Abstract
Background
Active tuberculosis (TB) significantly increases the risk of cardiovascular disease, but the underlying mechanisms remain unclear. This study aimed to investigate the association between inflammation biomarkers and dyslipidemia in patients with drug-resistant TB (DR-TB).
Methods
This was a secondary analysis of data from a cross-sectional multi-center study in Uganda conducted 2021. Participants underwent anthropometric measurements and laboratory tests included a lipid profile, full haemogram and serology for HIV infection. Dyslipidemia was defined as total cholesterol > 5.0 mmol/l and/or low-density lipoprotein cholesterol > 4.14 mmol/l, and/or triglycerides (TG) ≥ 1.7 mmol/l, and/or high density lipoprotein cholesterol (HDL-c) < 1.03 mmol/l for men and < 1.29 mmol/l for women. Biomarkers of inflammation were leukocyte, neutrophil, lymphocyte, monocyte, and platelet counts, as well as neutrophil/lymphocyte (NLR), platelet/lymphocyte, and lymphocyte/monocyte (LMR) ratios, mean corpuscular volume (MCV), and the systemic immune inflammation index (SII) (neutrophil × platelet/lymphocyte). Modified Poisson Regression analysis was used for determining the association of the biomarkers and dyslipidemia.
Results
Of 171 participants, 118 (69.0%) were co-infected with HIV. The prevalence of dyslipidemia was 70.2% (120/171) with low HDL-c (40.4%, 69/171) and hypertriglyceridemia (22.5%, 38/169) being the most common components. Patients with dyslipidemia had significantly higher lymphocyte (P = 0.008), monocyte (P < 0.001), and platelet counts (P = 0.014) in addition to a lower MCV (P < 0.001) than those without dyslipidemia. Further, patients with dyslipidemia had lower leucocyte (P < 0.001) and neutrophil (P = 0.001) counts, NLR (P = 0.008), LMR (P = 0.006), and SII (P = 0.049). The MCV was inversely associated with low HDL-C (adjusted prevalence ratio (aPR) = 0.97, 95% CI 0.94–0.99, P = 0.023) but was positively associated with hypertriglyceridemia (aPR = 1.04, 95% CI 1.00-1.08, P = 0.052).
Conclusions
Individuals with dyslipidemia exhibited elevated lymphocyte, monocyte, and platelet counts compared to those without. However, only MCV demonstrated an independent association with specific components of dyslipidemia. There is need for further scientific inquiry into the potential impact of dyslipidemia on red cell morphology and a pro-thrombotic state among patients with TB.
Similar content being viewed by others
Background
Tuberculosis (TB) remains the leading infectious cause of mortality globally [1]. Moreover, individuals with active TB face a significantly high risk of major adverse cardiovascular events, estimated at 51% greater than those without TB [2]. This translates to increased incidence of ischemic stroke, peripheral artery disease, and myocardial infarction in patients with TB [3,4,5,6]. The underlying mechanisms of cardiovascular disease (CVD) remain unclear, but several factors contribute.
Patients with TB often exhibit a higher prevalence of traditional CVD risk factors, which could play a causal role in this increased incidence. A recent systematic review of CVD risk factors among patients with TB in Africa reported 26% smoking, 21% hazardous alcohol use, 14% hypertension, 7% diabetes, and 4% obesity [7]. Additionally, we have previously shown that 63% of patients with drug resistant TB (DR-TB) have dyslipidemia [8].
Beyond these traditional CVD risk factors, TB infection triggers a potent cell-mediated immune response, marked by an over-expression of pro-inflammatory cytokines (interleukin [IL]-1, IL-2, IL-6, interferon-gamma [IFNγ], tumor necrotic factor-alpha [TNFα]), alongside monocyte/macrophage, CD4 + T-helper 1, and T-helper 17 cell activation [9]. These immune responses are believed to contribute to atherogenesis, offering a potential mechanistic link to the observed increase in CVD risk among patients with TB [9].
Dyslipidemia (hyperlipidaemia and hypolipidaemia), characterized by elevated blood total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c) and triglycerides (TG), and reduced high-density lipoprotein cholesterol (HDL-c) (hypolipidaemia) levels, presents a complex picture [10]. While canonically associated with CVD in the general population [11], elevated blood lipids in patients with TB have been linked to lower TB infection risk, less severe radiological disease, lower mycobacterial load, and lower all-cause mortality [12,13,14]. This association of hyperlipidemia and lower TB mortality further complicates the understanding of dyslipidemia and CVD-related mortality among patients with TB.
