Abstract
Background
Lipids, including phospholipids and bile acids, exert various signaling effects and are thought to contribute to the development of coronary artery disease (CAD). Here, we aimed to compare lipidomic and bile acid profiles in the blood of patients with and without CAD stratified by sex.
Methods
From 2015 to 2022, 3,012 patients who underwent coronary angiography were recruited in the INTERCATH cohort. From the overall cohort, subgroups were defined using patient characteristics such as CAD vs. no CAD, 1st vs. 3rd tertile of LDL-c, and female vs. male sex. Hereafter, a matching algorithm based on age, BMI, hypertension status, diabetes mellitus status, smoking status, the Mediterranean diet score, and the intake of statins, triglycerides, HDL-c and hs-CRP in a 1:1 ratio was implemented. Lipidomic analyses of stored blood samples using the Lipidyzer platform (SCIEX) and bile acid analysis using liquid chromatography with tandem mass spectrometry (LC‒MS/MS) were carried out.
Results
A total of 177 matched individuals were analyzed; the median ages were 73.5 years (25th and 75th percentile: 64.1, 78.2) and 71.9 years (65.7, 77.2) for females and males with CAD, respectively, and 67.6 years (58.3, 75.3) and 69.2 years (59.8, 76.8) for females and males without CAD, respectively. Further baseline characteristics, including cardiovascular risk factors, were balanced between the groups. Women with CAD had decreased levels of phosphatidylcholine and diacylglycerol, while no differences in bile acid profiles were detected in comparison to those of female patients without CAD. In contrast, in male patients with CAD, decreased concentrations of the secondary bile acid species glycolithocholic and lithocholic acid, as well as altered levels of specific lipids, were detected compared to those in males without CAD. Notably, male patients with low LDL-c and CAD had significantly greater concentrations of various phospholipid species, particularly plasmalogens, compared to those in high LDL-c subgroup.
Conclusions
We present hypothesis-generating data on sex-specific lipidomic patterns and bile acid profiles in CAD patients. The data suggest that altered lipid and bile acid composition might contribute to CAD development and/or progression, helping to understand the different disease trajectories of CAD in women and men.
Registration
https://clinicaltrials.gov/ct2/show/NCT04936438, Unique identifier: NCT04936438.
Similar content being viewed by others
Introduction
Atherosclerotic cardiovascular disease (ASCVD) and its manifestation within the coronary wall leading to coronary artery disease (CAD) are the main causes of death in Europe [1]. With regard to the pathophysiological evolution of CAD, low-density lipoprotein cholesterol (LDL-c) and other lipoproteins have been proven to lead to the development of atherosclerosis [2, 3]. The quantification of lipoproteins such as LDL-c and further ApoB-containing lipoproteins can predict ASCVD. In addition, changes in the lipidome, including lipid species such as sterol esters and phospho- and sphingolipids, are associated with the clinical development, presentation and outcome of CAD [4,5,6,7]. Quantifying the concentration of various lipids by employing mass spectrometry-based lipidomic analyses provides important insights into the diversity and composition of lipids and lipoproteins [8, 9].
Furthermore, rapidly increasing evidence suggests that bile acids (BAs) in combination with atherogenic lipoproteins are potential mediators of ASCVD, including coronary atherosclerosis [10, 11]. More recently, BAs were found to act as hormones mediating the development of ASCVD by signaling through G protein-coupled and nuclear receptors such as FXR, TGR5 and S1PR2, thereby demonstrating their role in health and disease [12].
Although some cardiovascular risk factors are similar for both female and male patients, sex-associated differences in the progression of CAD remain poorly understood [13,14,15]. We therefore aimed to investigate sex differences in the lipidome and BA profiles in a contemporary cohort.
Methods
Cohort definition and inclusion and exclusion criteria
The INTERCATH cohort is an observational single-center cohort of patients undergoing coronary angiography at the University Heart and Vascular Center Hamburg-Eppendorf, Germany, and has previously been described in detail [16]. In brief, all patients who underwent coronary angiography at our center were screened for inclusion, and a total of 3,012 individuals were recruited from 2015 to 2021. The study was performed in accordance with the Declaration of Helsinki, and an ethics vote was obtained from the local ethics committee (PV4303, Hamburg, Germany). Moreover, written informed consent was acquired from all participants. The study design and rationale are available at ClinicalTrials.gov (NCT04936438).
