Abstract
Background
A number of researches have evaluated the association between the ABCB1 polymorphism and the lipid-lowering response of statins, but the results have been inconclusive. To examine the lipid-lowering efficacy and safety associated with the ABCB1 C3435T polymorphism in hypercholesterolemic patients receiving statin, all available studies were included in this meta-analysis.
Methods
A systematic search for eligible studies in the Cochrane library database, Scopus and PubMed was performed. Articles meeting the inclusion criteria were comprehensively reviewed, and the available data were accumulated by the meta-analysis.
Results
The results indicated that the comparisons of CC+CT vs. TT were associated with a significant elevation of the serum HDL-C levels after statin treatment (CC+CT vs. TT: MD, 2.46; 95 % CI, 0.36 to 4.55; P = 0.02), and the ABCB1 C3435T variant in homozygotes was correlated with decreases in LDL-C (CC vs. TT: MD, 2.29; 95 % CI, 0.37 to 4.20; P = 0.02) as well as TC (CC vs. TT: MD, 3.05; 95 % CI, 0.58 to 5.53; P = 0.02) in patients treated with statin. However, we did not observe a significant association in the TG group or an association between other genetic models serum lipid parameters. In addition, statin treatment more than 5 months led to a higher risk of muscle toxicity.
Conclusions
The evidence from the meta-analysis demonstrated that the ABCB1 C3435T polymorphism may represent a pharmacogenomic biomarker for predicting treatment outcomes in patients on statins and that statin treatment for more than 5 months can increase the risk of myopathy.
Similar content being viewed by others
Introduction
Cardiovascular diseases are the leading cause of death worldwide, and atherosclerosis due to lipid metabolism is one of the main determinants of cardiovascular risk [1]. Statins, which inhibit 3-hydroxy-3-methylglutaryl co-enzyme A reductase (HMG-CoA reductase) are effective at reducing atherosclerosis and cardiovascular risks in clinical practice by lowering the levels low-density lipoprotein cholesterol (LDL-C) and total triglycerides (TG) [2–4]. However, the pharmacodynamic response to statins varies greatly among patients [5]. Although the mechanisms have not been fully clarified, genetic polymorphisms may play an important role in individual susceptibility to drug response, including the ABCB1 C3435T (rs1045642) genetic variant.
The ABCB1 (adenosine triphosphate -binding cassette, sub-family B, member 1) gene, which also called multidrug resistance 1 gene (MDR1), encodes the intestinal efflux transporter P-glycoprotein, which has been associated with the transport of cellular lipids and drugs [6]. More than 50 single-nucleotide polymorphisms (SNP) in the ABCB1 gene, located at 7, p21-21.1 [7], have been described in the literature. Among them, ABCB1 C3435T (rs1045642) is the extensively investigated, and many studies have shown that the ABCB1 C3435T genotype influences ability of P-glycoprotein to direct the absorption of statins [8].
Both the lipid-lowering response of statin and their safety has been investigated. Kajinamietal. [9] first analyzed the effect of the C3435T polymorphism on clinical outcomes in patients receiving atorvastatin and found that the C3435T polymorphism was significantly and independently related to a smaller reduction in LDL-C and a larger increase in high-density lipoprotein cholesterol (HDL-C) relative to variant allele carriers in a gender-specific manner. One study [10] reported that the ABCB1 C3435T polymorphism differed statistically between the ADR (muscle toxicity) and non-ADR groups treated with simvastatin. However, the results from different studies [9–16] have been inconsistent. Therefore, we performed a meta-analysis to determine the relationship between the ABCB1 C3435T polymorphism and the lipid-lowering efficacy and safety of statins.
Methods
Literature search
Three electronic databases (PubMed, Scopus and the Cochrane library) were comprehensively searched. The latest search was updated in February 2015 with the following terms: statin, ABCB1, MDR1, multidrug resistance, polymorphism, myopathy, myositis, and rhabdomyolysis. All the eligible studies were retrieved, and the bibliographies of the eligible studies and previous meta-analyses were checked for additional relevant articles.
Inclusion criteria
Studies that meet the following criteria were included: (1) published in English, (2) case- control studies on lipid-lowering therapy, (3) the evaluation of the ABCB1 C3435T polymorphism, the lipid-lowering efficacy and safety in patients receiving statin, (4) the availability of the genotype frequency in studied population, and (5) available data to calculate a mean difference (MD) or P-value with a 95 % confidence interval (CI).
Data extraction
Two reviewers reviewed all studies carefully, extracted the data independently, and reached a consensus on all aspects. The following information was obtained from each study: the first author’s name, the publication date, the country of studied population, sex, age, follow-up period, treatment protocol, serum lipids parameters, myopathy incidence, statin, and gene information.