Several cell indices on the full blood count (FBC) serve as inflammation surrogate markers and CVD predictors. Elevated leucocyte, neutrophil, platelet counts, neutrophil to leucocyte ratio (NLR), platelet to lymphocyte ratio (PLR), mean corpuscular volume (MCV), red cell distribution width (RDW), and mean platelet volume (MPV) associate with CVD incidence and/or severity [15]. Among patients with TB, Wei et al. demonstrated that MPV and elevated monocyte percentage correlated with incident ischemic stroke [4]. However, the interplay between these inflammatory markers and dyslipidemia in TB remains poorly understood. A recent Taiwanese study suggested an inverse correlation between HDL-c and TC with the leucocyte count-to- NLR ratio, suggesting a potential link between HDL-c and lower systemic inflammation in patients with TB [14]. Further research is needed to fully understand this intricate relationship.
This study aimed to explore the association between dyslipidemia and inflammatory markers on the FBC in patients with TB, exploring potential mechanisms that could explain increased CVD risk observed in patients with TB. The study hypothesized that dyslipidemia and specific lipid abnormalities, would be associated with increased levels of inflammatory markers in people with TB, potentially contributing to the high prevalence of CVD this population.
Materials and methods
Study population and study design
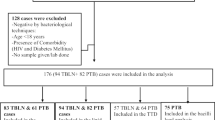
This was an exploratory analysis of data from a cross-sectional study among adults ≥ 18 years with bacteriologically confirmed pulmonary DR-TB receiving treatment at four large treatment centers in Uganda. The detailed methodology of the primary study has been described elsewhere [8, 16]. Briefly, people were diagnosed with rifampicin-resistant TB using the Mtb Xpert/RIF assay. Baseline sputum samples were subsequently sent to the national TB reference laboratory for a full drug susceptibility test for resistance to isoniazid, rifampicin, fluroquinolones, and second-line injectable aminoglycosides. Consecutive eligible participants were enrolled during routine clinic visits and underwent anthropometric measurements (weight, height, waist, and hip circumference), blood pressure measurements, and blood draw for a non-fasting lipid profile, FBC, glycated hemoglobin (HbA1c), random blood glucose (RBG), and HIV testing. In this secondary analysis, participants who had data for both non-fasting lipid profile and FBC parameters were included.
Data collection and study measurements
Participants provided informed consent and completed a questionnaire collecting sociodemographic information, smoking and alcohol use history, and any pre-existing medical conditions. A trained nurse performed anthropometric measurements, took blood pressure measurements and drew blood samples using standardized techniques.
Participants underwent anthropometric measurements of their weight, height and waist/hip ratio using a weighing scale (Seca 760®), stadiometer (Seca 213®) and tape measure respectively. The body mass index (BMI) was calculated using the formula; BMI = weight (kilograms)/height (in metres)2. Using a battery powered digital blood pressure (BP) machine (Omron®, Hem 7120), the BP was taken on two separate occasions, 20 min apart, at the DR-TB treatment centre. The average BP of the two measurements was considered as the participant’s BP. A study nurse drew 4 millilitres (mls) of blood which was tested for random blood glucose (RBG), glycated haemoglobin (HbA1c), non-fasting lipid profile, and a complete blood count. The HbA1c and blood lipids (TG, TC, LDL-c and HDL-c) were estimated using the Cobas® 6000 analyzer series (Roche Diagnostics, USA).
High body mass index (BMI) (weight [kilograms]/height2 [meters]) was defined as a BMI ≥ 25.0 kg/m2. Hyperglycemia was defined as HbA1c > 5.6% [17]. Central obesity was defined as waist circumference ≥ 102/88 cm and/or waist-hip ratio ≥ 0.90/0.85 for men/women, respectively [18].
Study outcome
The primary outcome was dyslipidemia defined according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines [19]: TC > 5.0 mmol/L and/or LDL-c > 4.14 mmol/L and/or TG ≥ 1.7 mmol/L and/or HDL-c < 1.03 mmol/L for men and < 1.29 mmol/L for women. Low HDL-c was an HDL-c < 1.03 mmol/l for men and < 1.29 mmol/l for women and hypertriglyceridemia was TG ≥ 1.7 mmol/l. While hyperlipidemia was defined as elevated TC, LDL-c and TG, hypolipidemia was defined as low HDL-c using the aforementioned cut-offs. The LDL-c/HDL-c ratio was considered elevated if > 2.5 [20, 21].
