Abstract
Background
Trans-fatty acids (TFAs) occur in small amounts in nature but became widely produced by the food industry. The hazardous effects of different TFA subtypes to human health are controversial. We aimed to evaluate the association of plasma TFAs levels (elaidic acid, vaccenic acid, palmitelaidic acid, and linoelaidic acid) with mortality.
Methods
Utilizing 1999–2000 Nutrition Examination Survey (NHANES) and linked mortality data, we performed a cohort study with 1456 participants and used Cox proportional hazards models and penalized smoothing spline plots to elucidate the relationships between TFAs and all-cause, cardiovascular diseases (CVD) and cancer mortality.
Results
During 16,034 person-years of follow-up, a total of 221 deaths occurred. In the multivariate model, including mutual adjustment for the 4 TFA subtypes, elaidic acid associated with higher all-cause mortality (hazard ratio (HR) = 2.00, 95% confidence interval (CI) = 1.18 to 3.40, fourth quartiles versus second quartiles) and CVD mortality (HR = 1.64, 95% CI = 1.07 to 2.50, per 10 units increase). Higher palmitelaidic acid levels were associated with increased cancer mortality (HR = 2.91, 95% CI = 1.09 to 7.81, fourth quartiles versus second quartiles). A J-shaped pattern was observed in the regression curve of elaidic acid and all-cause mortality, as well palmitelaidic acid and cancer mortality.
Conclusions
Plasma elaidic acid levels are associated with higher risk of all-cause and CVD mortality, and palmitelaidic acid levels are associated with higher cancer mortality in later life. Further studies are needed to investigate current inconsistent results in this field and the possible underlying mechanisms.
Similar content being viewed by others
Background
Trans-fatty acids (TFAs), a type of unsaturated fatty acid containing double bonds in the trans-configuration, are not commonly found in nature (ruminant TFAs). However, industrial production of TFAs from vegetable fats since the 1950s was widely increased for use in processed foods, margarine, frying fast foods and commercial baked goods [1]. In industrially-produced TFAs (IP-TFAs), a portion of cis-isomers are converted into trans-isomers during hydrogenation. This conversion stabilizes polyunsaturated oils, protects them against rancidification and keeps them solid at room temperature. TFAs, particularly IP-TFAs, consistently increases the risk for diseases associated with the modern Western lifestyle such as cardiovascular diseases (CVD) [2,3,4], stroke [5], diabetes [6], Alzheimer’s disease [7], and certain cancers [8]. Therefore, many measures have been adopted to reduce the general population’s intake of TFAs. The Food and Drug Administration announced a final rule that requires food manufacturers to list TFAs on nutrition facts labels in 2003 [9]. As a result of this rule, the US National Health and Nutrition Examination Survey (NHANES) 1999–2000 show that plasma TFA concentrations in American adults declined from 1999 to 2010 [10,11,12].
An abundance of studies have investigated the adverse health effects of different TFA subtypes and their specific isomers concentrations in blood and have found inconsistent results. For instance, some early studies suggested that high blood levels of TFAs or TFA (all types of isomers, include C18:1 t) intake was associated with increased risk of prostate cancer [13] or coronary heart disease (CHD) [14,15,16]. However, some studies argued that TFA C18:2 t, but not C18:1 t or C16:1 t, were associated with nonfatal acute myocardial infarction (MI) [17], CHD death [18], total mortality [19] and sudden cardiac arrest [20]. One explanation for these conflicting reports is that most prior studies evaluated only the sum of these TFAs. In addition, it is also debated whether variations of TFAs at low concentrations are associated with adverse outcomes risk. It is unknown how different individual isomers relate to total or cause-specific mortality. Therefore, it is necessary to relate to these different TFA subtypes to key outcomes, such as all-cause, CVD or cancer mortality.
