Abstract
Background
Subclinical hypothyroidism (SCH) is linked to dyslipidaemia and adverse pregnancy outcomes. However, the impact of dyslipidaemia on the outcome of pregnancy in SCH is unclear.
Methods
We enrolled 36,256 pregnant women and evaluated their pregnancy outcomes. The following data was gathered during the first trimester (≤ 13+ 6 weeks of gestation): total cholesterol (TC), low-density lipoprotein (LDL-C), triglyceride (TG), high-density lipoprotein (HDL-C), free thyroxine (FT4) and thyroid-stimulating hormone (TSH) concentrations. The reference ranges for lipids were estimated to range from the 5th to the 95th percentile. Logistic regression assessed the relationships between dyslipidaemia and adverse pregnancy outcomes, including abortion, preeclampsia/eclampsia, low birth weight, foetal growth restriction, premature rupture of foetal membranes, gestational hypertension, preterm birth, macrosomia and gestational diabetes mellitus (GDM). Additionally, the best thresholds for predicting adverse pregnancy outcomes based on TSH, FT4, and lipid levels were determined using receiver operating characteristic curves.
Results
In the first trimester, LDL-C > 3.24 mmol/L, TG > 1.92 mmol/L, HDL-C < 1.06 mmol/L, and TC > 5.39 mmol/L were used to define dyslipidaemia. In this cohort, 952 (3.56%) patients were diagnosed with SCH, and those who had dyslipidaemia in the first trimester had higher incidences of gestational hypertension (6.59% vs. 3.25%), preeclampsia/eclampsia (7.14% vs. 3.12%), GDM (22.53% vs. 13.77%), and low birth weight (4.95% vs. 2.08%) than did those without dyslipidaemia. However, after adjusting for prepregnancy body mass index (pre-BMI), dyslipidaemia was no longer related to these risks. Furthermore, elevated TG dyslipidaemia in SCH patients was connected to an enhanced potential of gestational hypertension (odds ratio [OR]: 2.687, 95% confidence interval [CI]: 1.074 ~ 6.722), and elevated LDL-C dyslipidaemia correlated with increased preeclampsia/eclampsia risk (OR: 3.172, 95% CI: 1.204 ~ 8.355) after accounting for age, smoking status, alcohol use, pre-BMI, and levothyroxine use. Additionally, the combination of TC, TG, LDL-C, pre-BMI, and TSH exhibited enhanced predictive capabilities for gestational hypertension, preeclampsia/eclampsia, and GDM. Values of 0.767, 0.704, and 0.706 were obtained from the area under the curve.
Conclusions
Among pregnant women with SCH, dyslipidaemia in early pregnancy was related to elevated risks of adverse pregnancy consequences. The combined consideration of age, pre-BMI, TSH, and lipid levels in the first trimester could be beneficial for monitoring patients and implementing interventions to reduce adverse pregnancy outcomes.
Similar content being viewed by others
Background
During pregnancy, lipids are physiologically elevated to accommodate the increased needs of both the mother and foetus. However, excessive lipid levels can lead to lipotoxicity, potentially impacting placental development, inflammation, and metabolism, thereby contributing to adverse perinatal outcomes and potential health issues for the offspring [1]. Elevated maternal triglyceride (TG) and apolipoprotein levels are related to an increased likelihood of macrosomia and preeclampsia [2, 3]. A meta-analysis of 31,402 pregnancies reveals that increased maternal TG also decreased high-density lipoprotein cholesterol (HDL-C) values during pregnancy particularly in overweight or obese women are connected to an increased probability of large for gestational age (LGA) infants and macrosomia and a decreased likelihood of small-for-gestational-age (SGA) infants [4]. Additionally, increased low-density lipoprotein cholesterol (LDL-C) concentrations are related to a higher likelihood of preterm birth [5]. Notably, maternal hypercholesterolemia during pregnancy leads to alterations in the foetal aorta, potentially predisposing the offspring to atherosclerosis later in life [6]. However, the underlying cause of dyslipidaemia during pregnancy remains unclear. Moreover, there are currently no established recommendations for lipid reference ranges during pregnancy, and the lack of safety data has hindered the development of effective medications for controlling dyslipidaemia during pregnancy [7].
Subclinical hypothyroidism (SCH) is the most commonly observed thyroid disorder during pregnancy; SCH is defined by normal free thyroxine (FT4) values and raised thyroid stimulating hormone (TSH) concentrations [8]. Elevated TSH levels are significantly correlated with an unfavourable lipid profile [9] and could serve as a risk factor for dyslipidaemia during pregnancy. Furthermore, several convincing meta-analyses have demonstrated a connection between SCH during pregnancy and an increased incidence of SGA infants, lower birthweights, preeclampsia, and preterm birth [10,11,12]. Two smaller studies have identified differences in lipid molecules and metabolites between pregnant women with SCH or hypothyroidism and euthyroid individuals, suggesting a potential link between lipid profiles and the pathogenesis of thyroid disorders during pregnancy, in addition to unfavourable pregnancy outcomes [13, 14].
