Abstract
Background
The preventive effect of cholesterol efflux capacity (CEC) on the progression of atherosclerotic lesions has been confirmed in animal models, but findings in the population are inconsistent. Therefore, this meta-analysis aimed to systematically investigate the relationship of CEC with coronary artery disease (CAD) and cardiovascular mortality in a general population.
Methods
Four electronic databases (PubMed, Embase database, Cochrane Library, Web of Science) were searched from inception to February 1st, 2022 for relevant studies, without any language restriction. For continuous variables, the mean and standard deviation (SD), maximum adjusted odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) and 95% confidence intervals (CIs) were extracted. The random-effects model was adopted to calculate the pooled results, and dose-response analyses were conducted. All pooled results were expressed by standardized mean difference (SMD) and ORs.
Results
Finally, 18 observational studies were included. Compared with the non-CAD group, the CAD group (SMD -0.48, 95% CI − 0.66 to − 0.30; I2 88.9%) had significantly lower CEC. In the high-CEC population, the risks of CAD (OR 0.52, 95% CI 0.37 to 0.71; I2 81%) significantly decreased, and a linear negative dose-response was detected. However, an association between CEC and the risk of cardiovascular mortality was not found (OR 0.44, 95% CI 0.18 to 1.06; I2 83.2%).
Conclusions
This meta-analysis suggests that decreased CEC is strongly associated with the risk of CAD, independent of HDL-C level. However, a decreased CEC seems not to be related to cardiovascular mortality. Meanwhile, CEC is linearly negatively correlated with the risk of CAD.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD), a manifestation of atherosclerosis, results in stable angina, unstable angina, myocardial infarction (MI), or sudden cardiac death [1]. Currently, CAD remains the leading cause of morbidity and mortality worldwide [2]. Atherosclerotic plaques are the hallmark of CAD, and its formation is closely associated with dyslipidemia and inflammatory processes [2,3,4]. During early plaque formation, endothelial cell damage is followed by inflammatory infiltration and abnormal accumulation of cholesterol in macrophages can result in foam cell formation and eventually atherosclerotic plaques [5, 6]. Considering these pathomechanisms, high-density lipoprotein (HDL) has been extensively studied due to its abilities of simultaneous reverse cholesterol transport (RCT) and anti-inflammatory effects [7, 8].
RCT is the process where excess cholesterol is transported via HDL from peripheral tissues to the liver and ultimately to the bile and feces for excretion [9]. In 1975, it was proposed that the concentration of HDL was negatively correlated with atherosclerosis, and this assumption was confirmed in animal models [10]. Subsequently, the Framingham study confirmed that HDL levels were inversely associated with the risk of CAD [11]. In the process of RCT, cholesterol is initially transported from arterial macrophages to HDL, thus maintaining intracellular cholesterol homeostasis, which is critical for the viability and function of macrophages [12]. Once macrophages fail to efficiently expel the remaining excess cholesterol, they will degenerate to foam cells. This triggers the local aggregation of pro-inflammatory cells, thereby providing an environment conducive to the progression of atherosclerotic plaques [13]. In view of the above, it is hypothesized that a higher HDL level may be associated with a lower cardiovascular risk.
To verify this hypothesis, several trials based on statin therapy, cholesteryl ester transfer protein (CETP)-inhibitors and niacin have been conducted, but their results are unsatisfactory [14,15,16,17]. Furthermore, the National Institute of Health (NIH) terminated the AIM-HIGH study 18 months earlier than planned, since niacin (the most effective drug on raising HDL levels) could not provide additional cardiovascular benefits when used in combination with statins [18]. To sum up, these studies show that a mere increase in HDL levels has no cardiovascular benefits.
Thus, the research focus has shifted towards HDL functionality rather than quantity. Cholesterol efflux capacity (CEC) indicates the ability of HDL to facilitate the removal of cholesterol from the lipid-filled macrophages [19]. CEC depends primarily on the cholesterol content in macrophages, the expression of various macrophage-mediated transporters and the lipid and protein compositions of HDL as an extracellular receptor [20]. Moreover, the preventive effect of CEC on the progression of atherosclerotic lesion has been confirmed in animal models [9]. Recently, CEC has also been measured in clinical cohorts. Several studies have suggested that physiological CEC is negatively correlated with the development of CAD, independent of the HDL cholesterol levels [21, 22]. This study systematically investigated the relationships of CEC with CAD and cardiovascular mortality. In addition, dose-response analyses were also conducted for CEC and the risks of CAD and cardiovascular mortality.
Methods
Literature search strategy
This study protocol and report were based on the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement [23] and were registered in the NPLASY-International Platform of Registered Systematic Review and Meta-analysis Protocols under the identifier of INPLASY202170006. Relevant studies were identified from four electronic databases (PubMed, Embase database, Cochrane Library, and Web of Science) from 1980 to February 1st, 2022, without any language restriction. Two groups of medical subject terms were applied, including “cardiovascular diseases” and “cholesterol efflux capacity “. Furthermore, to identify additional studies, previous systematic reviews or meta-analyses were reviewed as well. The detailed search strategy is presented in Supplementary Materials (Additional file 1).
