Abstract
Idiopathic pulmonary fibrosis (IPF) is considered an age-related disease. Age-related changes, along with other factors such as obesity, hormonal imbalances, and various metabolic disorders, lead to ectopic fat deposition (EFD). This accumulation of fat outside of its normal storage sites is associated with detrimental effects such as lipotoxicity, oxidative stress, inflammation, and insulin resistance. This narrative review provides an overview of the connection between ectopic and visceral fat deposition in aging, obesity, and IPF. It also elucidates the mechanism by which ectopic fat deposition in the airways and lungs, pericardium, skeletal muscles, and pancreas contributes to lung injury and fibrosis in patients with IPF, directly or indirectly. Moreover, the review discusses the impact of EFD on the severity of the disease, quality of life, presence of comorbidities, and overall prognosis in IPF patients. The review provides detailed information on recent research regarding representative lipid-lowering drugs, hypoglycemic drugs, and lipid-targeting drugs in animal experiments and clinical studies. This may offer new therapeutic directions for patients with IPF.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive disease that leads to the formation of lung scarring. The pathogenesis of IPF involves complex interactions between various cell types and signaling pathways, and the precise triggers and exact cause of IPF are still unknown. However, studies have reported that the development of IPF begins with alveolar epithelial injury in the context of predisposing factors, such as genetics, aging, environment, epigenetics, immune response, and comorbidities. Persistent injury leads to metabolic dysfunction, senescence, abnormal epithelial cell activation, and impaired epithelial repair in alveolar epithelial cells (AECs). Dysregulated AECs interact with mesenchymal cells, immune cells, and endothelial cells through multiple signaling mechanisms [1]. Molecular abnormalities involved in a series of profibrotic cellular interactions have been identified; the affected factors include reactive oxygen species (ROS), inflammatory cytokines, pulmonary surfactants, matrix remodeling factors, growth factors, and noncoding RNAs. Various cellular processes are also thought to promote lung fibrosis; such processes include cell apoptosis, oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress. These complex changes occur as a result of AEC injury, ultimately leading to the transformation of fibroblasts into myofibroblasts, excessive deposition of extracellular matrix (ECM), pulmonary interstitial fibrosis, progressive worsening of the disease, and eventually respiratory failure and death. Current treatment options for IPF have limited efficacy. Although two drugs, pirfenidone and nintedanib, approved by the Food and Drug Administration (FDA), have been reported to delay the decline in lung function in some IPF patients, the prognosis of IPF remains poor. The median survival of newly diagnosed adult IPF patients (typically over 60 years old) is less than 5 years [2]. Lung transplantation is an effective treatment option for patients with end-stage IPF, but it is limited to a relatively young and healthy subset of patients [3]. Therefore, a better understanding of the underlying systemic pathogenic factors and mechanisms involved in IPF is crucial for optimizing IPF management and treatment.
IPF has been demonstrated to be an age-related disease [4], and changes in body composition accompany the processes of aging and obesity. Alterations in the immune-metabolic characteristics of adipose tissue and the redistribution of fat have been identified as risk factors for various age-related diseases [5]. Fat tissue not only functions to regulate temperature and store energy, as recent findings have also revealed its active role as an endocrine and immune organ. Adipose-derived factors and immune cell populations within adipose tissue impact systemic immunity and metabolism. Different immune cell populations exist in adipose tissue, and their composition and immune responses vary based on nutritional and environmental conditions. Specifically, factors such as aging and obesity promote low-grade sterile inflammation within adipose tissue and excessive infiltration of immune cells. This is accompanied by a decline in the ability of adipose tissue to store lipids, leading to ectopic fat deposition (EFD). However, cold exposure resolves obesity-induced chronic inflammation [6]. Compared to subcutaneous fat, visceral adipose tissue (VAT) is more strongly associated with chronic inflammatory diseases such as coronary artery disease, nonalcoholic steatohepatitis, diabetes, and obesity. In fact, there is also increasing recognition of the relationship between VAT and various lung diseases, including IPF. The effects of excessive VAT on pulmonary diseases include its mechanical effects on the respiratory tract, lipotoxicity, pro-inflammatory properties, and oxidative stress. Recent evidence suggests that VAT could be a modifiable risk factor for IPF [7]. However, body composition analysis of IPF patients is often overlooked, and there is currently no comprehensive review on the complex relationship between fat deposition and IPF.
There is growing interest in the role of lipids in regulating the process of pulmonary fibrosis. However, whether ectopic and visceral fat deposition serves as a profibrotic factor in the development of fibrosis and as a clinically intervenable factor remains largely unknown. This review emphasizes the frequently overlooked role of fat deposition in pulmonary fibrosis and summarizes abundant basic experiments and clinical trials. This is the first review to summarize lipid-lowering drugs, hypoglycemic drugs, and lipid-targeting drugs as a therapeutic approach for pulmonary fibrosis. By using bioinformatics methods, this review reveals lipid metabolism-related genes (LMRGs) associated with pulmonary fibrosis, introduces IPF assessment tools that are easily applicable in clinical practice, and offers novel intervention approaches from a new perspective to improve fat deposition-associated pulmonary fibrosis.
Definition and causes of EFD
When adipose tissue dysfunction occurs or when the energy intake exceeds the storage capacity of subcutaneous adipose tissue (SAT), further calorie overload leads to excess lipid accumulation. Excess lipids accumulate in organs and tissues such as the liver, heart (pericardium, epicardium, and myocardium), lungs, intestines, pancreas, skeletal muscles, and blood vessels. This process is known as "EFD" [8]. One characteristic of EFD in humans is increased VAT accumulation, which is associated with abdominal obesity and is unrelated to body mass index (BMI) [8]. Obesity and aging significantly affect adipose tissue function by altering the spectrum of adipokines secreted by adipocytes, promoting adipocyte hypertrophy, changing the population and function of fibroadipogenic progenitor (FAP) cells, and increasing adipose tissue macrophage (ATM) infiltration [9]. These effects prevent SAT from proliferating and expanding to serve as a protective fat storage depot. In fact, several factors can contribute to increased fat deposition; these factors include high-fat diets, high-sugar diets, decreased physical activity, low serum albumin levels [10] (which binds and transports free fatty acids [FFAs]), male sex, and hormonal imbalance [11].
EFD in the lung induces alveolar structural and functional damage in IPF
Accumulating evidence indicates that a high-fat diet promotes lung fibrosis [12]. In obese individuals, fat can directly accumulate in the lung and airways; adipose tissue can be found in the outer walls of the larger airways, correlating with BMI, airway wall thickness, and higher neutrophil counts [13]. Studies on obese animal models have shown elevated levels of phospholipids and triglycerides in lung tissue [14]. Abundant lipid droplets can be observed in the pulmonary interstitium and lung macrophages, concomitant with the destruction of ultrastructural features of alveolar epithelial type II cells (AT2), expansion of rough endoplasmic reticulum, reduced cellular biosynthesis, impaired secretion of lung surfactant, and increased interstitial collagen [15]. Animal studies have revealed that obese diabetic rats exhibit a 136% increase in total lung triglyceride content, a 32% increase in interstitial collagen fibers, and a reduced diffusing capacity of the lungs for carbon monoxide (DLCO) [16].
EFD can also occur in lung lipofibroblasts (LIFs) of obese individuals. LIFs are important lung stromal cells that are commonly found adjacent to AT2 cells and support the self-renewal and differentiation of AT2 stem cells to AT1 cells. LIFs provide triglycerides to AT2 cells for the synthesis of pulmonary surfactant [17]. Fat deposition associated with diabetes, obesity, and aging leads to impaired function of lung LIFs, compromising their ability to aid in the renewal of AECs and maintain alveolar lipid homeostasis. Furthermore, dysfunctional LIFs can directly transdifferentiate into myofibroblasts, resulting in excessive ECM production and subsequent pulmonary fibrosis [18,19,20].
Lipotoxicity of fat deposition and IPF: direct cytotoxicity and indirect proinflammatory effects
Lipotoxicity of FFAs to AECs promotes pulmonary fibrosis
The profibrotic role of pulmonary EFD is associated with the lipotoxicity of excessive fatty acids on AECs. Enlarged adipocytes also exhibit enhanced lipolysis, leading to increased delivery of FFAs to other organs. Increased FFA levels can disrupt the integrity of biological membranes in EFD tissues and alter cellular acid‒base homeostasis. FFAs have been shown to activate Toll-like receptor 2 (TLR-2), TLR-4/nuclear factor-kappaB (NF-κB), and c-Jun N-terminal kinase (JNK) signaling pathways, thereby promoting inflammation and insulin resistance [21, 22]. Furthermore, FFAs serve as precursors for the synthesis of harmful bioactive lipids, particularly ceramides and diacylglycerols. Overall, the deleterious effects resulting from the secretion of adipokines, lipid molecules, and inflammatory factors from ectopic fat tissues are referred to as "lipotoxicity."
Elevated levels of palmitic acid esters (a saturated FFA) have been observed in the lungs of patients with IPF, leading to endoplasmic reticulum stress and apoptosis in AECs. This phenomenon has been confirmed in a bleomycin (BLM)-induced IPF mouse model fed different diets [23]. The lipotoxicity of AECs induced by a high-fat diet suggests that EFD contributes to the initiation of IPF and exacerbates fibrosis severity. In addition to inducing endoplasmic reticulum stress and AEC apoptosis, lung EFD has been associated with increased lipid levels in bronchoalveolar lavage fluid (BALF) in a BLM-induced model. Alveolar macrophages engulf extracellular oxidized phospholipids and transform into lipid-laden foam cells, releasing more transforming growth factor beta1 (TGF-β1) and further exacerbating pulmonary fibrosis [24]. Lipid-lowering agents and cluster of differentiation 36 (CD36, a fatty acid translocase) inhibitors or CD36 gene knockout reduced the differentiation of lung fibroblasts to myofibroblasts in BLM mice [25, 26]. This suggests that EFD plays a crucial role in pulmonary fibrosis through macrophage-CD36 oxidative lipid signaling.
Further metabolites of FFAs, known as bioactive sphingolipids, such as sphingosine-1-phosphate (S1P), play an important role in the pathogenesis of pulmonary fibrosis [27]. Under conditions of nutrient overload, S1P synthesis increases using neural-derived sphingolipids as substrates, and S1P acts as a second messenger by autocrine or paracrine binding to G protein-coupled receptors. Studies have shown that the levels of sphingosine kinase 1 (SPHK1, catalyzing the generation of S1P) are significantly increased in IPF patient lung tissues and strongly correlated with α-smooth muscle actin (α-SMA), vimentin, and type I collagen. S1P and SPHK1 levels in BALF, serum, and peripheral blood monocytes of IPF patients are negatively correlated with lung function and positively correlated with mortality rate [28]. Animal and cell experiments have shown that the SPHK1/S1P signaling pathway is associated with TGF-β signaling, promoting the activation of fibroblasts and their transformation into myofibroblasts through the activation of mitochondrial Rho kinase, the Hippo/YAP (Yes-associated protein) pathway, etc. [29,30,31].