There are complex interactions between thyroid-related hormones and lipids during pregnancy. However, it is unclear how dyslipidaemia affects outcomes in patients with SCH during pregnancy. For this reason, the study was designed to discuss the connection between dyslipidaemia in early pregnancy and unfavourable pregnancy outcomes in pregnant women with SCH.
Methods
Participants
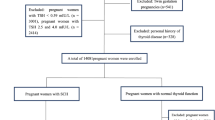
The cohort study enrolled pregnant women who established archives at the Beijing Obstetrics and Gynecology Hospital, Capital Medical University, from February 2018 to December 2020 and who had taken part in the China Birth Cohort Study (CBCS) [15]. All participants were asked to complete questionnaires, underwent clinical laboratory measurements, and were followed up with to assess pregnancy outcomes. To establish reference ranges for lipids in the first trimester, pregnant women who satisfied the following criteria were excluded: (1) dropped out after enrolment; (2) pregnancies with non-singletons; (3) were outside the range of 18 to 50 years old; (4) had incomplete lipid data in the first trimester; (5) had preexisting conditions before pregnancy (e.g., severe cardiac, hepatic, or renal disorders; hypertension; diabetes; thyroid disorders; malignant tumours or postoperative tumours; infectious diseases; or hyperlipidaemia); (6) had used of lipid-affecting medications prior to pregnancy; (7) had pregnancies achieved through assisted reproductive technologies; and (8) were lost to follow-up regarding pregnancy outcomes. Furthermore, during the screening process for pregnant women with SCH, who matched at least one of these conditions were excluded: had used medications affecting thyroid function before pregnancy (e.g., levothyroxine (L-T4), prednisone, budesonide, dexamethasone, propylthiouracil, labetalol, methylprednisolone); had TSH levels in the normal ranges of reference; had TSH levels lower than normal reference ranges; or had FT4 concentrations outside of the normal range. This study received approval from the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University on February 2nd, 2018 (reference number: 2018-KY-003-02). All participants provided informed consent.
Data acquisition
Baseline data were collected using the questionnaires from the CBCS and the medical records at the hospital. Age was calculated using the birthdate and enrolment date. Prepregnancy body mass index (pre-BMI) was obtained by dividing prepregnancy weight in kilograms by height in meters squared. Smoking status and alcohol consumption during pregnancy were categorized as “yes”. Education levels were classified into three groups: high school and below, undergraduate, and graduate. Additionally, prepregnancy health conditions and medication usage were verified through a combination of questionnaires and medical records. The use of L-T4 before and during pregnancy was confirmed through questionnaires, hospital records, and phone follow-ups.
During the first trimester (≤ 13+ 6 weeks gestation), serum samples were collected from those enrolled in the CBCS study and archived at Beijing Obstetrics and Gynecology Hospital, Capital Medical University. The samples were maintained at 4 °C for less than 24 h, and the test was completed on the day of sample collection. Then, if samples need extended storage, they were kept at -20 °C for 48 h or -80 °C for more than three months. TSH and FT4 serum levels were collected via electrochemiluminescence immunoassays (Siemens Healthcare Diagnostics, ADVIA Centaur XP, Tarrytown, NY/USA). A fully automated Integrated System Chemistry/Immunology Analyser (ARCHITECT ci16200, Abbott Park, IL, USA) was used to quantify lipid levels, comprising LDL-C, HDL-C, TG, and total cholesterol (TC) using the appropriate assay kits (reagent cat. No. H05119R02 for TC, G07893R02 for TG, G05251R03 for HDL-C, and G69452R13 for LDL-C, Abbott Park, IL, USA). Initial blood lipid and thyroid function test results in early pregnancy were utilized as key indicators in this investigation.
Definitions
During early pregnancy, SCH was diagnosed by normal concentrations of FT4 and TSH concentrations above 4.0 mIU/L, which is in accordance with the 2017 American Thyroid Association Guidelines [16]. As per our hospital’s standards, the normal reference range for FT4 was established to be 11.8–18.4 pmol/L.
Reference ranges for LDL-C, TG, HDL-C, and TC in early pregnancy were established in this study. Dyslipidaemia was identified by elevated TC, elevated TG, elevated LDL, and reduced HDL according to the reference range in this study. Adverse pregnancy outcomes included abortion (a pregnancy terminated before 28 weeks and with foetal weight less than 1000 g, including artificial abortion and spontaneous abortion), gestational hypertension (diastolic blood pressure ≥ 90 mmHg and/or systolic blood pressure ≥ 140 mmHg that appears after the 20th week of pregnancy and becomes normal within 12 weeks of postpartum, and accompanied by a negative urine protein test), gestational diabetes mellitus (GDM, diagnosed if any following ranges is exceeded: 2-hour post glucose load ≤ 8.5 mmol/L, 1-hour post glucose load ≤ 10.0 mmol/L, fasting blood glucose ≤ 5.1 mmol/L), preeclampsia/eclampsia (meeting criteria for gestational hypertension coupled with significant proteinuria or convulsions arising from preeclampsia without other explanatory causes), premature rupture of foetal membranes (PROM, identified as preterm membrane rupture before 37 weeks of gestation), foetal growth restriction (FGR, characterized by foetuses whose estimated foetal weight falls below the 10th gestational age percentile) [17], low birth weight (regardless of gestational age, birth weight below 2,500 g), macrosomia (neonatal birthweight is equal to or more than 4,000 g, irrespective of gestational age), preterm birth (described as a premature birth before 37 weeks of pregnancy), and birth defects (inclusive of structural deformities and functional abnormalities). The analyses also included modes of delivery, comprising vaginal delivery (encompassing both natural childbirth and operative vaginal birth) and caesarean section.