Parameters and outcomes of interest
The exposure of interest was HDL-CEC. All studies reporting CEC were included, regardless of the specific laboratory or calculation methods. According to the definitions of the included original studies, CAD was defined as coronary stenosis ≥50% confirmed by coronary angiography or multi-slice coronary computed tomography, or history of myocardial infarction or angina confirmed by hospital or autopsy records. Cardiovascular mortality was defined as death due to any cardiovascular cause or sudden cardiac death. In this study, our endpoints included CAD and cardiovascular mortality risk.
Inclusion and exclusion criteria
With reference to the PICOS (Population, Intervention, Control, Outcome, and Study) criteria [24], the study inclusion criteria were as follows:
-
1)
studies conducted on the general population with the age over 18 years;
-
2)
studies, whose exposure of interest was HDL-CEC;
-
3)
the CEC values of non-CAD population in case-control studies were regarded as the control group;
-
4)
the CEC values in cohort studies were divided by medians or into quartiles, and patients with the lowest CEC quartile or those with CEC below the median were enrolled into the reference group according to the calculation method of original publications;
-
5)
studies, that reported the risk of CAD or cardiovascular mortality;
-
6)
studies, that were limited to case-control, cohort studies, or randomized controlled trials;
-
7)
studies reporting relevant effect values such as odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) together with respective 95% confidence intervals (CIs).
The study exclusion criteria were:
-
1)
studies focusing on cancer, autoimmune diseases, familial hyperlipidemia, or renal failure;
-
2)
studies where the HDL-CEC values could not be obtained or converted;
-
3)
studies not reporting the risk of CAD or cardiovascular mortality;
-
4)
cross-sectional studies;
-
5)
studies with unavailable ORs, RRs, or HRs;
-
6)
conference abstracts, case reports, or case series.
Data extraction and quality assessment
A uniform list was prepared to collect related baseline characteristics, including first author, year of publication, country, sample size, proportion of males, age of patients on inclusion, follow-up, subjects, endpoints, donor cell line, labeled cholesterol, and cholesterol acceptor. In addition, the maximum covariate-adjusted ORs, RRs, or HRs were extracted. The quality of the included studies was assessed using the Newcastle Ottawa Scale (NOS), with a total score of 9 stars [24]. Studies with a score of ≥6 stars were considered to have a low risk of bias, while those with a score of < 6 stars were deemed to show a high risk of bias [25].
Statistical analysis
Firstly, we assessed the difference in CEC value between CAD and non-CAD populations. For this purpose, the sample numbers, mean CEC values, and SD of CEC in CAD and non-CAD groups were extracted from the selected studies. Considering the heterogeneities in the experimental measurement methods, the standardized mean difference (SMD) was utilized for assessment. For studies reporting median values and interquartile ranges, with a large sample size (> 100/group), the median values were treated as means, and SD was calculated by 75th minus 25th percentiles divided by 1.35 [26], otherwise SD could not be estimated.
Secondly, we assessed the difference in cardiovascular risk between the highest and the lowest CEC value groups. In cohort studies, CEC values were divided according to quartiles or medians, where the quartile 1 (Q1) or less-than median group was regarded as the lowest group, while the quartile 4 (Q4) or over median group as the highest group. Broadly speaking, the differences between the different effect values (e.g., ORs, RRs and HRs) were very minor if the incidence of the outcome is low (< 10%) [27]. The cumulative results were expressed as ORs for conservative assessment, while subgroup analyses were also performed to find any difference between the three measures of association (OR, RR or HR) [28]. On the other hand, statistical heterogeneity was assessed using the I2 statistic, where I2 values of 25, 50, and 75% indicated low, moderate, and high inconsistencies, respectively [29]. If there was a high heterogeneity between studies, subgroup analyses (number of studies > 10) were performed to explore the possible sources of heterogeneity between groups. Further, sensitivity analyses were carried out in parallel by progressively eliminating one study each time, so as to assess the potential sources of heterogeneity and the stability of our pooled results. Moreover, the DerSimonian and Laird (DL) method for random-effects model was employed to estimate the pooled standardized mean difference (SMD) and ORs, as the former evaluated the pooled results more conservatively compared to the fixed-effects model. Also, if sufficient studies (n ≥ 10) were included [18], Begg’s and Egger’s tests were utilized to assess the potential risk of publication bias. Later, trim and fill analyses were performed if there was evidence of publication bias.
Thirdly, this study focused on assessing the dose-response analyses between CEC and cardiovascular outcomes. To maximize the available studies, the robust error meta-regression approach proposed by Xu and Doi [30] was employed to establish the potential dose-response relationship between CEC and CAD and cardiovascular mortality. In this “one-stage” framework, each of the included studies was considered as a cohort across the entire population. In general, the lowest dose category was required as a reference for the included studies. In this analysis, the potential nonlinear trends in three knots were tested using the restricted cubic splines, and nonlinear p-values were calculated by testing whether the coefficient of the second spline was equal to zero. When the nonlinear p-value was < 0.05, the nonlinear model was adopted; otherwise, the linear model was adopted. Furthermore, when studying open intervals, the amplitudes were assumed to be the same as the adjacent categories. All statistical analyses were conducted using the statistical program Stata 12.0E.