To evaluate the association of plasma TFAs levels with mortality, we conducted a cohort study using data from the NHANES 1999–2000, a large, nationally representative, socioeconomically and ethnically diverse sample of U.S. adults [21]. Our primary goals were to address the following research questions: 1) Are plasma TFAs levels associated with all-cause or cause-specific mortality in adults aged 20–84? 2) What is the highest concentration of TFA that may be harmless? 3) Should the same thresholds be applied to both IP-TFAs and ruminant TFAs? Answering these questions could help comprehensively illuminate the association between TFAs and mortality risk.
Methods
Sample and procedures
Each NHANES participant undergoes a household interview and a physical examination in a Mobile Examination Center [21]. More details about NHANES and its methods have been published [22]. The study protocol was approved by the National Center for Health Statistics (NCHS) Ethics Review Board and written informed consent was obtained.
Baseline data for this study were obtained from the publicly released NHANES 1999–2000. It is a cross-sectional sample of about 9965 individuals aged 2 months and older. Of these, 2188 participants were eligible to have their plasma TFAs examined. Given the possibility of survival bias among the extreme elderly, we restricted our analysis to persons who were ≥20 years and <85 years at baseline. Next, 732 were excluded due to missing information on one or more covariates and/or mortality. Finally, 1456 subjects, who met the inclusion criteria, were included in our analysis.
Mortality
The de-identified and anonymized data of NHANES 1999–2000 participants were linked to longitudinal medicare and mortality data using the NHANES assigned sequence number [23, 24]. Mortality follow-up data are available from the date of survey participation until December 31, 2011. Person-years of follow-up from the interview date ranged from 0.01 to 12.7, with a mean of 11.0. We examined all-cause mortality, as well as mortality due to CVD, cancer, and other diseases including chronic lower respiratory diseases, influenza, pneumonia, liver disease, kidney disease, diabetes, and Alzheimer’s disease. Cause of death was determined using the International Classification of Diseases, Tenth Revision (ICD-10). Cardiovascular death was classified using ICD-10 codes I00-I78, and cancer death was classified using ICD-10 codes C00-C97.
Trans-fatty acids
Four TFAs (elaidic acid [C18:1 t9], vaccenic acid [C18:1 t11], palmitelaidic acid [C16:1 t9], and linoelaidic acid [C18:2 t9, 12]) were measured in plasma stored at −70 °C, following previously described procedures [25, 26]. Description of laboratory methodology and the quality control protocol is available at https://wwwn.cdc.gov/nchs/data/nhanes/1999-2000/labmethods/TFA_A_trans_fatty_acids_met.pdf.
The four major TFAs examined here are reasonably representative of TFAs in blood [27]. The C18:1 t isomers account for 80%–90% of total TFAs in foods [28], including elaidic acid and vaccenic acid. Elaidic acid (C18:1 9 t) is the major isomer in industrial sources of TFAs. The transisomer of vaccenic acid (C18:1 11 t), a precursor for conjugated linoleic acid, is the major TFA isomer in ruminant fat and is also less frequently found in IP-TFAs [29]. Other TFA isomers include C16:1 t, C18:2 t, C18:3 t, as well as long-chain polyunsaturated TFAs [29] including palmitoleic acid (C16:1 t9) and linolelaidic acid (C18:2 t9, 12). The major sources of palmitoleic acid are ruminant meat and milk, while linolelaidic acid partially comes from hydrogenated oils.
Other covariates
Demographic data and health history (diabetes, hypertension, coronary heart disease, myocardial infarction, and stroke) were determined by self-report. Race was classified as non-Hispanic white, non-Hispanic black, other Hispanic, Mexican American, or others. Body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), high density lipoprotein (HDL), uric acid (UA) and creatinine (Cr) were measured by standard protocol. Estimation glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) study equation [30]. Alcohol use was determined by self-reporting (yes or no) whether they have at least 12 servings of any type of alcoholic beverage in any 1 year. Smoking status was categorized as current smoker (frequent or occasional), former smoker, and nonsmoker. Prescription medication use was assessed by self-report and verified by interviewers through examination of medication containers. Diabetes was defined as a fasting glucose value ≥7.0 mmol/L or use of insulin or oral hypoglycemic agent. Hypertension was defined as systolic/diastolic blood pressure ≥ 140/90 mmHg or use of antihypertensive drugs.