Statistical analyses
Continuous variables with a normal distribution were displayed in both average and standard deviations; nonnormally distributed values were then in the form of medians and interquartile ranges. For categorical variables, standard deviation and median were used. Subsequently, reference ranges were then established for the first trimester of pregnancy using the 5th to 95th percentiles of lipid measurements. Differences between groups were investigated using Mann-Whitney U and chi-square tests. The relationships between dyslipidaemia and pregnancy consequences were investigated by logistic regression analysis. The adjusted model included age, pre-BMI, smoking status, alcohol use, and L-T4 use during pregnancy as sequential adjustment factors. Receiver operating characteristic (ROC) curve determined the threshold levels for TSH, FT4, lipid levels, age, and pre-BMI for predicting adverse outcomes in pregnant women with SCH. Prediction accuracy was analysed by area under the curve (AUC). Youden index was adopted to find the optimal trade-off between sensitivity and specificity. Analysis of data was carried out with SPSS 26.0 (SPSS, Inc., Chicago, IL, USA). P < 0.05 was seen as an indicator of statistical significance.
Results
First-trimester serum lipid reference range
Among the enrolled pregnant women, 26,776 were included in the analysis to establish reference values. Table 1 displays the average TC, HDL-C, TG, and LDL-C concentrations in the first trimester, and also the respective percentiles. As it is customary to use the 95th percentiles to define the normal reference range [18], the first-trimester reference ranges for serum LDL-C, TG, HDL-C, and TC were set between the 5th and 95th percentiles. Therefore, the following reference values for the first trimester: TC: 3.20 ~ 5.39 mmol/L; TG: 0.56 ~ 1.92 mmol/L; HDL-C: 1.06 ~ 2.01 mmol/L; and LDL-C: 1.37 ~ 3.24 mmol/L.
Baseline information
In total, 952 pregnant women were identified with SCH. The flowchart is depicted in Fig. 1. Among these pregnant women with SCH, 770 (80.88%) presented without dyslipidaemia, while 182 (19.12%) presented with dyslipidaemia. Pregnant women with dyslipidaemia were characterized by advanced maternal age (32.00 years vs. 30.00 years) and had a greater pre-BMI (23.03 kg/m2 vs. 20.76 kg/m2) than did those without dyslipidaemia. Additionally, a greater proportion of women in the dyslipidaemia group were classified as older (age ≥ 35 years) or obese (pre-BMI ≥ 28 kg/m2). In the first trimester of pregnancy, among those with SCH, those with dyslipidaemia exhibited a greater incidence of gestational hypertension (6.59% vs. 3.25%), preeclampsia/eclampsia (7.14% vs. 3.12%), GDM (22.53% vs. 13.77%), and low birth weight (4.95% vs. 2.08%). No significant disparities in other pregnancy outcomes were evident between the dyslipidaemia and the nondyslipidaemia group (Table 2).
Adverse pregnancy outcome risk in patients with dyslipidaemia
Compared to women with SCH who did not have dyslipidaemia, those with dyslipidaemia exhibited an increased risk of developing gestational hypertension (odds ratio [OR]: 2.104, 95% confidence interval [CI] [1.036 ~ 4.271], P = 0.040), preeclampsia/eclampsia (OR: 2.391, 95% CI: 1.193 ~ 4.792, P = 0.014), GDM (OR: 1.821, 95% CI: 1.216 ~ 2.727, P = 0.004), and low birth weight (OR: 2.452, 95% CI: 1.066 ~ 5.640, P = 0.035) in the crude model. However, after accounting for confounding factors, the association lacked statistical significance (Table 3).
As shown in Table 4, after adjusting for L-T4 use and age, dyslipidaemia still raised the risk of the above four undesirable pregnancy outcomes. After including smoking status and alcohol use as adjustment variables, dyslipidaemia in the first trimester was only linked to an enhanced risk of gestational hypertension (OR: 2.810, 95% CI: 1.321 ~ 5.975, P = 0.007) and GDM (OR: 1.702, 95% CI: 1.101 ~ 2.632, P = 0.017) in women with SCH. Moreover, after adjusting for pre-BMI, the correlation did not reach statistical significance.