Mechanism of adipose-derived adipokines in pulmonary fibrosis
In addition to lipid molecules such as FFAs, adipose-derived adipokines are also considered key participants in the development of pulmonary fibrosis in IPF patients and BLM-treated mice. Changes in the secretion levels of various adipokines, including hormones (such as leptin and adiponectin) and peptides (such as angiotensinogen, apelin, resistin, and plasminogen activator inhibitor-1 [PAI-1]), have been observed in obese and elderly patients [32, 33]. Leptin and adiponectin play a role in the pathogenesis of obesity-related lung diseases by affecting systemic inflammation, regulatory T (Treg) cell activity, and T helper cell 17 (Th17) and T helper cell 2 (Th2) immune responses [34]. It is known that aging, a high-fat diet, and adipose tissue dysfunction caused by obesity increase the leptin/adiponectin ratio, which is associated with lung function and fibrosis markers [35]. Serum leptin levels are positively correlated with body fat and negatively correlated with lung function. In contrast to leptin, adiponectin levels are decreased in subjects with impaired lung function and obesity [36].
Leptin is secreted by adipocytes in white adipose tissue, and leptin receptors are highly expressed on the surface of alveolar macrophages. The binding of leptin to its receptor drives the activation of the NOD (nucleotide oligomerization domain)-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome. This leads to the production of pro-inflammatory and pro-fibrotic cytokines, such as interleukin (IL)-1, IL-18, and TGF-β, promoting AEC mitochondrial stress, cellular apoptosis, and insulin resistance [37]. Activation of the NLRP3 inflammasome is also closely associated with increased collagen deposition and enhanced expression of connective tissue growth factor in pulmonary fibrosis [38]. Increased IL-1β signaling in the lungs promotes the expression of proinflammatory cytokines (such as IL-23 and IL-5) and recruits T cells, B cells, and eosinophils to produce IL-13 and TGF-β1, which are critical regulatory factors for fibroblast activation and excessive ECM production [39]. However, VAT has a stronger negative correlation with adiponectin than subcutaneous fat [40]. Adiponectin was identified as an initiator of AMP-activated protein kinase (AMPK)-dependent autophagy.
Deficiency of adiponectin, which is associated with EFD, can lead to the generation of ROS and potassium efflux. This induces mitochondrial dysfunction and results in lung injury and activation of the NLRP3 inflammasome [41]. Adiponectin has also been identified as an anti-atherosclerotic, anti-inflammatory, and anti-diabetic adipokine, and these protective effects are attributed to its impact on the activation of the NF-kB (nuclear factor kappa B) pathway in B cells, which enhances insulin sensitivity [37, 42].
Another important adipokine is angiotensinogen (AGT), which is produced by adipose tissue and accounts for one-third of the circulating AGT levels. In the obese state, adipose tissue-produced AGT increases [43], leading to excessive activation of the local adipose tissue and systemic renin-angiotensin system (RAS) [44,45,46]. Studies have revealed that patients with the ID/DD (insertion/deletion) polymorphism of angiotensin-converting enzyme (indicating higher levels of the enzyme) are prone to pulmonary fibrosis [47]. Angiotensin II (Ang II) has been identified as a pro-apoptotic and pro-fibrotic factor in experimental pulmonary fibrosis animal models. In human lung fibroblast cultures, Ang II induces the activation of TGF-β1/Smad2/3, promoting fibroblast-myofibroblast transition [48]. Elevated Ang II levels in the local or circulation of mouse lungs can induce progressive pulmonary fibrosis, while renin inhibitors such as aliskiren or angiotensin II type 1 receptor-specific antagonists, such as losartan, can block the production of ECM proteins and fibrogenic factors [49, 50].
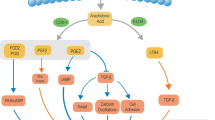
Similar to Ang II, the adipokine PAI-1 is also overexpressed and released by adipocytes in obesity; it has been shown to have a definite promoting effect on pulmonary fibrosis [51]. PAI-1 is a recognized inhibitor of fibrinolysis and can also affect the functionality of fibronectin, thereby interfering with cell adhesion [52]. Its overexpression contributes to the accumulation of ECM. PAI-1 is increased in the lungs of patients with pulmonary fibrosis. It not only promotes fibrosis but also activates alveolar macrophages to promote inflammation, and through TGF-β1, it strongly induces AT2 cell senescence [53]. However, it should be noted that the current research on the direct relationship among Ang II, PAI-1 sourced from excessive adipose tissues, and IPF in humans is still limited in terms of quantity. Considering that visceral fat is one of the main sources of fibrotic and inflammatory factors, further research into the mechanisms underlying the association between visceral fat and fibrosis is crucial. The changes in aging adipose tissue and the involvement of fat deposition in the occurrence and development of IPF are shown in Fig. 1.
Alterations in aging adipose tissue and the involvement of fat deposition in the occurrence and development of IPF. 1) During the aging process, excessive expansion of adipose tissue leads to hypoxia. This stimulates adipocytes and ATMs to secrete inflammatory chemokines, resulting in immune cell infiltration in aging adipose tissue. 2) Fibrosis in dysfunctional adipose tissue leads to lipotoxicity and an increased leptin/adiponectin ratio. This activates highly proinflammatory M1-type macrophages (M1 ATMs) through molecules such as leptin, PAI-1, FFA, and inflammatory cytokines, thereby exacerbating the inflammatory response. 3) Lipotoxicity and inflammation in aging adipose tissue leads to endoplasmic reticulum stress, mitochondrial dysfunction, apoptosis, autophagy and necrosis of AT2 cells. Subsequently, in the alveoli, cell debris, recruited immune cells, and foam cells (macrophages engulfing lipid droplets) participate in the inflammatory cascade response, resulting in fibroblast-to-myofibroblast (MYF) transformation and epithelial-mesenchymal transition (EMT). 4) Adipose factors such as Ang II, PAI-1, and S1P can also promote fibroblast-to-MYF transformation. 5) Lipotoxicity and inflammation not only promote the differentiation of LIFs into MYFs but also affect the supply of pulmonary surfactant precursors to AT2 cells. The figure was created using BioRender (www.biorender.com). Abbreviations: adipose tissue macrophages (ATMs), plasminogen activator inhibitor-1 (PAI-1), free fatty acids (FFA), alveolar epithelial type II cells (AT2), myofibroblast (MYF), epithelial-mesenchymal transition (EMT), Angiotensin II (Ang II), sphingosine-1-phosphate (S1P), lipofibroblasts (LIFs)
Insulin resistance and immune cell infiltration in the fat deposition of lungs promote IPF
Insulin resistance in fat deposition promotes IPF through TGF-β signaling
Insulin resistance caused by elevated levels of adipokines, resistin and retinol-binding protein 4 and reduced levels of adiponectin is another potential mechanism for the occurrence and development of IPF [54]. Additionally, enlarged fat cells release proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), IL-6, IL-8, and monocyte chemotactic protein-1 (MCP-1), leading to serine phosphorylation of insulin receptor substrate-1 (IRS-1) production and blocking insulin signal transduction. This consequently reduces insulin sensitivity and causes insulin resistance, which is a key feature of metabolic syndrome [55]. Compared to elderly patients without metabolic syndrome, elderly patients with metabolic syndrome have higher airway resistance. They also exhibit higher levels of proinflammatory mediators, such as leptin, IL-1β, IL-8, and TNF-α, lower levels of anti-inflammatory mediators, including adiponectin, IL-1 receptor antagonist, and IL-10, and increased expression levels of TGF-β1 and phosphorylated Smad-2/3 [35]. In mice, intranasal insulin administration enhances bronchial epithelial TGF-β1 expression, activating the TGF-β/Smad signaling pathway and causing fibrosis around the airways and blood vessels. TGF-β also stimulates the differentiation of Th0 cells into Th17 cells, which release IL-17 and contribute to airway hyperreactivity [54]. Serum vitamin D and NAD (nicotinamide adenine dinucleotide)-dependent deacetylase sirtuin (SIRT), an anti-aging factor, levels are decreased under conditions of insulin resistance. Vitamin D deficiency inhibits the phosphorylation of Smad-2/3, activates RAS activity, and subsequently activates TGF-β1 signaling, promoting pulmonary fibrosis [56]. SIRT-1 has been shown to inhibit NF-κB activity and reduce inflammation through various mechanisms, including inhibiting iNOS (inducible nitric oxide synthase) activity and downregulating COX-2 (Cyclooxygenase-2) expression, thereby alleviating oxidative stress. Aerobic exercise in obese mice improves insulin resistance, reduces neutrophil infiltration in the lungs, decreases pro-inflammatory, pro-oxidative stress, and pro-fibrotic factors in BALF, and upregulates the expression of anti-inflammatory factors IL-10 and SIRT-1 mRNA in the lungs [57]. Furthermore, studies have indicated that SIRT-1 acts as a target for anti-pulmonary fibrosis drugs and inhibits the EMT in BLM-induced pulmonary fibrosis in mice [58].
Fat deposition participates in the pathogenesis of IPF through immune cell infiltration
The presence of inflammatory cells in dysfunctional adipose tissue can affect adjacent tissues and organs [59]. As mentioned earlier, ectopic fat can be directly deposited in airways, alveolar interstitium, lung LIFs, and alveolar macrophages, indicating that the lungs can be directly influenced by inflammatory factors released from local adipose tissue and immune cell infiltration. Enlarged adipocytes and reduced capillary density in hypertrophic adipose tissue lead to a hypoxic state in adipocytes, characterized by abnormal preadipocyte differentiation, inflammation, altered secretion profile, increased oxidative stress and mitochondrial dysfunction in adipocytes, and accumulation of aged fat cells and fibrosis in adipose tissue [60]. The differentiation of preadipocytes to adipocytes is decreased, and instead, their differentiation to ATMs expressing surface markers, such as F4/80, CD80, and CD86, is increased. Moreover, adipocytes undergo hypoxic cell death, recruiting a large number of monocytes through MCP-1. These monocytes differentiate into proinflammatory M1 macrophages and form “crown-like structures,” a process activated through the NLRP3 pathway [61]. During the formation of crown-like structures, lipid metabolism increases in ATMs, leading to lipotoxicity, inflammation, and enhanced insulin resistance [62].
In obese and elderly VAT, ATMs are the most abundant immune cells. These cells account for 10% of immune cells in normal subjects and 50% of immune cells in obese individuals, and the ratio of M1 ATMs (proinflammatory characteristics) to M2 ATMs (anti-inflammatory characteristics) is significantly increased in obese individuals [63]. Hypoxia may induce inflammation through hypoxia-inducible factor 1-alpha (HIF-1α) gene expression, triggering the secretion of proinflammatory mediators such as TNF-α, IL-6, IL-8, MCP-1, adipokines, and retinol-binding protein by hypertrophic adipocytes and M1 ATMs and promoting further immune cell infiltration [64, 65]. Lymphocytes constitute the second most abundant immune cell population in the VAT of obese and elderly patients. There was a twofold increase in CD3 + T cells, predominantly CD8 + T cells (cytotoxic T cells), in aged mouse VAT compared to young animal VAT, and a similar trend was observed in obese mice [66, 67]. NLRP3 regulates IL-18 and interferon-γ (IFN-γ) in white adipose tissue and promotes the differentiation of effector CD8 + T cells, releasing proinflammatory and profibrotic molecules, such as IL-13 and IL-17, and M1 ATMs and alveolar macrophages. This leads to lung and systemic inflammation and insulin resistance [68]. Previous studies have shown a significant increase in the expression levels of IL-1β, IL-8, and IL-6 in BALF and lung tissue of pulmonary fibrosis patients and in animal models, and IL-1β or IL-6/IL-13 activation of JAK2 (Janus kinase 2) and STAT3 (Signal transducer and activator of transcription 3) stimulates primary AT2 and lung fibroblasts. This stimulates the release of TGF-β1 by immune cells and fibroblasts, which induces EMT and fibroblast-to-MYF transformation, and promotes AT2 cell aging and an apoptotic phenotype [69, 70]. In the BLM-induced lung fibrosis animal model, lung inflammation, fibrosis, and collagen deposition depend on the IL-1R1/MyD88 signaling pathway [71]. Elevated levels of IL-6 (> 25.20 pg/mL) are an independent risk factor for acute exacerbation (AE-IPF) (odds ratio [OR] 1.014, p = 0.036) and mortality (OR 1.007, p = 0.018) in patients with interstitial lung diseases [72]. IL-17A inhibits autophagy in bronchial epithelial cells through the PI3K/Akt/mTOR pathway [73]. It also promotes lung fibroblast proliferation and contributes to lung inflammation and fibrosis through the IL-17A-TGFβ axis. The primary function of IL-8 is to amplify the differentiation of mesenchymal stem cells to fibroblasts, promote lung fibroblast proliferation and migration, recruit and activate macrophages, and play a crucial role in airway fibrosis and remodeling [74].
