Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease. Metabolism-related genes significantly influence the onset and progression of the disease. Hence, it is necessary to screen metabolism-related biomarkers for the diagnosis and treatment of NAFLD patients.
Methods
GSE48452, GSE63067, and GSE89632 datasets including nonalcoholic steatohepatitis (NASH) and healthy controls (HC) analyzed in this study were retrieved from the Gene Expression Omnibus (GEO) database. First, differentially expressed genes (DEGs) between NASH and HC samples were obtained. Next, metabolism-related DEGs (MR-DEGs) were identified by overlapping DEGs and metabolism-related genes (MRG). Further, a protein–protein interaction (PPI) network was developed to show the interaction among MR-DEGs. Subsequently, the “Least absolute shrinkage and selection operator regression” and “Random Forest” algorithms were used to screen metabolism-related genes (MRGs) in patients with NAFLD. Next, immune cell infiltration and gene set enrichment analyses (GSEA) were performed on these metabolism-related genes. Finally, the expression of metabolism-related gene was determined at the transcription level.
Results
First, 129 DEGs related to NAFLD development were identified among patients with nonalcoholic steatohepatitis (NASH) and healthy control. Next, 18 MR-DEGs were identified using the Venn diagram. Subsequently, four genes, including AMDHD1, FMO1, LPL, and P4HA1, were identified using machine learning algorithms. Moreover, a regulatory network consisting of four genes, 25 microRNAs (miRNAs), and 41 transcription factors (TFs) was constructed. Finally, a significant increase in FMO1 and LPL expression levels and a decrease in AMDHD1 and P4HA1 expression levels were observed in patients in the NASH group compared to the HC group.
Conclusion
Metabolism-related genes associated with NAFLD were identified, containing AMDHD1, FMO1, LPL, and P4HA1, which provide insights into diagnosing and treating patients with NAFLD.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome. Metabolism associated with risk factors, including obesity, hypertension, dyslipidemia, and diabetes, are associated with NAFLD [1]. NAFLD is a major cause of chronic liver disease [2]. NAFLD can be divided based on disease progression profiles into the non-alcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), and liver fibrosis as well as cirrhosis [3]. Studies have shown that the incidence of NAFLD among adults in European and American countries is 20%-33% [4]. NAFLD has overtaken viral hepatitis as a major chronic liver disease. Moreover, the incidence of NAFLD is increasing year by year and becoming younger [3]. Globally, NAFLD is the most prevalent type of chronic liver disease [5]. Although treating patients with NAFLD at an early stage could help in complete recovery, approximately 20% of patients with NAFLD progress to cirrhosis and end-stage liver disease [4]. This seriously endangers the health of people [6]. Additionally, NAFLD is involved in the onset and progression of diabetes, arteriosclerosis, and several chronic liver diseases, respectively, seriously affecting the quality of life and life expectancy [7]. Due to the regulation of insulin resistance, there is an increased risk of developing cancers other than the liver, such as bladder cancer [8]. Therefore, identifying biomarkers for diagnosing patients with NAFLD is the need of the hour.
Metabolic dysfunction, such as hepatic steatosis, is an early indicator of NAFLD development. Studies have shown the interaction between metabolic pathways, intestinal flora, and immune systems in patients with NAFLD. Immune disorders, intestinal imbalances, and metabolic disorders promote liver inflammation and other aspects during late-stage NAFLD [9]. A study has shown that inflammation and metabolic processes in adipose tissue could accelerate NASH development and serve as therapeutic targets [10]. In fact, some studies have suggested that NAFLD could be referred to as metabolic (dysfunction) associated fatty liver disease (MAFLD) [11]. Therefore, determining the influence of metabolism on regulating NAFLD would aid in designing targeting therapies [12]. However, the role of metabolism-related genes (MRGs) in NAFLD is yet to be elucidated.
In this study, publicly available databases were searched, followed by bioinformatic analysis to identify MRGs in NAFLD onset and progression. Next, the regulatory network of MRGs and their correlation with the immune microenvironment were analyzed. The results of this research would aid in understanding the mechanisms of MRGs in NAFLD and identifying new therapeutic targets.
Materials and methods
Data acquisition
The GSE48452 and GSE63067 datasets consisting of data on clinical features and gene expression in liver tissue of patients with NASH and healthy controls (HC) were retrieved from the Gene Expression Omnibus (GEO) database based the source and size of samples. GSE48452 comprised 14 HC and 18 patients with NASH, and GSE63067 comprised seven HC and nine patients with NASH. Additionally, the GSE89632 dataset, comprising 24 HC and 19 patients with NASH, was used to verify the expression level of biomarkers. Finally, based on the background gene set “c2.cp.kegg.v7.4.symbols.gmt”, the gene set related to metabolism were obtained from The Molecular Signatures Database (MsigDB) database (v7.4, https://www.gsea-msigdb.org/gsea/msigdb/), and 948 metabolism-related genes (MRGs) were acquired by merging the genes from gene set and after de-duplicating. The flow chart of this study is shown in Supplementary Fig. 1.
Removing batch effects
To minimize the effects of sequencing platforms, experimental environments, sample processing and other factors, the Combat function in the “sva” package [13]was used to implement the de-batch of GSE48452 and GSE63067 datasets. Then, the merged dataset was used as a training cohort. Furthermore, the “ggplot2” package and UMAP algorithm were used to validate the correction results.