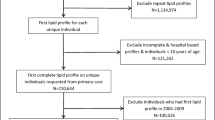
For current analyses, a subgroup of patients was selected via the use of a propensity score matching (PSM) algorithm. Details regarding PSM can be found in the statistical analysis section. Patients with myocardial infarction at the time of presentation, previous coronary artery bypass graft surgery, a history of malignancy, intake of any lipid-lowering medication other than statins, cardiac transplantation, or diabetes mellitus type I were excluded. In total, 192 patients were matched. From this cohort, 15 patients were removed from further analysis due to missing blood samples (n = 6), prevalent manifest hepatic diseases (n = 8), and/or misclassification of CAD (n = 1), leaving 177 patients for analysis (the study flow-chart is displayed in Figure S1).
Assessment of clinical parameters and baseline risk factors
We defined cardiovascular risk factors as follows: age, male sex, diabetes mellitus type II (self-reported/documented), current smoking status (self-reported), body mass index (BMI), and arterial hypertension status (self-reported/documented hypertension or self-reported intake of antihypertensive drugs). The diagnoses were based on patient charts, whereas smoking status was assessed by a standardized questionnaire. The use of statins was either self-reported or documented in patient charts. The established Mediterranean diet score (MDS) was derived as described previously (questionnaire-based assessment of consumption of six food groups and alcohol), aiming to quantify adherence to a Mediterranean diet [17]. Briefly, a score ranging from 0 to 28 points was calculated, where 0 points reflects nonadherence, and 28 points reflects the highest adherence. An MDS of < 12 (failure of MDS) has been proven to be predictive of severity and prognosis for patients with chronic coronary syndromes [21].
Laboratory measurements and coronary angiography
Blood samples were drawn before coronary angiography. Total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-c), LDL-c (measured using the Friedewald formula) and high-sensitivity C-reactive protein (hs-CRP) were determined using standard laboratory measures within clinical practice. Using stored blood samples, high-sensitivity troponin I (hsTnI) concentrations were quantified at our biomarker laboratory utilizing a commercially available immunoassay from Abbott Diagnostics (ARCHITECT STAT).
The obtained coronary angiograms were assessed by interventional cardiologists. In addition to the classical CAD classification, the residual Gensini and SYNTAX scores were calculated as previously published [18, 19].
Lipidomics
Lipidomic analysis was performed using the Lipidyzer platform™. A triple quadrupole mass spectrometer (QTRAP 5500; SCIEX, Darmstadt, Germany) equipped with a differential mobility spectrometer (DMS) interface operating with SelexION technology was coupled to an ultrahigh-pressure liquid chromatography system (Nexera X2, Shimadzu, Kyoto, Japan) serving as an autosampler. The lipidomics platform (LipidyzerTM) was operated with lipidomics algorithms (Analyst version 1.6.8 and Lipidomics workflow manager; SCIEX). The Lipidyzer™ Platform was tuned using the SelexION Tuning Kit (SCIEX), and a system suitability test was performed using the System Suitability Kit (SCIEX) according to the manufacturer’s instructions. A 50 µL aliquot of plasma from the biobanked blood samples was used for lipid extraction, which was performed as previously described [20,21,22]. Briefly, plasma samples were spiked with internal standards, and lipids were extracted by employing an adjusted MTBE/methanol extraction protocol [23]. The mass spectrometry-generated data obtained from Analyst software were converted from wiff files to mzML format using MSconvertGUI. Data processing and quantification were performed using the Shotgun Lipidomic Assistant (SLA) software, a python-based application according to Su et al. [23, 24].
Bile acid measurements
BAs were extracted from the plasma of the biobanked samples by methanol liquid‒liquid extraction. Briefly, 50 µL of plasma was spiked with internal standards, and methanol was added. After vigorous homogenization by vortexing, samples were cleared from debris by centrifugation for 10 min at 10,000 ×g and 4 °C. The supernatants were transferred to fresh tubes and evaporated until dryness. The residual BAs were resuspended in acetonitrile/methanol (3/1, v/v) containing 0.1% formic acid and 20 mM ammonium acetate. Quantitative measurement of BAs was performed using an ultrahigh-pressure liquid chromatography system (Nexera X2, Shimadzu, Kyoto, Japan) coupled to a triple quadrupole mass spectrometer (QTRAP 5500; SCIEX, Darmstadt, Germany) (UPLC-QqQ) system operated via Analyst software version 1.6.8 (Sciex). The BAs were separated on a Kinetex C18 column (100 Å, 150 mm × 2.1 mm i.d., Phenomenex, Torrance, CA, USA) run in gradient elution mode with water as eluent A and acetonitrile/methanol (3/1, v/v) as eluent B, both of which contained 0.1% formic acid and 20 mM ammonium acetate. The eluted analytes were ionized in the mass spectrometer with both positive and negative electrospray ionization techniques and analyzed in multiple reaction monitoring (MRM) scan mode. A detailed description of this approach has been previously reported [25]. Peak identification and quantification from the generated chromatograms were performed by comparing retention times, MRM transitions and peak areas to the corresponding standard chromatograms using Multiquant software version 3.0.3 (Sciex). All quantified bile acids are displayed in Supplementary Table S1.