Statistical analysis
We used the R survey package [31] to appropriately weight analyses for the complex, multistage sampling design of NHANES. The sample weight, WTMEC2YR (full sample 2-year MEC exam weight) was used to obtain unbiased national estimates. We included cluster and strata variables from demographic datasets in our weighted analyses to account for survey design.
First, we conducted penalized smoothing spline by R package pspline [32] to develop hazards ratio (HR) curves to explore the possible nonlinear relationships between TFAs and mortality based on Cox proportional hazards models. In these models, the covariates were adjusted and TFA subtypes were used as continuous variables.
Second, we applied Cox proportional hazards models to explore the relationships between TFA levels and all-cause, CVD and cancer mortality among whole cohorts, with time-at-risk until the first event of interest, censoring at other causes of death in analyses of cause-specific mortality or December 31, 2011. We performed models with baseline TFAs levels as a continuous predictor and as categorical predictor (according to quartiles and used the second quartiles as reference category). In these models, per standard deviation (sd) increase TFAs measure was also to reflect increased risk of mortality. Cox proportional hazards models were first age, and gender adjusted and then adjusted for the covariates listed above. Because different TFA subtypes shared some common dietary sources and were partly intercorrelated [19, 33], associations for one subtype could be confounded by the influence of other subtypes [34]. Therefore, we further conducted analyses with mutual adjustment for the various TFA subtypes.
All P values were derived from 2-tailed analyses, with significance accepted at P ≤ 0.05. Data management and all analyses were performed using R software program, version 3.1.2 (http://www.R-project.org).
Results
Baseline characteristics
At baseline, the mean age ± standard deviation was 49.9 ± 17.9 years. Of the cohort, 701 (48.1%) participants were men and 695 (47.7%) were non-Hispanic white. Baseline TFA concentrations significantly differed by population characteristics (Table 1). In unadjusted cross-sectional analyses, older, overweight or obese, diabetic and non-Hispanic white patients tended to have higher TFA concentrations. Compared with individuals assumed alive, participants assumed deceased had higher elaidic acid and palmitelaidic acid. These two TFAs were associated with lower alcohol consumption. In additoin, elaidic acid and linolelaidic acid associated with smoking status, and participants diagnosed with hypertension had higher linolelaidic acid than those without hypertension (Table 1).
Intercorrelations between TFA subtypes
Distribution of TFAs and intercorrelations between TFA subtypes are presented in Fig. 1. Highly relevant intercorrelations between TFA subtypes were observed and were lowest for elaidic acid and linolelaidic acid (correlation coefficient = 0.68).
Correlations between TFAs and all-cause mortality
A median follow-up of 11.6 years was obtained from the 1456 subjects. During 16,034 person-years of follow-up, a total of 221 deaths occurred, including 39 and 60 deaths attributed to CVD and cancer, respectively.
Displayed in Fig. 2, penalized smoothing splines in the multivariate proportional hazards models revealed a nonlinear association between TFA levels and mortality. Also shown in Fig. 2a, the risks of all-cause mortality sharply raised as elaidic acid levels increased after the relatively low level of approximately 25 μmol/L (μM). This risk slightly leveled off, displaying a J-shaped pattern. Figure 2b revealed an approximately linear and positive association between vaccenic acid and all-cause mortality. While a U-shaped curve with a threshold of 6.0 (palmitelaidic acid) μM/2.0 (linolelaidic acid) μM were observed in Fig. 2c, d.