Analysis of differential genes
First, the “linear models for microarray data” R package [14] were used to identify differentially expressed genes (DEGs) based on “P < 0.05” and “|log2fold change (FC)|> 0.5” criteria in the NASH and HC groups in the training cohort. Next, a volcano plot and heatmap were constructed to show DEGs in NAFLD. Metabolism-related DEGs (MR-DEGs) were identified by intersecting DEGs and key module genes using the “VennDiagram” package [15].
Functional enrichment analysis and Protein–protein interaction (PPI) network
Gene Ontology (GO) and “Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were conducted on MR-DEGs using the “clusterProfiler” package [16] with “P < 0.05” as screening criteria. Next, a PPI network was constructed to present the interaction between MR-DEGs using the STRING (https://string-db.org) database. Finally, the topological features of the network were visualized using the “Cytoscape (version 3.7.2)” software [17].
Machine learning methods
The “Least absolute shrinkage and selection operator (LASSO)” regression analysis was performed, and the random forest (RF) algorithm was implemented using the “randomForest” package to screen important genes in the training cohort. The LASSO algorithm was performed by “glmnet” package [18] (version4.0–2), with parameters set to: family = binomial, type.measure = class, nfold = 10, to reduce the feature dimensions. Biomarkers were identified by intersecting genes identified using these algorithms. Moreover, the diagnostic significance of these genes was evaluated by constructing receiver operating characteristic (ROC) curves using the “pROC” package [19]. GSE89632 served as the external validation cohort for verifying these biomarkers. Based on the expression of biomarkers and sample grouping information in the training set, the nomogram was established by “RMS” [20] package (version6.4–1). To assess the predictive power of the nomogram, the calibration curve was plotted and the consistency index calculated using the calibrate function in the “RMS” package.
Immune cell infiltration (ICI) and Gene set enrichment analysis (GSEA)
The single-sample GSEA (ssGSEA) algorithm was used for calculating the relative abundance of 28 immune cells infiltrating the immune microenvironment of patients with NAFLD. Next, the correlation between diagnosis-related MRGs and differentially infiltrating immune cells was calculated and visualized. Finally, GSEA was performed to identify MRG-enriched KEGG pathways and GO terms using the “clusterProfiler” package [21].
Constructing biomarker regulatory networks
Biomarker-related micro-RNAs (miRNAs) were screened using the miRwalk3.0 database as per the following criteria: “binding probability ≥ 0.95” and “binding site position at 3’ untranslated region (UTR).” In addition, the transcription factors (TFs) targeting these biomarkers were predicted using the “hTFtarget” database. Finally, the TF-miRNA-mRNA network was constructed with the aid of the “Cytoscape (version 3.8.2)” software.
Analysis of MRG expression
Quantitative reverse transcriptase PCR (RT-qPCR) was performed to determine MRG expression profiles among patients with NAFLD. Blood samples were collected from five HC and five patients with NASH. Liver tissue and serum samples were collected from patients undergoing liver biopsy between April 2019 and July 2020, and categorized based on histopathological diagnosis into hepatic steatosis (HS), non-alcoholic steatohepatitis (NASH), and hepatitis control (HC). Participants with predetermined abnormal imaging parameters were offered a liver biopsy. Biopsies were read in a blinded fashion with results based on the consensus by 2 expert pathologists.The prevalence of NASH was defined by biopsy. The demographic information of the included participants was shown in Supplementary Table 1.
All participants provided informed consent to participate in the study. This study was approved by the Medical Ethics Committee of Yan'an Hospital, affiliated with the Kunming Medical University ethics committee. First, total RNA was isolated from ten blood samples using TRIzol (Ambion, Austin, USA). Next, cDNA was synthesized by reverse transcribing total RNA into cDNA using the First-strand-cDNA-synthesis-kit (Servicebio, Wuhan, China). Finally, qRT-PCR was performed using the 2xUniversal Blue SYBR Green qPCR Master Mix (Servicebio, Wuhan, China). All these experiments were performed based on the protocol provided by the manufacturer. The sequences of primer used for PCR were designed based on the length of primer (17–25 bp), Tm value (58–62℃), GC content (40–60%), the size of the product (100–200 bp) and so on (Table 1). GAPDH served as an internal reference gene. Gene expression was calculated using the 2-ΔΔCt method [22].
Results
Subject characteristics
The demographic and clinical characteristics of the participants are summarized in supplementary Table 1. There were no significant differences in age, sex, race, diabetes and hypertension prevalence between the NASH and HC groups. The levels of TC and BMI in NASH group were higher than those in HC group, but there was no significant difference in TG, LDL-C, HDL-C, ALT, AST, GGT and WC between the two groups.