The main exposure was inflammatory markers measured in the FBC known to predict CVD. Specific hemogram inflammatory markers analyzed were total leucocyte, neutrophil, lymphocyte, and monocyte counts; NLR, PLR, lymphocyte/monocyte ratio (LMR), MCV, RDW, and systemic immune-inflammation index (SII) (neutrophil × platelet/lymphocyte) [15, 22].
Statistical analysis
Statistical analyses were performed using Stata version 17.0. Medians and the corresponding interquartile ranges were used to summarise continuous data while categorical variables were summarized as frequencies and percentages. Baseline characteristics were compared by dyslipidemia status using the Chi-square test (categorical variables) and the Kruskal-Wallis test (continuous variables). Because of the high prevalence of dyslipidemia, univariate and multivariate modified Poisson Regression models were employed to assess the association between inflammatory markers and dyslipidemia, as well as its most prevalent components, low HDL-c and hypertriglyceridemia. The multivariable models were adjusted for potential confounders: hyperglycemia, high BMI, HIV status, smoking history, and central obesity, selected based on their established relevance to CVD risk in existing literature. The data were tested for collinearity by calculating the variance inflation factor (VIF) of the variables in the model. Variables with a high VIF (that is, VIF > 10) were removed from the model. Specifically, the leucocyte and neutrophil counts, PLR and SII were removed from the multivariable model. Further evaluation of the correlation between the individual cell counts and the derived indices found weak correlation between these variables save for the NLR and PLR (Pearson correlation coefficient of 0.895) (Appendix 1). Hyperglycemia, high BMI, HIV status, smoking history, and central obesity were assessed whether they are potential modifiers by introducing interaction terms for each biomarker and no effect modification was found. Statistical significance was set at P < 0.05. A power analysis was not conducted for this secondary analysis as sample size requirements were estimated based on the primary study.
Results
Out of 212 participants in the original study, 171 (80.7%) had data available for both lipid profiles and full blood counts.
Characteristics of study participants
Among the 171 participants, 118 (69.0%) were co-infected with HIV (all on antiretroviral therapy), while 69 (40.4%) had central obesity and 60 (35.1%) reported a history of smoking. Notably, 15 (8.9%) had hyperglycemia and 14 (8.6%) had a high BMI (Table 1). The mean (SD) RBG was 5.02 (1.54) mmol/l and was weakly positively correlated with the HbA1c (Pearson’s correlation coefficient = 0.11). The prevalence of dyslipidemia was 70.2% (95% CI 62.7–76.9). A total of 69 (40.4%) had low HDL-c (hypolipidemia), 38 (22.5%) had hypertriglyceridemia, 24 (16.9%) had elevated TC and, 11 (6.4%) had elevated LDL-c. As such, hypolipidaemia was the commonest form of dyslipidaemia. Notably, 158 (92.4%) had elevated LDL-c/HDL-c ratio. Patients with dyslipidemia had significantly higher lymphocyte (P = 0.008), monocyte (P < 0.001) and platelet counts (P = 0.014) in addition to a lower MCV (P < 0.001) than those without dyslipidemia. Further, patients with dyslipidemia had lower leucocyte (P < 0.001) and neutrophil (P = 0.001) counts, NLR (P = 0.008), LMR (P = 0.006) and SII (P = 0.049). There were no differences in age (P = 0.519) and sex (P = 0.358) distribution between the two groups.
Association between biomarkers of inflammation and dyslipidemia
In the multivariable analysis, no biomarker as independently associated with dyslipidemia on the overall (Table 2). Among the specific dyslipidemia components, the MCV was inversely associated with low HDL-C (adjusted prevalence ratio (aPR) = 0.97, 95% CI 0.94–0.99, P = 0.023) but was positively associated with hypertriglyceridemia (aPR = 1.04, 95% CI 1.00-1.08, P = 0.052). The association between the biomarkers of inflammation and the individual lipid abnormalities are shown in Table 2.
Discussion
The aim of this study was to explore the association between dyslipidemia and inflammatory markers on the FBC in patients with TB. The study found a high prevalence of dyslipidemia among patients with TB (70%) that was predominantly due to low HDL-c and hypertriglyceridemia. Patients with dyslipidemia had elevated platelets, lymphocyte, and monocyte counts. The study also found that an increase in the MCV was positively associated with hypertriglyceridemia but inversely associated with low HDL-c. CVD is emerging as a significant cause of morbidity and mortality among patients with TB and TB survivors [2, 23]. While dyslipidemia and chronic inflammation are a cornerstone in atherogenesis [24], the association between markers of inflammation and dyslipidemia is not well characterized in TB. The study fills this crucial gap.