In recent years, it has been demonstrated that ectopic adipose tissue outside the lungs is also involved in the pathogenesis of IPF. Excessive pericardial adipose tissue is a rich source of proinflammatory mediators in the systemic circulation and has been associated with higher levels of inflammatory markers (IL-6, TNF-α, MCP-1, CD11c, and iNOS) and fibrotic markers (collagen levels, TGF-β, matrix metalloproteinase-3) in various cardiovascular and pulmonary diseases, such as COVID-19 (Coronavirus Disease 2019), COPD, pulmonary arterial hypertension, sleep apnea syndrome, heart failure, coronary heart disease, and lung transplant recipients. Therefore, excessive pericardial adipose tissue indicates a poor prognosis of these diseases. In 2021, Anderson MR and his colleagues found that for each doubling in pericardial adipose tissue volume, the odds of interstitial lung abnormalities increased by 20%, while the FVC (forced vital capacity) percentage predicted a decreased of 5.5%. The study also identified the involvement of IL-6 and leptin in the association between adipose tissue and lung fibrosis [75]. These findings suggest that proinflammatory cytokines and adipokines from ectopic adipose tissue outside the lungs can enter the pulmonary circulation and cause lung injury. In addition to inflammatory factors and adipokines, the neutrophil-to-lymphocyte ratio (NLR) and serum hs-CRP levels have also demonstrated a positive correlation with pericardial adipose tissue volume, and a high NLR has been shown to independently influence the occurrence of IPF [76, 77].
Fat deposition aggravates lung function loss in IPF
Mechanism of sarcopenia caused by fat infiltration
Studies suggest that fat deposition aggravates lung function loss in IPF, including but not limited to increased fat infiltration in skeletal muscle, airway and pericardial adipose tissues. Skeletal muscle fat infiltration and skeletal muscle atrophy are considered to be harmful to muscle mass, strength, activity, and muscle metabolism [78]. The most common cause of death in IPF patients is chronic respiratory failure, and skeletal muscle atrophy and skeletal muscle fat deposition are very common in patients with respiratory failure requiring mechanical ventilation and malnutrition [79]. Additionally, these factors have been shown to increase the risks of hospitalization and death in IPF [80]. Chun-wei Li et al. proposed that dysfunction of adipocytes caused by aging and obesity is the earliest driving factor of local inflammation and insulin resistance [81]. This is followed by a systemically expanded vicious loop called “the metabaging cycle,” in which excessive lipids can “spill over” into skeletal muscle tissue. These lipids accumulate in the form of intermuscular lipids, intramyocellular lipids, and lipid droplets within muscle cells, leading to the accumulation of toxic lipids such as diacylglycerol and ceramides in skeletal muscle tissue [81]. Ceramides directly induce insulin resistance in skeletal muscle cells by blocking downstream signaling of insulin, such as the translocation of glucose transporter-4 (the main glucose transporter for glucose uptake in skeletal muscle) [82]. Various other obesity-related lipid metabolites, such as homocysteine, free fatty acids, ROS, uric acid, and cholesterol crystals, activate the NLRP3 inflammasome to induce the production of IL-1β and IL-18 by macrophages. These cytokines can then further promote inflammation in T cells, impairing skeletal muscle insulin sensitivity [83]. Muscle tissue is one of the primary effectors of insulin. Insulin resistance in muscle leads to restricted glucose uptake and synthesis of muscle glycogen, as well as limited lipid uptake by muscle tissue. As a result, blood glucose is directed toward the synthesis of more fat in adipocytes, leading to the further elevation of free fatty acid concentrations and local hyperlipidemia. The increased blood glucose load contributes to systemic endogenous free radicals and inflammation, perpetuating the metabaging cycle [84].
In obesity, factors such as TNF-α, IL-18, IL-6, and iNOS are released by M1 ATMs, leading to reactive atrophy of skeletal muscle tissue and a decrease in the number of muscle cells [85]. As a population of mesenchymal stem cells, FAPs (fibro-adipogenic progenitors) possess multipotent differentiation potential, including the ability to differentiate to fibroblasts, adipocytes, chondrocytes, and osteoblasts [86]. When regulated by paracrine signals from adipose tissue proinflammatory factors, FAPs in skeletal muscle can differentiate to a fat cell-like phenotype, leading to reduced muscle cell regeneration and increased skeletal muscle fat infiltration. TNF-α, released by M1 ATM1s, plays a crucial role in the process of muscle wasting and fat infiltration within skeletal muscle. Studies have shown that high levels of TNF-α directly impair mitochondrial biogenesis in muscle cells and disrupt myotube formation in human primary myoblasts [87]. Additionally, TNF-α, through the activation of TNF receptor 1, triggers the activation of the caspase cascade, increasing apoptosis of muscle cells and FAPs. This subsequently increases the release of TNF-α and exacerbates the vicious cycle. TNF-α not only induces programmed cell death in skeletal muscle cells but also upregulates ROS directly or indirectly through adipocyte necrosis and lipotoxicity. This, in turn, activates the NF-κB pathway and upregulates the expression of muscle-specific E3 ubiquitin ligase, muscle RING-finger protein-1 (MuRF1), promoting proteolysis of myofibrillar proteins and muscle wasting [88]. In summary, the deposition of intramuscular lipids demonstrates significant lipotoxicity, leading to the induction and aggravation of mitochondrial dysfunction, oxidative stress, insulin resistance, and inflammation. These molecular changes interact with each other, resulting in a vicious cycle that impairs muscle regeneration and ultimately increases the risk of systemic muscle wasting or cachexia [51].
Muscle fat infiltration is associated with lung function loss
The mechanisms underlying muscle wasting due to EFD can explain the prognostic differences observed in different nutritional phenotypes in IPF patients. In a prospective study of 90 IPF patients, the proportions of normally nourished, nonsarcopenic obese, sarcopenic and sarcopenic obese (muscle loss with increased visceral fat) patients were 67.8%, 25.3%, 4.6%, and 2.3%, respectively [89]. Compared to patients with nonsarcopenic obesity or sarcopenia, patients with sarcopenic obesity showed decreased protein synthesis and increased protein breakdown in respiratory muscles. These patients also exhibited a reduction in respiratory muscle mitochondria and mitochondrial dysfunction compared to healthy control individuals [90]. This suggests a synergistic amplification of adverse consequences through the metabaging cycle formed by increased EFD and skeletal muscle loss, leading to maximized metabolic damage, decreased quality of life, and increased morbidity and mortality rates of IPF. IPF is a restrictive lung disease, and there is strong evidence from large-sample studies suggesting that sarcopenic obesity is primarily associated with an increased risk of restrictive lung disease in the elderly (OR 2.81, 95% confidence interval [CI]: 1.72–4.59). The sarcopenic obesity group had a significantly lower FVC than the normal control group, while the FEV1/FVC ratio (an indicator of obstructive ventilation) was not significantly different between the two groups [91]. The distribution of visceral fat and changes in muscle mass also explain the contradictory observations of BMI in the prognosis of IPF. Evidence suggests that weight loss in IPF indicates an increased risk of hospitalization and worse prognosis [92, 93]. However, some studies have also revealed a protective effect of high BMI on survival in respiratory disease patients [94]. This “obesity paradox” is partly due to the limitations of using BMI to measure visceral obesity [95]. This suggests that weight, BMI, or other body composition indicators may not be suitable prognostic indicators for IPF, and more direct measures of body composition need to be determined. Quantification of skeletal muscle, visceral fat, and lean body mass has become a new hotspot in research [96, 97]. Studies have demonstrated that sarcopenia (decreased quantity and poor physical performance) in patients with IPF is associated with high severity, poor quality of life and poor prognosis [98,99,100,101,102,103]. Inspiratory muscle training in IPF patients who can tolerate pulmonary rehabilitation is beneficial because it partially offsets muscle fat infiltration and muscle mass reduction associated with aging and improves disuse muscle atrophy [104, 105].
In conclusion, the damage inflicted by muscle fat deposition in IPF patients is multifactorial, including its impact on respiratory muscle dysfunction contributing to respiratory failure, systemic inflammation, oxidative stress, and cachexia. These findings may have substantial implications for the management of IPF patients, and the assessment of body composition, including muscle and visceral fat, should become a routine clinical practice in IPF. Future research can evaluate nutritional interventions based on patients' nutritional phenotypes and develop personalized respiratory muscle training and other pulmonary rehabilitation programs.
Other factors lead to a negative effect on lung function
In addition to sarcopenia and respiratory weakness caused by respiratory muscle fat infiltration, there are at least three other factors that contribute to the negative impact of fat deposition on lung function in IPF patients. 1) Fat deposition in the visceral cavity produces mechanical obstructive effects on the respiratory tract and restrictive effects on the diaphragm. 2) Lipotoxicity resulting from fat deposition damages alveolar ultrastructure, reduces surfactant production, and promotes lung tissue fibrosis, leading to pulmonary diffusion dysfunction. It also leads to mild systemic inflammation that impairs lung immune responses and increases airway hyperresponsiveness (as discussed in Sects. " EFD in the lung induces alveolar structural and functional damage in IPF" and " Lipotoxicity of fat deposition and IPF: direct cytotoxicity and indirect proinflammatory effects" of this review). 3) Fat deposition is involved in various IPF complications, including OSAS, pulmonary hypertension, COPD, and hemodynamic disturbance caused by increased pericardial fat [106]. EFD in the mediastinum and abdominal cavity limits lung expansion, leading to a significant decrease in expiratory reserve volume and functional residual capacity. The reduction in functional residual capacity is directly proportional to the severity of obesity, with overweight, mildly obese, and severely obese subjects presenting reduction rates of 10%, 22%, and 33%, respectively [107]. Fat deposition in the airways, extrapleural space, and chest wall reduces lung compliance and increases respiratory resistance, resulting in a direct mechanical impact on respiratory function. Chronic lipotoxicity primarily affects lung diffusing capacity, while the cardiopulmonary complications of IPF mainly lead to ventilation/perfusion (V/Q) mismatch. Compared to patients with other chronic lung diseases, IPF patients often experience more common hypoxemia and accompanying pulmonary hypertension due to impaired V/Q balance, which also limits tolerance to pulmonary rehabilitation therapy in IPF. The one-year incidence of AE-IPF is approximately 16.5%, and EFD-related IL-6 and IL-8 are predictive factors for the early onset of AE-IPF [108]. The pulmonary function impairment caused by EFD is of considerable importance in lethal AE-IPF cases [109]. In multivariate analysis, resting hypoxemia requiring oxygen therapy ([hazard ratio]HR 2.44, 95% CI: 1.45–4.10), every 10% decrease in FVC percentage predicted (HR 1.28, 95% CI: 1.10–1.49), and every 10% decrease in DLCO percentage predicted (HR 1.25, 95% CI: 1.04–1.51) were significantly associated with an increased risk of death or lung transplantation in IPF patients [110].