Identifying MR-DEGs in Patients with NAFLD
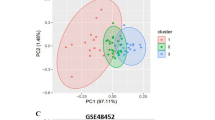
Principal component analysis (PCA) plots show GSE48452 and GSE63067 before and after eliminating batch effects between datasets (Fig. 1A-D). In total, 129 DEGs among patients with NASH and HC in the merged dataset were identified. Of which 100 genes were upregulated, and 29 genes were downregulated genes (Fig. 2A). These DEGs were visualized using a heatmap (Fig. 2B). Finally, DEGs and 948 MRGs were overlapped to identify 18 MR-DEGs (Fig. 2C). Subsequently, functional enrichment analysis was performed to identify the underlying mechanisms of NAFLD-associated MR-DEGs. Figure 2D shows the top ten GO terms under each classification. The results revealed that these MR-DEGs were primarily enriched in the “isoprenoid metabolic process,” “acylglycerol biosynthetic process,” and “fatty acid metabolic process.” The KEGG pathways enriched by these MR-DEGs primarily included the “biosynthesis of steroid hormones” as well as the “fatty acid and glycerolipid metabolism” pathways (Fig. 2E). Finally, a PPI network of MR-DEGs was constructed, which included 12 nodes (11 up-regulated genes and 1 down-regulated genes) and 14 edges (Fig. 2F). The PPI network revealed that LPL had interactions with PLA2G7, ME1, FADS2, ACSL4, and DGAT2. Meanwhile, FMO1 interacted with CYP1A1 and UGT2A3.
Identifying metabolism-related differentially expressed genes (MR-DEGs) and functional enrichment analysis. A, B The volcano map (A) and heat map (B) show 129 differentially expressed genes (DEGs) between the nonalcoholic steatohepatitis (NASH) and healthy controls (HC) groups in the merged dataset. C The Venn diagram of 18 MR-DEGs was obtained by overlapping DEGs and 948 metabolism-related genes (MRGs). D, E Gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enriched by MR-DEGs. BP, biological progress; CC, cellular component; MF, molecular function. F The protein–protein interaction (PPI) network of MR-DEGs
Screening of biomarkers related to metabolism
To further dig out the key genes, LASSO regression analysis was performed on 18 MR-DEGs to screen for key genes, and 10 feature genes were identified, including ACSL4, Amidolytic domain1 (AMDHD1), CA14, DGAT2, Flavin-Containing Dimethylaniline Monooxygenase 1 (FMO1), IDO2, Lipoprotein lipase (LPL), (Proline 4-hydroxylase subunit α1) P4HA1, PKLR, and TREH (Fig. 3A-B). Meanwhile, 10 feature genes were identified, including CYP1A1, UGT2A3, P4HA1, ME1, AMDHD1, PDE11A, PLA2G7, FMO1, LPL, and FADS2 using the RF algorithm (Fig. 3C-D). Subsequently, four overlapping genes, AMDHD1, FMO1, LPL, and P4HA1, were obtained using these algorithms (Fig. 3E) and defined as metabolism-related biomarkers (MRBs) in NAFLD. The AUC values of the MRBs were > 0.8, thus indicating that these MRBs had good diagnostic accuracy (Fig. 3F). Next, the diagnostic value of biomarkers in the external validation set (GSE89632) was further validated, and the results were consistent with the training set (Fig. 3G). Subsequently, the nomogram (C-index = 0.95) was created to further explore the clinical value of biomarkers (Fig. 3H). The calibration curve showed that the error between the actual and predicted risk was small, indicating that the nomogram model has high prediction accuracy for NASH samples (Fig. 3I).
Identification of biomarkers for non-alcoholic fatty liver disease (NAFLD). A, B 18 key genes were screened using least absolute shrinkage and selection operator (LASSO). C, D Ten feature genes were retained by the random forest (RF) algorithm. E The Venn diagram of four biomarkers. F, G The receiver operating characteristic (ROC) curves of biomarkers in the training and external validation sets. AUC, the area under the curve. H The nomogram of biomarkers. I The calibration curve of the nomogram
Immune infiltration and functional enrichment analysis
To analyze the immune microenvironment of patients with NAFLD, the relative abundance of 28 immune cell types was compared between both groups in the training cohort. The results revealed significant differences in the abundance of eight immune cells, including activated and central memory CD4 T cells, immature and activated dendritic cells (DCs), effector memory CD8 T cells, mast cells, myeloid-derived suppressor cells (MDSCs), and T follicular helper (Tfh) cells (Fig. 4A). Figure 4B shows the correlation between MRBs and differential ICI. A significant negative correlation between AMDHD1 and Tfh cells as well as a positive correlation between LPL and activated CD4 T cells was observed (Fig. 4C). Next, ssGSEA was performed on biomarkers to determine the possible functions of AMDHD1, FMO1, LPL, and P4HA1 in NAFLD. The results showed that AMDHD1 was mainly enriched in the ‘cytoplasmic translation’ and ‘ECM − receptor interaction’ (Supplementary Fig. 2A). Additionally, FMO1 was primarily enriched in the intrinsic apoptotic and FoxO signaling pathways (Supplementary Fig. 2B). “Chromosome segregation” and “complement and coagulation pathways” were enriched primarily by LPL (Supplementary Fig. 2C). P4HA1 was primarily enriched in the “organic acid catabolic process” and “the interactions between neuroactive ligand receptors” (Supplementary Fig. 2D).