Results
Search results and study characteristics
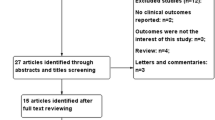
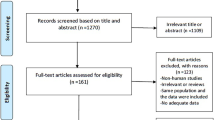
Initially, a total of 2524 studies were identified from the four databases. Among them, 646 records were identified more than once and consequently excluded, leaving 1878 studies. After screening the titles and abstracts of these 1878 studies, 1814 were excluded due to irrelevance, and 8 studies were excluded because of being not retrievable, leaving 56 studies for full-text review. Finally, 18 observational studies were included for the present meta-analysis. The specific reasons for study exclusion were as follows, a) reviews (n = 8); b) studies not reporting CEC (n = 12); c) studies not including the risk of CAD or cardiovascular mortality (n = 14); d) cross-sectional studies (n = 4). Further, a “snowball search” was performed based on the citation list of all included studies. In the 18 studies initially identified, there were 614 citations, whereas 523 were irrelevant for this meta-analysis, 55 were duplicates, and 36 were reviews. Ultimately, no additional studies were included in the analysis. The detailed flow chart of the study retrieval process is presented in Fig. 1.
Our meta-analysis included 14 case-control and four cohort studies [21, 22, 31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] involving altogether 6298 cases. Eleven studies [21, 31,32,33, 37,38,39,40, 42, 43, 45], including 3521 cases, mentioned CEC differences between CAD and non-CAD groups. Additionally, nine studies [21, 22, 32,33,34,35, 40, 42, 46], involving 5023 cases, mentioned a relationship of CEC with CAD susceptibility, with seven studies [21, 22, 33, 34, 40, 42, 46] showing positive results and two studies [32, 35] showing no significant association. Four articles [22, 36, 41, 46], including 916 cases, reported the correlation between CEC and cardiovascular mortality risk, with two studies [36, 46] showing negative correlation and two studies [22, 41] showing no significant correlation. In the dose-response analyses, three studies [21, 22, 42] mentioned the dose-relationship between CEC and CAD risk, while another three [22, 36, 41] indicated the dose-relationship between CEC and cardiovascular mortality susceptibility. The detailed baseline information is presented in Table 1. The results of all the included studies were adjusted by HDL-C levels. Studies with a score of ≥6 stars were considered as high-quality studies (Supplementary Table 1).
CEC values in CAD compared with non-CAD groups
CEC values of CAD group were significantly lower than those of non-CAD group (SMD = − 0.48, 95% CI − 0.66 to − 0.30; I2 = 88.9%) (Fig. 2). Sensitivity analyses were conducted by excluding one study each time, and the pooled results of CAD were slightly changed (Supplementary Fig. 1). Moreover, a funnel plot, which was prepared to assess the potential publication bias for CAD, exhibited asymmetry pointing towards a bias (Supplementary Fig. 2). Besides, there was no obvious evidence of publication bias (p = 0.350) detected by Begg’s test, whereas Egger’s tests suggested that there might be a publication bias (p = 0.007). Trim and fill analyses were thereby conducted because there were no additional studies. The pooled results obtained from the random model were the same.
Forest plots of random-effects meta-analysis (between-study variance estimator: DerSimonian and Laird (DL)) for cholesterol efflux capacity (CEC) differences between coronary artery disease (CAD) vs. non-CAD group. Shown is the standardized mean difference (SMD) together with its 95% confidence interval (95% CI) as effect measure
CEC and the risk of CAD (highest vs. lowest)
The risk of CAD decreased by 48% in the highest CEC group compared with the lowest CEC group (OR = 0.52, 95% CI 0.37 to 0.71; I2 = 81%), and similar results were obtained after subgroup analysis by OR (OR = 0.45, 95% CI 0.32 to 0.63; I2 = 63%) and HR (HR = 0.51, 95% CI 0.26 to 1.0; I2 = 89.2%). Similarly, the risk of CAD decreased by 19% per 1-SD CEC increment (OR = 0.81, 95% CI 0.71 to 0.93; I2 = 68.2%), and similar results were observed from the subgroup analysis according to OR (OR = 0.69, 95% CI 0.52 to 0.92; I2 = 68.4%) and HR (HR = 0.83, 95% CI 0.71 to 0.96; I2 = 59.1%) (Fig. 3). In the sensitivity analysis, the pooled results remained stable after excluding one study each time (Supplementary Fig. 3A and B). No obvious evidence of publication bias was detected by Funnel plot (Supplementary Fig. 4), Begg’s test (p = 0.371) or Egger’s test (p = 0.283).
Forest plots of random-effects meta-analysis (between-study variance estimator: DerSimonian and Laird (DL)) for the differences of coronary artery disease (CAD) risk between highest cholesterol efflux capacity (CEC) vs. lowest CEC groups. Shown is the odds ratio (OR)/ relative risks (RR)/hazard ratio (HR) together with their 95% confidence interval (95% CI) as effect measure
As shown in Fig. 4A, a linear model was adopted to explore the association between CEC and the risk of CAD. As a result, the risk of CAD gradually decreased with the increase in CEC. Meanwhile, as CEC increased by 20%, the risk of CAD decreased by 10% (OR = 0.90; 95% CI 0.82–0.99).