Statistical analyses
Matching was conducted using the nearest neighbors method, a Mahalanobis distance and a caliper of 0.8, as well as without the reuse of controls and a variable ratio of cases and controls, using R (version 4.1.0) and the “MatchIt” package (version 4.2.0). From the overall cohort, subgroups were defined according to the presence or absence of CAD, 1st vs. 3rd third of LDL-c, and female vs. male sex. Within these subgroups, propensity score matching in a 1:1 fashion was carried out using the following covariables: age, BMI, arterial hypertension status, diabetes mellitus status, current smoking status, MDS status, and the intake of statins, triglycerides, HDL-c, and hs-CRP. For each sex, further analyses were conducted with the first LDL-c third as the reference, stratified by the presence or absence of CAD.
Categorical variables are shown as absolute numbers and percentages and were compared by Fisher’s exact test. Continuous variables are described as medians, 25th percentiles and 75th percentiles and were compared by the Mann‒Whitney test. Baseline plasma lipid and BA characteristics were compared using the Kruskal‒Wallis test. For visualization of lipid concentration/composition in the subgroups using volcano plots, the negative logarithm of the p value calculated by Student’s t test on the base 10 is plotted against the logarithm of the fold change between the two conditions. The concentration and composition of all lipid species investigated, the fold changes and p values are given in the Supplementary Tables. A two-sided P value of < 0.05 was considered to indicate statistical significance. All statistical analyses, including volcano plot analysis, were carried out utilizing R statistical software, version 4.1.0 (R Foundation for Statistical Computing), GraphPad Prism, version 10.3.2, and Excel 2016.
Results
Baseline characteristics
After propensity score matching, 177 patients were included in the current analysis. The median ages were 73.5 years (25th and 75th percentiles: 64.1, 78.2) and 71.9 years (65.7, 77.2) for females and males with CAD, respectively, and 67.6 years (58.3, 75.3) and 69.2 years (59.8, 76.8) for females and males without CAD, respectively. Across the subgroups, all baseline characteristics, including classical cardiovascular risk factors such as diabetes, intake of statins, and crude lipoprotein levels such as LDL-c, were evenly distributed. Detailed information about baseline characteristics is provided in Table 1 and Supplementary Table S2 (stratified according to sex, presence or absence of CAD and LDL-c third).
Lipid class characteristics
Analysis of 13 lipid classes at baseline (CE: cholesteryl esters, CER: ceramides, DAG: diacylglycerols, FFA: free fatty acids, HCER: hexosylceramides, LCER: lactosylceramides, LPC: lysophosphatidylcholines, LPE: lysophosphatidylethanolamines, PC: phosphatidylcholines, SM: sphingomyelins, TAG: triacylglycerols) revealed a similar pattern in lipid class concentrations in the female and male subgroups. In line with the selection criteria that were based on LDL-c plasma levels, for both female and male patients within the 3rd LDL-c third, a significantly higher concentration of cholesteryl esters was observed. No significant differences in BA concentrations were noted between the sexes. The detailed baseline lipid class characteristics are included in Fig. 1 (females) and Fig. 2 (males).
Baseline lipid class characteristics in females. Lipid classes were quantified in plasma by mass spectrometry. Individual data points are presented in µMol/l, and vertical lines represent median values. CE: cholesteryl esters, CER: ceramides, DAG: diacylglycerols, FFA: free fatty acids, HCER: hexosylceramides, LCER: lactosylceramides, LPC: lysophosphatidylcholines, LPE: lysophosphatidylethanolamines, PC: phosphatidylcholines, SM: sphingomyelins, TAG: triacylglycerols, BA: bile acids. *P < 0.05, ** P < 0.01, *** P < 0.001 compared to the 1st third LDL-no CAD group by the Kruskal‒Wallis test
Baseline lipid class characteristics in males. Lipid classes were quantified in plasma by mass spectrometry. Individual data points are presented in µMol/l, and vertical lines represent median values. CE: cholesteryl esters, CER: ceramides, DAG: diacylglycerols, FFA: free fatty acids, HCER: hexosylceramides, LCER: lactosylceramides, LPC: lysophosphatidylcholines, LPE: lysophosphatidylethanolamines, PC: phosphatidylcholines, SM: sphingomyelins, TAG: triacylglycerols, BA: bile acids. * P < 0.05, ** P < 0.01, *** P < 0.001 compared to the 1st third LDL-no CAD group by the Kruskal‒Wallis test
A volcano plot of differentially altered lipid species revealed significantly more cholesterol ester species, such as CEs (18:2), when comparing women (Fig. 3A-B) to men (Fig. 3C-D) in the 1st versus 3rd LDL-c tertiles. In addition to the cholesteryl ester species, a number of sphingomyelin species were substantially more abundant in both females and males in the third LDL-c group. Overall, the grouping of patients according to LDL-c levels was confirmed by lipidome analysis, which demonstrated the validity and reliability of the methodology used. All analyzed lipid and BA species and the respective concentrations as well as p values used for Fig. 3 are included in the Supplemental Table for Fig. 3.