Immune infiltration and functional enrichment analyses. A The relative abundance of 28 immune cells in the immune microenvironment of patients with NAFLD. B The correlation between MRBs and differential immune cells. C Correlation between hub gene expression and differential immune cells in patients with NAFLD
Four MRB-based regulatory network
A regulatory network integrating AMDHD1, FMO1, LPL, and P4HA1 was constructed to identify the underlying regulatory mechanism of these MRBs. In addition, mRNA-miRNA and TF-mRNA pairs were matched to construct an “mRNA-miRNA-TF” regulatory network consisting of 25 miRNAs, four mRNAs, 41 TFs, 37 nodes, and 51 edges (Fig. 5). The results revealed that AMDHD1, P4HA1, and FMO1 could have common regulatory factors, such as PAD21 and CTCF.
Validating MRB expression
Prognostic gene expression in patients in the training set and GSE89632 was determined, respectively. The results revealed an increase in FMO1 and LPL expression levels and a decrease in AMDHD1 and P4HA1 expression levels among patients in the NASH group compared to the HC group in the training cohort (Fig. 6A). In GSE89632, a significant decrease in AMDHD1 and P4HA1 expression level and a significant increase in FMO1 and LPL expression level were observed in patients in the NASH group compared to the HC group (Fig. 6B). Finally, the expression of these MRBs in the blood samples of the patients was determined using qRT-PCR. The results were consistent with the results obtained by analyzing publicly available databases (Fig. 7).
Discussion
Adipocyte dysfunction affects NAFLD onset and progression and could be a therapeutic target for treating patients with NAFLD. However, there were significant challenges in addressing the global epidemic of NAFLD, metabolic, and hepatic complications [23]. In this study, four NAFLD-associated MRBs were identified to determine the correlation between MRGs and differential ICI.
AMDHD1 is localized in the cytoplasm, which catalyzes the non-peptide carbon–nitrogen bond hydrolysis in cyclic amides. AMDHD1 encodes a 426 amino acid protein. It is involved in the catabolism of histidine, including glutamic acid and formamide, glutamic acid, and Formate [24]. AMDHD1 is highly expressed in liver-specific [25]. A study showed a significant increase in AMDHD1 expression level in the regenerating liver compared to the developing liver, thereby indicating that AMDHD1 is involved in the renewal and repair of the liver [26]. Our results revealed a decrease in AMDHD1 expression level in patients with NAFLD, which suggest inhibition in the regeneration and development of the liver among patients with NAFLD. The failure of liver tissues to adequately repair after damage due to inflammation leads to NAFLD onset and progression. A study performed with integrated proteomics and bioinformatic analysis showed that AMDHD1 could predict the prognosis of patients with hepatocellular carcinoma (HCC) [27].
FMOs are involved in the metabolism of small-molecule drugs. In humans, five FMOs, such as FMO1, FMO3, and FMO5, are involved in drug metabolism in the liver. FMO1 and FMO3 convert Trimethylamine N-oxide (TMAO), a metabolite, to trimethylamine in the liver [28]. Because it was associated with insulin resistance, the formation of atherosclerotic plaques, cancer, diabetes, heart failure, and Hepatic steatosis was positively correlated. TMAO is produced by the fermentation of dietary nutrients, such as choline and carnitine, in the gut microbiota. In metabolic syndromes, an increase in TMAO levels occurs along with an increase in body mass, visceral adiposity, and fatty liver indices [29]. Our results revealed that FMO1 is a hub gene in NAFLD; however, the involvement of FMO1 in NAFLD pathogenesis is yet to be determined. A study used marker-free quantitative proteomics to demonstrate the effect of NASH on biological processes and functions in rats. The results showed a decrease in FMO1 expression level in rats in the NASH group, indicating significant involvement of FMO1 in NAFLD progression [30].
LPL encodes for lipoprotein lipase, detected in cardiac, muscle, and adipose tissue. LPL is a homodimer and acts as a triglyceride hydrolase and a ligand-bridging factor involved in the uptake of lipoproteins mediated by the receptors. LPL binds to glycerol tripolyester. It participates in catalysis, the process associated with changes in cellular state or activity (such as motility, secretion, enzyme production, gene expression, etc.), and the internal homeostasis of cholesterol. Gain, loss, or modification of proteins or lipids in chylomicrons, including lipoprotein lipase hydrolysis of triglycerides and subsequent loss of free fatty acids. LPL activates or enhances the frequency, rate, or degree of adipocyte differentiation. Severe mutations due to LPL deficiency cause type I hyperlipoproteinemia. In fact, LPL mutations are involved in multiple lipoprotein metabolism diseases. Moreover, rs328 polymorphism in the G allele of LPL could reduce the risk of abdominal obesity [31]. Fibrin and omega-3 fatty acids reduce TG levels. However, ideal therapeutic strategies for patients with high TG and TGRL levels and low HDL levels are still unavailable. LPL is a key regulator of lipids. In lipoprotein particles, lipids into glycerol and free fatty acids are hydrolyzed by LPL for the storage and consumption of lipids by peripheral organs. With an enhancement in our comprehension of human genetics, proteins regulating LPL activity, including the apolipoprotein and angiopoietin-like families, have been identified.
