Abstract
Background
Bile acids (BAs) not only play an important role in lipid metabolism and atherosclerosis but also have antiapoptotic and neuroprotective effects. However, few studies have focused on the relationship of the total bile acid (TBA) levels with the severity and prognosis of acute ischemic stroke (AIS).
Objectives
The aim of this study was to investigate the potential associations of the fasting serum TBA levels on admission with the stroke severity, in-hospital complication incidence and 3 -month all-cause mortality in patients with AIS.
Methods
A total of 777 consecutive AIS patients were enrolled in this study and were divided into four groups according to the quartiles of the serum TBA levels on admission. Univariate and multivariate logistic regression analyses were used to explore the relationship between the fasting TBA levels and the stroke severity, in-hospital complications, and 3-month mortality in AIS patients.
Results
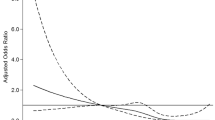
Patients in group Q3 had the lowest risk of severe AIS (NIHSS > 10) regardless of the adjustments for confounders (P < 0.05). During hospitalization, 115 patients (14.8%) had stroke progression (NIHSS score increased by ≥ 2), and 222 patients (28.6%) developed at least one complication, with no significant difference among the four groups (P > 0.05). There was no significant difference in the incidence of pneumonia, urinary tract infection (UTI), hemorrhagic transformation (HT), gastrointestinal bleeding (GIB), seizures or renal insufficiency (RI) among the four groups (P > 0.05). A total of 114 patients (14.7%) died from various causes (including in-hospital deaths) at the 3-month follow-up, including 42 (21.3%), 26 (13.3%), 19 (9.9%) and 27 (13.9%) patients in groups Q1, Q2, Q3 and Q4 respectively, with significant differences (P = 0.013). After adjusting for confounding factors, the risk of death decreased (P -trend < 0.05) in groups Q2, Q3, and Q4 when compared with group Q1, and the OR values were 0.36 (0.16-0.80), 0.30 (0.13-0.70), and 0.29 (0.13-0.65), respectively.
Conclusions
TBA levels were inversely associated with the 3-month mortality of AIS patients but were not significantly associated with the severity of stroke or the incidence of complications.
Similar content being viewed by others
Introduction
As the population ages, stroke has become the second leading cause of death(11.6% [10.8–12.2] of the total deaths in 2019) worldwide after ischemic heart disease [1] and is also associated with a high rate of disability and recurrence, which brings a great burden to society and families, especially in low- and middle-income countries [1, 2]. Ischemic stroke is the most prevailing type of stroke event. In 2019, acute ischemic stroke (AIS) was reported to account for 62.4% of all stroke events globally [1]. Primary intracerebral hemorrhage (PICH) accounted for approximately 27.9% of strokes, and subarachnoid hemorrhage (SAH) accounted for 9.7% of strokes [1]. Treatments such as early intravenous thrombolysis and endovascular treatment can allow the occluded blood vessels to be recanalized leading to blood reperfusion, which may reduce the infarct volume and effectively improve the overall prognosis of stroke patients. In addition, the therapeutic time window of reperfusion for AIS has been gradually extended owe to the development of neuroimaging techniques [3,4,5,6,7,8,9]. Unfortunately, the majority of AIS patients still fail to receive reperfusion treatment because they are outside of the time window, which affects the prognosis. Moreover, patients suffering from AIS, especially elderly and critically ill patients, commonly experience certain complications, such as poststroke pneumonia and gastrointestinal bleeding, which leads to a higher risk of early death, which is the result of a joint effect together with AIS [10,11,12].
Lipid metabolism disorders can cause cholesterol overload, leading to excessive deposition of lipid substances, such as low-density lipoprotein cholesterol (LDL-C), within the intima of the large and medium-sized arteries, which is considered the cause of the atherosclerosis incidence and the main risk factor for coronary heart disease, stroke, peripheral vascular disease, aortic aneurysm, and renal artery stenosis [13,14,15,16,17]. Studies have shown that excessive cholesterol in the human body can be converted into bile acids (BAs) and can be excreted from feces in the form of bile salts [13, 18, 19]. A large amount of bile acid excretion can prevent the development of atherosclerosis, while the reduction can lead to an increased risk of atherosclerosis and coronary heart disease [20,21,22]. Researchers have also found that ursodeoxycholic acid (UDCA) facilitates the prevention of the occurrence of atherosclerosis and promotes plaque regression with dissolved cholesterol crystals [23]. Additionally, a 20-year prospective follow-up study showed that reduced bile acid excretion was an independent risk factor for stroke incidence and death [24].