Volcano plot for plasma lipid and bile acid species in comparison between patients with low and high LDL-c. (A, C) The x-axis represents the log fold change, and the y-axis represents the –log10 p value. Values of differentially abundant lipid species with p < 0.01 (-log P > 2) in (A) females and (C) males are highlighted in red. (B, D) Plasma levels of the ten most significantly different plasma lipid species in the (B) female subgroup and (D) male subgroup
CAD-associated differences in plasma lipid and bile acid concentrations and composition in women
PC(18:0/20:1) and DAG(18:1/22:4) were lower in women with CAD than in women without CAD (Fig. 4A). Furthermore, the lipid components HCER (22:0) and DCER (24:1) were lower, and TAG52:3-FA14:0 and PE (P-16:0/20:4) were greater in women with CAD than in those without CAD (Fig. 4B), while no differences in the BA profile were noted according to the presence or absence of CAD. Further analysis suggested that plasma lipid levels did not differ among women in the subgroup with high LDL-c levels (Fig. 5A-B). All analyzed lipid species and BA species and the respective concentrations as well as p values used for Figs. 4 and 5 are included in the Supplemental Table for Figs. 4 and 5, respectively.
Volcano plot for plasma lipid and bile acid species concentrations and compositions in patients with and without CAD. The x-axis represents the log fold change, and the y-axis represents the –log10 p value. (A) Differentially abundant lipid species in females with an FDR > 1.3 are highlighted in red. (B) Differentially abundant lipid species in females with an FDR > 1.3 are highlighted in red. (C) Differentially abundant lipid species in males; FDRs > 1.3 are highlighted in red. (D) Differentially abundant lipid species in females; FDRs > 1.3 are highlighted in red
Volcano plot for plasma lipid and bile acid species concentrations according to LDL-c thirds in patient subgroups without and with CAD. The x-axis represents the log fold change, and the y-axis represents the –log10 p value. Values of p < 0.05 (-log P > 1.3) are highlighted in red. Differentially abundant lipid species (A) in females in the 1st LDL-c third group, (B) in females in the 3rd LDL-c third group, (C) in males in the 1st LDL-c third group, and (D) in males in the 3rd LDL-c third group
CAD-associated differences in plasma lipid and bile acid concentration and composition in men
The lipid components DAG(18:0/22:6), DAG(16:0/16:0), DAG(14:0/16:0), DAG(16:0/18:0) and DAG(16:1/18:0) were lower, and CE(24:0), CE(20:0) and DAG(20:0/20:0) were greater in men with CAD than in men without CAD (Fig. 4C-D). In contrast to those in women, the concentrations of the secondary BAs glycolithocholic acid (GLCA) and lithocholic acid (LCA) were lower, and the concentrations of PE (P-18:0/20:4), PE (O-18:0/20:4) and PE (P-18:1/20:4) were greater in men with CAD than in those without CAD (Fig. 4C-D). Several plasmalogen lipids (PE-P) were greater in men with low LDL-c (Fig. 5C), an effect not observed in males with high LDL-c (Fig. 5D).
Discussion
In our study, a comparison between female and male CAD and non-CAD patients accounting for possible confounders through the implementation of a matching algorithm revealed a sex-specific pattern of both lipidomic and BA profiles. Although females with CAD had no changes in BA concentrations in contrast to non-CAD females, males with CAD had significantly lower levels of the secondary BAs GLCA and LCA than the control group without CAD. These hypothesis-generating findings identify potential drivers of sex-specific differences in the development of CAD.
In recent years, population-based studies have shown sex differences in both metabolic and lipidomic parameters [26, 27]. Recently, in a study from Tabassum and colleagues, sex-specific changes in 141 lipid species were demonstrated. This finding was shown to be age dependent since opposite age-related changes were observed in 39 lipid species, and sex differences were attenuated with increasing age. Interestingly, and confirmatory of our results concerning the reported changes in some lipid levels, Tabassum et al. demonstrated that both age- and sex-associated changes in lipid concentrations and composition extended to PCs, PEs and CER, which were deflected in our study cohort [28].