It has been shown that obesity-induced impaired lung function in patients can be effectively reversed through weight loss surgery [111]. In experimental animals that underwent gastric sleeve surgery, an improvement in alveolar structure, a reduction in collagen fiber and lipid deposition, an inhibition of the excessive proliferation of chronic hypoxia-induced capillary basement membrane, and an increase in capillary blood supply were observed [15]. Fortunately, fat deposition and lung function impairment caused by aging can be partially improved through dasatinib and quercetin. Senolytics are a class of drugs that selectively induce the death of senescent cells [5], and dasatinib and quercetin constitute the first combination of senolytic drugs. Dasatinib can eliminate aged adipocyte progenitor cells [112] and reduce the secretion of inflammatory mediators in aging VAT. With senolytics treatment, the BLM-induced lung fibrosis mice showed downregulation of the inflammatory pathway in lung tissue and significant improvements in lung function and physical fitness [2]. In the first human trial, treatment with dasatinib and quercetin resulted in an average improvement of 21.5 m in the 6-min walking distance of elderly patients [113]. With the progress of preclinical and phase I clinical trials, senolytics have shown great therapeutic prospects in IPF [114].
Fat deposition promotes complications in IPF, intensifies the pathogenicity of environmental factors in IPF, and aggravates IPF prognosis and lung transplant outcomes
Fat deposition contributes to complications of IPF
The majority of IPF patients have pulmonary and/or extrapulmonary complications. Only 60–70% of deaths are directly attributable to IPF-related conditions, with the cause of death in the remaining patients likely being other comorbidities present in the elderly population [115]. In a meta-analysis that included 126 studies, pulmonary complications in IPF included pulmonary hypertension (prevalence rate 3–86%), COPD (6–67%), OSAS (6–91%), and lung cancer (3–48%), and the nonpulmonary diseases included type 2 diabetes (10–42%), ischemic heart disease (3–68%), congestive heart failure, gastroesophageal reflux disease (0–94%), sarcopenia, anxiety and/or depression [116]. These complications have been shown to be related to the functional status, quality of life, and survival time of IPF. This is particularly true for lung cancer and pulmonary hypertension, which have the most substantial impact on the survival and lung transplant outcomes of IPF patients [117, 118]. Additionally, the cumulative number of complications is negatively correlated with IPF survival rates. Pulmonary hypertension before transplantation is associated with poor posttransplant survival [HR 4.832, p = 0.039] and increases the risk of posttransplant complications [119]. The EMPIRE registry study included 3,580 IPF patients from multiple countries, and at the time of enrollment, 91.3% of patients had been diagnosed with at least one comorbidity, with over one-third (37.8%) reporting four or more comorbidities. The 5-year survival rates for patients without common complications and with 1, 2, 3, and ≥ 4 complications were 53.7%, 48.4%, 47.0%, 43.8%, and 41.1%, respectively [120].
Fat deposition increases the risk of complications in IPF
These comorbidities share common risk factors with IPF, and one of these factors is fat deposition (Fig. 2). Furthermore, fat deposition exacerbates the pathogenicity of environmental factors (such as exposure to cigarette smoke and pathogens) on IPF and its comorbidities. For instance, fat deposition not only plays a role in the development of IPF through mechanisms such as lipotoxicity, inflammation, oxidative stress, and fibrogenesis but also has direct evidence of fat deposition in the pancreas, leading to pancreatic fat infiltration. This pancreatic fat infiltration contributes to the occurrence of diabetes, which is a common pulmonary comorbidity in IPF patients [121]. Adipocytes mainly infiltrate the pancreatic parenchyma and accumulate near islets. The number of D68-positive cells in islets is positively correlated with homeostatic model assessment of insulin resistance (HOMA-IR) and the area of pancreatic adipocytes and leads to intensified local inflammation, β-cell apoptosis promotion, and alterations to insulin secretion and glucose tolerance [122], which are well-known mechanisms of diabetes. A case‒control study showed that type 2 diabetes is an independent risk factor for IPF, with a higher incidence of diabetes in IPF patients than in patients without IPF (11.3% vs. 2.9%) [119, 123]. A meta-analysis of 260,000 individuals revealed that the odds of having diabetes were increased by 1.54 times in IPF patients compared to patients without IPF (95% CI, 1.30–1.84; P < 0.001) [124]. Another meta-analysis of nine case‒control studies also reported similar results (OR 1.65, P < 0.0001) [125]. In a cohort study, the presence of diabetes (HR 2.5, 95% CI 1.04–5.9) was identified to increase mortality in the IPF cohort [126]. Based on this evidence, the co-occurrence and connection of pancreatic and pulmonary pathologies in IPF can be partially explained by EFD. In addition to extrapulmonary comorbidities, EFD is also involved in respiratory system comorbidities in IPF. The mechanical effects of EFD on airway caliber, lung capacity, and cardiac diastole are mainly associated with COPD, OSA, and pulmonary hypertension, while its promotion of inflammation and airway hyperresponsiveness is mainly associated with asthma and increased pathogenicity of environmental factors (such as COPD and pulmonary infections) [127, 128]. Fat deposition leads to decreased numbers and functional defects of natural killer cells, resulting in impaired malignant cell clearance and an increased risk of lung cancer [129]. Its impact on respiratory muscle depletion is mainly related to respiratory failure and cachexia in COPD and lung cancer [130]. Long-term hypoxemia contributes to the occurrence of pulmonary heart disease.
Alterations in adipose tissue distribution in aging individuals contribute to the development of IPF. The left part illustrates the distribution of white adipose tissue and brown adipose tissue in the healthy human body. The right part shows a list of comorbidities associated with an excessive accumulation of ectopic fat and visceral adipose tissue in elderly individuals. The figure was created using BioRender (www.biorender.com). Abbreviations: alveolar epithelial type II cells (AT2), chronic obstructive pulmonary disease (COPD), insulin resistance (IR), lipofibroblast (LIF), myofibroblast (MYF), obstructive sleep apnea syndrome (OSAS), pulmonary arterial hypertension (PAH), surfactant protein A (SPA)
Drugs of hypoglycemic or lipid-lowering and targeted lipid-mediated pathways for pulmonary fibrosis
An increasing number of researchers believe that hyperglycemia and lipid deposition may be risk factors for pulmonary fibrosis, which is closely associated with systemic inflammation and oxidative stress. In recent years, various drugs for glycemic regulation and lipid modulation have shown antifibrotic properties. Among them, hypoglycemic drugs, including empagliflozin (a sodium-glucose cotransporter-2 inhibitor), liraglutide (a glucagon-like peptide 1 receptor agonist), metformin, and rosiglitazone, have been shown to have good effects in alleviating pulmonary fibrosis in various animal models (see Table 1). Lipid-lowering drugs have also attracted attention, and studies have shown that fenofibrate, pravastatin, atorvastatin, ezetimibe and probucol can significantly reduce the development of pulmonary fibrosis in animal models (see Table 1, Ref. [131,132,133,134,135,136,137,138,139]).
Moreover, based on preclinical and clinical research data, three major lipid-targeting drugs have been tested in patients with IPF (Table 2. Ref. [140,141,142,143,144,145,146]). First, mTOR inhibitors or PI3K/mTOR inhibitors, such as sirolimus (rapamycin) and omipalisib (GSK2126458), have completed randomized, double-blind phase I clinical trials for patients with IPF. Another lipid target of interest is LPA1, which has been shown to mediate fibroblast recruitment [147]. In a phase II clinical trial, the first-generation LPA1 receptor antagonist BMS986020 significantly slowed the decline rate of FVC in patients with IPF, but this trial was prematurely terminated due to an increased risk of hepatic enzyme abnormalities. The second-generation LPA1 receptor antagonist BMS986278 has demonstrated good properties in various preclinical animal experiments [148] and is currently in a phase II clinical trial. The third potential lipid target is ATX. Phase III clinical trials of the ATX antagonist GLPG1690 (ISBELA 1 and 2) to treat IPF were terminated because the benefit-risk profile no longer supported continuing the study. However, other ATX antagonists are still under investigation, such as the drugs BBT-877 and cudetaxestat (BLD-0409), which are poised to enter phase II clinical trials to evaluate their efficacy and safety in patients with IPF.
In addition, other lipid-targeting drugs are currently being tested in clinical trials for IPF, such as PBI4050 (a GPR40 agonist and GPR84 antagonist). This drug has completed an open-label phase II clinical trial in IPF patients, demonstrating its safety when used alone or in combination with nintedanib or pirfenidone. Furthermore, GPLG1250 (a functional antagonist of GPR84) has shown antifibrotic effects in animal models and has completed phase II clinical trials. In addition to the targets that have entered testing, many lipid metabolism-related genes that are under investigation, such as Thy-1 (a glycophosphatidylinositol anchored cell surface glycoprotein), SphK1, and S1PL (S1P lyase), have shown promising antifibrotic effects in in vitro or animal experiments and may become new therapeutic targets [149, 150]. In conclusion, further research on the mechanisms of glycemic regulation, lipid modulation and lipid-targeting drugs in pulmonary fibrosis may provide new treatment options for patients with IPF.
LMRGs are associated with poor prognosis of IPF
To further discuss the relationship between lipid metabolism and IPF prognosis at the gene level, this review provides prognostic analysis results according to LMRGs. These results suggest that high expression levels of multiple LMRGs, which promote lipid accumulation, were associated with a poor survival prognosis in IPF patients. An additional file shows this in more detail (see Additional file 1).
The EFD-related alterations in fat metabolism and secretion explain the negative correlation between excessive VAT and IPF progression, quality of life, and prognosis. This review highlights the benefits of interventions such as NLRP3 inflammasome-targeted therapy to improve ectopic fat tissue dysfunction, anti-aging treatments, aerobic exercise, respiratory muscle strength training, dietary modifications, and even bariatric surgery for IPF patients. Additionally, this review summarized that fat deposition is a common risk factor for both IPF and its pulmonary and extrapulmonary comorbidities. The reported findings suggest that in the majority of IPF patients who currently have limited drug treatment options and are unable to tolerate pulmonary rehabilitation, improving ectopic and visceral fat deposition and managing IPF comorbidities play a key role in optimizing survival quality and extending survival time for IPF patients.