CEC and the risk of cardiovascular mortality (highest vs lowest)
It was observed from Fig. 5 that, the risk of cardiovascular mortality did not decrease in the highest CEC group compared with the lowest CEC group (OR = 0.44, 95% CI 0.18 to 1.06; I2 = 83.2%), similar results were obtained after subgroup analysis by HR (HR = 0.52, 95% CI 0.19 to 1.43; I2 = 85.7%). However, when the study by Ritsch et al. (the incidence of outcome was 18%>10%) was removed, the pooled results showed a weak correlation (OR = 0.33, 95% CI 0.11 to 0.96; p = 0.042) (Supplementary Fig. 5).
Forest plots of random-effects meta-analysis (between-study variance estimator: DerSimonian and Laird (DL)) for the differences of cardiovascular mortality risk between highest cholesterol efflux capacity (CEC) vs. lowest CEC groups. Shown is the odds ratio (OR)/ hazard ratio (HR) together with their 95% confidence interval (95% CI) as effect measure
In Fig. 4B, the linear model was utilized to assess the correlation between CEC and the risk of cardiovascular mortality. As a result, the risk of cardiovascular mortality did not decrease with the increase in CEC.
Subgroup analyses
To investigate the potential sources of heterogeneity in our study, subgroup analyses were conducted. However, due to the small number of studies included, we only performed subgroup analyses on the pooled results covering more than 10 studies, which included the following clinical characteristics: study design, country, year of publication, sample size, and study quality scores (Supplementary Table 1). Thereafter, the heterogeneity between studies was converted to zero based on subgroup analyses of the donor cell line and cholesterol acceptor suggesting that these factors might potentially contribute to the heterogeneity in the comparison of CEC between CAD and non-CAD groups. However, potential sources of heterogeneity between CEC and CAD risk were not identified.
Discussion
The results of the meta-analysis can be summarized as follows: 1). CEC values were significantly lower in the CAD group compared to the non-CAD group. 2). The reduced CEC values were significantly associated with the risk of CAD. 3). There was no significant correlation between CEC values and the risk of cardiovascular mortality.
To the best of our knowledge, four previous systemic reviews and meta-analyses have systematically investigated the relationship of HDL-CEC with cardiovascular disease (CVD) [47,48,49,50]. Our meta-analysis includes seven [21, 22, 33, 35, 36, 42, 46], four [27, 33, 35],14 studies [21, 22, 31, 33, 36,37,38,39,40, 42,43,44,45,46] and 8 studies [21, 22, 32, 33, 35, 36, 42, 46] that were also included in these four meta-analyses, respectively. For instance, the study by Qiu et al. examined the associations of HDL-CEC with the risk of CVD and all-cause mortality. The results indicated that CEC was negatively correlated with the risk of CVD and possibly independent of the HDL level. The study by Lee et al. investigated that CEC is associated with adverse cardiovascular outcomes but does not increase the risk of all-cause and cardiovascular mortality, which is similar to our results. However, both of them also included cross-sectional studies, which might diminish the quality [51], as patients with renal failure and familial hyperlipidemia were also included, leading to a high cohort heterogeneity. Therefore, in view of this limitation, we restricted our analysis to cohort studies, case-control studies or randomized controlled trials. Patients in these case-control and cohort studies did not have a specific disease (cancer, autoimmune diseases, familial hyperlipidemia, or renal failure), and ORs were adjusted for covariates. Soria-Florido et al. investigated the potential associations of HDL function, including CEC values, the antioxidant and anti-inflammatory activities with the risks of CVD and mortality. They reported that higher CEC and anti-inflammatory activity were associated with the lower risks of CVD and mortality. These findings were in line with those obtained by Qiu et al. In addition, Ye et al. suggested that decreased CEC was an independent risk factor for CAD, which predicted the all-cause and cardiovascular mortality among CAD patients. Further, we added the information that CEC in the CAD population was generally reduced compared with that in the non-CAD population. Moreover, the risk of CAD significantly decreased in the high-CEC populations independent of HDL-C levels. However, the study by Li et al. reported the opposite result. In their study, two cohorts were included, one was an angiographic cohort and the other was an outpatient cohort. After 3 years of follow-up, the risk of myocardial infarction/stroke/death was inversely associated with CEC levels in the outpatient cohort and positively associated with CEC levels in the stable angiographic cohort, and these correlations remained significant even after adjustment for traditional cardiovascular risk factors. They considered that the presence of such results was related to specific cohort populations, that is, the relationship between CEC and CAD might differ significantly in different populations. Hence, the relationship between CEC and CAD might vary from person to person and more studies are necessary to prove this assumption.
HDL may delay the formation of atherosclerotic lesions through various mechanisms [52]. It is well known, that genetic variants that alter HDL-C levels are not necessarily associated with CAD risk, and that interventions aiming to increase HDL levels do not necessarily reduce cardiovascular events in CAD patients [53]. Thus, simply quantifying HDL levels is not a sufficient method to assess HDL function [54]. Whereas, CEC is involved in the reverse cholesterol transport from macrophages in the arterial wall to the liver, an important step in the process of HDL retardation of arterial plaque formation [55]. We thus suggest that HDL-CEC is indicative of plasma HDL function and can be taken as a biomarker to predict cardiovascular risk [56].