Associations between different types of dyslipidaemia and pregnancy outcomes
According to the crude model, among pregnant women with SCH, elevated TC dyslipidaemia was correlated with increased preeclampsia/eclampsia risk, while elevated TG dyslipidaemia was linked to increased risks of gestational hypertension, GDM, and low birth weight. Elevated LDL-C dyslipidaemia was correlated with an elevated incidence of GDM and preeclampsia/eclampsia, and reduced HDL-C dyslipidaemia was associated with low birth weight. However, after adjusted for age, L-T4 use, smoking status, and alcohol use, there were still the significant associations between elevated TG dyslipidaemia and an increased gestational hypertension risk (OR: 4.064, 95% CI: 1.691 ~ 9.763, P = 0.002) and GDM (95% CI: 1.071 ~ 3.341, P = 0.028), as well as between elevated LDL-C dyslipidaemia and increased preeclampsia/eclampsia risk (OR: 3.870, 95% CI: 1.502 ~ 9.966, P = 0.005) and between reduced HDL-C dyslipidaemia and increased low birth weight incidence (OR: 3.581, 95% CI: 1.012 ~ 12.666, P = 0.048). Furthermore, after incorporating pre-BMI as an adjustment variable, only the associations between elevated TG dyslipidaemia and the risk of gestational hypertension (OR: 2.687, 95% CI: 1.074 ~ 6.722, P = 0.035) and between elevated LDL-C dyslipidaemia and preeclampsia/eclampsia (OR: 3.172, 95% CI: 1.204 ~ 8.355, P = 0.019) continued to be significant (Table 5).
Baseline information for pregnant women with SCH predicts adverse pregnancy outcomes
As depicted in Table 6, among pregnant women with SCH, TSH was predictive capability for preterm birth (AUC = 0.624, 95% CI: 0.535 ~ 0.714, P = 0.017) and FGR (AUC = 0.608, 95% CI: 0.546 ~ 0.669, P = 0.001), as showed by ROC curve analysis. The established thresholds were 4.565 mIU/L and 5.575 mIU/L, respectively.
Together with pre-BMI data, the TC, TG, and LDL-C concentrations during pregnancy in women with SCH exhibited predictive value for gestational hypertension, preeclampsia/eclampsia, and GDM (TC: AUCs were 0.638, 0.610, and 0.585, respectively; TG: AUCs were 0.616, 0.629, and 0.662, respectively; LDL-C: AUCs were 0.637, 0.659, and 0.593, respectively; pre-BMI: 0.734, 0.632, and 0.625, respectively).
Furthermore, a combination of factors proved more effective in predicting certain adverse outcomes. Specifically, combining pre-BMI, TC, TG, LDL-C, TSH, and TPOAb demonstrated better predictive power for the occurrence of gestational hypertension (AUC = 0.767 95% CI: 0.661 ~ 0.873, P < 0.001, Fig. 2a). Combining pre-BMI, TC, TG, LDL-C, and TSH was suitable for predicting preeclampsia/eclampsia (AUC = 0.704 95% CI: 0. 615 ~ 0.793, P < 0.001, Fig. 2b). Additionally, combining TSH, TC, TG, LDL-C, pre-BMI, and age was found to be more suitable for predicting the occurrence of GDM (AUC = 0.706 95% CI: 0.662 ~ 0.749, P < 0.001, Fig. 2c), and combining TSH, TG, and pre-BMI was more suitable for predicting the occurrence of preterm birth (AUC = 0.672 95% CI: 0.570 ~ 0.774, P = 0.001, Fig. 2d).
ROC for metabolic markers in the first trimester in predicting adverse outcomes in subclinical hypothyroid. (a) ROC curve in predicting gestational hypertension. Combination 1: TC, TG, LDL-C, pre-BMI, TSH and TPOAb. (b) ROC curve in predicting preeclampsia/eclampsia. Combination 2: TC, TG, LDL-C, pre-BMI, and TSH. (c) ROC curve in predicting GDM. Combination 3: TC, TG, LDL-C, pre-BMI, TSH, and age. (d) ROC curve in predicting preterm birth. Combination 4: TG, pre-BMI, and TSH. Abbreviations: GDM gestational diabetes mellitus; TSH, thyroid-stimulating hormone; TC, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; Pre-BMI, pre-pregnancy body mass index
Discussion
This study, which concentrated on women with SCH in their first-trimester pregnancy, indicated that those with dyslipidaemia exhibited elevated incidences of gestational hypertension, preeclampsia/eclampsia, GDM, and low birth weight compared to those without dyslipidaemia. Furthermore, elevated TG dyslipidaemia was correlated with elevated gestational hypertension risk, and elevated LDL-C dyslipidaemia was correlated with elevated preeclampsia/eclampsia risk independent of age, pre-BMI, smoking status, alcohol use, and L-T4 use. Additionally, the combination of lipid and TSH levels had good predictive power for the above pregnancy outcomes.