Strengths and limitations
The major strength of this review is that it provides a new perspective on the pathogenesis and prognosis of IPF. Improving ectopic and visceral fat can contribute to the prevention and treatment of this fatal disease. Furthermore, understanding the molecular mechanisms and signaling pathways of excessive fat deposition-related pulmonary fibrosis is crucial for researchers and drug developers to identify new therapeutic targets. Moreover, the biomarkers, clinical assessment tools, treatments, complications, and prognosis of IPF discussed in this review can improve clinical management. However, there are some limitations in this review. First, differences in study designs and participants make it challenging to extract data for meta-analysis or to give recommendations and guidance based on reliable evidence. Second, it is necessary to continuously track the outcomes of ongoing clinical trials to determine the safety and efficacy of these drugs (hypoglycemic or lipid-lowering drugs, lipid-targeting drugs) in the treatment of IPF. Last, due to a lack of relevant studies, this review cannot provide quantitative thresholds and changes in blood glucose, lipid levels, and fat deposition during the occurrence and development of pulmonary fibrosis.
Conclusions
In summary, the impact of ectopic and visceral fat deposition on IPF is complex and involves multiple factors, including mechanical injury, lipotoxicity, inflammatory mediators, and insulin resistance. Additionally, ectopic and visceral fat deposition plays a role in various stages of IPF, from onset and exacerbation to complications and prognosis. Current research indicates that medications aimed at improving sugar and lipid metabolism may slow the rate of decline in lung function and reduce the extent of pathological lung fibrosis. Potential therapeutic targets associated with abnormal adipose tissue function have been identified; these targets include the NLRP3 inflammasome, SIRT, and important lipid-related genes linked to IPF.
This review holds great relevance for clinical practice, as it highlights a noticeable correlation between fat deposition and pulmonary fibrosis based on clinical observations. While the six-minute walk test is a commonly employed method in clinical practice to evaluate cardiopulmonary function and prognosis in IPF, it may not be feasible for patients in advanced stages or experiencing acute exacerbations. The review introduces various indicators and tools of body composition analysis that have demonstrated a robust association with lung function and prognosis in pulmonary fibrosis. These noninvasive, easily quantifiable assessment methods offer potential alternatives for evaluating IPF conditions. They pave the way for identifying the necessity for improvements in body fat distribution and exercise capacity, especially in high-risk pulmonary fibrosis patients. Furthermore, this review emphasizes the importance of focusing on the mechanisms of excessive fat deposition in IPF and the latest clinical evidence, which holds promising prospects for the future. This suggests that physicians can potentially prevent and treat IPF by intervening in obesity (through lifestyle interventions and lipid-targeting drugs), addressing sarcopenia (through exercise and pulmonary rehabilitation), and targeting inflammation and LMRGs (via inflammasome modulation and potential gene therapies). However, to gain a deeper understanding of the role of excessive fat deposition in IPF, it is necessary to provide simultaneous assessments of ectopic fat deposition, metabolic status, and the degree of lung fibrosis. Experimental validation of key mechanisms is also essential in future studies. Ultimately, these efforts may lead to the development of novel management or treatment strategies for IPF, the formulation of personalized nutritional and rehabilitation plans, and the significant assessment of lung transplantation risks.
Availability of data and materials
Not applicable.
Abbreviations
- IPF:
-
Idiopathic pulmonary fibrosis
- EFD:
-
Ectopic fat deposition
- NOD:
-
Nucleotide oligomerization domain
- NLRP3:
-
NOD-like receptor thermal protein domain associated protein 3
- AECs:
-
Alveolar epithelial cells
- ROS:
-
Reactive oxygen species
- ECM:
-
Extracellular matrix
- FDA:
-
Food and Drug Administration
- VAT:
-
Visceral adipose tissue
- SAT:
-
Subcutaneous adipose tissue
- BMI:
-
Body mass index
- FAP:
-
Fibro-adipogenic progenitor
- ATMs:
-
Adipose tissue macrophages
- FFAs:
-
Free fatty acids
- COPD:
-
Chronic obstructive pulmonary disease
- IR:
-
Insulin resistance
- LIF:
-
Lipofibroblast
- MYF:
-
Myofibroblast
- OSAS:
-
Obstructive sleep apnea syndrome
- PH:
-
Pulmonary hypertension
- SPA:
-
Surfactant protein A
- AT2:
-
Alveolar epithelial type II cells
- DLCO:
-
Diffusing capacity of the lungs for carbon monoxide
- TLR-2:
-
Toll-like receptor 2
- JNK:
-
C-Jun N-terminal kinase
- NF-κB:
-
Nuclear factor-kappaB
- BLM:
-
Bleomycin
- BALF:
-
Bronchoalveolar lavage fluid
- TGF-β1:
-
Transforming growth factor beta1
- S1P:
-
Sphingosine-1-phosphate
- SPHK1:
-
Sphingosine kinase 1
- α-SMA:
-
α-Smooth muscle actin
- YAP:
-
Yes-associated protein
- PAI-1:
-
Plasminogen activator inhibitor-1
- EMT:
-
Epithelial-mesenchymal transition
- MAPKs:
-
Mitogen-activated protein kinases
- Th17:
-
T helper cell 17
- IL:
-
Interleukin
- AMPK:
-
AMP-activated protein kinase
- AGT:
-
Angiotensinogen
- RAS:
-
Renin-angiotensin system
- Ang II:
-
Angiotensin II
- MYF:
-
Myofibroblast
- TNF-α:
-
Tumor necrosis factor-alpha
- MCP-1:
-
Monocyte chemotactic protein-1
- IRS-1:
-
Insulin receptor substrate-1
- SIRT:
-
Sirtuin
- IFN-γ:
-
Interferon-γ
- STAT3:
-
Signal transducer and activator of transcription 3 (STAT3)
- CI:
-
Confidence interval
- HR:
-
Hazard Ratio
- FVC:
-
Forced vital capacity
- NLR:
-
Neutrophil-to-lymphocyte ratio
- MuRF1:
-
Muscle-specific E3 ubiquitin ligase, muscle RING-finger protein-1
- HOMA-IR:
-
Homeostatic Model Assessment of Insulin Resistance
- PI3K/mTOR:
-
Phosphoinositide 3-kinase/ mammalian target of rapamycin
- FAPs:
-
Fibro-Adipogenic Progenitors
- LPA1:
-
Lysophosphatidic acid receptor type1
- ATX:
-
Autotaxin
- LMRGs:
-
Multiple lipid metabolism-related genes
References
Moss BJ, Ryter SW, Rosas IO. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Ann Rev Pathol. 2022;17:515–46. https://doi.org/10.1146/annurev-pathol-042320-030240.
Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. https://doi.org/10.1038/ncomms14532.
Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, et al. Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2021;40:1349–79. https://doi.org/10.1016/j.healun.2021.07.005.
Cho SJ, Stout-Delgado HW. Aging and lung disease. Ann Rev Physiol. 2020;82:433–59. https://doi.org/10.1146/annurev-physiol-021119-034610.
Tylutka A, Morawin B, Walas Ł, Michałek M, Gwara A, Zembron-Lacny A. Assessment of metabolic syndrome predictors in relation to inflammation and visceral fat tissue in older adults. Sci Rep. 2023;13:89. https://doi.org/10.1038/s41598-022-27269-6.
Sugimoto S, Mena HA, Sansbury BE, Kobayashi S, Tsuji T, Wang CH, et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat Metab. 2022;4:775–90. https://doi.org/10.1038/s42255-022-00590-0.
Selvan K, Adegunsoye A. The progression to interstitial lung disease: vicarious facts about visceral fat. Chest. 2021;160:400–2. https://doi.org/10.1016/j.chest.2021.05.002.
Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–25. https://doi.org/10.1016/s2213-8587(19)30084-1.
Laforest S, Labrecque J, Michaud A, Cianflone K, Tchernof A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Critical Rev Clin Lab Sci. 2015;52:301–13. https://doi.org/10.3109/10408363.2015.1041582.
Visser M, Kritchevsky SB, Newman AB, Goodpaster BH, Tylavsky FA, Nevitt MC, et al. Lower serum albumin concentration and change in muscle mass: the health, aging and body composition study. Am J Clin Nutr. 2005;82:531–7. https://doi.org/10.1093/ajcn.82.3.531.
Schorr M, Dichtel LE, Gerweck AV, Valera RD, Torriani M, Miller KK, et al. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. 2018;9:28. https://doi.org/10.1186/s13293-018-0189-3.
Ge XN, Greenberg Y, Hosseinkhani MR, Long EK, Bahaie NS, Rao A, et al. High-fat diet promotes lung fibrosis and attenuates airway eosinophilia after exposure to cockroach allergen in mice. Exp Lung Res. 2013;39:365–78. https://doi.org/10.3109/01902148.2013.829537.
Elliot JG, Donovan GM, Wang KCW, Green FHY, James AL, Noble PB. Fatty airways: implications for obstructive disease. Eur Respir J. 2019;54.https://doi.org/10.1183/13993003.00857-2019.
Inselman LS, Chander A, Spitzer AR. Diminished lung compliance and elevated surfactant lipids and proteins in nutritionally obese young rats. Lung. 2004;182:101–17. https://doi.org/10.1007/s00408-003-1048-4.
Ruze R, Li J, Xu Q, Zhong M, Xiong Y, Yan Z, et al. Sleeve gastrectomy ameliorates alveolar structures and surfactant protein expression in lungs of obese and diabetic rats. Int J Obes. 2005;2020(44):2394–404. https://doi.org/10.1038/s41366-020-0647-y.
Foster DJ, Ravikumar P, Bellotto DJ, Unger RH, Hsia CC. Fatty diabetic lung: altered alveolar structure and surfactant protein expression. Am J Physiol Lung Cell Mol Physiol. 2010;298:L392-403. https://doi.org/10.1152/ajplung.00041.2009.
Lv YQ, Dhlamini Q, Chen C, Li X, Bellusci S, Zhang JS. FGF10 and lipofibroblasts in lung homeostasis and disease: insights gained from the adipocytes. Front Cell Dev Biol. 2021;9:645400. https://doi.org/10.3389/fcell.2021.645400.
Wu J, Chu X, Chen C, Bellusci S. Role of fibroblast growth factor 10 in mesenchymal cell differentiation during lung development and disease. Front Genet. 2018;9:545. https://doi.org/10.3389/fgene.2018.00545.
McGowan SE. The lipofibroblast: more than a lipid-storage depot. Am J Physiol Lung Cell Mol Physiol. 2019;316:L869-l71. https://doi.org/10.1152/ajplung.00109.2019.
Rehan M, Deskin B, Kurundkar AR, Yadav S, Matsunaga Y, Manges J, et al. Nicotinamide N-methyltransferase Mediates Lipofibroblast-Myofibroblast Transition and Apoptosis Resistance. J Biol Chem. 2023:105027.https://doi.org/10.1016/j.jbc.2023.105027.
Chueire VB, Muscelli E. Effect of free fatty acids on insulin secretion, insulin sensitivity and incretin effect - a narrative review. Arch Endocrinol Metab. 2021;65:24–31. https://doi.org/10.20945/2359-3997000000313.
Kochumon S, Wilson A, Chandy B, Shenouda S, Tuomilehto J, Sindhu S, et al. Palmitate Activates CCL4 Expression in Human Monocytic Cells via TLR4/MyD88 Dependent Activation of NF-κB/MAPK/ PI3K Signaling Systems. Cell Physiol Biochem. 2018;46:953–64. https://doi.org/10.1159/000488824.
Chu SG, Villalba JA, Liang X, Xiong K, Tsoyi K, Ith B, et al. Palmitic Acid-rich high-fat diet exacerbates experimental pulmonary fibrosis by modulating endoplasmic reticulum stress. Am J Respir Cell Mol Biol. 2019;61:737–46. https://doi.org/10.1165/rcmb.2018-0324OC.