Further, HDL-CEC can exert anti-inflammatory and anti-atherosclerotic effects by inhibiting inflammatory processes in macrophages and activation of the inflammasome [57]. In this study, we observed that the risk of CAD obviously increased with the decrease of CEC, which may be related to the impaired HDL-CEC function in CAD patients. CAD patients frequently suffer from metabolic diseases [58]. Importantly, animal and in vitro experiments have shown that inflammation and metabolic disorders can convert HDL to a dysfunctional form. This not only impairs HDL’s CEC from macrophages and thus anti-atherosclerotic properties [59,60,61], but also can convert HDL to act pro-inflammatory, further increasing the risk of CAD [60, 62,63,64]. Therefore, alterations in HDL-CEC may be both a cause and a consequence in the development and progression of CAD. As outlined for CAD, also the incidence of cardiovascular mortality involves a multitude of traditional cardiovascular risk factors and genetic factors. CEC is only one factor amongst others and actually, in our study, we found no association between CEC and cardiovascular mortality. However, it is worth mentioning that CEC is a long-term stable trait being modestly independently heritable and independent of the HDL particle number, particle size and apolipoprotein A-II levels [56], thus functioning as a possible target for therapeutic intervention in the future.
Although CEC is a key indicator for the anti-atherosclerotic function of HDL and a strong predictor for CAD outcomes, measurement of HDL-CEC is not a part of routine lipid tests in CVD patients. This is partly explained by the lack of standardized procedures and the complexity of CEC mechanisms, because cholesterol efflux involves plenty of enzymes, like ATP-binding cassette transporter A1 (ABCA1), ATP-binding cassette transporter G1 (ABCG1), scavenger receptor class-BI (SR-BI), and aqueous diffusion [65]. Meanwhile, the determination of CEC value involves in-vitro testing, while standardized measurement of HDL-3 and HDL-2 levels can help to overcome technical limitations in routine testing. Cholesterol efflux to the HDL-3 subpopulation (350 kDa) is more efficient than to HDL-2 particles (175 kDa). Further, HDL-3 particles possess greater anti-oxidative and anti-apoptotic activities than HDL-2 particles. Finally, the increased HDL-2-to-HDL-3 ratio is associated with a higher risk of CAD [66, 67]. Therefore, determining the HDL-3-to-HDL-2 ratio may provide a simple standardized routine test to identify CAD manifestation or to predict outcome.
Compared to previous studies, the present study has the following strengths. To our knowledge, this is the first meta-analysis that systematically analyzes the association of CEC with the risks of CAD and cardiovascular mortality by dose-response analyses.
Nonetheless, certain limitations should also be noted. 1). The search and screening of this paper were done by the first author twice in July 2021 and February 2022, respectively. No kappa statistics were prepared. 2). There were high heterogeneities in the pooled cohorts used to generate the results of this paper, representing a limitation of this study. Heterogeneity is a consequence of the variability in experimental procedures, methods, study quality, and the differences in the characteristics of the studied patients. To address this problem, the donor cell line, labeled cholesterol, and cholesterol acceptors were identified as the possible sources of heterogeneity through subgroup analyses. Future experiments should consider the potential impact of experimental materials on the study. 3). Due to the insufficient number of existing studies, some pooled results were not further analyzed by subgroup analyses to detect the potential sources of heterogeneity. 4). A high percentage of patients were from the study by Ritsch et al., where the incidence of cardiovascular mortality was exceptionally high (18%). This might be explained by the comparably long follow-up period (approximately 10 years) and the advanced age of the population. Except for the study by Ritsch et al., the other included cohort studies had a low incidence of cardiovascular outcomes (< 10%). Besides, although all the effect values of ORs/RRs included in this analysis were adjusted for covariates by the original authors (Supplementary Table 3), the effect of residual covariates on the results of this paper was not excluded. 5). The number of studies included in dose-response analyses was relatively small, and the results of this study should be interpreted with caution. Notably, results of dose-response analyses represent the trends in risk rather than precise quantification. 6). Most of the studies included in this paper are based on the assessment of serum efflux capacity, which may differ from the in vitro situation. Meanwhile, the CEC assay was performed in a closed system and ignored the dynamic comprehensive aspects of cholesterol flux through the RCT pathway. 7). Most of the cohorts included US and Asian patients, with only few Europeans and none from other regions. Considering the different genetic backgrounds, more multi-regional studies are warranted in the future. 8). The NOS score was used to evaluate the quality of cohort studies and case-control studies. Although the NOS score is currently widely accepted in meta-analysis, it is also criticized to deliver arbitrary results [68].
Conclusions
Based on the current evidence, we suggest that a decreased CEC is strongly associated with the risk of CAD, independent of HDL-C level. However, a decreased CEC seems not to be related to cardiovascular mortality. In addition, CEC is negatively correlated with the risk of CAD in a linear manner. Nonetheless, further multi-center, multi-regional, and large sample studies are warranted in the future to complement and further validate these findings.
Availability of data and materials
All data generated or analyzed during the current study are included in this published article and its supplementary information files.