A previous study showed an increase in LPL expression levels among patients with NASH, consistent with our results. LPL is critically involved in incorporating plasma lipids into tissues, regulates the metabolism of lipids, and energy balance in the body [32]. The hepatic stellate cells (HSC) activation pathway mediated by LPL could be targeted for treating liver fibrosis in patients with NASH [33]. During fasting, high insulinemia and blood glucose could reduce the LPL-mediated catabolism of triglyceride-rich lipoproteins and increases postprandial levels [34]. LPL regulates lipid metabolism by hydrolyzing triglycerides and very low-density lipoproteins in chylomicrons. In high-fat diet-fed mice, a high LPL expression level could attenuate lipid droplet accumulation in the liver and improve the metabolism of glucose. These results could aid in designing new drugs to treat metabolic syndromes, such as type 2 diabetes and NAFDL [35]. Studies have shown that targeting the LPL/FABP4/CPT1 axis could be a promising strategy for preventing NASH-related HCC [36].
P4HA1 encodes for a component of proline 4-hydroxylase, a key enzyme in the synthesis of collagen and composed of two. The protein encoded by P4HA1 contains several different alpha subunit types and serves as a major portion of the catalytic site of an active enzyme. P4HA1 also acts as a cofactor and antioxidant in several species [37]. P4HA1 is an essential rate-limiting enzyme and a P4H (also called PHD) isoenzyme [38]. P4H acts as an oxygen sensor in cells and regulates the degradation of hypoxia-inducible factor (HIF) in proteasomes in an oxygen-dependent manner. HIF is activated by P4H hydroxylation and regulates adaptive hypoxic responses [39]. A study has shown the involvement of P4H in the pathogenesis of NAFLD [36]. Our results showed that P4HA1 was a hub gene in NAFLD. Furthermore, a study has shown differential expression of P4HA1 in the liver of patients with morbid obesity [40]. Our results revealed a decrease in P4HA1 expression level in patients with NASH.
In the KEGG enrichment analysis, FOXO1 is involved in both the insulin signaling pathway, and FOXO1 was the characteristic target gene for NAFLD. A study showed that Lut/ZnO NPs could activate the PI3K/AKT signaling pathway, thereby inactivating FOXO1. Thus, Lut/ZnO NPs could alleviate NAFLD progression by reducing insulin resistance and antioxidant levels, as well as by regulating the insulin signaling pathway [41]. Furthermore, LPL was mainly enriched in chromosome segregation. A paradoxical response to DNA damage occurs in HCC, which leads to errors in chromosome segregation [42]. Error in mitosis occurs in metabolic disorders and causes numerical and structural chromosome aberrations during cell division [43]. FMO1 and P4HA1 involved in these pathways are closely associated with HCC [44]. Inflammation and ICI are critically involved in NALFD development. P4HA1 was primarily enriched in the interaction between neuroactive ligand receptors was a close relationship of NALFD [45].
Activated innate immune cells are involved in NAFLD pathogenesis. In fact, NAFLD progression involves recognizing immune cells, such as Kupffer cells, neutrophils, DCs, and natural killer T cells, by pattern recognition. This leads to oxidative imbalance, which promotes the production of cytokines and new reactive species by innate immune cells, thereby promoting inflammation induced by adaptive immune cells. Recent studies suggest that the activation of innate and adaptive immune cells causes inflammation and fibrosis in the liver. Tfh cells cause dysregulation of humoral immunity in patients with liver cirrhosis [46] and are involved in virus-induced liver fibrosis [47]. The results of this study revealed a significant negative correlation between AMDHD1 and Tfh cells. Therefore, it is speculated that AMDHD1 could aid in preventing liver fibrosis and cirrhosis among patients with NAFLD. In addition, HSC activation induces liver fibrogenesis in NAFLD. CD4 + T cells suppressed Th9 cell differentiation and reduced IL-9 expression, thus promoting the activation of hepatic stellate cells (HSCs) by activating the Raf/MEK/ERK signaling pathway [48].
TFs and miRNAs regulate gene expression and its downstream targets. MiRNAs regulate genes associated with normal metabolism in the liver. MiRNA dysregulation is involved in the development and progression of NAFLD [49]. microRNA-432-5p suppresses E2F3 translation by binding to the 3' UTR of E2F3, thereby influencing the invasion and migratory abilities of liver cancer cells [50]. miR-124-3p is involved in NAFLD development by directly targeting preadipocyte factor-1 [51]. miR-124-3p modulates sirtuin 1 expression in liver cancer, thereby attenuating the growth of liver cancer cells [52].
The results of this study showed that CTCF was regulated by AMDHD1, P4HA1, and FMO1. CTCF could alleviate NAFLD. Specific deletion of CTCF in the liver causes augments PPARγ-DNA binding activity, which increases downstream lipid MRG expression, thus causing hepatosteatosis [53].
Study strengths and limitations
For the first time, MRBs in patients with NAFLD were identified using GEO to discover new targets for treating patients with NAFLD. In addition, these biomarkers and differential ICI would aid in exploring the correlation between NAFLD and the immune microenvironment. Nevertheless, the present study has a few limitations. First, this is a retrospective study, and the data were obtained from publicly available databases. Hence, the results should be validated using additional clinical samples and data. Second, the mechanism of action of these MRBs in NAFLD progression needs to be determined.
Conclusion
In summary, four MRBs, including AMDHD1, FMO1, LPL, and P4HA1, were identified and demonstrated good ability in distinguishing patients with NAFLD from HCs. The results of this study would aid in determining the involvement of metabolism in the onset and development of NAFLD. This would help identify new targets for diagnosing and treating patients with NAFLD.