The high prevalence of dyslipidemia among patients with TB begs the question whether lipid lowering drugs have a role in reducing CVD risk among patients with TB. Compelling evidence suggests that statins not only reduce cholesterol levels (critical for Mycobacterium tuberculosis metabolism and cell wall integrity), thereby reducing active TB risk and severity, but also exert immune modulatory effects on monocytes, NK cells, and lymphocytes [25,26,27,28,29]. More studies are needed to establish whether statins prevent the initiation, progression and instability of atheroma plaques among people with TB; thereby reducing CVD risk. From the bivariate comparisons (Table 1), patients with dyslipidemia had higher lymphocyte and monocyte counts, suggesting that these cells could have a role in the interplay between dyslipidemia and inflammation. Few studies have reported the prevalence of dyslipidemia in patients with DR-TB. Similar to the current findings, Biranu and colleagues [30] found low HDL-c (65.5%) and hypertriglyceridemia (14.2%) to be the most prevalent forms of dyslipidemia in patients with multi-drug resistant TB. The mechanisms explaining the high prevalence are not apparent. However, in addition to inflammation and traditional CVD risk factors, it could be partly attributed to DR-TB treatment which alters gut microbiota to a phenotype that engenders elevated TC and LDL-c [31].
In the current study population, low HDL-c was the most predominant form of dyslipidemia. HDL plays a crucial role in the reverse cholesterol transport system, shuttling cholesterol from peripheral tissues back to the liver for excretion, thereby preventing the formation of atherosclerotic plaques [32]. Beyond this, HDL-c also boasts anti-oxidant, anti-thrombotic, and anti-inflammatory properties, including suppressing the expression of endothelial adhesion molecules and regulating the platelet activating factor, a potent activator of platelets, monocytes, and leukocytes [32]. In Mycobacterium tuberculosis infection, low HDL-c levels could exacerbate systemic inflammation, which high HDL-c has been shown to counter by suppressing tumor necrosis factor production [33]. However, the story of HDL-c is not as straightforward as it seems. While low levels are associated with increased CVD risk, indiscriminately raising HDL-c through pharmacotherapy has yielded disappointing results [34,35,36]. Emerging evidence suggests that specific sub-classes, particularly HDL3, have a more profound effect in protecting against CVD [37]. This shows a critical need for exploring drugs like fibrates, which selectively enhance HDL3 functionality, but their long-term association with mortality in patients with TB requires cautious consideration [32, 38].
The MCV is a measure of the average size of red blood cells and has been traditionally used for typing anemia [39]. Beyond its traditional role, emerging research links elevated MCV with endothelial dysfunction, increased severity of coronary artery disease, arterial stiffness, and a higher likelihood of major adverse cardiovascular events [40,41,42,43,44,45]. The current study delineates a nuanced relationship between MCV and dyslipidemia, revealing an inverse association with low HDL-c and a positive association with hypertriglyceridemia. Specifically, the study found that an elevated MCV corresponds to higher levels of both HDL-c and triglycerides. Though hypertriglyceridemia is a known culprit in CVD, it’s crucial to remember that high HDL-c, while often deemed protective, can be a double-edged sword. In fact, HDL-c levels exceeding 80 mg/100 ml have been associated with an increased risk of mortality, challenging the conventional understanding of its protective role in CVD [46].
The observation of an association between high MCV and elevated HDL-c levels, mirrors results from the National Health and Nutrition Examination Survey 2005–2006 [47] and studies involving overweight/obese individuals in Iran and non-anemic elderly populations [48, 49]. One plausible explanation is the role of plasma HDL-c as a cholesterol source for red blood cell membranes, leading to changes in red cell membrane cholesterol content and diameter. Such alterations can decrease red cell fluidity, stiffen the lipid shell, increase membrane density, and modify red cell morphology [50]. The potential role of the HDL-c on red cell membrane cholesterol is also supported by the positive association of the RDW, a measure of red cell size variability, and low HDL-c in the bivariate analysis [51]. The RDW has been associated with myocardial infarction, heart failure, stroke, atrial fibrillation, coronary artery disease, peripheral artery disease and hypertension [52]. The observed association between elevated RDW and HDL-c in coronary artery disease patients could be indicative of chronic inflammation and oxidative stress as well [53]. The results from this study collectively suggest that alterations in HDL-c levels can significantly impact red cell membrane characteristics, potentially affecting blood rheology and predisposing individuals to CVD. Although patients with dyslipidemia had significantly elevated platelet counts than those without, further investigation is needed to determine if this, along with red blood cell membrane alterations, contributes to a pro-thrombotic state in this population.