Abbreviations
- CEC:
-
Cholesterol efflux capacity
- CAD:
-
Coronary artery disease
- RCT:
-
Reverse cholesterol transport
- CETP:
-
Cholesteryl ester transfer protein
- NIH:
-
National Institute of Health
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SD:
-
Standard deviation
- OR:
-
Odds ratios
- RRs:
-
Relative risks
- HRs:
-
Hazard ratios
- Cis:
-
Confidence intervals
- SMD:
-
Standardized mean difference
- HDL-C:
-
High-density lipoprotein cholesterol
- CVD:
-
Cardiovascular disease
- NOS:
-
Newcastle Ottawa Scale
- ABCA1:
-
ATP-binding cassette transporter A1
- ABCG1:
-
ATP-binding cassette transporter G1
- DL:
-
DerSimonian and Laird
- Q1:
-
Quartile 1
References
Álvarez-Álvarez MM, Zanetti D, Carreras-Torres R, Moral P, Athanasiadis G. A survey of sub-Saharan gene flow into the Mediterranean at risk loci for coronary artery disease. Eur J Hum Genet. 2017;25:472–6. https://doi.org/10.1038/ejhg.2016.200.
Kumar A. Potential biomarkers to detect inflammation leading to coronary artery disease. J Nat Sci Biol Med. 2020;11:1. https://doi.org/10.4103/0976-9668.280267.
Abid H, Abid Z, Abid S. Atherogenic indices in clinical practice and biomedical research: a short review. Baghdad J Biochem Appl Biol Sci. 2021;2:60–70. https://doi.org/10.47419/bjbabs.v2i02.52.
Patil V, Avhad A, Kulkarni A, Pandere K. High-sensitive C-reactive protein in patients with coronary artery disease. J Nat Sc Biol Med. 2020;11:39. https://doi.org/10.4103/jnsbm.JNSBM_159_19.
Maguire EM, Pearce SWA, Xiao Q. Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease. Vasc Pharmacol. 2019;112:54–71. https://doi.org/10.1016/j.vph.2018.08.002.
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–67. https://doi.org/10.1089/ars.2012.5149.
Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport. Circ Res. 2019;124:1505–18. https://doi.org/10.1161/CIRCRESAHA.119.312617.
Säemann MD, Poglitsch M, Kopecky C, Haidinger M, Hörl WH, Weichhart T. The versatility of HDL: a crucial anti-inflammatory regulator. Eur J Clin Investig. 2010;40:1131–43. https://doi.org/10.1111/j.1365-2362.2010.02361.x.
Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–94. https://doi.org/10.1194/jlr.R800088-JLR200.
Fuller J, Jarrett R, Keen H, Pinney S, Avogaro P, Cazzolato G, et al. High-density lipoprotein and atherosclerosis. Lancet. 1975;305:691–2. https://doi.org/10.1016/S0140-6736(75)91801-2.
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14. https://doi.org/10.1016/0002-9343(77)90874-9.
Adorni MP, Ronda N, Bernini F, Zimetti F. High density lipoprotein cholesterol efflux capacity and atherosclerosis in cardiovascular disease: pathophysiological aspects and pharmacological perspectives. Cells. 2021. https://doi.org/10.3390/cells10030574.
Hafiane A. Vulnerable plaque, characteristics, detection, and potential therapies. J Cardiovasc Dev Dis. 2019. https://doi.org/10.3390/jcdd6030026.
Nicholls SJ, Puri R, Wolski K, Ballantyne CM, Barter PJ, Brewer HB, et al. Effect of the BET protein inhibitor, RVX-208, on progression of coronary atherosclerosis: results of the phase 2b, randomized, double-blind, multicenter, ASSURE Trial. Am J Cardiovasc Drugs. 2016;16:55–65. https://doi.org/10.1007/s40256-015-0146-z.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. https://doi.org/10.1056/NEJMoa1206797.
Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–15. https://doi.org/10.1056/NEJMoa1009744.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. https://doi.org/10.1056/NEJMoa0706628.
Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. https://doi.org/10.1056/NEJMoa1107579.
Rothblat GH, La Llera-Moya M, de, Atger V, Kellner-Weibel G, Williams DL, Phillips MC. Cell cholesterol efflux: integration of old and new observations provides new insights. J Lipid Res. 1999;40:781–96.
Niisuke K, Kuklenyik Z, Horvath KV, Gardner MS, Toth CA, Asztalos BF. Composition-function analysis of HDL subpopulations: influence of lipid composition on particle functionality. J Lipid Res. 2020;61:306–15. https://doi.org/10.1194/jlr.RA119000258.
Khera AV, Cuchel M, La Llera-Moya M, de Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. https://doi.org/10.1056/NEJMoa1001689.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93. https://doi.org/10.1056/NEJMoa1409065.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii-x, 1–173. https://doi.org/10.3310/hta7270.
Zhang C, Wang S, Chen S, Yang S, Wan L, Xiong B. Association between pretreatment Glasgow prognostic score and gastric cancer survival and clinicopathological features: a meta-analysis. Onco Targets Ther. 2016;9:3883–91. https://doi.org/10.2147/OTT.S103996.
Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0: The Cochrane Collaboration; 2011. www.cochrane-handbook.org.
Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. https://doi.org/10.1001/jama.280.19.1690.
Khan AR, Golwala H, Tripathi A, Bin Abdulhak AA, Bavishi C, Riaz H, et al. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2017;38:3082–9. https://doi.org/10.1093/eurheartj/ehx418.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.
Xu C, Doi SAR. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Healthc. 2018;16:138–44. https://doi.org/10.1097/XEB.0000000000000132.