Study outcomes
Two endpoints—the lipid-lowering efficacy and safety—were studied. The efficacy was evaluated by the change in TC (total cholesterol), TG, HDL-C, and LDL-C after statin treatment. The lipid-lowering safety in this study was reflected in the number of adverse effects of muscle toxicity, which comprised myalgia, myopathy, rhabdomyolysis or statin-induced elevations in serum Creatinekinase(CK) [17].
Statistical analysis
The observed genotype frequencies in the controls were compared with the expected genotype frequencies using the Hardy-Weinberg equilibrium (HWE). The mean difference (MD) and 95 % confidence interval (CI) in each study was used to assess the strength of the association between the ABCB1 C3435T polymorphism and lipid-lowering efficacy and safety in patients who received statin treatment. The pooled MDs were assessed for the dominant genetic model (CT+TT vs.CC), recessive genetic model (TT vs. CC+CT) and homozygote comparison (TT vs. CC) according to the method reported by Woolf [18]; significance was evaluated using the Z-test. Heterogeneity between studies was examined through the use of the χ2-based Q statistic test and was considered significant if p <0.1 [19]. The I2statistic was used to efficiently test for the heterogeneity [20]. If the effects appeared to be homogeneous, the fixed-effect method was used. Otherwise the random-effect model was adopted.
The heterogeneity was tested by subgroup analyses. Sensitivity analyses were performed to identify the potential influence of each study set on the pooled MDs by the sequential omission of individual studies. Publication bias was also detected by the funnel plot, and the symmetry of the plot distribution indicated the absence of publication bias [21]. All statistical tests were conducted using Review Manager (v.5.1, The Cochrane Collaboration), and were considered significant if the two-tailed P value was less than 0.05.
Result
Study characteristics
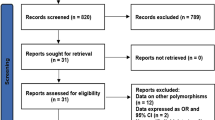
A total of 140 studies on the ABCB1 C3435T polymorphism and the lipid-lowering efficacy and safety of statins were identified, and 23 replicated studies were excluded. Additionally, 24 reviews, 1 meta-analysis, 2 case reports and 4 studies not involving human beings were excluded. Meanwhile, 64 irrelevant studies were excluded after reviewing the title and abstract. Furthermore, seven studies were excluded because of their insufficient genetic information, and seven trials were excluded because the serum lipid parameters (TC, TG, HDL-C, and LDL-C) and the number of cases of myopathy were not available. Finally, eight studies, including seven studies (930 patients) of the lipid-lowering efficacy and three of statin-associated myopathy (58 cases and 239 controls), met the inclusion criteria (Fig. 1). The characteristics of the included studies are summarized in Table 1 and Table 2. All the studies were performed on a representative sample of the target group. Various statin treatment protocols were applied, including simvastatin 20 mg/day [10], atorvastatin 80 mg/day [15], atorvastatin arm 40 mg/day [14], and atorvastatin arm 10 mg/day [9, 12, 13], among others (atorvastatin, rosuvastatin or simvastatin) which we could not be determined precisely based on the original articles [11, 16]. The frequencies of the genotypes in each study (Tables 1 and 2) all followed the HWE.
Meta-analysis results
Association with lipid-lowering therapy
HDL-C
The comparison of CC+CT vs. TT was associated with a significant elevation in the serum HDL-C levels upon statin treatment (CC+CT vs. TT: MD, 2.46; 95 % CI, 0.36 to 4.55; P = 0.02; I2 = 22 %; Fig. 2 and Table 3). No significant relationship between the change in the serum HDL-C level and other genetic models of the ABCB1 C3435T polymorphism were observed, and no heterogeneity was identified.
LDL-C
Seven studies provided data on LDL-C levels, and the heterogeneity was low for all comparisons. The overall meta-analysis demonstrated that LDL-C lowering effects were associated with the ABCB1 C3435T variation in homozygotes (CC vs. TT: MD, 2.29; 95 % CI, 0.37 to 4.20; P = 0.02; Fig. 3 and Table 3).
TC
When all eligible studies were pooled, the homozygous ABCB1 C3435T polymorphism was correlated with decreased TC levels in patients on statin treatment (CC vs. TT: MD, 3.05; 95 % CI, 0.58 to 5.53; P = 0.02 Fig. 4 and Table 3). No between-study heterogeneity was detected.