Study strengths and weaknesses
The merits of the study lie in the multi-center nature of the study and the use of readily available markers of inflammation. Despite its valuable insights, the current study has limitations that warrant consideration. Due to its snapshot nature, changes in blood lipids and inflammation markers over time could not be assessed, particularly regarding the influence of TB treatment. This limits the understanding of any dynamic interplay between these factors and their long-term implications. Although some studies suggest HDL-c and total cholesterol remain stable during TB treatment [54], the participants in the current study had been on TB treatment for a median of 6 months. This raises the possibility that baseline lipid and inflammation marker levels may have differed from those at study entry, potentially affecting the observations. Longitudinal studies would be better suited to capture these dynamics over time. Another concern is the generalizability of the results. The research focused exclusively on patients with DR-TB, raising questions about the applicability of the findings to patients with drug-susceptible TB. While the prevalence of traditional CVD risk factors is reportedly similar in both DR-TB and drug-susceptible TB populations [7], it remains unclear whether the interplay between lipids and biomarkers of inflammation would differ according to TB drug resistance status. Genetically diverse strains of Mycobacterium tuberculosis exhibit varied propensity to metabolize host cholesterol but the effect on overall lipid levels of the host is unclear [55]. This aspect warrants further investigation. While the study evaluated readily available markers on a full haemogram known to predict CVD, we did not investigate the interaction of blood lipids with established inflammatory biomarkers such as C-reactive protein, erythrocyte sedimentation rate, interleukin-6, and tumor necrosis factor. These data were not collected from the study population. Future investigations could explore the potential synergistic effects of these inflammatory markers with blood lipids on CVD risk.
Conclusion
In conclusion, the study highlights a significant prevalence of dyslipidemia among patients with DR-TB, primarily characterized by low HDL-c levels and hypertriglyceridemia. Notably, the results showed elevated monocytes, platelets and lymphocytes among patients with dyslipidemia as well as an association between the MCV with elevated HDL-c and hypertriglyceridemia. These findings underscore the intricate interplay between lipid metabolism and hematological changes in patients with TB. Crucially, the associations with MCV suggest that dyslipidemia may influence red blood cell morphology, potentially leading to alterations in blood rheology. This observation, coupled with the noted changes in platelet count, points towards a possible pro-thrombotic state in patients with TB with dyslipidemia. Such hematological alterations could have significant implications for the cardiovascular health of these patients.
Appendix 1
Correlation between cell counts and derived cell ratios
Neutrophil count | NLR | Lymphocyte count | PLR | Platelet count | Monocyte count | LMR | |
|---|---|---|---|---|---|---|---|
Neutrophil count | 1.000 | ||||||
NLR | 0.244 | 1.000 | |||||
Lymphocyte count | -0.301 | -0.483 | 1.000 | ||||
PLR | 0.133 | 0.895 | -0.433 | 1.000 | |||
Platelet count | -0.101 | -0.046 | 0.294 | 0.070 | 1.000 | ||
Monocyte count | -0.394 | -0.318 | 0.652 | -0.286 | 0.4168 | 1.000 | |
LMR | 0.331 | -0.113 | 0.192 | -0.113 | -0.213 | -0.273 | 1.000 |
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CVD:
-
cardiovascular disease
- LDL-c:
-
low density lipoprotein cholesterol
- HDL-c:
-
high density lipoprotein cholesterol
- HbA1c:
-
glycated haemoglobin
- BMI:
-
body mass index
- TB:
-
tuberculosis
- DRTB:
-
drug resistant tuberculosis
- IQR:
-
interquartile range
- RDW:
-
red cell distribution width
- NLR:
-
neutrophil lymphocyte ratio
- MCV:
-
Mean corpuscular volume
- MPV:
-
mean platelet volume
- SII:
-
systemic inflammation index
- LMR:
-
lymphocyte/monocyte ratio
References
World Health Organisation. Global tuberculosis report 2023. Geneva, Switerzland: WHO; 2023 Nov.
Basham CA, Smith SJ, Romanowski K, Johnston JC. Cardiovascular morbidity and mortality among persons diagnosed with tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2020;15:e0235821.
Lee HR, Yoo JE, Choi H, Han K, Jung JH, Park J, et al. Tuberculosis and risk of ischemic stroke: a Nationwide Cohort Study. Stroke. 2022;53:3401–9.
Wei Y, Tang S, Xie Z, He Y, Zhang Y, Xie Y, et al. Pulmonary tuberculosis-related ischemic stroke: a retrospective Case Control Study. J Inflamm Res. 2022;15:4239–49.