Agarwala AP, Rodrigues A, Risman M, McCoy M, Trindade K, Qu L, et al. High-density lipoprotein (HDL) phospholipid content and cholesterol efflux capacity are reduced in patients with very high HDL cholesterol and coronary disease. Arterioscler Thromb Vasc Biol. 2015;35:1515–9. https://doi.org/10.1161/ATVBAHA.115.305504.
Cahill LE, Sacks FM, Rimm EB, Jensen MK. Cholesterol efflux capacity, HDL cholesterol, and risk of coronary heart disease: a nested case-control study in men. J Lipid Res. 2019;60:1457–64. https://doi.org/10.1194/jlr.P093823.
Ishikawa T, Ayaori M, Uto-Kondo H, Nakajima T, Mutoh M, Ikewaki K. High-density lipoprotein cholesterol efflux capacity as a relevant predictor of atherosclerotic coronary disease. Atherosclerosis. 2015;242:318–22. https://doi.org/10.1016/j.atherosclerosis.2015.06.028.
Kuusisto S, Holmes MV, Ohukainen P, Kangas AJ, Karsikas M, Tiainen M, et al. Direct estimation of HDL-mediated cholesterol efflux capacity from serum. Clin Chem. 2019;65:1042–50. https://doi.org/10.1373/clinchem.2018.299222.
Li X-M, Tang WHW, Mosior MK, Huang Y, Wu Y, Matter W, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–705. https://doi.org/10.1161/ATVBAHA.113.301373.
Liu C, Zhang Y, Ding D, Li X, Yang Y, Li Q, et al. Cholesterol efflux capacity is an independent predictor of all-cause and cardiovascular mortality in patients with coronary artery disease: a prospective cohort study. Atherosclerosis. 2016;249:116–24. https://doi.org/10.1016/j.atherosclerosis.2015.10.111.
Luo M, Liu A, Wang S, Wang T, Hu D, Wu S, et al. ApoCIII enrichment in HDL impairs HDL-mediated cholesterol efflux capacity. Sci Rep. 2017;7:2312. https://doi.org/10.1038/s41598-017-02601-7.
Luo M, Zhang Z, Peng Y, Wang S, Peng D. The negative effect of ANGPTL8 on HDL-mediated cholesterol efflux capacity. Cardiovasc Diabetol. 2018;17:142. https://doi.org/10.1186/s12933-018-0785-x.
Norimatsu K, Kuwano T, Miura S-I, Shimizu T, Shiga Y, Suematsu Y, et al. Significance of the percentage of cholesterol efflux capacity and total cholesterol efflux capacity in patients with or without coronary artery disease. Heart Vessel. 2017;32:30–8. https://doi.org/10.1007/s00380-016-0837-7.
Patel PJ, Khera AV, Wilensky RL, Rader DJ. Anti-oxidative and cholesterol efflux capacities of high-density lipoprotein are reduced in ischaemic cardiomyopathy. Eur J Heart Fail. 2013;15:1215–9. https://doi.org/10.1093/eurjhf/hft084.
Ritsch A, Duerr A, Kahler P, Hunjadi M, Stojakovic T, Silbernagel G, et al. Cholesterol efflux capacity and cardiovascular disease: the Ludwigshafen risk and cardiovascular health (LURIC) study. Biomedicines. 2020. https://doi.org/10.3390/biomedicines8110524.
Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–13. https://doi.org/10.1016/S2213-8587(15)00126-6.
Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–42. https://doi.org/10.1161/CIRCRESAHA.114.303454.
Shea S, Stein JH, Jorgensen NW, McClelland RL, Tascau L, Shrager S, et al. Cholesterol mass efflux capacity, incident cardiovascular disease, and progression of carotid plaque. Arterioscler Thromb Vasc Biol. 2019;39:89–96. https://doi.org/10.1161/ATVBAHA.118.311366.
Wang G, Mathew AV, Yu H, Li L, He L, Gao W, et al. Myeloperoxidase mediated HDL oxidation and HDL proteome changes do not contribute to dysfunctional HDL in Chinese subjects with coronary artery disease. PLoS One. 2018;13:e0193782. https://doi.org/10.1371/journal.pone.0193782.
Zhang J, Xu J, Wang J, Wu C, Xu Y, Wang Y, et al. Prognostic usefulness of serum cholesterol efflux capacity in patients with coronary artery disease. Am J Cardiol. 2016;117:508–14. https://doi.org/10.1016/j.amjcard.2015.11.033.
Ye H, Xu G, Ren L, Peng J. Cholesterol efflux capacity in coronary artery disease: a meta-analysis. Coron Artery Dis. 2020;31:642–9. https://doi.org/10.1097/MCA.0000000000000886.
Qiu C, Zhao X, Zhou Q, Zhang Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: a systematic review and meta-analysis. Lipids Health Dis. 2017;16:212. https://doi.org/10.1186/s12944-017-0604-5.
Soria-Florido MT, Schröder H, Grau M, Fitó M, Lassale C. High density lipoprotein functionality and cardiovascular events and mortality: a systematic review and meta-analysis. Atherosclerosis. 2020;302:36–42. https://doi.org/10.1016/j.atherosclerosis.2020.04.015.
Lee JJ, Chi G, Fitzgerald C, Kazmi SHA, Kalayci A, Korjian S, et al. Cholesterol efflux capacity and its association with adverse cardiovascular events: a systematic review and Meta-analysis. Front Cardiovasc Med. 2021;8:774418. https://doi.org/10.3389/fcvm.2021.774418.