In addition to being associated with lipid metabolism, bile acids were also reported to play a beneficial role in cellular protection and anti-apoptosis in rats with acute stroke and acute myocardial infarction [25,26,27,28], as well as in the reduction of glial cell activation in animal models of acute neuroinflammation [29]. A clinical trial found that there is a potential relationship between increased serum total bile acid (TBA) levels and a smaller hematoma volume during cerebral hemorrhage as well as a better outcome [30]. UDCA can be used to treat chronic heart failure by improving peripheral blood flow [31], while tauroursodeoxycholic acid (TUDCA) has antiapoptotic effects on a number of neurodegenerative diseases, including amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease and Huntington’s disease [32].
To our knowledge, no study has evaluated serum TBA levels for associations with the clinical manifestations and early prognosis of patients with AIS. Here, we attempted to fill this gap by initially exploring the relationship between fasting TBA levels on admission and several AIS-related targets including stroke severity, in-hospital complications, and 3-month mortality.
Materials and methods
Study population
A total of 777 consecutive AIS patients treated in the Department of Neurology, Zhangjiagang Hospital of Traditional Chinese Medicine (TCM) affiliated to Nanjing University of Chinese Medicine in China from April 2012 to January 2016 were eventually included in the study. The detailed inclusion and exclusion criteria are shown in Table 1.
The diagnosis of AIS was made by two or more neurologists after admission to our hospital based on the patient’s medical history, clinical presentation, and brain computed tomography (CT) or magnetic resonance imaging (MRI) manifestations, according to World Health Organization (WHO) standards as follows: the development of a sudden focal or a complete neurological deficit, a neurological deficit lasting more than 24 h, exclusion of brain dysfunction caused by other nonvascular factors, and a diagnosis based on brain CT or MRI. All enrolled patients (n=983) had stable vital signs on admission without any severe disturbance of consciousness or any severe dysfunction of other organs. Patients who had more than 72 h from the onset to the admission (n=102) and those without TBA measurements within 24 h of admission (n=45) were excluded. In addition, patients who had severe hepatobiliary or renal disease prior to or on admission (n=17), underlying blood disease or cancer (n=16), any current infections or immune system disease (n=12) or who were lost to follow-up at 3 months of admission (n=14) were excluded as well (Fig. 1).
Ethics statement
Approval of the Ethics Committee of Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine in China was obtained before starting the study (No. 2020-77-1), while the requirement for written informed consent was waived as this is a retrospective study and the data are anonymous. The study fully complied with the Declaration of Helsinki and obtained the required data from the clinical records without any clinical intervention for the protection of patient privacy.
Data collection
Baseline information was comprised of the demographic characteristics (such as sex, age) and known risk factors for cerebrovascular disease (such as stroke, hypertension, diabetes, atrial fibrillation, coronary heart disease, heart failure, smoking and drinking history). The time from onset to admission, stroke severity (National Institutes of Health Stroke Scale, NIHSS), previous thrombolytic therapy, clinical data and laboratory indexes on admission (such as systolic blood pressure, diastolic blood pressure, blood routine, serum TBA, liver function, blood glucose, blood lipids, creatinine, and uric acid) and in-hospital complications were recorded. The laboratory data were obtained in the emergency department before hospital admission or in the ward within 24 h after hospital admission. Blood routine data were obtained with XE-5000 (Mindray, Shenzhen, China). Biochemical data were obtained from fasting blood samples with Olympus AU5400 Automatic Analyzer (First Chemical Co., Ltd, Tokyo, Japan). All tests were completed by experts from the Laboratory Department of our hospital.
Outcome evaluation
The NIHSS score on admission was used to represent the severity of stroke on admission. A NIHSS score greater than 10 was defined as a severe stroke, and a NIHSS score that had increased by more than 2 points was defined as stroke progression during hospitalization. Six complications of relatively high incidence, including pneumonia, urinary tract infection (UTI), hemorrhagic transformation (HT), gastrointestinal bleeding (GIB), seizures, and renal insufficiency, were included in the study. The definitions for these complications are described in Table 2. The three-month death rate was determined by telephone interviews of the patients or their families three months after the onset.