Concerning lipids and lipoproteins, we reported downregulated concentrations of PC (18:0/20:1) in female patients with CAD, while no changes in PCs were noted in male patients. The predictive utility of circulating PCs has been demonstrated via integration in the so-called ceramide test score (CERT2), which consists of one ceramide/ceramide ratio, two ceramide/PC ratios and a single PC. In contrast to our results, during the development of the CERT2 score, PC (14:0/22:6), PC (16:0/22:5) and PC (16:0/16:0) were identified to predict cardiovascular outcomes [29]. Various associations of PC-esters have been described in the literature, with both elevated and decreased levels of PC being associated with CAD [6, 30]. Hence, the broader utility of PCs in cardiovascular disease manifestation still needs to be clarified further. Interestingly, ceramides, including DCER (24:1), were significantly decreased in terms of lipid composition in female CAD patients in our study. This is in contrast to a previous report in which the levels of all sphingolipids measured, except for 3 glucosylceramides, were elevated in patients with CAD, although no sex-specific analysis was performed [5]. Similarly, between the sexes, DAG levels were lower in both subgroups without CAD. DAGs have been described as components of cellular membranes, as well as lipid second messengers with signaling properties linked to metabolism [41]. However, previous reports have noted inconsistent results with regard to the impact of DAG, as both up- and downregulated levels of DAG have been described in different disease entities [4, 7, 31, 32].
In this study, we found higher plasmalogen levels specifically in male CAD patients with low LDL-c levels. Chronic inflammatory diseases have been reported to correlate with lower plasmalogen levels, which is contrary to our observation [33]. On the other hand, plasmalogens can initiate both anti- and proinflammatory responses. The latter seems to be associated with the release of arachidonic acid, which, in turn, serves as a precursor to produce proinflammatory lipid mediators [33]. Additional studies will be necessary to mechanistically decipher the relevance of plasmalogens for CAD progression, especially in the context of hypo- and hypercholesterolemic conditions.
In recent years, a multitude of potential pathways/mechanisms related to sex-specific changes in the lipidome have been investigated. These include the effects of sex hormones (estrogen and testosterone through various signaling pathways), autosomal genetic variants, incomplete X chromosome inactivation, and male-specific Y chromosome mutations, as well as external factors, including diet and physical activity [15]. However, the precise mechanisms underlying sex-specific lipid profiles remain to be elucidated.
Moreover, in our study, no BA perturbations according to CAD status were detected in female patients. However, specific changes in the secondary BAs GLCA and LCA were noted in the male CAD subgroup. Recently, BAs have been investigated as potential mediators in the development of atherosclerosis, with lower serum BA concentrations being present in patients with CAD in both large-scale cohorts and smaller studies [11, 34]. Here, lower total BA concentrations were associated with CAD, MI and the severity of coronary lesions, as measured by the Gensini score, irrespective of patient sex [34]. More recently, Nguyen and colleagues confirmed the association of lower BA serum concentrations in patients with CAD, and it was demonstrated that glycochenodeoxycholic acid, a conjugated primary BA, was predictive of CAD [11].
One of the potential atheroprotective effects of BAs has been hypothesized to be attributed to their signaling properties through molecular action targeting the TGR5 and FXR receptors, potentially via anti-inflammatory or direct metabolic effects [35, 36]. Building on these observations, BA receptors have been used as therapeutic target in patients with steatohepatitis, where obeticholic acid, a receptor ligand for the farnesoid X nuclear receptor, has been shown to improve histological measures [37, 38]. Hence, our finding that changes in BA patterns are sex specific has potential clinical implications for these newly developed methods in broader applications, including in cardiovascular disease [39].
Our study did not provide mechanistic insights into the observed differences in BA metabolism. However, differences in the BA pool and synthesis have been described in other disease entities [40, 41]. Here, the effects of synthetic and endogenous estrogens have been implicated in the observed group differences both for cardiovascular and other disease manifestations [14, 42, 43]. Additionally, as LCA and GLCA are secondary BAs and are thus derived from gut microbiota, the changes in their concentrations might also be products of altered gut flora in CVD patients.
Although prior studies have investigated either the lipidome or BA in patients with CAD, we believe that several aspects of our work are novel. First, we carried out parallel quantification of the lipidome as well as BAs in our cohort, aiming to unravel the potential interplay between these mediators of atherosclerosis. Second, using a propensity score matching algorithm, we aimed to account for a multitude of confounding factors for both lipid and BA levels, which are commonly not considered in other studies [44, 45]. Finally, our results represent the first sex-specific analysis of the lipidome and BAs in patients with CAD in comparison to a non-CAD control cohort.