Romero F, Shah D, Duong M, Penn RB, Fessler MB, Madenspacher J, et al. A pneumocyte-macrophage paracrine lipid axis drives the lung toward fibrosis. Am Respir Cell Mol Biol. 2015;53:74–86. https://doi.org/10.1165/rcmb.2014-0343OC.
Summer R, Mora AL. Lipid metabolism: a new player in the conundrum of lung fibrosis. Am J Respir Cell Mol Biol. 2019;61:669–70. https://doi.org/10.1165/rcmb.2019-0098ED.
Kou L, Kou P, Luo G, Wei S. Progress of Statin Therapy in the Treatment of Idiopathic Pulmonary Fibrosis. Oxid Med Cell Longev. 2022;2022:6197219. https://doi.org/10.1155/2022/6197219.
Suryadevara V, Ramchandran R, Kamp DW, Natarajan V. Lipid mediators regulate pulmonary fibrosis: potential mechanisms and signaling pathways. Int J Mol Sci. 2020;21.https://doi.org/10.3390/ijms21124257.
Huang LS, Berdyshev E, Mathew B, Fu P, Gorshkova IA, He D, et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB J. 2013;27:1749–60. https://doi.org/10.1096/fj.12-219634.
Milara J, Navarro R, Juan G, Peiró T, Serrano A, Ramón M, et al. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–56. https://doi.org/10.1136/thoraxjnl-2011-200026.
Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, et al. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol. 2007;37:395–404. https://doi.org/10.1165/rcmb.2007-0065OC.
Huang LS, Sudhadevi T, Fu P, Punathil-Kannan PK, Ebenezer DL, Ramchandran R, et al. Sphingosine Kinase 1/S1P Signaling Contributes to Pulmonary Fibrosis by Activating Hippo/YAP Pathway and Mitochondrial Reactive Oxygen Species in Lung Fibroblasts. Int J Mol Sci. 2020;21.https://doi.org/10.3390/ijms21062064.
Liu L, Shi Z, Ji X, Zhang W, Luan J, Zahr T, et al. Adipokines, adiposity, and atherosclerosis. Cell MolLife Sci. 2022;79:272. https://doi.org/10.1007/s00018-022-04286-2.
Becerril S, Rodríguez A, Catalán V, Ramírez B, Mentxaka A, Neira G, et al. Sex- and Age-Dependent Changes in the Adiponectin/Leptin Ratio in Experimental Diet-Induced Obesity in Mice. Nutrients. 2022;15.https://doi.org/10.3390/nu15010073.
Kiernan K, MacIver NJ. The role of the Adipokine leptin in immune cell function in health and disease. Front Immunol. 2020;11:622468. https://doi.org/10.3389/fimmu.2020.622468.
Brandao-Rangel MAR, Moraes-Ferreira R, Oliveira-Junior MC, Santos-Dias A, Bachi ALL, Gabriela-Pereira G, et al. Pulmonary function changes in older adults with and without metabolic syndrome. Sci Rep. 2021;11:17337. https://doi.org/10.1038/s41598-021-96766-x.
d’Alessandro M, Bergantini L, Refini RM, Cameli P, Perillo F, Landi C, et al. Adiponectin and leptin levels in idiopathic pulmonary fibrosis: A new method for BAL and serum assessment. Immunobiology. 2020;225:151997. https://doi.org/10.1016/j.imbio.2020.151997.
Han H, Chung SI, Park HJ, Oh EY, Kim SR, Park KH, et al. Obesity-induced vitamin D deficiency contributes to lung fibrosis and airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2021;64:357–67. https://doi.org/10.1165/rcmb.2020-0086OC.
Unamuno X, Gómez-Ambrosi J, Ramírez B, Rodríguez A, Becerril S, Valentí V, et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol Immunol. 2021;18:1045–57. https://doi.org/10.1038/s41423-019-0296-z.
Zhou X, Hu H, Huynh ML, Kotaru C, Balzar S, Trudeau JB, et al. Mechanisms of tissue inhibitor of metalloproteinase 1 augmentation by IL-13 on TGF-beta 1-stimulated primary human fibroblasts. J Allergy Clin Immunol. 2007;119:1388–97. https://doi.org/10.1016/j.jaci.2007.02.011.
Lee JJ, Britton KA, Pedley A, Massaro JM, Speliotes EK, Murabito JM, et al. Adipose Tissue Depots and Their Cross-Sectional Associations With Circulating Biomarkers of Metabolic Regulation. J Am Heart Assoc. 2016;5.https://doi.org/10.1161/jaha.115.002936.
Lasithiotaki I, Giannarakis I, Tsitoura E, Samara KD, Margaritopoulos GA, Choulaki C, et al. NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. Eur Respir J. 2016;47:910–8. https://doi.org/10.1183/13993003.00564-2015.
Qiu W, Wu H, Hu Z, Wu X, Tu M, Fang F, et al. Identification and characterization of a novel adiponectin receptor agonist adipo anti-inflammation agonist and its anti-inflammatory effects in vitro and in vivo. Br J Pharmacol. 2021;178:280–97. https://doi.org/10.1111/bph.15277.
Yvan-Charvet L, Quignard-Boulangé A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011;79:162–8. https://doi.org/10.1038/ki.2010.391.
Okada S, Kozuka C, Masuzaki H, Yasue S, Ishii-Yonemoto T, Tanaka T, et al. Adipose tissue-specific dysregulation of angiotensinogen by oxidative stress in obesity. Metabolism. 2010;59:1241–51. https://doi.org/10.1016/j.metabol.2009.11.016.
Frigolet ME, Torres N, Tovar AR. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J Nutr Biochem. 2013;24:2003–15. https://doi.org/10.1016/j.jnutbio.2013.07.002.
Engeli S, Gorzelniak K, Kreutz R, Runkel N, Distler A, Sharma AM. Co-expression of renin-angiotensin system genes in human adipose tissue. J Hypertension. 1999;17:555–60. https://doi.org/10.1097/00004872-199917040-00014.
Tan WSD, Liao W, Zhou S, Mei D, Wong WF. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol. 2018;40:9–17. https://doi.org/10.1016/j.coph.2017.12.002.
Montes E, Ruiz V, Checa M, Maldonado V, Melendez-Zajgla J, Montaño M, et al. Renin is an angiotensin-independent profibrotic mediator: role in pulmonary fibrosis. Eur Respir J. 2012;39:141–8. https://doi.org/10.1183/09031936.00130310.
Wang J, Chen L, Chen B, Meliton A, Liu SQ, Shi Y, et al. Chronic activation of the renin-angiotensin system induces lung fibrosis. Sci Rep. 2015;5:15561. https://doi.org/10.1038/srep15561.
Li X, Zhuang J, Uhal BD. Local activation of the pulmonary extravascular angiotensin system induces epithelial apoptosis and lung fibrosis. J Lung Pulmonary Respir Res. 2018;5:192–200. https://doi.org/10.15406/jlprr.2018.05.00191.
Skurk T, Lee YM, Röhrig K, Hauner H. Effect of angiotensin peptides on PAI-1 expression and production in human adipocytes. Hormone Metab Res = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2001;33:196–200. https://doi.org/10.1055/s-2001-14948.
Courey AJ, Horowitz JC, Kim KK, Koh TJ, Novak ML, Subbotina N, et al. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood. 2011;118:2313–21. https://doi.org/10.1182/blood-2010-12-324574.
Rana T, Jiang C, Liu G, Miyata T, Antony V, Thannickal VJ, et al. PAI-1 Regulation of TGF-β1-induced Alveolar Type II Cell Senescence, SASP Secretion, and SASP-mediated Activation of Alveolar Macrophages. Am J Respir Cell Mol Biol. 2020;62:319–30. https://doi.org/10.1165/rcmb.2019-0071OC.
Park YH, Oh EY, Han H, Yang M, Park HJ, Park KH, et al. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-β1 pathway. ExpMol Med. 2019;51:1–12. https://doi.org/10.1038/s12276-019-0258-7.
Salmenniemi U, Ruotsalainen E, Pihlajamäki J, Vauhkonen I, Kainulainen S, Punnonen K, et al. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation. 2004;110:3842–8. https://doi.org/10.1161/01.Cir.0000150391.38660.9b.
Zhu T, Zhao J, Zhuo S, Hu Z, Ouyang S, Wunier, et al. High Fat Diet and High Cholesterol Diet Reduce Hepatic Vitamin D-25-Hydroxylase Expression and Serum 25-Hydroxyvitamin D(3) Level through Elevating Circulating Cholesterol, Glucose, and Insulin Levels. Mol Nutr Food Res. 2021;65:e2100220. https://doi.org/10.1002/mnfr.202100220.
Wang X, Yi X, Tang D. Aerobic exercise improves pulmonary fibrosis by improving insulin resistance and inflammation in obese mice. Front Physiol. 2021;12:785117. https://doi.org/10.3389/fphys.2021.785117.
Rong L, Wu J, Wang W, Zhao RP, Xu XW, Hu D. Sirt 1 activator attenuates the bleomycin-induced lung fibrosis in mice via inhibiting epithelial-to-mesenchymal transition (EMT). Eur Rev Med Pharmacol Sci. 2016;20:2144–50.
David AS, Jeste DV, Folstein MF, Folstein SE. Voluntary movement dysfunction in Huntington’s disease and tardive dyskinesia. Acta neurologica Scandinavica. 1987;75:130–9. https://doi.org/10.1111/j.1600-0404.1987.tb07907.x.
Zamboni M, Nori N, Brunelli A, Zoico E. How does adipose tissue contribute to inflammageing? Exp Gerontol. 2021;143:111162. https://doi.org/10.1016/j.exger.2020.111162.
Kotzbeck P, Giordano A, Mondini E, Murano I, Severi I, Venema W, et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res. 2018;59:784–94. https://doi.org/10.1194/jlr.M079665.
Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci. 2019; 20.https://doi.org/10.3390/ijms20092358.
Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol (Baltimore, Md : 1950). 2011;187:6208–16. https://doi.org/10.4049/jimmunol.1102188.
Bremer AA, Devaraj S, Afify A, Jialal I. Adipose tissue dysregulation in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E1782-8. https://doi.org/10.1210/jc.2011-1577.
Bergantini L, d'Alessandro M, Gangi S, Bianchi F, Cameli P, Perea B, et al. Predictive role of cytokine and adipokine panel in hospitalized COVID-19 patients: evaluation of disease severity, survival and lung sequelae. Int J Mol Sci. 2023;24.https://doi.org/10.3390/ijms241612994.
Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–5. https://doi.org/10.1038/s41586-021-03547-7.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. https://doi.org/10.1038/nm.1964.
O’Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–90. https://doi.org/10.1007/s00125-011-2103-y.
Milara J, Hernandez G, Ballester B, Morell A, Roger I, Montero P, et al. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respir Res. 2018;19:24. https://doi.org/10.1186/s12931-018-0728-9.
Bolourani S, Brenner M, Wang P. The interplay of DAMPs, TLR4, and proinflammatory cytokines in pulmonary fibrosis. J Mol Med (Berlin, Germany). 2021;99:1373–84. https://doi.org/10.1007/s00109-021-02113-y.
Kolahian S, Fernandez IE, Eickelberg O, Hartl D. Immune Mechanisms in Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2016;55:309–22. https://doi.org/10.1165/rcmb.2016-0121TR.