Relationship between baseline TFA subtypes (μM) and incident all-cause mortality and their 95% confidence intervals (shade scope), based on Cox proportional hazards regression adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, triglyceride, total cholesterol, high density lipoprotein, uric acid, estimation glomerular filtration rate, alcohol use, smoking, and self-reported cardiovascular diseases history at baseline. Histogram of TFA subtypes present in right vertical axis. a Elaidic acid (C18:1 t9), b Vaccenic acid (C18:1 t11), c Palmitelaidic acid (C16:1 t9), d Linolelaidic acid (C18:2 t9, 12)
Considering nonlinear correlation patterns between TFA levels and all-cause mortality, we defined the second quartile as the reference category. In age and gender adjusted analysis, elaidic acid was associated with higher all-cause mortality, with 63% higher risk among participants in the top quartile, compared with the second quartile (HR = 1.63, 95%CI = 1.12 to 2.38). Other TFA subtypes were not significantly associated with all-cause mortality upon age and gender adjustment. Further adjusted for potential confounders (race, BMI, SBP, DBP, FPG, TG, TC, HDL, UA, eGFR, alcohol use, smoking, and self-reported CVD history at baseline) did not diminish the effect sizes of elaidic acid (HR = 2.01, 95%CI = 1.30 to 3.08). Also, palmitelaidic acid (HR = 1.82) and linolelaidic acid (HR = 1.49, quartile 3 vs. quartile 2) became significantly associated with all-cause mortality after adjustments. Considering intercorrelations of the TFA subtypes (Fig. 1), we performed further analyses with mutual adjustment to evaluate independent associations. After mutual adjustment, elaidic acid remained associated with all-cause mortality (HR = 2.00, 95%CI = 1.18 to 3.40). Other TFA subtypes were not significantly associated with all-cause mortality with mutual adjustment. In addition, the lowest TFAs quartiles, except for vaccenic acid, were also related to increased mortality. This tendency was fit for all model analysis (Table 2).
To assess generalizability, we also presented linear relationships between TFA levels and mortality per (10) unit(s)/sd increase. Results showed that elaidic acid positively associated with all-cause mortality in all three models (Per 10 units increase age and gender adjusted HR = 1.07, multivariable adjusted HR = 1.13 in model 2, and equal to 1.30 after mutual adjustment, all P-value <0.05). Vaccenic acid was significantly associated with all-cause mortality only observed in model 2 (Per 10 units increase HR = 1.08, 95%CI = 1.01 to 1.15). The other two TFA subtypes were not significant (P-value >0.05) (Table 2).
Correlations between TFAs and CVD and cancer mortality
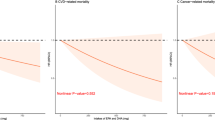
When we evaluated TFA correlations with CVD and cancer mortality, different nonlinear associations were obtained (Additional file 1: Figure S1 and Additional file 2: Figure S2) for most TFA subtypes. It is worth mentioning that the J-shaped pattern with a threshold of 5.5 μM was more obvious among relationships between palmitelaidic acid and cancer mortality.
In multivariable adjusted analysis, due to fewer outcomes for CVD and cancer mortality, the correlations between TFAs and CVD and cancer mortality became non-significant in most stratification analyses (Tables 3 and 4). The association of elaidic acid with CVD mortality as a continuous variable was statistically significant in model1 and model 3 (Table 3). Palmitelaidic acid was statistically associated with higher cancer mortality, the multivariable adjusted HR was equal to 2.56 among participants in the top quartile, compared with the second quartile, even after mutual adjustment (HR = 2.91, 95%CI = 1.09 to 7.81) (Table 4).
Discussion
We analyzed the association between plasma TFA levels and mortality in the U.S. population. After an average 11.0-year follow-up, we obtained three main findings. 1) Elaidic acid was most strongly associated with higher risk of all-cause or CVD-related mortality, even after adjustment for potential confounders or other TFA subtypes. Regression between elaidic acid and all-cause mortality revealed a J-shaped curve with a threshold elaidic acid level of 25 μM. 2) Vaccenic acid, palmitelaidic acid and linolelaidic acid levels were all significantly associated with all-cause mortality only after multivariate adjustment, but not after mutual adjustment for other TFAs. 3) The J-shaped pattern with a threshold of 5.5 μM is most obvious in the relationship between palmitelaidic acid levels and cancer mortality.