Availability of data and materials
The raw data of this study are derived from the TCGA database (https://portal.gdc.cancer.gov/) and the GEO data portal (https://www.ncbi.nlm.nih.gov/geo/), which are publicly available databases.
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- HC:
-
Healthy control
- GEO:
-
Gene Expression Omnibus
- DEGs:
-
Differentially expressed genes
- MRGs:
-
Metabolism-related genes
- MR-DEGs:
-
Metabolism-related differentially expressed genes
- PPI:
-
Protein–protein interaction
- LASSO:
-
Least absolute shrinkage and selection operator
- RF:
-
Random forest
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- ROC:
-
Receiver operating characteristic
- GSEA:
-
Gene set enrichment analysis
- TF:
-
Transcription factor
- qRT-PCR:
-
Quantitative reverse transcriptase PCR
References
Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17(4):748-55.e3. https://doi.org/10.1016/j.cgh.2018.05.057.
Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47-64. https://doi.org/10.1016/j.jhep.2014.12.012.
Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156(5):1264-1281.e4. https://doi.org/10.1053/j.gastro.2018.12.036.
The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases.Clin Liver Dis (Hoboken). 2018;67(1):328-357. https://doi.org/10.1002/cld.722.
Arshad, T. , Paik, J. M. , Biswas, R. , Alqahtani, S. , Henry, L. , & Younossi, Z. M. Trends in the prevalence of nonalcoholic fatty liver disease (nafld) among adolescents and young adults in the united states, 2007–2016. Hepatology: Official Journal of the American Association for the Study of Liver Diseases(Suppl.1), 72. https://doi.org/10.1002/hep4.1760.
Tang A, Ng CH, Phang PH, Chan KE, Chin YH, Fu CE, et al. Comparative Burden of Metabolic Dysfunction in Lean NAFLD vs Non-lean NAFLD - A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;S1542–3565(22):00669–3. https://doi.org/10.1016/j.cgh.2022.06.029.
Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AA-O, Gores GJ, et al. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71(3):907–16. https://doi.org/10.1002/hep.30858.
Lazarus JV, Mark HE, Villota-Rivas M, Palayew A, Carrieri P, Colombo M, et al. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J Hepatol. 2022;76(4):771–80. https://doi.org/10.1016/j.jhep.2021.10.025.
arantino G, Crocetto F, Di Vito C, Creta M, Martino R, Pandolfo SD, Pesce S, Napolitano L, Capone D, Imbimbo C. Association of NAFLD and Insulin Resistance with Non Metastatic Bladder Cancer Patients: A Cross-Sectional Retrospective Study. J Clin Med. 2021;18(2):346. https://doi.org/10.3390/jcm10020346.
He Z, Bin Y, Chen G, Li Q, Fan W, Ma Y, Yi J, Luo X, Tan Z, Li J. Identification of MAP3K4 as a novel regulation factor of hepatic lipid metabolism in non-alcoholic fatty liver disease. J Transl Med. 2022;20(1):529. https://doi.org/10.1186/s12967-022-03734-8.
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882–3. https://doi.org/10.1093/bioinformatics/bts034.
Eslam M, Sanyal AJ, George J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158(7):1999-2014.e1. https://doi.org/10.1053/j.gastro.2019.11.312.
Colaprico A, Silva TC, Olsen C, Garofano LA-O, Cava C, Garolini D, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8):e71. https://doi.org/10.1093/nar/gkv1507.
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential Clinical Characteristics and Mortality Outcomes in Persons With NAFLD and/or MAFLD. Clin Gastroenterol Hepato. 2021;19(10):2172-2181.e6. https://doi.org/10.1016/j.cgh.2021.05.029.
Wu J, Lu P, Yang T, Wang L. Meta-analysis of the differentially expressed breast cancer-related microRNA expression profiles. J Obstet Gynaecol. 2014;34(7):630–3. https://doi.org/10.3109/01443615.2014.920782.
Yu G, Wang Lg Fau - Han Y, Han Y Fau - He Q-Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7. https://doi.org/10.1089/omi.2011.0118.
Gupta A, Elfiky A. Novel findings from determination of common expressed plasma exosomal microRNAs in patients with psoriatic arthritis, psoriasis vulgaris, rheumatoid arthritis, and gouty arthritis.Discov Med. 2019;28(152):113–122. PMID: 31926583.
Wang S, Su W, Zhong C, Yang T, Chen W, Chen G, Liu Z, Wu K, Zhong W, Li B, Mao X, Lu J. An Eight-CircRNA Assessment Model for Predicting Biochemical Recurrence in Prostate Cancer. Front Cell Dev Biol. 2020;10(8):599494.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;17(12):77. https://doi.org/10.1186/1471-2105-12-77.
Liu TT, Li R, Huo C, Li JP, Yao J, Ji XL, Qu YQ. Identification of CDK2-Related Immune Forecast Model and ceRNA in Lung Adenocarcinoma, a Pan-Cancer Analysis. Front Cell Dev Biol. 2021;30(9):682002.
Kumar L, Futschik ME. Mfuzz: a software package for soft clustering of microarray data.Bioinformation. Bioinformation. 2007;2(1):5–7. https://doi.org/10.6026/97320630002005.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab Cell Metab. 2018;27(1):22–41. https://doi.org/10.1016/j.cmet.2017.08.002.