Study strengths and limitations
Strengths of the study include angiographic assessment for coronary artery disease in all patients and state-of-the-art quantification of lipid and bile components in their blood. Although we describe a very well-defined contemporary all-comers CAD cohort with broad matching criteria, some limitations merit consideration. First, the number of patients was low, limiting our ability to correct for multiple testing. Second, serial measurements of BAs at different timepoints and lipidomic profiles were not carried out. Natural variations in the concentrations of the measured components were therefore not captured. Additionally, while our patient cohort was precisely characterized concerning CAD severity and baseline characteristics, selective screening with regard to potential confounding disease entities such as steatohepatitis and fatty liver disease, which have been shown to be associated with circulating BA levels, was not implemented. However, using a matching algorithm, we aimed to account for metabolic abnormalities, including diabetes and obesity, which are associated with the development of liver disease, limiting the confounding potential of these disorders. Finally, due to the relatively small sample size in our present investigation, which accounts for a limited proportion of the overall INTERCATH cohort, our current analysis delivers hypothesis-generating data only. Additionally, the identification of lipidomic or bile acid predictors of CAD is limited and requires further exploration in larger-scale studies.
Conclusion
Our study adds to the growing body of literature showing that a sex-specific pattern in circulating lipid species as well as BAs is present in patients with ASCVD. To further understand the potential causative changes in the sex-specific pathophysiology of atherosclerosis, mechanistic studies are needed. Nonetheless, our findings have clinical implications concerning the utilization of lipidomic measurements in the risk prediction of patients with cardiovascular disease and the potential targeting of BA receptors as treatment in patients with CAD.
Data availability
The dataset supporting the conclusions of this article is included within the article and its additional files.
Abbreviations
- ASCVD:
-
Atherosclerotic cardiovascular disease
- BA:
-
Bile acid
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CE:
-
Cholesteryl esters
- CER:
-
Ceramides
- DAG:
-
Diacylglycerols
- FFA:
-
Free fatty acids
- GLCA:
-
Glycolithocholic acid
- HCER:
-
Hexosylceramide
- HDL-c:
-
High-density lipoprotein cholesterol
- hs-CRP:
-
High-sensitivity C-reactive protein
- hsTnI:
-
High-sensitivity troponin I
- LCER:
-
Lactosylceramide
- LDL-c:
-
Low-density lipoprotein cholesterol
- LPC:
-
Lysophosphatidylcholine
- LPE:
-
Lysophosphatidyethanolamines
- MDS:
-
Mediterranean diet score
- PC:
-
Phosphatidylcholine
- PSM:
-
Propensity score matching
- SM:
-
Sphingomyelins
- TAG:
-
Triacylglycerols
- LCA:
-
Lithocholic acid
References
Townsend N, Kazakiewicz D, Lucy Wright F, Timmis A, Huculeci R, Torbica A, Gale CP, Achenbach S, Weidinger F, Vardas P. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. 2022;19:133–43.
Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41:2313–30.
Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, Ference BA, Gaudet D, Hegele RA, Kersten S, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42:4791–806.
Meikle PJ, Wong G, Tsorotes D, Barlow CK, Weir JM, Christopher MJ, MacIntosh GL, Goudey B, Stern L, Kowalczyk A, et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:2723–32.
Poss AM, Maschek JA, Cox JE, Hauner BJ, Hopkins PN, Hunt SC, Holland WL, Summers SA, Playdon MC. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J Clin Invest. 2020;130:1363–76.
Cavus E, Karakas M, Ojeda FM, Kontto J, Veronesi G, Ferrario MM, Linneberg A, Jorgensen T, Meisinger C, Thorand B, et al. Association of circulating metabolites with risk of Coronary Heart Disease in a European Population: results from the biomarkers for Cardiovascular Risk Assessment in Europe (BiomarCaRE) Consortium. JAMA Cardiol. 2019;4:1270–9.
Ottosson F, Emami Khoonsari P, Gerl MJ, Simons K, Melander O, Fernandez C. A plasma lipid signature predicts incident coronary artery disease. Int J Cardiol. 2021;331:249–54.
Ding M, Rexrode KM. A review of Lipidomics of Cardiovascular Disease highlights the importance of isolating lipoproteins. Metabolites 2020, 10.
Joshi A, Rienks M, Theofilatos K, Mayr M. Systems biology in cardiovascular disease: a multiomics approach. Nat Rev Cardiol. 2021;18:313–30.
Chiang JYL, Ferrell JM, Wu Y, Boehme S. Bile acid and cholesterol metabolism in atherosclerotic Cardiovascular Disease and Therapy. Cardiol Plus. 2020;5:159–70.
Chong Nguyen C, Duboc D, Rainteau D, Sokol H, Humbert L, Seksik P, Bellino A, Abdoul H, Bouazza N, Treluyer JM, et al. Circulating bile acids concentration is predictive of coronary artery disease in human. Sci Rep. 2021;11:22661.
Perino A, Demagny H, Velazquez-Villegas L, Schoonjans K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and aging. Physiol Rev. 2021;101:683–731.