Wang S-H, Chien W-C, Chung C-H, Lin F-H, Peng C-K, Chian C-F, et al. Tuberculosis increases the risk of peripheral arterial disease: a nationwide population-based study. Respirology. 2017;22:1670–6.
HUAMAN MA, KRYSCIO RJ, FICHTENBAUM CJ, STERLING HENSONDSALTE. Tuberculosis and risk of acute myocardial infarction: a propensity score-matched analysis. Epidemiol Infect. 2017;145:1363–7.
Baluku JB, Ronald O, Bagasha P, Okello E, Bongomin F. Prevalence of cardiovascular risk factors in active tuberculosis in Africa: a systematic review and meta-analysis. Sci Rep. 2022;12:16354.
Baluku JB, Nabwana M, Nalunjogi J, Muttamba W, Mubangizi I, Nakiyingi L, et al. Cardiovascular risk factors among people with drug-resistant tuberculosis in Uganda. BMC Cardiovasc Disord. 2022;22:464.
Huaman MA, Henson D, Ticona E, Sterling TR, Garvy BA. Tuberculosis and cardiovascular disease: linking the epidemics. Trop Dis Travel Med Vaccines. 2015;1:10.
Cabezas MC, Burggraaf B, Klop B. Dyslipidemias in clinical practice. Clin Chim Acta. 2018;487:117–25.
Soppert J, Lehrke M, Marx N, Jankowski J, Noels H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. 2020;159:4–33.
Mani AP, Shanmugapriya K, Yadav S, Shanmugapriya A, Deepakkanna K. Assessment of lipid Profile in patients with Pulmonary Tuberculosis: an observational study. Cureus. 2023;15.
Deniz O, Gumus S, Yaman H, Ciftci F, Ors F, Cakir E, et al. Serum total cholesterol, HDL-C and LDL-C concentrations significantly correlate with the radiological extent of disease and the degree of smear positivity in patients with pulmonary tuberculosis. Clin Biochem. 2007;40:162–6.
Chidambaram V, Zhou L, Ruelas Castillo J, Kumar A, Ayeh SK, Gupte A, et al. Higher serum cholesterol levels are Associated with reduced systemic inflammation and Mortality during Tuberculosis Treatment Independent of Body Mass Index. Front Cardiovasc Med. 2021;8:696517.
Seo I-H, Lee Y-J. Usefulness of Complete Blood Count (CBC) to assess Cardiovascular and metabolic diseases in clinical settings: a Comprehensive Literature Review. Biomedicines. 2022;10:2697.
Baluku JB, Nakazibwe B, Wasswa A, Naloka J, Ntambi S, Waiswa D, et al. Prevalence of intestinal helminth coinfection in drug-resistant tuberculosis in Uganda. Open Forum Infect Dis. 2022;9:ofac541.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43:14–31.
Uganda Ministry of Health. Uganda Clinical guidelines. Kampala, Uganda: Ministry of Health; 2016.
Haynes JW, Barger EV, National Cholesterol Education Program. : Adult Treatment Panel III Guidelines and the 2004 Update. Hyperlipidemia Management for Primary Care: An Evidence-Based Approach. 2008;15–38.
Sun T, Chen M, Shen H, PingYin, Fan L, Chen X, et al. Predictive value of LDL/HDL ratio in coronary atherosclerotic heart disease. BMC Cardiovasc Disord. 2022;22:273.
Chen Q-J, Lai H-M, Chen B-D, Li X-M, Zhai H, He C-H, et al. Appropriate LDL-C-to-HDL-C ratio Cutoffs for categorization of Cardiovascular Disease Risk factors among Uygur adults in Xinjiang, China. Int J Environ Res Public Health. 2016;13:235.
Ye Z, Hu T, Wang J, Xiao R, Liao X, Liu M et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: A systematic review and meta-analysis. Frontiers in Cardiovascular Medicine [Internet]. 2022 [cited 2023 Aug 27];9. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fcvm.2022.933913.
Romanowski K, Baumann B, Basham CA, Ahmad Khan F, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:1129–37.
Malekmohammad K, Bezsonov EE, Rafieian-Kopaei M. Role of lipid Accumulation and inflammation in atherosclerosis: focus on Molecular and Cellular mechanisms. Front Cardiovasc Med. 2021;8:707529.
Davuluri KS, Singh AK, Singh AV, Chaudhary P, Raman SK, Kushwaha S, et al. Atorvastatin potentially reduces mycobacterial severity through its action on Lipoarabinomannan and Drug Permeability in Granulomas. Microbiol Spectr. 2023;11:e03197–22.