Statistical analysis
The quartiles of the TBA levels on admission were referenced to divide patients into four groups (Q1, ≤3.0 µmol/L; Q2, 3.0-5.7 µmol/L; Q3, 5.7-9.5 µmol/L; Q4, >9.5 µmol/L). SPSS software (Version 23.0; IBM, Armonk, NY, USA) was used for statistical analysis, and a two-tailed P value <0.05 was considered statistically significant.
Since four groups were generated with a total sample size ≥200 (each >100), continuous variables were analyzed in normality with the Kolmogorov-Smirnov test, and were represented by the mean (standard deviation) via one-way ANOVA in cases of all four groups are in normal distribution or the median (interquartile range) via Kruskal-Wallis test when one of the four groups did not conform to the normal distribution. Categorical variables were compared by the Chi-square test or Fisher’s exact probability method.
The correlation analysis for the serum TBA with severe AIS on admission and the 3-month all-cause mortality was evaluated on univariate and multivariate logistic regression models. In the multivariate logistic regression model, the independence of TBA was identified after adjusting for covariates. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each group using the lowest quartile (Q1) of TBA as a reference. The potential confounders included age, sex, thrombolytic therapy, history of atrial fibrillation, the admission white blood cell (WBC) count and the platelet count for the TBA level and stroke severity. Age, sex, the NIHSS score on admission, stroke progression and at least one complication during hospitalization, history of atrial fibrillation, and the admission WBC count are potential confounders for the TBA level and 3-month mortality.
Results
Baseline characteristics
In total, 777 eligible patients (420 males and 357 females) with AIS were enrolled in the study, and they had a mean age of 71 (62-78) years and a mean NIHSS score of 4 (3-8) on admission. The patients were assigned into groups Q1 (≤ 3.0 µmol/L, n = 197), Q2 (3.0-5.7 µmol/L, n = 195), Q3 (5.7-9.5 µmol/L, n = 191) and Q4 (> 9.5 µmol/L, n = 194) according to the quartiles of fasting serum TBA concentrations on admission, which were associated with NIHSS scores of 5, 5, 4 and 4, respectively, and there were no significant differences (P = 0.389) (Table 3). No significant differences were noted in the baseline demographic, clinical and laboratory parameters (including blood lipids) (P > 0.05), except for the history of atrial fibrillation (AF) and the admission white blood cell (WBC) count (P < 0.05), among the four groups. Multiple comparisons showed that there was no significant difference in the AF rate between the Q1 and Q2 groups, while there was a significant difference between the other groups. A posthoc analysis found that the WBC count difference between the Q1 group and Q4 group was statistically significant (P < 0.05) and the WBC count in Q4 group was lower than in Q1 group (6.3 (5.1-7.9) vs. 6.8 (5.6-8.5) ×109/L).
Correlation between TBA and AIS severity
The numbers and proportions of severe AIS cases (NIHSS > 10) among the four groups were significantly different (P = 0.029), and they were much higher in group Q1 (n = 41, 20.8%) and group Q4 (n = 36, 18.6%), and were lower in group Q2 (n = 28, 14.4%) and group Q3 (n = 20, 10.5%) (Table 4). A binary logistic regression analysis showed that patients in group Q3 had a significantly lower risk of severe AIS than those in group Q1 (OR, 0.45; 95% CI, 0.25-0.79) before adjustments. In multivariate-adjusted models (Model 1 for age and sex, and Model 2 for age, sex, thrombolytic therapy, history of AF, WBC count, platelet count), compared to group Q1, patients in groups Q2 and Q3 had a lower risk of severe AIS, which was not reflected in group Q4. In addition, the p-trend was greater than 0.05 regardless of the adjustment for other confounding factors, and no significant trend was displayed.
Association between TBA and in-hospital complications
During hospitalization, 115 (14.8%) of the 777 patients had stroke progression (NIHSS score increased by ≥ 2 points), but there was no significant difference among the four groups (P = 0.584). There were 222 (28.6%) patients that developed at least one complication, with no significant difference among the groups (P = 0.906), and the incidence rates of pneumonia, UTI, HT, GIB, seizures, and renal insufficiency were 11.7%, 9.1%, 9.5%, 2.1%, 0.9%, and 2.4%, respectively, still with no significant difference among the four groups (all P > 0.05). The detailed results are shown in Table 5.