Spence JD, Pilote L. Importance of sex and gender in atherosclerosis and cardiovascular disease. Atherosclerosis. 2015;241:208–10.
Phelps T, Snyder E, Rodriguez E, Child H, Harvey P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol Sex Differ. 2019;10:52.
Tabassum R, Widen E, Ripatti S. Effect of biological sex on human circulating lipidome: an overview of the literature. Atherosclerosis. 2023;384:117274.
Waldeyer C, Seiffert M, Staebe N, Braetz J, Kohsiack R, Ojeda F, Schofer N, Karakas M, Zeller T, Sinning C, et al. Lipid management after first diagnosis of coronary artery disease: contemporary results from an Observational Cohort Study. Clin Ther. 2017;39:2311–e23202312.
Stewart RA, Wallentin L, Benatar J, Danchin N, Hagstrom E, Held C, Husted S, Lonn E, Stebbins A, Chiswell K, et al. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high-risk patients with stable coronary heart disease. Eur Heart J. 2016;37:1993–2001.
Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606.
Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–27.
Dieckmann S, Strohmeyer A, Willershauser M, Maurer SF, Wurst W, Marschall S, de Angelis MH, Kuhn R, Worthmann A, Fuh MM, et al. Susceptibility to diet-induced obesity at thermoneutral conditions is independent of UCP1. Am J Physiol Endocrinol Metab. 2022;322:E85–100.
Evangelakos I, Schwinge D, Worthmann A, John C, Roeder N, Pertzborn P, Behrens J, Schramm C, Scheja L, Heeren J. Oxysterol 7-alpha Hydroxylase (CYP7B1) Attenuates Metabolic-Associated Fatty Liver Disease in Mice at Thermoneutrality. Cells 2021, 10.
Fischer AW, Jaeckstein MY, Gottschling K, Heine M, Sass F, Mangels N, Schlein C, Worthmann A, Bruns OT, Yuan Y, et al. Lysosomal lipoprotein processing in endothelial cells stimulates adipose tissue thermogenic adaptation. Cell Metab. 2021;33:547–e564547.
Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008;49:1137–46.
Su B, Bettcher LF, Hsieh WY, Hornburg D, Pearson MJ, Blomberg N, Giera M, Snyder MP, Raftery D, Bensinger SJ, Williams KJ. A DMS Shotgun Lipidomics Workflow Application to Facilitate High-Throughput, Comprehensive Lipidomics. J Am Soc Mass Spectrom. 2021;32:2655–63.
Wegner K, Just S, Gau L, Mueller H, Gerard P, Lepage P, Clavel T, Rohn S. Rapid analysis of bile acids in different biological matrices using LC-ESI-MS/MS for the investigation of bile acid transformation by mammalian gut bacteria. Anal Bioanal Chem. 2017;409:1231–45.
Beyene HB, Olshansky G, AA TS, Giles C, Huynh K, Cinel M, Mellett NA, Cadby G, Hung J, Hui J, et al. High-coverage plasma lipidomics reveals novel sex-specific lipidomic fingerprints of age and BMI: evidence from two large population cohort studies. PLoS Biol. 2020;18:e3000870.
Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215.
Tabassum R, Ruotsalainen S, Ottensmann L, Gerl MJ, Klose C, Tukiainen T, Pirinen M, Simons K, Widen E, Ripatti S. Lipidome- and genome-wide study to Understand Sex differences in circulatory lipids. J Am Heart Assoc. 2022;11:e027103.
Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schottker B, Laaperi M, Kauhanen D, Koistinen KM, et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J. 2020;41:371–80.
Djekic D, Pinto R, Repsilber D, Hyotylainen T, Henein M. Serum untargeted lipidomic profiling reveals dysfunction of phospholipid metabolism in subclinical coronary artery disease. Vasc Health Risk Manag. 2019;15:123–35.
Eichmann TO, Lass A. DAG tales: the multiple faces of diacylglycerol–stereochemistry, metabolism, and signaling. Cell Mol Life Sci. 2015;72:3931–52.
Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91.
Bozelli JC Jr., Azher S, Epand RM. Plasmalogens and Chronic Inflammatory diseases. Front Physiol. 2021;12:730829.
Li W, Shu S, Cheng L, Hao X, Wang L, Wu Y, Yuan Z, Zhou J. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis. 2020;292:193–200.
Mencarelli A, Renga B, Distrutti E, Fiorucci S. Antiatherosclerotic effect of farnesoid X receptor. Am J Physiol Heart Circ Physiol. 2009;296:H272–281.
Miyazaki-Anzai S, Masuda M, Levi M, Keenan AL, Miyazaki M. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS ONE. 2014;9:e108270.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65.
Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–e582571.