Duan H, Liu T, Zhang X, Yu A, Cao Y. Statin use and risk of tuberculosis: a systemic review of observational studies. Int J Infect Dis. 2020;93:168–74.
Meregildo-Rodriguez ED, Chunga-Chévez EV, Gianmarco R-AL, Vásquez-Tirado GA. Further insights into to the role of statins against active tuberculosis: systematic review and meta-analysis. Infez Med. 2022;30:194–203.
Guerra-De-Blas PDC, Bobadilla-Del-Valle M, Sada-Ovalle I, Estrada-García I, Torres-González P, López-Saavedra A et al. Simvastatin Enhances the Immune Response Against Mycobacterium tuberculosis. Frontiers in Microbiology [Internet]. 2019 [cited 2023 Sep 3];10. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2019.02097.
Lü H-Z, Li B-Q. Effect of HMG-CoA reductase inhibitors on activation of human gammadeltaT cells induced by Mycobacterium tuberculosis antigens. Immunopharmacol Immunotoxicol. 2009;31:485–91.
Biranu E, Wolde M, Negesso AE, Sisay MM, Tola HH. Lipid profile, abnormality of serum glucose levels and their associated factors in multidrug-resistant tuberculosis patients. Global J Obes Diabetes Metabolic Syndrome. 2021;8:018–28.
Wang J, Xiong K, Zhao S, Zhang C, Zhang J, Xu L et al. Long-Term Effects of Multi-Drug-Resistant Tuberculosis Treatment on Gut Microbiota and Its Health Consequences. Frontiers in Microbiology [Internet]. 2020 [cited 2024 Jan 14];11. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2020.00053.
Woudberg NJ, Pedretti S, Lecour S, Schulz R, Vuilleumier N, James RW et al. Pharmacological Intervention to Modulate HDL: What Do We Target? Frontiers in Pharmacology [Internet]. 2018 [cited 2023 Nov 24];8. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fphar.2017.00989.
Inoue M, Niki M, Ozeki Y, Nagi S, Chadeka EA, Yamaguchi T, et al. High-density lipoprotein suppresses tumor necrosis factor alpha production by mycobacteria-infected human macrophages. Sci Rep. 2018;8:6736.
Jung E, Kong SY, Ro YS, Ryu HH, Shin SD. Serum cholesterol levels and risk of Cardiovascular Death: a systematic review and a dose-response Meta-analysis of prospective cohort studies. Int J Environ Res Public Health. 2022;19:8272.
Abedi F, Sadeghi M, Omidkhoda N, Kelesidis T, Ramezani J, Samadi S, et al. HDL-cholesterol concentration and its association with coronary artery calcification: a systematic review and meta-analysis. Lipids Health Dis. 2023;22:60.
Kaur N, Pandey A, Negi H, Shafiq N, Reddy S, Kaur H, et al. Effect of HDL-Raising drugs on Cardiovascular outcomes: a systematic review and Meta-regression. PLoS ONE. 2014;9:e94585.
Wang F, Wang X, Ye P, Cao R, Zhang Y, Qi Y, et al. High-density lipoprotein 3 cholesterol is a predictive factor for arterial stiffness: a community-based 4.8-year prospective study. Lipids Health Dis. 2018;17:5.
Liu C-L, Lu Y-T, Tsai I-F, Wu L-C, Chien W-C, Chung C-H, et al. Fenofibrate Facilitates Post-active Tuberculosis Infection in macrophages and is Associated with higher mortality in patients under long-term treatment. J Clin Med. 2020;9:337.
Schop A, Stouten K, Riedl JA, van Houten RJ, Leening MJG, Bindels PJE, et al. The accuracy of mean corpuscular volume guided anaemia classification in primary care. Fam Pract. 2021;38:735–9.
Cheng L, Zhang L, Liu J, Li W, Bai X, Li R, et al. Prognostic Value of Admission Mean Corpuscular volume for major adverse Cardiovascular events following Stent Implantation in Nondiabetic and Diabetic patients with Acute Coronary Syndrome. Dis Markers. 2020;2020:e7054596.
Wu T-H, Fann JC-Y, Chen SL-S, Yen AM-F, Wen C-J, Lu Y-R, et al. Gradient relationship between increased Mean Corpuscular volume and Mortality Associated with cerebral ischemic stroke and ischemic heart disease: a longitudinal study on 66,294 Taiwanese. Sci Rep. 2018;8:16517.