TFAs and mortality
Several studies report conflicting results regarding associations between plasma TFAs levels and total or CVD mortality [18, 19, 35, 36]. Recently, a sponsored, community-based and multicenter prospective cohort study of older American adults, called Cardiovascular Health Study (CHS) [19], assessed whether plasma phospholipid compositions of TFA subtypes and their isomers are associated with total, cardiovascular and nonvascular mortality. The 31,494 person-years of follow-up from CHS shows the C18:2 t isomer is significantly associated with increased risk of total mortality (extreme quintile HR = 1.23). This association may principally be due to its strong positive association with CVD-related death (HR = 1.40). Interestingly, neither C16:1 t nor C18:1 t isomers were significantly associated with study outcomes. Additionally, no significant or inverse associations between blood TFA levels (total, C16:1 t or C18:1 t) and CVD or all-cause mortality has been observed in other cohort studies [35, 36].
In contrast to previous cohort findings, our study shows elaidic acid (C18:1 9 t) is most strongly associated with higher risks of all-cause and CVD-related mortality. Our findings are biologically plausible. TFA C18:1 t is the major contributor to total TFA consumption from partially hydrogenated vegetable oil, which has been consistently linked to adverse lipid effects in trials [2, 37]. Also, TFAs can increase markers of inflammation [38, 39], promote thrombogenesis and impair flow-mediated dilatation [40]. More recently, human endothelial cells treated with TFAs were found to decrease the production of the vasodilator nitric oxide [41]. Self-reported dietary questionnaires show TFA C18:1 t can elevate the CHD risk [14,15,16]. Nevertheless, prior biomarker studies [17,18,19, 34, 42] show no significant or inverse associations of plasma C18:1 t levels with sudden cardiac arrest, CHD or mortality. These contradictory findings may be partially due to the health effects of C18:1 t evaluated in sum in most previous studies that probably mask the effects. It must be noted, the high intercorrelation of C18:1 t isomers preclude their separate evaluation.
Although several studies [17,18,19, 34, 42, 43] show total C18:2 t relates to higher risk of adverse outcomes, the associations of vaccenic acid, palmitelaidic acid and linolelaidic acid with all-cause mortality found in the present study may be masked by other TFAs or may be a chance finding. Together with previous research, we cautiously conclude that IP-TFAs compared to ruminant TFAs may have greater health hazards in all-cause and CVD-related mortality.
Our study observed a relationship between palmitelaidic acid and cancer mortality. This finding may be supported by the results from North Carolina Colon Cancer Study II [44], which suggests high-TFA consumption is positively associated with distal colorectal cancer among white people. Relative to the lowest quartile, the adjusted odds ratio of the fourth quartile of C16:1 t consumption is 1.57 (95%CI: 1.14 to 2.17). Potential mechanisms may be TFAs could promote colonic mucosa irritation [45], insulin sensitivity [46] and cell proliferation [47, 48], which are positively associated with the incidence of cancers. Nevertheless, further research is needed to uncover why palmitelaidic acid, rather than other TFAs, is associated with cancer mortality.
Higher mortality rates are not restricted to people within the third or fourth quartile of TFA levels, but are also extended to those with lower TFA concentrations. J-shaped or U-shaped associations between plasma TFA levels and all-cause mortality were observed in all tested TFAs, except vaccenic acid. For instance, subjects within the first quartile of elaidic acid levels have 1.35-fold higher risk of all-cause mortality compared to those with median levels. One possible explanation is that low TFA levels are linked to malnutrition, economic poverty or inadequate medical services, which may increase the risk of death. These J- and U-shaped associations should be investigated in detail.
Strengths and limitations
This study’s strengths lie in its prospective design and a reasonable follow-up duration (11.0 years on average) in the non-institutionalized U.S. population. Second, the measurements of TFA exposure, mortality and other covariates are generally reliable, thanks to the guidance of the U.S. National Center for Health Statistics. Third, measurements of blood TFA levels are generally more reliable and comprehensive than self-reported intake assessment, which are limited in the ability to accurately reflect the nutrient consumption of individuals due to measurement error, recall bias, selective report, and incompleteness of food composition databases [49]. Fourth, we separately evaluated 4 major TFAs and their specific isomers, and thereby provided an accurate and reasonable proof of relationship between specific TFAs and mortality. Furthermore, a penalized smoothing spline model was conducted to develop hazard ratio curves and thereby explore the possible nonlinear relationships between TFAs and mortality. In addition, our ability to adjust confounders increased by prospectively collecting primary covariates including blood lipid parameters using standard methods.