Correlation between TBA and 3-month all-cause mortality
The 3-month follow-up visits revealed that there were 114 deaths (14.7%) from various causes (including hospital deaths), and there were 42 (21.3%), 26 (13.3%), 19 (9.9%) and 27 (13.9%) deaths in groups Q1, Q2, Q3 and Q4, respectively, indicating significant differences (P = 0.013) (Table 6). In Model 2, with adjustments for sex, age, the NIHSS score on admission, stroke progression and the occurrence of at least one complication during hospitalization, the 3-month all-cause mortality decreased with the increase in serum TBA content. The OR values of groups Q2, Q3, and Q4 as compared to group Q1 were 0.36 (0.16-0.80), 0.35 (0.16-0.78), and 0.30 (0.14-0.66), respectively. In addition to the factors adjusted in Model 2, history of AF and the baseline WBC count were finally included in Model 3. In this case, the OR values of groups Q2, Q3, and Q4 were 0.36 (0.16-0.80), 0.30 (0.13-0.70), and 0.29 (0.13-0.65), respectively, compared to group Q1. In Model 2 and Model 3, both of the P-trend values were less than 0.05, indicating a decreased risk of 3-month mortality in reaction to the increase in serum TBA levels.
Discussion
In many animal experiments, bile acids, in addition to being a regulator of blood lipid and cholesterol content by participating in lipid metabolism, also act as signal molecules that activate different nuclear receptors, such as the farnesoid X receptor (FXR), pregnane X receptor (PXR), vitamin D receptor (VDR), and transmembrane G protein-coupled receptor 5 (TGR5), which reduce the risk of atherosclerosis via a variety of metabolic pathways in diverse tissues [15, 18, 33,34,35]. Bile acid chelates, such as coleswelen hydrochloride, can not only reduce the LDL-C levels, but also decrease the levels of hypersensitive C-reactive protein (hs-CRP) to prevent the development of atherosclerosis [36].
Bile acids also have anti-apoptosis and cellular protection effects. Andrew L. Rivard et al. [27] found reduced apoptosis and improved cardiac function in rats by TUDCA administration before myocardial infarction. In a rat model of acute stroke, bile acid TUDCA showed neuroprotective effects, and the underlying mechanism was proven with the involvement of enhanced cell apoptosis in response to inhibited mitochondrial disturbance and subsequent caspase activation [25]. In addition, TUDCA was found to negatively regulate Nrf2 signaling pathway to decrease lipid peroxidation, inflammation and apoptosis in acute cerebral infarction (ACI) rats [37]. TUDCA can not only reduce the cell apoptosis of rats with acute hemorrhagic stroke and protect the nerve from being damaged [26], but also reduce the activation of glial cells in animal models of acute neuroinflammation [29]. Joana D. Amaral et al. [28] reviewed the role of bile acids in the regulation process of apoptosis, which highlighted the anti-apoptotic effects of UDCA and TUDCA, as well as their potential application as new and alternative drugs for the treatment of apoptosis-related diseases. All these certain evidences provide some basis for the conjecture that serum TBA may have a protective effect on AIS.
Comparisons with other studies and what does the current work add to the existing knowledge
An article published by Gideon Charach et al. in 2018 showed that diminished bile acid excretion is a risk factor for coronary artery disease [22]. At the same time, they also studied the in-hospital bile acid excretion of 68 men and 35 women admitted to the hospital between 1996 and 1998 for chest pain and suspected cardiac events and who were followed for up to 20 years [24]. They found a significantly higher average bile acid excretion in patients without stroke relative to those with stroke, while those with lower bile acid excretion had higher stroke incidence and mortality, suggesting that reduced bile acid excretion was also an independent risk factor for stroke incidence and death. A population-based cohort study in Taiwan demonstrated that cholecystectomy is related to a reduced risk of overall stroke, ischemic stroke, and hemorrhagic stroke [38]. Gallstones can cause bile excretion disorders and inflammation that is characterized by bile retention in the gallbladder. Lipid accumulation caused by decreased bile acid excretion, together with chronic inflammation, increases the risk of atherosclerosis, thereby increasing the risk of cerebral infarction [38]. Wenyuan Li et al. [34] analyzed the relationship between the fasting serum TBA levels and the occurrence and severity of coronary heart disease in a total of 7438 consecutive patients with suspected CAD, who had undergone coronary angiography. They revealed that patients with CAD had lower fasting serum TBA levels than individuals without CAD. This indirectly established a link between the serum total bile acid levels and ischemic stroke, and this provides some support for the hypothesis that the serum total bile acid levels may play a protective role in ischemic stroke.