Lee JH, Jang JH, Park JH, Jang HJ, Park CS, Lee S, et al. The role of interleukin-6 as a prognostic biomarker for predicting acute exacerbation in interstitial lung diseases. PloS One. 2021;16:e0255365. https://doi.org/10.1371/journal.pone.0255365.
Cong LH, Li T, Wang H, Wu YN, Wang SP, Zhao YY, et al. IL-17A-producing T cells exacerbate fine particulate matter-induced lung inflammation and fibrosis by inhibiting PI3K/Akt/mTOR-mediated autophagy. J Cell Mol Med. 2020;24:8532–44. https://doi.org/10.1111/jcmm.15475.
Yang L, Herrera J, Gilbertsen A, Xia H, Smith K, Benyumov A, et al. IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity. AmJ Physiol Lung Cell Molecular Physiol. 2018;314:L127-l36. https://doi.org/10.1152/ajplung.00200.2017.
Anderson MR, Kim JS, Allison M, Giles JT, Hoffman EA, Ding J, et al. Adiposity and interstitial lung abnormalities in community-dwelling adults: The MESA cohort study. Chest. 2021;160:582–94. https://doi.org/10.1016/j.chest.2021.03.058.
Zinellu A, Paliogiannis P, Sotgiu E, Mellino S, Mangoni AA, Zinellu E, et al. Blood cell count derived inflammation indexes in patients with idiopathic pulmonary fibrosis. Lung. 2020;198:821–7. https://doi.org/10.1007/s00408-020-00386-7.
Sung KT, Kuo R, Sun JY, Hung TC, Chang SC, Liu CC, et al. Associations between CT-determined visceral fat burden, hepatic steatosis, circulating white blood cell counts and neutrophil-to-lymphocyte ratio. PloS One. 2018;13:e0207284. https://doi.org/10.1371/journal.pone.0207284.
Girousse A, Gil-Ortega M, Bourlier V, Bergeaud C, Sastourné-Arrey Q, Moro C, et al. The Release of adipose stromal cells from subcutaneous adipose tissue regulates ectopic intramuscular adipocyte deposition. Cell Rep. 2019;27:323-33.e5. https://doi.org/10.1016/j.celrep.2019.03.038.
Grillot J, D’Engremont C, Parmentier AL, Lakkis Z, Piton G, Cazaux D, et al. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin Nutr (Edinburgh, Scotland). 2020;39:3024–30. https://doi.org/10.1016/j.clnu.2020.01.001.
Jalaber C, Lemerre-Poincloux J, Jouneau S, Rousseau C, Dolou B, Rouag E, et al. Usefulness of body composition CT analysis in patients with idiopathic pulmonary fibrosis: a pilot study. Acad Radiol. 2022;29(Suppl 2):S191-s201. https://doi.org/10.1016/j.acra.2021.07.020.
Li CW, Yu K, Shyh-Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022;13:781–94. https://doi.org/10.1002/jcsm.12901.
Knudsen JR, Persson KW, Henriquez-Olguin C, Li Z, Di Leo N, Hesselager SA, et al. Microtubule-mediated GLUT4 trafficking is disrupted in insulin-resistant skeletal muscle. eLife. 2023;12.https://doi.org/10.7554/eLife.83338.
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. https://doi.org/10.1038/nm.2279.
Ma S, Shyh-Chang N. The Metabaging Cycle. Cell Prolif. 2022;55:e13197. https://doi.org/10.1111/cpr.13197.
Giuliani G, Rosina M, Reggio A. Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease. FEBS J. 2022;289:6484–517. https://doi.org/10.1111/febs.16080.
Hogarth MW, Defour A, Lazarski C, Gallardo E, Diaz Manera J, Partridge TA, et al. Fibroadipogenic progenitors are responsible for muscle loss in limb girdle muscular dystrophy 2B. Nat Commun. 2019;10:2430. https://doi.org/10.1038/s41467-019-10438-z.
Sente T, Van Berendoncks AM, Fransen E, Vrints CJ, Hoymans VY. Tumor necrosis factor-α impairs adiponectin signalling, mitochondrial biogenesis, and myogenesis in primary human myotubes cultures. Am J physiol Heart Circ Physiol. 2016;310:H1164-75. https://doi.org/10.1152/ajpheart.00831.2015.
Chen W, You W, Valencak TG, Shan T. Bidirectional roles of skeletal muscle fibro-adipogenic progenitors in homeostasis and disease. Ageing Res Rev. 2022;80:101682. https://doi.org/10.1016/j.arr.2022.101682.
Faverio P, Fumagalli A, Conti S, Madotto F, Bini F, Harari S, et al. Nutritional assessment in idiopathic pulmonary fibrosis: a prospective multicentre study. ERJ Open Res. 2022;8.https://doi.org/10.1183/23120541.00443-2021.
Kim KW, Baek MO, Yoon MS, Son KH. Deterioration of mitochondrial function in the human intercostal muscles differs among individuals with sarcopenia, obesity, and sarcopenic obesity. Clin Nutr (Edinburgh, Scotland). 2021;40:2697–706. https://doi.org/10.1016/j.clnu.2021.03.009.
Lee SE, Park JH, Kim KA, Kang YS, Choi HS. Association between sarcopenic obesity and pulmonary function in Korean elderly: results from the Korean National Health and nutrition examination survey. Calcified Tissue Int. 2020;106:124–30. https://doi.org/10.1007/s00223-019-00623-z.
Kim HJ, Snyder LD, Adegunsoye A, Neely ML, Bender S, White ES, et al. Hospitalizations in patients with idiopathic pulmonary fibrosis. Respir Res. 2021;22:257. https://doi.org/10.1186/s12931-021-01851-4.
Pugashetti J, Graham J, Boctor N, Mendez C, Foster E, Juarez M, et al. Weight loss as a predictor of mortality in patients with interstitial lung disease. Eur Respir J. 2018;52.https://doi.org/10.1183/13993003.01289-2018.
Lee JH, Yoon YC, Kim HS, Cha MJ, Kim JH, Kim K, et al. Obesity is associated with improved postoperative overall survival, independent of skeletal muscle mass in lung adenocarcinoma. J Cachexia Sarcopenia Muscle. 2022;13:1076–86. https://doi.org/10.1002/jcsm.12956.
Liu C, Wong PY, Chung YL, Chow SK, Cheung WH, Law SW, et al. Deciphering the “obesity paradox” in the elderly: a systematic review and meta-analysis of sarcopenic obesity. Obes Rev. 2023;24:e13534. https://doi.org/10.1111/obr.13534.
Jouneau S, Lederlin M, Vernhet L, Thibault R. Malnutrition in idiopathic pulmonary fibrosis: the great forgotten comorbidity! Eur Respir J. 2019;53.https://doi.org/10.1183/13993003.00418-2019.
He S, Yang J, Li X, Gu H, Su Q, Qin L. Visceral adiposity index is associated with lung function impairment: a population-based study. Respir Res. 2021;22:2. https://doi.org/10.1186/s12931-020-01599-3.
Faverio P, Fumagalli A, Conti S, Madotto F, Bini F, Harari S, et al. Sarcopenia in idiopathic pulmonary fibrosis: a prospective study exploring prevalence, associated factors and diagnostic approach. Respir Res. 2022;23:228. https://doi.org/10.1186/s12931-022-02159-7.
Fujita K, Ohkubo H, Nakano A, Mori Y, Fukumitsu K, Fukuda S, et al. Frequency and impact on clinical outcomes of sarcopenia in patients with idiopathic pulmonary fibrosis. Chronic Respir Dis. 2022;19:14799731221117298. https://doi.org/10.1177/14799731221117298.
Chandel A, Pastre J, Valery S, King CS, Nathan SD. Derivation and validation of a simple multidimensional index incorporating exercise capacity parameters for survival prediction in idiopathic pulmonary fibrosis. Thorax. 2023;78:368–75. https://doi.org/10.1136/thoraxjnl-2021-218440.
Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kataoka K, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J. 2010;36:1067–72. https://doi.org/10.1183/09031936.00152609.
Moon SW, Choi JS, Lee SH, Jung KS, Jung JY, Kang YA, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res. 2019;20:35. https://doi.org/10.1186/s12931-019-1001-6.
Nakano A, Ohkubo H, Taniguchi H, Kondoh Y, Matsuda T, Yagi M, et al. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci Rep. 2020;10:2312. https://doi.org/10.1038/s41598-020-59100-5.
Hanada M, Kasawara KT, Mathur S, Rozenberg D, Kozu R, Hassan SA, et al. Aerobic and breathing exercises improve dyspnea, exercise capacity and quality of life in idiopathic pulmonary fibrosis patients: systematic review and meta-analysis. J Thorac Dis. 2020;12:1041–55. https://doi.org/10.21037/jtd.2019.12.27.
Vainshelboim B, Oliveira J, Yehoshua L, Weiss I, Fox BD, Fruchter O, et al. Exercise training-based pulmonary rehabilitation program is clinically beneficial for idiopathic pulmonary fibrosis. Respir Int Rev Thorac Dis. 2014;88:378–88. https://doi.org/10.1159/000367899.
Hickson DA, Liu J, Bidulescu A, Burchfiel CM, Taylor HA, Petrini MF. Pericardial fat is associated with impaired lung function and a restrictive lung pattern in adults: the Jackson Heart Study. Chest. 2011;140:1567–73. https://doi.org/10.1378/chest.11-0258.
Palma G, Sorice GP, Genchi VA, Giordano F, Caccioppoli C, D'Oria R, et al. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int J Mol Sci. 2022;23.https://doi.org/10.3390/ijms23137349.
Papiris SA, Tomos IP, Karakatsani A, Spathis A, Korbila I, Analitis A, et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine. 2018;102:168–72. https://doi.org/10.1016/j.cyto.2017.08.019.
Arai T, Kagawa T, Sasaki Y, Sugawara R, Sugimoto C, Tachibana K, et al. Heterogeneity of incidence and outcome of acute exacerbation in idiopathic interstitial pneumonia. Respirology (Carlton, Vic). 2016;21:1431–7. https://doi.org/10.1111/resp.12862.
Snyder L, Neely ML, Hellkamp AS, O’Brien E, de Andrade J, Conoscenti CS, et al. Predictors of death or lung transplant after a diagnosis of idiopathic pulmonary fibrosis: insights from the IPF-PRO Registry. Respir Res. 2019;20:105. https://doi.org/10.1186/s12931-019-1043-9.
Umemura A, Sasaki A, Nikai H, Yanari S, Ishioka H, Takahashi N, et al. Improvements of lung volumes and respiratory symptoms after weight loss through laparoscopic sleeve gastrectomy. Langenbeck’s Arch Surg. 2022;407:2747–54. https://doi.org/10.1007/s00423-022-02549-x.
Gasek NS, Kuchel GA, Kirkland JL, Xu M. Strategies for targeting senescent cells in human disease. Nature Aging. 2021;1:870–9. https://doi.org/10.1038/s43587-021-00121-8.
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–56. https://doi.org/10.1038/s41591-018-0092-9.
Nambiar A, Kellogg D 3rd, Justice J, Goros M, Gelfond J, Pascual R, et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. eBioMedicine. 2023;90:104481. https://doi.org/10.1016/j.ebiom.2023.104481.
