Arrellín-Rosas G, et al. Aqueous fraction from Cucumis sativus Aerial Parts attenuates Angiotensin II-Induced endothelial dysfunction in vivo by activating Akt. Nutrients. 2023;15(21):4680. https://doi.org/10.3390/nu15214680.
Singh A, Srivastava N, Amit S, Prasad SN, Misra MP, Ateeq B. Association of AGTR1 (A1166C) and ACE (I/D) polymorphisms with breast Cancer Risk in North Indian Population. Transl Oncol. 2018;11(2):233–42. https://doi.org/10.1016/j.tranon.2017.12.007.
Atiku SM, Kasozi D, Campbell K. Single nucleotide variants (SNVs) of angiotensin-converting enzymes (ACE1 and ACE2): a plausible explanation for the global variation in COVID-19 prevalence. J Renin Angiotensin Aldosterone Syst. 2023;2023:9668008. https://doi.org/10.1155/2023/9668008.
Rajkumari S, Ningombam SS, Chhungi V, Newmei MK, Devi NK, Mondal PR, et al. Association of ACE I/D gene polymorphism and related risk factors in impaired fasting glucose and type 2 diabetes: a study among two tribal populations of North-East India. Mol Biol Rep. 2022;49(2):1037–44. https://doi.org/10.1007/s11033-021-06924-7.
Amara A, Mrad M, Sayeh A, Lahideb D, Layouni S, Haggui A, et al. The effect of ACE I/D polymorphisms alone and with concomitant risk factors on coronary artery disease. Clin Appl Thromb Hemost. 2018;24(1):157–63. https://doi.org/10.1177/1076029616679505.
Li L, Momma H, Chen H, Nawrin SS, Xu Y, Inada H, et al. Dietary patterns associated with the incidence of hypertension among adult Japanese males: application of machine learning to a cohort study. Eur J Nutr. 2024;63(4):1293–314. https://doi.org/10.1007/s00394-024-03342-w.
Musso G, Saba F, Cassader M, Paschetta E, De Michieli F, Pinach S, et al. Angiotensin II type 1 receptor rs5186 gene variant predicts Incident NAFLD and Associated Hypertension: role of Dietary Fat-Induced pro-inflammatory cell activation. Am J Gastroenterol. 2019;114(4):607–19. https://doi.org/10.14309/ajg.0000000000000154.
Holven KB, van Roeters J. Sex differences in lipids: a life course approach. Atherosclerosis. 2023;384:117270. https://doi.org/10.1016/j.atherosclerosis.2023.117270.
Baspinar O, Kocer D, Kiraz A, Tokmak TT, Dizdar OS. Osteopontin as an early predictor of atherosclerosis in attack-free Familial Mediterranean fever patients. Med (Baltim). 2023;102(39):e35137. https://doi.org/10.1097/md.0000000000035137.
Lin LJJ, Saliba B, Adams J, Peng W. Prevalence and risk factors contributing to the occurrence of diabetes mellitus in Chinese international migrants: a narrative review. Diabetes Res Clin Pract. 2023;197:110560. https://doi.org/10.1016/j.diabres.2023.110560.
Heurtebize MA, Faillie JL. Drug-induced hyperglycemia and diabetes. Therapie. 2024;79(2):221–38. https://doi.org/10.1016/j.therap.2023.09.010.
Acknowledgements
This work was supported by the Postdoctoral Research and Development Foundation of Sichuan University[20826041G4039]. Qi Wei Guo is the recipient of the grant.
Funding
This work was supported by the Postdoctoral Research and Development Foundation of Sichuan University[20826041G4039].
Author information
Authors and Affiliations
Contributions
J.L., Q.W.G., D.Z.F. and H.H. were responsible for conception, study design, data interpretation and manuscript finalization. J.Y L. was responsible for lab experiments, statistical analysis and manuscript preparation. Y.Z.Y. was involved in evolving the ideas and revising the manuscript. K.X.J., X.C.L., J.J.C. and Y.L.S. were involved in lab experiments and statistical analysis. G.M.S., X. C. and X.Y.Z. participated in performing the experiments and drafting the manuscript. All authors have contributed to and have approved the final manuscript.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Human Research Ethics Committee of Chongqing Orthopedic Hospital of Traditional Chinese Medicine (approval number: 202103) and informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J.Y., Yi, Y.Z., Guo, Q.W. et al. Associations of ACE I/D and AGTR1 rs5182 polymorphisms with diabetes and their effects on lipids in an elderly Chinese population. Lipids Health Dis 23, 231 (2024). https://doi.org/10.1186/s12944-024-02222-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02222-w