Dyslipidaemia significantly impacts the long-term health of both mothers and offspring [19, 20]. Elevated TG levels in early pregnancy were shown in previous studies to be correlated with raised risks of gestational hypertension, preeclampsia, GDM, caesarean section, preterm birth, macrosomia, and LGA infants [21, 22]. Additionally, elevated concentrations of LDL-C have been related to an elevated risk of GDM, preterm birth, macrosomia, and intrahepatic cholestasis in pregnancy [23, 24]. Li et al. [25] reported that in women with GDM, TG levels were elevated and HDL-C levels were reduced, and this was combined with higher incidences of caesarean section and macrosomia. Furthermore, within the context of metabolic syndrome, the presence of dyslipidaemia was found to elevate susceptibility to preeclampsia [26]. Notably, this investigation revealed an association between elevated TG and LDL-C dyslipidaemia in early pregnancy and gestational hypertension and preeclampsia/eclampsia in SCH patients.
Among the causes of dyslipidaemia, hypothyroidism is regarded as the most common secondary cause [27]. Thyroid hormones and TSH are significant factors in lipid metabolism disorders [28]. TSH enhances the activation of key enzymes, including sterol regulatory element binding protein 2 and 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, thereby enhancing the accumulation and synthesis of TC and TG within both hepatocytes and adipocytes through distinct signalling pathways [29, 30]. Additionally, an increase in the serum TSH concentrations coincides with the synchronous upregulation of proprotein convertase subtilisin/kexin type 9, causing a reduced LDL-C clearance [31]. A meta-analysis demonstrated an association between elevated values of TG, LDL-C and TC and SCH, whereas the association with HDL-C levels was less clear [32].
Dyslipidaemia constitutes a pivotal risk factor for cardiovascular ailments. The accumulation of lipids, which results in foam cell formation, along with hyperlipidaemic stress-induced inflammation and oxidative stress, are significant precursors to atherosclerotic cardiovascular diseases [33]. The nuclear receptor peroxisome proliferator-activated receptor-γ, which is present in preeclamptic placentas, is stimulated by high levels of TG and free fatty acids, and it ultimately results in abnormal placental vasculature [34]. SCH also frequently coincides with alterations in myocardial function [35], vascular abnormalities [36], and shifts in lipid metabolism [32], potentially heightening the risk of cardiovascular events. Thus, pregnant women with SCH and dyslipidaemia face an elevated risk of developing gestational hypertension and preeclampsia/eclampsia. It is recommended that hypothyroidism is screened for in individuals with dyslipidaemia, particularly those with hypercholesterolemia [37]. Recent studies have suggested that treatment with L-T4 can lead to improvements in TC and LDL-C concentrations in patients with SCH during pregnancy [38]. However, another study reported that using L-T4 in SCH patients to mitigate the risk of cardiovascular disease may not yield unequivocal benefits [39]. In addition, the risk of GDM increases in patients with SCH. TSH can directly reduce the synthesis and secretion of insulin in pancreatic β cells and can also affect insulin resistance [40]. Additionally, thyroid marker levels in early pregnancy are linked to an elevated incidence of GDM. This risk is largely influenced by specific lipid species [41]. Moreover, lipid biomarkers in the first trimester are associated with GDM and have been shown to be strongly predictive of GDM [42].
Notably, pre-BMI has a significant impact on pregnancy outcomes. After accounting for pre-BMI, the association between dyslipidaemia and adverse pregnancy outcomes also changed. Prospective data showed that individuals who were obese (BMI ≥ 25 kg/m2) or overweight (23 kg/m2 ≤ BMI ≤ 24.9 kg/m2) before pregnancy were at increased risk of GDM, preeclampsia, caesarean section, preterm, stillbirth, macrosomia, and LGA but were at decreased risk SGA [43]. Furthermore, a retrospective observational study showed that LGA was independently associated with BMI only in women without GDM [44]. This study demonstrated that pre-BMI independently predicted gestational hypertension during pregnancy in women with SCH, and the ideal cut-off point was 22.048 kg/m2. Moreover, age and pre-BMI during pregnancy are both important influencing factors on lipid metabolism. Studies have shown that pre-BMI is the main factor determining the rate of change of TC and LDL-C concentrations [45], and active intervention before or during the first trimester is more effective [46]. In addition, elevated TSH levels have been correlated with an increased BMI [47]. Furthermore, considering lipid levels, thyroid function, age and pre-BMI in pregnant women with SCH had good predictive effects on pregnancy outcomes.
Study strengths and limitations
As a cohort study during pregnancy, the present study had a large sample size and concentrated on how dyslipidaemia affects unfavourable pregnancy outcomes in women with SCH. Normal reference ranges for lipids in early pregnancy were established in this study and L-T4 use during pregnancy was included as an adjusting variable in the analyses. However, several limitations warrant consideration. First, the research focused on a specific geographical area, potentially introducing bias. Second, thyroid-related hormones and lipids were assessed only in the first trimester, overlooking the dynamic interplay between SCH and lipids throughout pregnancy. Third, thyroid autoimmune-related indicators, such as thyroid peroxidase antibodies and thyroglobulin antibodies, were incorporated into this study, potentially bearing relevance to the mechanistic underpinnings of dyslipidaemia in SCH during pregnancy and its associated adverse outcomes. Consequently, larger sample sizes and well-designed multicentre studies are imperative to corroborate the present findings and to gain a comprehensive understanding of the intricate interplay among dyslipidaemia, thyroid dysfunction, and unfavourable pregnancy outcomes.