Grimes DA, Atkins D. The U.S. Preventive services task force: putting evidence-based medicine to work. Clin Obstet Gynecol. 1998;41:332–42. https://doi.org/10.1097/00003081-199806000-00013.
Rye K-A, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2009;50(Suppl):S195–200. https://doi.org/10.1194/jlr.R800034-JLR200.
Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–25. https://doi.org/10.1016/S0140-6736(14)61217-4.
Fogacci F, Borghi C, Cicero AFG. New evidences on the association between HDL-C and cardiovascular risk: a never ending research story. Eur J Prev Cardiol. 2022. https://doi.org/10.1093/eurjpc/zwac015.
Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–55. https://doi.org/10.1161/CIRCULATIONAHA.104.475715.
Koekemoer AL, Codd V, Masca NGD, Nelson CP, Musameh MD, Kaess BM, et al. Large-scale analysis of determinants, stability, and heritability of high-density lipoprotein cholesterol efflux capacity. Arterioscler Thromb Vasc Biol. 2017;37:1956–62. https://doi.org/10.1161/ATVBAHA.117.309201.
Groenen AG, Halmos B, Tall AR, Westerterp M. Cholesterol efflux pathways, inflammation, and atherosclerosis. Crit Rev Biochem Mol Biol. 2021;56:426–39. https://doi.org/10.1080/10409238.2021.1925217.
Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–7. https://doi.org/10.1242/dmm.001180.
Vaisar T, Shao B, Green PS, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase and inflammatory proteins: pathways for generating dysfunctional high-density lipoprotein in humans. Curr Atheroscler Rep. 2007;9:417–24. https://doi.org/10.1007/s11883-007-0054-z.
McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–45. https://doi.org/10.1161/CIRCULATIONAHA.108.810721.
Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid a during metabolism of acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol. 2000;20:763–72. https://doi.org/10.1161/01.atv.20.3.763.
Vaisar T, Tang C, Babenko I, Hutchins P, Wimberger J, Suffredini AF, et al. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res. 2015;56:1519–30. https://doi.org/10.1194/jlr.M059089.
Annema W, Nijstad N, Tölle M, Boer JF, de Buijs RVC, Heeringa P, et al. Myeloperoxidase and serum amyloid a contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase a(2). J Lipid Res. 2010;51:743–54. https://doi.org/10.1194/jlr.M000323.
Barbosa CJ, Maranhão RC, Barreiros RS, Freitas FR, Franci A, Strunz CMC, et al. Lipid transfer to high-density lipoproteins in coronary artery disease patients with and without previous cerebrovascular ischemic events. Clin Cardiol. 2019;42:1100–5. https://doi.org/10.1002/clc.23259.
Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–9. https://doi.org/10.1074/jbc.R114.583658.
Du X-M, Kim M-J, Hou L, Le Goff W, Chapman MJ, van Eck M, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–42. https://doi.org/10.1161/CIRCRESAHA.116.305485.
Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. https://doi.org/10.1161/CIRCULATIONAHA.112.108753.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. https://doi.org/10.1007/s10654-010-9491-z.
Acknowledgements
WKC is funded by China Scholarship Council (CSC No. 202009370095). Many thanks the China Scholarship Council for its support to my study and project. Meanwhile, many thanks to my mentor HT and supervisor PB, as well as every colleague in the lab, for all the support and encouragement along the way.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
WKC and PB contributed to the conception or design of the work. WKC, MR and PB contributed to the acquisition, analysis, or interpretation of data in the work. WKC drafted the manuscript. WKC, HT, MR, JB, AJ, SD and PB critically revised the manuscript. All authors gave final approval.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
The sensitivity analyses of CEC difference in coronary artery disease (CAD) and non-CAD group. CEC, cholesterol efflux capacity. The figure depicts pooled results of random-effect meta-analyses considering the remaining studies after excluding named study. Supplementary Figure 2. The funnel of CEC difference in coronary artery disease (CAD) and non-CAD group. CEC, cholesterol efflux capacity. Supplementary Figure 3. A The sensitivity analyses of CEC and the risk of coronary artery disease (CAD). CEC, cholesterol efflux capacity. The figure indicates the pooled results of the remaining studies after excluding named study (Study of Li MX includes two cohort). B. The sensitivity analyses of CEC and CAD risk by per 1-SD increasement. CEC, cholesterol efflux capacity. SD, standard deviation. Supplementary Figure 4. The funnel of CEC and CAD risk. CEC, cholesterol efflux capacity. CAD, coronary artery disease. Supplementary Figure 5. The sensitivity analyses of CEC and the risk of cardiovascular mortality. CEC, cholesterol efflux capacity. Supplementary Table 1. Subgroup Analyses. Supplementary Table 2. Quality Assessment of the 18 observational Studies. Supplementary Table 3. Characteristics of 18 Observational Studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cheng, W., Rosolowski, M., Boettner, J. et al. High-density lipoprotein cholesterol efflux capacity and incidence of coronary artery disease and cardiovascular mortality: a systematic review and meta-analysis. Lipids Health Dis 21, 47 (2022). https://doi.org/10.1186/s12944-022-01657-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-022-01657-3