Torrisi SE, Ley B, Kreuter M, Wijsenbeek M, Vittinghoff E, Collard HR, et al. The added value of comorbidities in predicting survival in idiopathic pulmonary fibrosis: a multicentre observational study. European Respir J. 2019;53.https://doi.org/10.1183/13993003.01587-2018.
Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015;46:1113–30. https://doi.org/10.1183/13993003.02316-2014.
Moon SW, Park MS, Kim YS, Jang J, Lee JH, Lee CT, et al. Combined pulmonary fibrosis and emphysema and idiopathic pulmonary fibrosis in non-small cell lung cancer: impact on survival and acute exacerbation. BMC Pulmonary Med. 2019;19:177. https://doi.org/10.1186/s12890-019-0951-2.
Nemoto M, Nei Y, Bartholmai B, Yoshida K, Matsui H, Nakashita T, et al. Automated computed tomography quantification of fibrosis predicts prognosis in combined pulmonary fibrosis and emphysema in a real-world setting: a single-centre, retrospective study. Respir Res. 2020;21:275. https://doi.org/10.1186/s12931-020-01545-3.
Kim CY, Park JE, Leem AY, Song JH, Kim SY, Chung KS, et al. Prognostic value of pre-transplant mean pulmonary arterial pressure in lung transplant recipients: a single-institution experience. J Thorac Dis. 2018;10:1578–87.
Jovanovic DM, Šterclová M, Mogulkoc N, Lewandowska K, Müller V, Hájková M, et al. Comorbidity burden and survival in patients with idiopathic pulmonary fibrosis: the EMPIRE registry study. Respiry Res. 2022;23:135. https://doi.org/10.1186/s12931-022-02033-6.
Tang SC, Baeyens L, Shen CN, Peng SJ, Chien HJ, Scheel DW, et al. Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia. 2018;61:168–81. https://doi.org/10.1007/s00125-017-4409-x.
Horii T, Fujita Y, Ishibashi C, Fukui K, Eguchi H, Kozawa J, et al. Islet inflammation is associated with pancreatic fatty infiltration and hyperglycemia in type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8.https://doi.org/10.1136/bmjdrc-2020-001508.
García-Sancho Figueroa MC, Carrillo G, Pérez-Padilla R, Fernández-Plata MR, Buendía-Roldán I, Vargas MH, et al. Risk factors for idiopathic pulmonary fibrosis in a Mexican population. A case-control study. Respir Med. 2010;104:305–9. https://doi.org/10.1016/j.rmed.2009.08.013.
Li C, Xiao Y, Hu J, Hu Z, Yan J, Zhou Z, et al. Associations between diabetes and idiopathic pulmonary fibrosis: a study-level pooled analysis of 26 million people. J Clin Endocrino Metab. 2021;106:3367–80. https://doi.org/10.1210/clinem/dgab553.
Bai L, Zhang L, Pan T, Wang W, Wang D, Turner C, et al. Idiopathic pulmonary fibrosis and diabetes mellitus: a meta-analysis and systematic review. Respir Res. 2021;22:175. https://doi.org/10.1186/s12931-021-01760-6.
Hyldgaard C, Hilberg O, Bendstrup E. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respir Med. 2014;108:647–53. https://doi.org/10.1016/j.rmed.2014.01.008.
Ather JL, Van Der Vliet KE, Mank MM, Reed LF, Dixon AE, Poynter ME. Obese adipose tissue modulates proinflammatory responses of mouse airway epithelial cells. Am J Physiol Regul Integr Comp Physiol. 2021;321:R79-r90. https://doi.org/10.1152/ajpregu.00316.2020.
Tkacova R. Systemic inflammation in chronic obstructive pulmonary disease: may adipose tissue play a role? Review of the literature and future perspective. Mediat Inflamm. 2010;2010:585989. https://doi.org/10.1155/2010/585989.
Spielmann J, Naujoks W, Emde M, Allweyer M, Kielstein H, Quandt D, et al. High-Fat Diet and Feeding Regime Impairs Number, Phenotype, and Cytotoxicity of Natural Killer Cells in C57BL/6 Mice. Front Nutr. 2020;7:585693. https://doi.org/10.3389/fnut.2020.585693.
de Jesus JCR, Murari ASP, Radloff K, de Moraes RCM, Figuerêdo RG, Pessoa AFM, et al. Activation of the adipose tissue NLRP3 inflammasome pathway in cancer cachexia. Fronti Immunol. 2021;12:729182. https://doi.org/10.3389/fimmu.2021.729182.
El-Horany HE, Atef MM, Abdel Ghafar MT, Fouda MH, Nasef NA, Hegab, II, et al. Empagliflozin Ameliorates Bleomycin-Induced Pulmonary Fibrosis in Rats by Modulating Sesn2/AMPK/Nrf2 Signaling and Targeting Ferroptosis and Autophagy. Int J Mol Sci. 2023;24.https://doi.org/10.3390/ijms24119481.
Fandiño J, Toba L, González-Matías LC, Diz-Chaves Y, Mallo F. GLP-1 receptor agonist ameliorates experimental lung fibrosis. Sci Rep. 2020;10:18091. https://doi.org/10.1038/s41598-020-74912-1.
Ji H, Dong H, Lan Y, Bi Y, Gu X, Han Y, et al. Metformin attenuates fibroblast activation during pulmonary fibrosis by targeting S100A4 via AMPK-STAT3 axis. Front Pharmacol. 2023;14:1089812. https://doi.org/10.3389/fphar.2023.1089812.
Zhang H, You L, Zhao M. Rosiglitazone attenuates paraquat-induced lung fibrosis in rats in a PPAR gamma-dependent manner. Eur J Pharmacol. 2019;851:133–43. https://doi.org/10.1016/j.ejphar.2019.02.037.
Samah M, El-Aidy Ael R, Tawfik MK, Ewais MM. Evaluation of the antifibrotic effect of fenofibrate and rosiglitazone on bleomycin-induced pulmonary fibrosis in rats. Eur J Pharmacol. 2012;689:186–93. https://doi.org/10.1016/j.ejphar.2012.05.026.
Kim JW, Rhee CK, Kim TJ, Kim YH, Lee SH, Yoon HK, et al. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin Exp Pharmacol Physiol. 2010;37:1055–63. https://doi.org/10.1111/j.1440-1681.2010.05431.x.
Yildirim M, Kayalar O, Atahan E, Oztay F. Atorvastatin attenuates pulmonary fibrosis in mice and human lung fibroblasts, by the regulation of myofibroblast differentiation and apoptosis. J Biochem Mol Toxicol. 2022;36:e23074. https://doi.org/10.1002/jbt.23074.
Seenak P, Kumphune S, Prasitsak T, Nernpermpisooth N, Malakul W. Atorvastatin and ezetimibe protect against hypercholesterolemia-induced lung oxidative stress, inflammation, and fibrosis in rats. Front Med. 2022;9:1039707. https://doi.org/10.3389/fmed.2022.1039707.
Zhang HX, Li YN, Wang XL, Ye CL, Zhu XY, Li HP, et al. Probucol ameliorates EMT and lung fibrosis through restoration of SIRT3 expression. Pulmonary PharmacolTher. 2019;57:101803. https://doi.org/10.1016/j.pupt.2019.101803.
Gomez-Manjarres DC, Axell-House DB, Patel DC, Odackal J, Yu V, Burdick MD, et al. Sirolimus suppresses circulating fibrocytes in idiopathic pulmonary fibrosis in a randomized controlled crossover trial. JCI Insight. 2023;8.https://doi.org/10.1172/jci.insight.166901.
Lukey PT, Harrison SA, Yang S, Man Y, Holman BF, Rashidnasab A, et al. A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur Respir J. 2019;53.https://doi.org/10.1183/13993003.01992-2018.
Palmer SM, Snyder L, Todd JL, Soule B, Christian R, Anstrom K, et al. Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial of BMS-986020, a Lysophosphatidic Acid Receptor Antagonist for the Treatment of Idiopathic Pulmonary Fibrosis. Chest. 2018;154:1061–9. https://doi.org/10.1016/j.chest.2018.08.1058.
Corte TJ, Lancaster L, Swigris JJ, Maher TM, Goldin JG, Palmer SM, et al. Phase 2 trial design of BMS-986278, a lysophosphatidic acid receptor 1 (LPA(1)) antagonist, in patients with idiopathic pulmonary fibrosis (IPF) or progressive fibrotic interstitial lung disease (PF-ILD). BMJ Open Respir Res. 2021;8.https://doi.org/10.1136/bmjresp-2021-001026.
Maher TM, Kreuter M, Lederer DJ, Brown KK, Wuyts W, Verbruggen N, et al. Rationale, design and objectives of two phase III, randomised, placebo-controlled studies of GLPG1690, a novel autotaxin inhibitor, in idiopathic pulmonary fibrosis (ISABELA 1 and 2). BMJ Open Respir Res. 2019;6:e000422. https://doi.org/10.1136/bmjresp-2019-000422.
Khalil N, Manganas H, Ryerson CJ, Shapera S, Cantin AM, Hernandez P, et al. Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. Eur Respir Jo. 2019;53.https://doi.org/10.1183/13993003.00663-2018.
Strambu IR, Seemayer CA, Fagard LMA, Ford PA, Van der Aa TAK, de Haas-Amatsaleh AA, et al. GLPG1205 for idiopathic pulmonary fibrosis: a phase 2 randomised placebo-controlled trial. Eur Respir J. 2023;61.https://doi.org/10.1183/13993003.01794-2022.
Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. https://doi.org/10.1038/nm1685.
Cheng PTW, Kaltenbach RF 3rd, Zhang H, Shi J, Tao S, Li J, et al. Discovery of an Oxycyclohexyl Acid Lysophosphatidic Acid Receptor 1 (LPA(1)) Antagonist BMS-986278 for the Treatment of Pulmonary Fibrotic Diseases. J Med Chem. 2021;64:15549–81. https://doi.org/10.1021/acs.jmedchem.1c01256.
Liu X, Wong SS, Taype CA, Kim J, Shentu TP, Espinoza CR, et al. Thy-1 interaction with Fas in lipid rafts regulates fibroblast apoptosis and lung injury resolution. Lab Investig. 2017;97:256–67. https://doi.org/10.1038/labinvest.2016.145.
Huang LS, Natarajan V. Sphingolipids in pulmonary fibrosis. Adv Biol Regul. 2015;57:55–63. https://doi.org/10.1016/j.jbior.2014.09.008.
Acknowledgements
Thanks to all the peer reviewers and editors for their opinions and suggestions.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82270079 and No. 82070070).
Author information
Authors and Affiliations
Contributions
All authors contributed to the completion of this study. Conceptualization, J.M. and W.X.; methodology, XY.C.; software SH.J. and BY.P.; validation, W.X. and BY.P.; formal analysis, W.X.; investigation, XY.C.; data curation, SH.J.; writing---original draft preparation, XY.C. and SH.J.; writing---review and editing, J.M.; visualization, SH.J. and XY.C.; supervision, J.M.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals.
performed by the authors.
Consent for publication
All authors give consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Fig. 1. The flowchart of literature research selection. Supplementary Fig. 2. Bioinformatics evidence of the association between lipid accumulation and poor IPF prognosis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cheng, X., Jiang, S., Pan, B. et al. Ectopic and visceral fat deposition in aging, obesity, and idiopathic pulmonary fibrosis: an interconnected role. Lipids Health Dis 22, 201 (2023). https://doi.org/10.1186/s12944-023-01964-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01964-3