Conclusions
Dyslipidaemia in the first trimester was positively linked to gestational hypertension, preeclampsia/eclampsia, GDM, and low birth weight, which was more affected by pre-BMI. Analysing unfavourable pregnancy outcomes for women with SCH requires a comprehensive assessment of both thyroid function and lipid profiles. In addition, improving the pre-BMI in women with SCH may be beneficial for reducing the risk of adverse pregnancy outcomes.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SCH:
-
Subclinical hypothyroidism
- TSH:
-
Thyroid stimulating hormone
- FT4:
-
Free thyroxine
- L-T4:
-
Levothyroxine
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-C:
-
High density lipoprotein cholesterol
- LDL-C:
-
Low density lipoprotein cholesterol
- BMI:
-
Body mass index
- CBCS:
-
China Birth Cohort Study
- GDM:
-
Gestational diabetes mellitus
- PROM:
-
Premature rupture of fetal membranes
- FGR:
-
Fetal growth restriction
- LGA:
-
Large for gestational age
- SGA:
-
Small for gestational age
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
References
Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond). 2010;119(3):123–9. https://doi.org/10.1042/cs20090640.
Serrano NC, Guio-Mahecha E, Quintero-Lesmes DC, Becerra-Bayona S, Paez MC, Beltran M, et al. Lipid profile, plasma apolipoproteins, and pre-eclampsia risk in the GenPE case-control study. Atherosclerosis. 2018;276:189–94. https://doi.org/10.1016/j.atherosclerosis.2018.05.051.
Barbour LA, Hernandez TL. Maternal non-glycemic contributors to fetal growth in obesity and gestational Diabetes: spotlight on lipids. Curr Diab Rep. 2018;18(6):37. https://doi.org/10.1007/s11892-018-1008-2.
Wang J, Moore D, Subramanian A, Cheng KK, Toulis KA, Qiu X, et al. Gestational dyslipidaemia and adverse birthweight outcomes: a systematic review and meta-analysis. Obes Rev. 2018;19(9):1256–68. https://doi.org/10.1111/obr.12693.
Wild R, Weedin EA, Wilson D. Dyslipidemia in pregnancy. Endocrinol Metab Clin North Am. 2016;45(1):55–63. https://doi.org/10.1016/j.ecl.2015.09.004.
Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: fate of early lesions in children (FELIC) study. Lancet. 1999;354(9186):1234–41. https://doi.org/10.1016/s0140-6736(99)02131-5.
Lewek J, Banach M. Dyslipidemia Management in pregnancy: why is it not covered in the guidelines? Curr Atheroscler Rep. 2022;24(7):547–56. https://doi.org/10.1007/s11883-022-01030-w.
Teng W, Shan Z, Patil-Sisodia K, Cooper DS. Hypothyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013;1(3):228–37. https://doi.org/10.1016/s2213-8587(13)70109-8.
van Vliet NA, Bos MM, Thesing CS, Chaker L, Pietzner M, Houtman E, et al. Higher thyrotropin leads to unfavorable lipid profile and somewhat higher Cardiovascular Disease risk: evidence from multi-cohort mendelian randomization and metabolomic profiling. BMC Med. 2021;19(1):266. https://doi.org/10.1186/s12916-021-02130-1.
Derakhshan A, Peeters RP, Taylor PN, Bliddal S, Carty DM, Meems M, et al. Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2020;8(6):501–10. https://doi.org/10.1016/s2213-8587(20)30061-9.
Toloza FJK, Derakhshan A, Männistö T, Bliddal S, Popova PV, Carty DM, et al. Association between maternal thyroid function and risk of gestational Hypertension and pre-eclampsia: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2022;10(4):243–52. https://doi.org/10.1016/s2213-8587(22)00007-9.
Korevaar TIM, Derakhshan A, Taylor PN, Meima M, Chen L, Bliddal S, et al. Association of thyroid function test abnormalities and thyroid autoimmunity with Preterm Birth: a systematic review and Meta-analysis. JAMA. 2019;322(7):632–41. https://doi.org/10.1001/jama.2019.10931.
Li J, Xu Y, Sun Z, Cai Y, Wang B, Zhang M, et al. Differential lipids in pregnant women with subclinical hypothyroidism and their correlation to the pregnancy outcomes. Sci Rep. 2021;11(1):19689. https://doi.org/10.1038/s41598-021-99252-6.
Cai Y, Xu Y, Ban Y, Li J, Sun Z, Zhang M, et al. Plasma lipid Profile and intestinal microflora in pregnancy women with hypothyroidism and their correlation with pregnancy outcomes. Front Endocrinol (Lausanne). 2021;12:792536. https://doi.org/10.3389/fendo.2021.792536.