Assié G, Guillaud-Bataille M Fau - Ragazzon B, Ragazzon B Fau - Bertagna X, Bertagna X Fau - Bertherat J, Bertherat J Fau - Clauser E, Clauser E. The pathophysiology, diagnosis and prognosis of adrenocortical tumors revisited by transcriptome analyses. Trends Endocrinol Metab. 2010;21(5):325–34. https://doi.org/10.1016/j.tem.2009.12.009.
Chang J, Nicolas E Fau - Marks D, Marks D Fau - Sander C, Sander C Fau - Lerro A, Lerro A Fau - Buendia MA, Buendia Ma Fau - Xu C, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1.RNA Biol. 2004;1(2):106–13. https://doi.org/10.4161/rna.1.2.1066.
Song Y, Ahn J Fau - Suh Y, Suh Y Fau - Davis ME, Davis Me Fau - Lee K, Lee K. Identification of novel tissue-specific genes by analysis of microarray databases: a human and mouse model.PLoS One. 2013;8(5):e64483. https://doi.org/10.1371/journal.pone.0064483.
Zhang Q, Xiao Z, Sun S, Wang K, Qian J, Cui Z, et al. Integrated Proteomics and Bioinformatics to Identify Potential Prognostic Biomarkers in Hepatocellular Carcinoma. Cancer Manag Res Cancer Manag Res. 2021;13:2307–17. https://doi.org/10.2147/CMAR.S291811.
Coutinho-Wolino KA-O, de FCLFMA-O, de Oliveira Leal VA-O, Mafra DA-O, Stockler-Pinto MA-O. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur J Nutr. Eur J Nutr. 2021;60(7):3567–3584. https://doi.org/10.1007/s00394-021-02491-6.
Gatarek P, Kaluzna-Czaplinska JA-O. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021;20:301–19. https://doi.org/10.17179/excli2020-3239.
Moreto FA-O, Ferron AA-O, Francisqueti-Ferron FA-O, D’Amato AA-O, Garcia JA-OX, Costa MA-O, et al. Differentially expressed proteins obtained by label-free quantitative proteomic analysis reveal affected biological processes and functions in Western diet-induced steatohepatitis. J Biochem Mol Toxicol. 2021;35(6):1–11. https://doi.org/10.1002/jbt.22751.
Camacho-Muñoz DA-O, Kiezel-Tsugunova M, Kiss O, Uddin M, Sundén M, Ryaboshapkina M, et al. Omega-3 carboxylic acids and fenofibrate differentially alter plasma lipid mediators in patients with non-alcoholic fatty liver disease. FASEB J. 2021;35(11):e21976. https://doi.org/10.1096/fj.202100380RRR.
Yang YY, Hong SY, Xun Y, Liu CQ, Sun JX, Xu JZ, Xu MY, An Y, He D, Xia QD, Wang SG. Characterization of the Lipid Metabolism in Bladder Cancer to Guide Clinical Therapy. J Oncol. 2022;12(2022):7679652. https://doi.org/10.1155/2022/7679652.
Teratani T, Tomita K, Furuhashi H, Sugihara N, Higashiyama M, Nishikawa M, et al. Lipoprotein Lipase Up-regulation in Hepatic Stellate Cells Exacerbates Liver Fibrosis in Nonalcoholic Steatohepatitis in Mice. Hepatol Commun. 2019;3(8):1098–112. https://doi.org/10.1002/hep4.1383.
Cassader M, Gambino R Fau - Musso G, Musso G Fau - Depetris N, Depetris N Fau - Mecca F, Mecca F Fau - Cavallo-Perin P, Cavallo-Perin P Fau - Pacini G, et al. Postprandial triglyceride-rich lipoprotein metabolism and insulin sensitivity in nonalcoholic steatohepatitis patients.Lipids. 2001;36(10):1117–24. https://doi.org/10.1007/s11745-001-0822-5.
Shimizu KA-O, Nishimuta S, Fukumura Y, Michinaga S, Egusa Y, Hase T, et al. Liver-specific overexpression of lipoprotein lipase improves glucose metabolism in high-fat diet-fed mice. PLoS One. 2022;17(9):e0274297. https://doi.org/10.1371/journal.pone.0274297.
Yang H, Deng Q, Ni T, Liu Y, Lu L, Dai H, et al. Targeted Inhibition of LPL/FABP4/CPT1 fatty acid metabolic axis can effectively prevent the progression of nonalcoholic steatohepatitis to liver cancer. Int J Biol Sci. 2021;17(15):4207–22. https://doi.org/10.7150/ijbs.64714.
Jia X, Zhai T, et al. Integrated Analysis of Multiple Microarray Studies to Identify Novel Gene Signatures in Non-alcoholic Fatty Liver Disease. Front Endocrinol (Lausanne). 2019;10:599. https://doi.org/10.3389/fendo.2019.00599.
Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45(2):106–24. https://doi.org/10.3109/10409231003627991.
Koivunen P, Kietzmann T. Hypoxia-Inducible Factor Prolyl 4-Hydroxylases and Metabolism. Trends Mol Med. 2018;24(12):1021–35. https://doi.org/10.1016/j.molmed.2018.10.004.