Most of the previous studies focused on the relationship between TBA and the occurrence and severity of coronary heart disease, cerebral infarction or other diseases. To our knowledge, this is the first clinical study to investigate the relationship between the admission serum TBA levels and stroke severity, in-hospital complication incidence, or short-term clinical outcomes in patients with AIS. In this study, the fasting serum TBA on admission showed a certain relationship with the 3-month clinical outcome, and low serum TBA levels were an independent risk factor for death within 3 months in patients with AIS. We speculated that the protective effect of TBA in this study may be related to cholesterol metabolism and its involvement as a signaling molecule in regulating various metabolic pathways in various tissues, as well as its neuroprotective and antiapoptotic effects. Bile acids can not only downregulate CYP7A1 expression by binding FXR but can also restrict the continuous synthesis of bile acids and maintain the homeostasis of bile acids through a feedback mechanism [39]. Moreover, FXR and TGR5 can regulate glucose and lipid metabolism, activate the AKT pathway to stimulate glycogen synthesis and inhibit gluconeogenesis to mimic insulin regulation of glucose metabolism [18]. In addition, TGR5 activation can also reduce chronic inflammation, improve insulin resistance and inhibit atherosclerosis by inhibiting systemic inflammation and macrophage infiltration in adipose tissue [18, 40].
We also found that patients in the Q4 group with the highest bile acid had lower WBC counts than those in the Q1 group, indicating that reduced inflammation may play a role in the protective effects of bile acids. We can further clarify the relevant mechanisms through animal experiments and the measurement of biomarkers in patients, such as interleukin, hs-CRP and other inflammatory indicators. At the same time, we can continue to carry out longer term follow-up studies, including the evaluation of patient survival, functional prognosis, recurrence of stroke and occurrence of cardiovascular events, for further research.
Study strengths and limitations
This study has the following strengths: (1) This study is the first to identify an association between high fasting serum TBA levels on admission and reduced mortality within three months after stroke in patients with AIS. (2) Although the serum TBA levels were not significantly correlated with the blood lipid levels (including triglycerides, total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol), the stroke severity, or in-hospital complication incidence, they were correlated with the incidence of AF and the WBC count, which may be a direction for research in future studies.
However, there are still some limitations: (1) This is only a single-center retrospective study with a small sample size limited to Chinese patients, and some results may vary among different populations. Though studies have shown that the characteristics and prevalence of cerebrovascular and cardiovascular risk factors in the Asian population are similar to those of other large contemporary trials and real-world registries that also include other ethnicities [41,42,43], further studies involving different populations and more centers are needed to support our findings. (2) Although our model was adjusted for several covariates that might have an impact on the outcomes, there are still some possible influencing factors that have not been collected. (3) This study did not follow up on the functional outcomes in patients with AIS who survived more than 3 months; thus, this study was unable to determine the effect of serum TBA on the functional recovery. (4) As long-term follow-up has not yet been completed, the long-term effects of serum TBA cannot be determined in this study. (5) In this study, we only measured the serum total fasting TBA, without specific components of bile acids.
Conclusions
This study shows that the admission fasting serum TBA levels were inversely associated with the 3-month mortality of AIS patients but were not significantly associated with the severity of stroke or the incidence of complications. This suggests that serum TBA levels may be a simple, cost-effective and readily available biomarker with additional predictive value for the prognosis of patients with AIS. Bile acids measurement at admission can help clinicians predict the prognosis of AIS patients, supplementation with bile acids during hospitalization, such as UDCA, may be beneficial to the prognosis of AIS patients.
Availability of data and materials
All data generated or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BAs:
-
Bile acids
- AIS:
-
acute ischemic stroke
- PICH:
-
primary intracerebral hemorrhage
- SAH:
-
subarachnoid hemorrhage
- TBA:
-
total bile acid
- LDL-C:
-
low density lipoprotein cholesterol
- HDL-C:
-
high density lipoprotein cholesterol
- UDCA:
-
Ursodeoxycholic acid
- TUDCA:
-
tauroursodeoxycholic acid
- CT:
-
computed tomography
- MRI:
-
magnetic resonance imaging
- WHO:
-
World Health Organization
- NIHSS:
-
National Institutes of Health Stroke Scale
- UTI:
-
urinary tract infection
- HT:
-
hemorrhagic transformation
- GIB:
-
gastrointestinal bleeding
- OR:
-
Odds ratios
- 95%CI:
-
95% confidence intervals
- AF:
-
atrial fibrillation
- WBC:
-
admission white blood cell
- FXR:
-
farnesoid X receptor
- PXR:
-
pregnane X receptor
- VDR:
-
vitamin D receptor
- TGR5:
-
transmembrane G protein-coupled receptor 5
References
Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820.
Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38(2):208–11.
Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi E, Investigators SE. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368(10):904–13.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N Engl J Med. 2015;372(11):1019–30.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Roman L, Serena J, Abilleira S, Ribo M, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378(8):708–18.
Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med. 2018;379(7):611–22.
Puig J, Shankar J, Liebeskind D, Terceno M, Nael K, Demchuk AM, Menon B, Dowlatshahi D, Leiva-Salinas C, Wintermark M, et al. From “Time is Brain” to “Imaging is Brain”: A Paradigm Shift in the Management of Acute Ischemic Stroke. J Neuroimaging. 2020;30(5):562–71.
Mohamed W, Bhattacharya P, Shankar L, Chaturvedi S, Madhavan R. Which Comorbidities and Complications Predict Ischemic Stroke Recovery and Length of Stay? Neurologist. 2015;20(2):27–32.
Schwarzbach CJ, Grau AJ. Complications after stroke: Clinical challenges in stroke aftercare. Nervenarzt. 2020;91(10):920–5.
Gattringer T, Posekany A, Niederkorn K, Knoflach M, Poltrum B, Mutzenbach S, Haring HP, Ferrari J, Lang W, Willeit J, et al. Predicting Early Mortality of Acute Ischemic Stroke. Stroke. 2019;50(2):349–56.
Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–12.
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res. 2016;118(4):535–46.
Schaftenaar F, Frodermann V, Kuiper J, Lutgens E. Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol. 2016;27(3):209–15.
Kruth HS. Lipoprotein cholesterol and atherosclerosis. Curr Mol Med. 2001;1(6):633–53.
Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122(18):1837–45.
Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3(3):1191–212.
Marin JJ, Macias RI, Briz O, Banales JM, Monte MJ. Bile Acids in Physiology, Pathology and Pharmacology. Curr Drug Metab. 2015;17(1):4–29.
Charach G, Grosskopf I, Rabinovich A, Shochat M, Weintraub M, Rabinovich P. The association of bile acid excretion and atherosclerotic coronary artery disease. Therap Adv Gastroenterol. 2011;4(2):95–101.
Charach G, Rabinovich A, Argov O, Weintraub M, Rabinovich P. The role of bile Acid excretion in atherosclerotic coronary artery disease. Int J Vasc Med. 2012;2012:949672.
Charach G, Argov O, Geiger K, Charach L, Rogowski O, Grosskopf I. Diminished bile acids excretion is a risk factor for coronary artery disease: 20-year follow up and long-term outcome. Therap Adv Gastroenterol. 2018;11:1756283X17743420.
Bode N, Grebe A, Kerksiek A, Lutjohann D, Werner N, Nickenig G, Latz E, Zimmer S. Ursodeoxycholic acid impairs atherogenesis and promotes plaque regression by cholesterol crystal dissolution in mice. Biochem Biophys Res Commun. 2016;478(1):356–62.
Charach G, Karniel E, Novikov I, Galin L, Vons S, Grosskopf I, Charach L. Reduced bile acid excretion is an independent risk factor for stroke and mortality: A prospective follow-up study. Atherosclerosis. 2020;293:79–85.
Rodrigues CMP, Spellman SR, Solá S, Grande AW, Linehan-Stieers C, Low WC, Steer CJ. Neuroprotection by a Bile Acid in an Acute Stroke Model in the Rat. J Cereb Blood Flow Metab. 2002;22(4):463–71.
Rodrigues CM, Sola S, Nan Z, Castro RE, Ribeiro PS, Low WC, Steer CJ. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc Natl Acad Sci U S A. 2003;100(10):6087–92.
Rivard AL, Steer CJ, Kren BT, Rodrigues CM, Castro RE, Bianco RW, Low WC. Administration of tauroursodeoxycholic acid (TUDCA) reduces apoptosis following myocardial infarction in rat. Am J Chin Med. 2007;35(2):279–95.
Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009;50(9):1721–34.
Yanguas-Casas N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramirez L. Tauroursodeoxycholic acid reduces glial cell activation in an animal model of acute neuroinflammation. J Neuroinflammation. 2014;11:50.
Wang K, Zhang Y, Zhong C, Zheng D, Xu J, Zhang Y, Shi J, Xiao G, Zhang X, Liu H, et al. Increased Serum Total Bile Acids can be Associated with a Small Hematoma Volume and Decreased Clinical Severity During Acute Intracerebral Hemorrhage. Curr Neurovasc Res. 2018;15(2):158–63.
von Haehling S, Schefold JC, Jankowska EA, Springer J, Vazir A, Kalra PR, Sandek A, Fauler G, Stojakovic T, Trauner M, et al. Ursodeoxycholic acid in patients with chronic heart failure: a double-blind, randomized, placebo-controlled, crossover trial. J Am Coll Cardiol. 2012;59(6):585–92.
Gronbeck KR, Rodrigues CM, Mahmoudi J, Bershad EM, Ling G, Bachour SP, Divani AA. Application of Tauroursodeoxycholic Acid for Treatment of Neurological and Non-neurological Diseases: Is There a Potential for Treating Traumatic Brain Injury? Neurocrit Care. 2016;25(1):153–66.
Ding L, Chang M, Guo Y, Zhang L, Xue C, Yanagita T, Zhang T, Wang Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018;17(1):286.
Li W, Shu S, Cheng L, Hao X, Wang L, Wu Y, Yuan Z, Zhou J. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis. 2020;292:193–200.
Meissner M, Wolters H, de Boer RA, Havinga R, Boverhof R, Bloks VW, Kuipers F, Groen AK. Bile acid sequestration normalizes plasma cholesterol and reduces atherosclerosis in hypercholesterolemic mice. No additional effect of physical activity. Atherosclerosis. 2013;228(1):117–23.
Bays HE, Davidson M, Jones MR, Abby SL. Effects of colesevelam hydrochloride on low-density lipoprotein cholesterol and high-sensitivity C-reactive protein when added to statins in patients with hypercholesterolemia. Am J Cardiol. 2006;97(8):1198–205.
Bian K-Y, Jin H-F, Sun W, Sun Y-J. DCA can improve the ACI-induced neurological impairment through negative regulation of Nrf2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(1):343–51.
Wei CY, Chuang SH, Lin CL, Kung WM, Tai HC, Tsai KW, Kao CH, Chen CH, Yeh YH, Hsu CY. Reduced risk of stroke following cholecystectomy: A nationwide population-based study. J Gastroenterol Hepatol. 2019;34(11):1992–8.
Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–44.
Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45(4):802–16.
Calabro P, Gragnano F, Di Maio M, Patti G, Antonucci E, Cirillo P, Gresele P, Palareti G, Pengo V, Pignatelli P, et al. Epidemiology and Management of Patients With Acute Coronary Syndromes in Contemporary Real-World Practice: Evolving Trends From the EYESHOT Study to the START-ANTIPLATELET Registry. Angiology. 2018;69(9):795–802.
Valgimigli M, Gragnano F, Branca M, Franzone A, Baber U, Jang Y, Kimura T, Hahn JY, Zhao Q, Windecker S, et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: individual patient level meta-analysis of randomised controlled trials. BMJ. 2021;373:n1332.
Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C, Ren Y, Qian Z, Vaughn MG, McMillin SE, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2021;6(12):e897–906.
Acknowledgements
N/A.
Funding
This work was supported by Special research of Suzhou industrial technology Innovation (SYSD2017006).
Author information
Authors and Affiliations
Contributions
RZ and YS provided funding and designed the study. GX, FL and YW collected the data. LH, FL and JJ were involved in data cleaning, follow-up and verification. YS revised the article. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The retrospective cohort study was approved by the Ethics Committee of the Zhangjiagang TCM Hospital affiliated to the Nanjing University of Chinese Medicine (No. 2020-77-1).
Consent for publication
All authors agree to publish this article in the journal of Lipids in Health and Disease.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, L., Xu, G., Zhang, R. et al. Increased admission serum total bile acids can be associated with decreased 3-month mortality in patients with acute ischemic stroke. Lipids Health Dis 21, 15 (2022). https://doi.org/10.1186/s12944-021-01620-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-021-01620-8