This study has some potential limitations that deserve consideration. First, the measurements of plasma TFA levels were cross-sectional at baseline and may not accurately reflect the long-term blood TFA status. Second, we were unable to illustrate the influences of TFA status on the mortality caused by specific diseases (e.g. Alzheimer’s disease and bowel cancer) due to insufficient data. Finally, given a low event rate, the statistical power in some analyses were low.
Conclusion
In the current study, we did observe plasma elaidic acid levels are associated with higher risk of all-cause and CVD mortality, and palmitelaidic acid levels are associated with higher cancer mortality in later life. TFA levels are nonlinearly associated with mortality. These findings highlight scientific knowledge that different TFA subtypes may influence total and cause-specific mortality. Further studies are needed to investigate current inconsistent findings in this field and the underlying mechanisms.
Abbreviations
- BMI:
-
Body mass index
- CHD:
-
Coronary heart disease
- CI:
-
Confidence interval
- Cr:
-
Creatinine
- CVD:
-
Cardiovascular diseases
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimation glomerular filtration rate
- FPG:
-
Fasting plasma glucose
- HDL:
-
High density lipoprotein
- HR:
-
Hazard ratio
- ICD:
-
International Classification of Diseases
- IP-TFAs:
-
Industrially produced TFAs
- MI:
-
Myocardial infarction
- NHANES:
-
Nutrition Examination Survey
- SBP:
-
Systolic blood pressure
- sd:
-
Standard deviation
- TC:
-
Total cholesterol
- TFAs:
-
Tran-fatty acids
- TG:
-
Triglyceride
- UA:
-
Uric acid
- μM:
-
μmol/L
References
Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, et al. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med. 1995;24:308–15.
Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13.
Guasch-Ferre M, Babio N, Martinez-Gonzalez MA, Corella D, Ros E, Martin-Pelaez S, et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015;102:1563–73.
de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978.
Kiage JN, Merrill PD, Judd SE, He K, Lipworth L, Cushman M, et al. Intake of trans fat and incidence of stroke in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Am J Clin Nutr. 2014;99:1071–6.
Schwenke DC, Foreyt JP, Miller ER 3rd, Reeves RS, Vitolins MZ. Plasma concentrations of trans fatty acids in persons with type 2 diabetes between September 2002 and April 2004. Am J Clin Nutr. 2013;97:862–71.
Grimm MO, Rothhaar TL, Grosgen S, Burg VK, Hundsdorfer B, Haupenthal VJ, et al. Trans fatty acids enhance amyloidogenic processing of the Alzheimer amyloid precursor protein (APP). J Nutr Biochem. 2012;23:1214–23.
Hidaka BH, Carlson SE, Kimler BF, Fabian CJ. Dietary associations with a breast cancer risk biomarker depend on menopause status. Nutr Cancer. 2016;68:1115–22.
Food, Drug Administration HHS. Food labeling: trans fatty acids in nutrition labeling, nutrient content claims, and health claims. Final rule Fed Regist. 2003;68:41433–506.
Restrepo BJ. Further decline of trans fatty acids levels among us adults between 1999-2000 and 2009-2010. Am J Public Health. 2017;107:156–8.
Vesper HW, Kuiper HC, Mirel LB, Johnson CL, Pirkle JL. Levels of plasma trans-fatty acids in non-Hispanic white adults in the United States in 2000 and 2009. JAMA. 2012;307:562–3.
Vesper HW, Caudill SP, Kuiper HC, Yang Q, Ahluwalia N, Lacher DA, et al. Plasma trans-fatty acid concentrations in fasting adults declined from NHANES 1999-2000 to 2009-2010. Am J Clin Nutr. 2017;105:1063–9.