Chen X, Zhang L, Wang Y, Li R, Yang M, Gao L. Identification of Key Target Genes and Pathway Analysis in Nonalcoholic Fatty Liver Disease Via Integrated Bioinformatics Analysis. Balkan J Med Genet. 2023;25(1):25–34. https://doi.org/10.2478/bjmg-2022-0006.
Ahmed EA-O, Mohamed HE, Farrag MA. Luteolin loaded on zinc oxide nanoparticles ameliorates non-alcoholic fatty liver disease associated with insulin resistance in diabetic rats via regulation of PI3K/AKT/FoxO1 pathway. Int J Immunopathol Pharmacol. 2022;36:3946320221137435. https://doi.org/10.1177/03946320221137435.
Su T, Qin XA-O, Dohmae N, Wei F, Furutani Y, Kojima S, et al. Inhibition of Ganglioside Synthesis Suppressed Liver Cancer Cell Proliferation through Targeting Kinetochore Metaphase Signaling. Metabolites. 2021;11(3):167. https://doi.org/10.3390/metabo11030167.
Sucularli C. Identification of BRIP1, NSMCE2, ANAPC7, RAD18 and TTL from chromosome segregation gene set associated with hepatocellular carcinoma. Cancer Gene. 2022;268–269:28–36. https://doi.org/10.1016/j.cancergen.2022.09.003.
Qiang R, Zhao Z, Tang L, Wang Q, Wang Y, Huang QA-O. Identification of 5 Hub Genes Related to the Early Diagnosis, Tumour Stage, and Poor Outcomes of Hepatitis B Virus-Related Hepatocellular Carcinoma by Bioinformatics Analysis. Comput Math Methods Med. 2021;2021:9991255. https://doi.org/10.1155/2021/9991255.
Wang A, Li Z, Sun Z, Wang Y, Fu S, Zhang D, et al. Heart failure with preserved ejection fraction and non-alcoholic fatty liver disease: new insights from bioinformatics. ESC Heart Fail. 2023;10(1):416–31. https://doi.org/10.1002/ehf2.14211.
Zhao J, Shi J, Qu M, Zhao X, Wang H, Huang M, et al. Hyperactive Follicular Helper T Cells Contribute to Dysregulated Humoral Immunity in Patients With Liver Cirrhosis. Front Immunol. 2019;13(10):1915. https://doi.org/10.3389/fimmu.2019.01915.
Wang W, Li F, Lai X, Liu H, Wu S, Han Y, et al. Exosomes secreted by palmitic acid-treated hepatocytes promote LX-2 cell activation by transferring miRNA-107. Cell Death Discov. 2021;7(1):174. https://doi.org/10.1038/s41420-021-00536-7.
Pan J, Tian Y, Hu F, Xu J, Tan N, Han Y, et al. Exploration of immune infiltration and feature genes in viral hepatitis-associated liver fibrosis using transcriptome data. Ann Transl Med. 2022;10(19):1051. https://doi.org/10.21037/atm-22-2205.
Pervez MA-O, Khan DA, Gilani SA-O, Fatima S, Ijaz AA-O, Nida S. Hepato-Protective Effects of Delta-Tocotrienol and Alpha-Tocopherol in Patients with Non-Alcoholic Fatty Liver Disease: Regulation of Circulating MicroRNA Expression. Int J Mol Sci. 2022;24(1):79. https://doi.org/10.3390/ijms24010079.
Liu G, Ouyang X, Gong L, Yao L, Liu S, et al. E2F3 promotes liver cancer progression under the regulation of circ-PRKAR1B. Int J Mol Sci. 2022;24(1):79. https://doi.org/10.1016/j.omtn.2021.07.002
Wang G, Zou H, Lai C, Huang X, Yao Y, Xiang G. Repression of MicroRNA-124–3p Alleviates High-Fat Diet-Induced Hepatosteatosis by Targeting Pref-1. Front Endocrinol (Lausanne). 2020;11:589994.
Xiang H, Luo M, Hou P, Xiao Z, Huang Z, Feng Q, et al. miR-124-3p combined with miR-506-3p delay hepatic carcinogenesis via modulating sirtuin 1. Biomarkers. 2021;26(3):196–206. https://doi.org/10.1080/1354750X.2020.1854856.
Choi Y, Song MJ, Jung WJ, Jeong H, Park S, Yang B, et al. Liver-Specific Deletion of Mouse CTCF Leads to Hepatic Steatosis via Augmented PPARγ Signaling. Cell Mol Gastroenterol Hepatol. 2021;12(5):1761–87. https://doi.org/10.1016/j.jcmgh.2021.07.016.
Funding
Supported by Shenzhen Science and Technology Innovation Commission (JCYJ20220530163602006).
Author information
Authors and Affiliations
Contributions
Hua Jiang and Yang Hu designed experiments, analyzed data and wrote the manuscript. Zhibo Zhang and Xujia Chen prepared the fifigures and tables. Jianpeng Gao reviewed and revised the manuscript. All authors read and approved the fifinal manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the procedures involving human participants were approved by the Medical Ethics Committee of Yan 'an Hospital Affiliated to Kunming Medical University (2023–019-01).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, H., Hu, Y., Zhang, Z. et al. Identification of metabolic biomarkers associated with nonalcoholic fatty liver disease. Lipids Health Dis 22, 150 (2023). https://doi.org/10.1186/s12944-023-01911-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01911-2