Li C, Yang J, Wang Y, Qi Y, Yang W, Li Y. Farnesoid X receptor agonists as therapeutic target for Cardiometabolic diseases. Front Pharmacol. 2020;11:1247.
Bennion LJ, Drobny E, Knowler WC, Ginsberg RL, Garnick MB, Adler RD, Duane WC. Sex differences in the size of bile acid pools. Metabolism. 1978;27:961–9.
Galman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. J Intern Med. 2011;270:580–8.
Brady CW. Liver disease in menopause. World J Gastroenterol. 2015;21:7613–20.
Bhupathy P, Haines CD, Leinwand LA. Influence of sex hormones and phytoestrogens on heart disease in men and women. Womens Health (Lond). 2010;6:77–95.
Zhu C, Sawrey-Kubicek L, Beals E, Hughes RL, Rhodes CH, Sacchi R, Zivkovic AM. The HDL lipidome is widely remodeled by fast food versus Mediterranean diet in 4 days. Metabolomics. 2019;15:114.
Moszak M, Szulinska M, Bogdanski P. You are what you eat-the relationship between Diet, Microbiota, and metabolic Disorders-A Review. Nutrients 2020, 12.
Acknowledgements
We thank Laura Ehlen and Meike Kroeger for their excellent technical assistance.
Funding
We acknowledge financial support from the Open Access Publication Fund of UKE - Universitätsklinikum Hamburg-Eppendorf. BB is supported by a grant from the German Heart Foundation (S/06/23). This work was supported by the State of Hamburg (LFF-FV75 to JH) and the Gertaud and Heinz Rose-Stiftung (awarded to BB and JR).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
BB: Formal analysis, Methodology, Data curation, Investigation, Visualization, Writing – original draft, preparation, Writing – review & editing. MMF: Methodology, Data curation, Investigation, Visualization, Writing – original draft, preparation, Writing – review & editing. JR: Data curation, Investigation, Visualization, Writing – review & editing. AW: Supervision, Project administration. AG: Formal analysis, Investigation, Writing – review & editing. NA: Data curation, Investigation, Writing – review & editing. LK: Data curation, Investigation, Writing – review & editing. TL: Data curation, Investigation, Writing – review & editing. CB: Data curation, Investigation, Writing – review & editing. PK: Supervision, Project administration. SB: Supervision, Project administration. MS: Data curation, Investigation, Writing – review & editing. FJB: Supervision, Project administration, Writing – review & editing. CW: Conceptualization, Writing – original draft, preparation, Writing – review & editing, Supervision, Project administration. JH: Conceptualization, Formal analysis, Visualization, Writing – original draft, preparation, Writing – review & editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
An ethics vote was obtained from the local ethics committee (PV4303, Hamburg, Germany), and written and informed consent was acquired from all participants.
Consent for publication
Not applicable.
Competing interests
NA reports grant and personal fees from Novartis outside of the submitted work. PK receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last three years. PK is listed as inventor on two issued patents held by University of Hamburg (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). SB reports research funding/grants from Abbott Diagnostics, Siemens, AMGEN, Astra Zeneca, Novartis and Daiichi Sankyo; Honoraria for lectures/chairs from Abbott, AMGEN, Bayer, Boehringer Ingelheim, Bristol Meyer Squib, Daiichi Sankyo, GlaxoSmithKline, LumiraDx, Novartis, Roche Diagnostics and Thermo Fisher; and is a member of the advisory board/consultant for Thermo Fisher. MS reports personal fees from Abiomed, AMGEN, AstraZeneca, Bristol-Myers Squibb, Daichii Sankyo, Inari Medical, Pfizer, Shockwave Medical, and Siemens Healthineers, personal fees and non-financial support from Abbott Vascular, Edwards Lifesciences, and Medtronic, and grants, personal fees and non-financial support from Boston Scientific and Philips, all outside the submitted work. FB reports grants from Daiichi Sankyo, Pfizer, and Sanofi, non-financial support from Abbott, ASAHI INTECC, and Inari Medical, personal fees from Amgen and Novartis outside of the submitted work. CW reports lecture and consulting fees from AMGEN, Novartis, Daiichi Sankyo, Sanofi and AstraZeneca, all outside of the submitted work. JH received honoraria for lectures from AMGEN, Pfizer, Daichii Sankyo and Eli Lilly, all outside of the submitted work. All other authors have nothing to declare.
Previous presentation
Part of this work has previously been presented at the 91st Congress of the European Atherosclerosis Society, 21st -23rd May 2023, Mannheim, Germany.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bay, B., Fuh, M.M., Rohde, J. et al. Sex differences in lipidomic and bile acid plasma profiles in patients with and without coronary artery disease. Lipids Health Dis 23, 197 (2024). https://doi.org/10.1186/s12944-024-02184-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02184-z