Chavarro JE, Stampfer MJ, Campos H, Kurth T, Willett WC, Ma J. A prospective study of trans-fatty acid levels in blood and risk of prostate cancer. Cancer Epidemiol Biomark Prev. 2008;17:95–101.
Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses' health study. Am J Epidemiol. 2005;161:672–9.
Oomen CM, Ocké MC, Feskens EJM, M-AJv E-B, Kok FJ, Kromhout D. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746–51.
Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–9.
Baylin A, Kabagambe EK, Ascherio A, Spiegelman D, Campos H. High 18:2 trans-fatty acids in adipose tissue are associated with increased risk of nonfatal acute myocardial infarction in costa rican adults. J Nutr. 2003;133:1186–91.
Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH, et al. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation. 2006;114:209–15.
Wang Q, Imamura F, Lemaitre RN, Rimm EB, Wang M, King IB, et al. Plasma phospholipid trans-fatty acids levels, cardiovascular diseases, and total mortality: the cardiovascular health study. J Am Heart Assoc. 2014;3
Lemaitre RN. Cell Membrane Trans-Fatty Acids and the Risk of Primary Cardiac Arrest. Circulation. 2001;105:697–701.
Centers for Disease Control and Prevention. NHANES. Public Data Release File Documentation. 1999-2000; Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/general_data_release_doc.pdf
National Center for Health Statistics. National Health and Nutrition Examination Survey. Introduction to NHANES. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013-14_overview_brochure.pdf
National Center for Health Statistics. Linkage Methods and Analytical Support for NCHS Linked Mortality Data: 2011 NDI Records Matching Process Flow Chart for NCHS Surveys. Available at: https://www.cdc.gov/nchs/data/datalinkage/2011_ndi_records-_matching_mortality_flow_chart.pdf
Centers for Disease Control and Prevention. NCHS Surveys Linked to NDI Mortality Data: 2011 Public-Use Linked Mortality Files. Available at: https://www.cdc.gov/nchs/data/datalinkage/public_use_linked_mortality_file_readme_text_1_2015.pdf
Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab. 2001;73:38–45.
Mossoba MM, Kramer JK, Delmonte P, Yurawecz MP, Rader JI. Official methods for the determination of trans fat. Urbana: AOCS Press; 2009.
Gebauer SK, Psota TL, Kris-Etherton PM. The diversity of health effects of individual trans fatty acid isomers. Lipids. 2007;42:787–99.
Allison DB, Egan SK, Barraj LM, Caughman C, Infante M, Heimbach JT. Estimated Intakes of Trans Fatty and Other fatty acids in the US population. J Am Diet Assoc. 1999;99:166–74.
Weggemans RM, Rudrum M, Trautwein EA. Intake of ruminant versus industrial trans fatty acids and risk of coronary heart disease–what is the evidence? Eur J Lipid Sci Technol. 2004;106:390–7.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1–19.
Chajes V, Biessy C, Ferrari P, Romieu I, Freisling H, Huybrechts I, et al. Plasma elaidic acid level as biomarker of industrial trans fatty acids and risk of weight change: report from the EPIC study. PLoS One. 2015;10:e0118206.
Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, et al. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr. 2010;91:883–93.
Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, et al. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701.
Borgeraas H, Hertel JK, Seifert R, Berge RK, Bohov P, Ueland PM, et al. Serum trans fatty acids, asymmetric dimethylarginine and risk of acute myocardial infarction and mortality in patients with suspected coronary heart disease: a prospective cohort study. Lipids Health Dis. 2016;15:38.
Kleber ME, Delgado GE, Lorkowski S, Marz W, von Schacky C. Trans-fatty acids and mortality in patients referred for coronary angiography: the Ludwigshafen Risk and Cardiovascular Health Study. Eur Heart J. 2016;37:1072–8.
Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55.
Kondo K, Ishida T, Yasuda T, Nakajima H, Mori K, Tanaka N, et al. Trans-fatty acid promotes thrombus formation in mice by aggravating antithrombogenic endothelial functions via Toll-like receptors. Mol Nutr Food Res. 2015;59:729–40.
Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–6.
de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol. 2001;21:1233–7.
Iwata NG, Pham M, Rizzo NO, Cheng AM, Maloney E, Kim F. Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS One. 2011;6:e29600.
Roberts TL, Wood DA, Riemersma RA, Gallagher PJ, Lampe FC. trans isomers of oleic and linoleic acids in adipose tissue and sudden cardiac death. Lancet. 1995;345:278–82.
Sun Q, Ma J, Campos H, Hankinson SE, Manson JE, Stampfer MJ, et al. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation. 2007;115:1858–65.
Vinikoor LC, Millikan RC, Satia JA, Schroeder JC, Martin CF, Ibrahim JG, et al. trans-Fatty acid consumption and its association with distal colorectal cancer in the North Carolina Colon Cancer Study II. Cancer Causes Control. 2009;21:171–80.
Colandre ME, Diez RS, Bernal CA. Metabolic effects of trans fatty acids on an experimental dietary model. Br J Nutr. 2003;89:631–9.
Riserus U. Trans fatty acids and insulin resistance. Atheroscler Suppl. 2006;7:37–9.
O'Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51–8.
Lee DY, Lupton JR, Aukema HM, Chapkin RS. Dietary fat and fiber alter rat colonic mucosal lipid mediators and cell proliferation. J Nutr. 1993;123:1808–17.
Bingham SA, Luben R, Welch A, Wareham N, Khaw KT, Day N. Are imprecise methods obscuring a relation between fat and breast cancer? Lancet. 2003;362:212–4.
Acknowledgements
The authors are thankful to MogoEdit for proofreading the manuscript.
Funding
This work was supported, in part, by Young and Middle-Aged Natural Science Foundation of Wannan Medical College (contract number WK201502).
Availability of data and materials
The datasets generated and/or analysed during the current study are from the corresponding author on reasonable request, and also available in the National Center for Health Statistics website: https://www.cdc.gov/nchs/nhanes/index.htm.
Author information
Authors and Affiliations
Contributions
HL, QZ, JS, AW, YZ, LD and YW all contributed to the design and conduct of the research; HL, QZ, LD and YW analyzed the data; and HL, QZ, JS, AW, YZ, LD and YW wrote the initial drafts of the paper and had responsibility for the final content All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the National Center for Health Statistics (NCHS) Ethics Review Board and written informed consent was obtained.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: Figure S1.
Relationship between baseline TFA subtypes (μM) and incident cardiovascular diseases mortality and their 95% confidence intervals (shade scope), based on Cox proportional hazards regression adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, triglyceride, total cholesterol, high density lipoprotein, uric acid, estimation glomerular filtration rate, alcohol use, smoking, and self-reported cardiovascular diseases history at baseline. Histogram of TFA subtypes present in right vertical axis. (A) Elaidic acid (C18:1 t9), (B) Vaccenic acid (C18:1 t11), (C) Palmitelaidic acid (C16:1 t9), (D) Linolelaidic acid (C18:2 t9, 12). (TIFF 1842 kb)
Additional file 2: Figure S2.
Relationship between baseline TFA subtypes (μM) and incident cancer mortality and their 95% confidence intervals (shade scope), based on Cox proportional hazards regression adjusted for age, gender, race, body mass index, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, triglyceride, total cholesterol, high density lipoprotein, uric acid, estimation glomerular filtration rate, alcohol use, smoking, and self-reported cardiovascular diseases history at baseline. Histogram of TFA subtypes present in right vertical axis. (A) Elaidic acid (C18:1 t9), (B) Vaccenic acid (C18:1 t11), (C) Palmitelaidic acid (C16:1 t9), (D) Linolelaidic acid (C18:2 t9, 12). (TIFF 1820 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, H., Zhang, Q., Song, J. et al. Plasma trans-fatty acids levels and mortality: a cohort study based on 1999–2000 National Health and Nutrition Examination Survey (NHANES). Lipids Health Dis 16, 176 (2017). https://doi.org/10.1186/s12944-017-0567-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-017-0567-6