Yue W, Zhang E, Liu R, Zhang Y, Wang C, Gao S, et al. The China birth cohort study (CBCS). Eur J Epidemiol. 2022;37(3):295–304. https://doi.org/10.1007/s10654-021-00831-8.
Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 guidelines of the American thyroid Association for the diagnosis and management of thyroid Disease during pregnancy and the Postpartum. Thyroid. 2017;27(3):315–89. https://doi.org/10.1089/thy.2016.0457.
Fetal Growth Restriction. ACOG Practice Bulletin, Number 227. Obstet Gynecol. 2021;137(2):e16–e28. https://doi.org/10.1097/aog.0000000000004251.
Piechota W, Staszewski A. Reference ranges of lipids and apolipoproteins in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992;45(1):27–35. https://doi.org/10.1016/0028-2243(92)90190-a.
Busso D, Rigotti A. Blood lipids during pregnancy: a progressively appreciated subject in basic and clinical research. Atherosclerosis. 2018;276:163–5. https://doi.org/10.1016/j.atherosclerosis.2018.06.884.
De Assis SM, Seguro AC, Helou CM. Effects of maternal hypercholesterolemia on pregnancy and development of offspring. Pediatr Nephrol. 2003;18(4):328–34. https://doi.org/10.1007/s00467-003-1082-8.
Zhu SM, Zhang HQ, Li C, Zhang C, Yu JL, Wu YT, et al. Maternal lipid profile during early pregnancy and birth weight: a retrospective study. Front Endocrinol (Lausanne). 2022;13:951871. https://doi.org/10.3389/fendo.2022.951871.
Jiang XF, Wang H, Wu DD, Zhang JL, Gao L, Chen L, et al. The impact of Gestational Weight Gain on the risks of adverse maternal and infant outcomes among normal BMI women with high triglyceride levels during early pregnancy. Nutrients. 2021;13(10). https://doi.org/10.3390/nu13103454.
Wang C, Zhu W, Wei Y, Su R, Feng H, Hadar E, et al. The associations between early pregnancy lipid profiles and pregnancy outcomes. J Perinatol. 2017;37(2):127–33. https://doi.org/10.1038/jp.2016.191.
Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW. Maternal lipids at mid-pregnancy and the risk of preterm delivery. Acta Obstet Gynecol Scand. 2012;91(6):726–35. https://doi.org/10.1111/j.1600-0412.2012.01391.x.
Li Y, Wang X, Jiang F, Chen W, Li J, Chen X. Serum lipid levels in relation to clinical outcomes in pregnant women with gestational Diabetes Mellitus: an observational cohort study. Lipids Health Dis. 2021;20(1):125. https://doi.org/10.1186/s12944-021-01565-y.
Ellerbrock J, Hubers E, Ghossein-Doha C, Schiffer V, Alers RJ, Jorissen L, et al. Second-trimester constituents of the metabolic syndrome and pregnancy outcome: an Observational Cohort Study. Nutrients. 2022;14(14). https://doi.org/10.3390/nu14142933.
Biondi B, Cappola AR, Cooper DS, Subclinical Hypothyroidism. Rev Jama. 2019;322(2):153–60. https://doi.org/10.1001/jama.2019.9052.
Liu H, Peng D. Update on dyslipidemia in hypothyroidism: the mechanism of dyslipidemia in hypothyroidism. Endocr Connect. 2022;11(2). https://doi.org/10.1530/ec-21-0002.
Zhang X, Song Y, Feng M, Zhou X, Lu Y, Gao L, et al. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J Lipid Res. 2015;56(5):963–71. https://doi.org/10.1194/jlr.M047654.
Ma S, Jing F, Xu C, Zhou L, Song Y, Yu C, et al. Thyrotropin and obesity: increased adipose triglyceride content through glycerol-3-phosphate acyltransferase 3. Sci Rep. 2015;5:7633. https://doi.org/10.1038/srep07633.
Gong Y, Ma Y, Ye Z, Fu Z, Yang P, Gao B, et al. Thyroid stimulating hormone exhibits the impact on LDLR/LDL-c via up-regulating hepatic PCSK9 expression. Metabolism. 2017;76:32–41. https://doi.org/10.1016/j.metabol.2017.07.006.
Liu XL, He S, Zhang SF, Wang J, Sun XF, Gong CM, et al. Alteration of lipid profile in subclinical hypothyroidism: a meta-analysis. Med Sci Monit. 2014;20:1432–41. https://doi.org/10.12659/msm.891163.
Hurtubise J, McLellan K, Durr K, Onasanya O, Nwabuko D, Ndisang JF. The different facets of Dyslipidemia and Hypertension in Atherosclerosis. Curr Atheroscler Rep. 2016;18(12):82. https://doi.org/10.1007/s11883-016-0632-z.
McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC, et al. Evidence implicating peroxisome proliferator-activated receptor-γ in the pathogenesis of preeclampsia. Hypertension. 2011;58(5):882–7. https://doi.org/10.1161/hypertensionaha.111.179440.
Chen X, Zhang N, Cai Y, Shi J. Evaluation of left ventricular diastolic function using tissue Doppler echocardiography and conventional doppler echocardiography in patients with subclinical hypothyroidism aged < 60 years: a meta-analysis. J Cardiol. 2013;61(1):8–15. https://doi.org/10.1016/j.jjcc.2012.08.017.
Owen PJ, Sabit R, Lazarus JH. Thyroid Disease and vascular function. Thyroid. 2007;17(6):519–24. https://doi.org/10.1089/thy.2007.0051.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. Circulation. 2014;129(25 Suppl 2):1–45. https://doi.org/10.1161/01.cir.0000437738.63853.7a.
Yang Y, Yuan H, Wang X, Zhang Z, Liu R, Yin C. Significance of levothyroxine treatment on serum lipid in pregnant women with subclinical hypothyroidism. BMC Pregnancy Childbirth. 2022;22(1):623. https://doi.org/10.1186/s12884-022-04950-2.
Sue LY, Leung AM. Levothyroxine for the treatment of subclinical hypothyroidism and Cardiovascular Disease. Front Endocrinol (Lausanne). 2020;11:591588. https://doi.org/10.3389/fendo.2020.591588.
Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and Diabetes Mellitus: two closely Associated disorders. Endocr Rev. 2019;40(3):789–824. https://doi.org/10.1210/er.2018-00163.
Wang Y, Sun F, Wu P, Huang Y, Ye Y, Yang X, et al. A prospective study of early-pregnancy thyroid markers, lipid species, and risk of gestational Diabetes Mellitus. J Clin Endocrinol Metab. 2022;107(2):e804–e14. https://doi.org/10.1210/clinem/dgab637.
Wang Y, Huang Y, Wu P, Ye Y, Sun F, Yang X, et al. Plasma lipidomics in early pregnancy and risk of gestational Diabetes Mellitus: a prospective nested case-control study in Chinese women. Am J Clin Nutr. 2021;114(5):1763–73. https://doi.org/10.1093/ajcn/nqab242.
Chen Y, Wan K, Gong Y, Zhang X, Liang Y, Wang X, et al. Assessing the relationship between pregravid body mass index and risk of adverse maternal pregnancy and neonatal outcomes: prospective data in Southwest China. Sci Rep. 2021;11(1):7591. https://doi.org/10.1038/s41598-021-87135-9.
Cosson E, Cussac-Pillegand C, Benbara A, Pharisien I, Nguyen MT, Chiheb S, et al. Pregnancy adverse outcomes related to pregravid body mass index and gestational weight gain, according to the presence or not of gestational Diabetes Mellitus: a retrospective observational study. Diabetes Metab. 2016;42(1):38–46. https://doi.org/10.1016/j.diabet.2015.06.001.
Farias DR, Franco-Sena AB, Vilela A, Lepsch J, Mendes RH, Kac G. Lipid changes throughout pregnancy according to pre-pregnancy BMI: results from a prospective cohort. BJOG. 2016;123(4):570–8. https://doi.org/10.1111/1471-0528.13293.
Catalano P. Maternal pre-pregnancy BMI: harbinger of late-pregnancy maternal lipid profile. BJOG. 2016;123(4):579. https://doi.org/10.1111/1471-0528.13296.
Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019–24. https://doi.org/10.1210/jc.2004-2225.
Acknowledgements
We thank all pregnant women participated in CBCS and the medical staff for collecting clinical information. We also thank to all the researchers who created the CBCS database.
Funding
This work was supported by The National Key Research and Development Program of China under Grant (2016YFC1000101); Beijing Municipal Science & Technology Commission under Grant (Z181100001718076); and Capital’s Funds for Health Improvement and Research under Grant (2022-1-2111); and Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital ‘Discipline Backbone’ Plan Special Funds under Grant (XKGG201803).
Author information
Authors and Affiliations
Contributions
CHY, WTY, RXL, EJZ, YZ, SG, SFS, SHX, JHL, YYL, and YSY participated in the establishment of CBCS. XRW, EJZ, RXL, SG, SFS, SHX and JHL contributed to the conception and design of the study. ZZ, KKH, ZYT and RZ provided consultation on clinical issues and statistical methods. XRW, EJZ, and RXL drafted the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Beijing Obstetrics and Gynecology Hospital, Capital Medical University Ethics Committee gave its approval to the study protocol on February 2nd, 2018 (reference number: 2018-KY-003-02). All participants provided their informed consent to be a part of this study and approved the sharing of their data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Zhang, E., Tian, Z. et al. The association between dyslipidaemia in the first trimester and adverse pregnancy outcomes in pregnant women with subclinical hypothyroidism: a cohort study. Lipids Health Dis 23, 13 (2024). https://doi.org/10.1186/s12944-023-01998-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01998-7