Wang H, Yang G, Zhao J, Wang M. Association between mean corpuscular volume and severity of coronary artery disease in the Northern Chinese population: a cross-sectional study. J Int Med Res. 2020;48:0300060519896713.
Kwon H, Park B. Borderline-High Mean Corpuscular volume levels are Associated with arterial stiffness among the apparently healthy Korean individuals. Korean J Fam Med. 2020;41:387–91.
Solak Y, Yilmaz MI, Saglam M, Demirbas S, Verim S, Unal HU, et al. Mean corpuscular volume is associated with endothelial dysfunction and predicts composite cardiovascular events in patients with chronic kidney disease. Nephrology. 2013;18:728–35.
Overmars LM, Mekke JM, van Solinge WW, De Jager SCA, Hulsbergen-Veelken CAR, Hoefer IE, et al. Characteristics of peripheral blood cells are independently related to major adverse cardiovascular events after carotid endarterectomy. Atherosclerosis Plus. 2023;52:32–40.
Liu C, Dhindsa D, Almuwaqqat Z, Sun YV, Quyyumi AA. Very high high-density lipoprotein cholesterol levels and Cardiovascular Mortality. Am J Cardiol. 2022;167:43–53.
Fessler MB, Rose K, Zhang Y, Jaramillo R, Zeldin DC. Relationship between serum cholesterol and indices of erythrocytes and platelets in the US population[S]. J Lipid Res. 2013;54:3177–88.
Kohsari M, Moradinazar M, Rahimi Z, Najafi F, Pasdar Y, Moradi A, et al. Association between RBC Indices, Anemia, and obesity-related diseases affected by body Mass Index in Iranian kurdish Population: results from a Cohort Study in Western Iran. Int J Endocrinol. 2021;2021:e9965728.
Noori M, Azadpour S, Valipour AA, Igder S, Malihi R. Relationship of lipid profile and erythrocyte indices in non-anaemic elderly. New Z J Med Lab Sci. 2021;75:206–10.
Loyola-Leyva A, Loyola-Rodríguez JP, Terán-Figueroa Y, González FJ, Atzori M, Camacho-Lopez S. Hypercholesterolemia associated with erythrocytes morphology assessed by scanning electron microscopy in metabolically unhealthy individuals with normal-weight and obesity. Obes Med. 2020;20:100292.
Tziakas D, Chalikias G, Grapsa A, Gioka T, Tentes I, Konstantinides S. Red blood cell distribution width: a strong prognostic marker in cardiovascular disease: is associated with cholesterol content of erythrocyte membrane. Clin Hemorheol Microcirc. 2012;51:243–54.
Arkew M, Gemechu K, Haile K, Asmerom H. Red blood cell distribution Width as Novel Biomarker in Cardiovascular diseases: a Literature Review. JBM. 2022;13:413–24.
Avci E, Kiris T, Demirtas AO, Kadi H. Relationship between high-density lipoprotein cholesterol and the red cell distribution width in patients with coronary artery disease. Lipids Health Dis. 2018;17:53.
Niki M, Yoshiyama T, Nagai H, Miyamoto Y, Niki M, Oinuma K, et al. Nutritional status positively impacts humoral immunity against its Mycobacterium tuberculosis, disease progression, and vaccine development. PLoS ONE. 2020;15:e0237062.
Moopanar K, Nyide ANG, Senzani S, Mvubu NE. Clinical strains of Mycobacterium tuberculosis exhibit differential lipid metabolism-associated transcriptome changes in in vitro cholesterol and infection models. Pathogens Disease. 2023;81:ftac046.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JBB – conceptualisation, formal analysis, methodology, drafting manuscript, revising manuscript, final approval. RN – formal analysis, methodology, revising manuscript, final approval. AK, EN, NM, FM, RO, FB - methodology, revising manuscript, final approval.
Ethics declarations
Ethics committee and consent to participate
All study procedures were conducted in accordance with the Declaration of Helsinki. The study was approved by the Mulago Hospital Research and Ethics Committee (MHREC-2020-23), and the Uganda National Council of Science and Technology (HS1521ES) prior to participant recruitment. Study participants provided written informed consent before study procedures were performed. For participants who couldn’t read the consent form, the document was orally presented to them or their legally authorized representative, in the presence of an independent witness.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baluku, J.B., Nalwanga, R., Kazibwe, A. et al. Association between biomarkers of inflammation and dyslipidemia in drug resistant tuberculosis in Uganda. Lipids Health Dis 23, 65 (2024). https://doi.org/10.1186/s12944-024-02063-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02063-7