TG
As described in Table 3, although no heterogeneity could be identified, the meta-analysis illustrated that the association between the change in serum TG after lipid-lowering therapy and the ABCB1 C3435T variation was not significant (CC vs. CT+TT: MD, 0.1; 95 % CI, −3.99 to 4.18; P = 0.96; CC+CT vs. TT: MD, 1.14; 95 % CI, −2.97 to 5.26; P = 0.59; CC vs. TT: MD, 1.51; 95 % CI, −3.68 to 6.70; P = 0.57).
Association with myopathy
When the three eligible studies were pooled, the association between statin-related myopathy and the ABCB1 C3435T variation was not significant. The I2 statistic indicated between-study heterogeneity (Table 4).
The effect of the ABCB1 C3435T polymorphism was further evaluated in a stratification analyses according to the treatment duration. The ABCB1 C3435T polymorphism was found to be associated with a risk of myopathy in patients treated with statins more than 5 months in four models (C vs. T: OR, 0.42; 95 % CI, 0.23 to 0.75; P = 0.004; CC vs. TT: OR, 0.20; 95 % CI, 0.06 to 0.70; P = 0.01; CC+CT vs. TT: OR, 0.30; 95 % CI, 0.12 to 0.75; P = 0.01; CC vs. CT+TT: OR, 0.33; 95 % CI, 0.11 to 0.98; P = 0.05). Meanwhile, the between-study heterogeneity in the subgroup was decreased (Table 4).
Test of heterogeneity
No significant heterogeneity was observed in three genetic comparisons with respect to HDL, LDL, TC, or TG. However, significant heterogeneity was present in all the four genetic models of the association of ABCB1 C3435T (Table 4) with myopathy. We evaluated the source of heterogeneity based on the treatment protocol. When we stratified the trials by treatment duration, the heterogeneity was not obvious in the subgroup treated for more than 5 months (Table 4).
Sensitivity analysis
Sensitivity analysis was performed in the genetic model producing the positive results. For the lipid-lowering comparisons, the significance of the pooled MDs was not significantly affected by the omission of the individual studies with the exception of one study [10] in the TC and LDL groups as well as in the female subgroup of Kajinami’s study [9] in the LDL and HDL-C group. Given the limited number of studies on statin-associated myopathy, we were unable to perform the sensitivity analysis in this section.
Publication bias
A funnel plot was created to assess the publication bias of our included studies. As indicated in Figs. 5, 6 and 7, the asymmetry was not statistically significant. Therefore, no significant publication bias was detected.
Discussion
Cholesterol-lowering therapy is a keystone in the primary and secondary prevention of CVD [22]. Statins, which are HMG-CoA reductase inhibitors, are used to improve the lipid profile in dyslipidemic patients. The ABCB1 gene is only involved in cellular drug excretion [23] and the absorption of some drugs (for example clopidogrel [24]) but also possesses other functions: cholesterol redistribution [25]; intestinal cholesterol re-absorption [26]; regulation of cholesterol cellular trafficking; and cholesterol redistribution in cholesterol-rich microdomains of the cell membrane [27, 28]. Hence, the ABCB1 gene and its polymorphisms may play vital roles in lipid-lowering response of statins. On the basis of varying response in the lipid-lowering efficacy and safety profiles, several genetic studies have been performed to evaluate the association between the ABCB1 C3435T polymorphism and the lipid-lowering response in patients on statin treatment, but the results are inconclusive. There is no published GWAS studying the association examined in this meta-analysis. A former meta-analysis [29] reported that there were no significant differences in the efficacy of statin therapy between the “CC” and “CT+TT” groups.
In the present meta-analysis, we included the latest research and conducted the study more meticulously than past studies, including different genetic models and more detailed data that the former meta-analysis had not collected; our analysis produced some significant results. First, this meta-analysis indicated that the reduction in LDL-C and TC levels was associated with the ABCB1 C3435T variation in homozygotes. Second, individuals of genotype CC+CT were more likely to exhibit an elevation in serum HDL-C upon statin treatment. However, no significant association in the TG group and other genetic models was detected with respect to the above serum lipid parameters. The results are inconsistent with those of the previous study. Although the between-study heterogeneity in the above comparison was very limited, it should also be noted. In addition to genetic polymorphisms, many factors can inference the results of this research, including the following: differences in ethnicity, age, gender, genotyping method, study period, the primary clinical characteristics, and the treatment protocol (the type of statin, the dose, and the treatment duration). Despite the best effort to perform subgroup analyses according to diverse variables, no significant outcome was identified. Recently, an investigation reported that rosuvastatin (20 mg/day) is more effective with respect to the ability to increase HDL-C levels than atorvastatin (80 mg/day) in patients with ST elevation myocardial infarction (STEMI) [30]. Hence, because of the variables listed above, the association might have been biased.
Despite the proven efficacy, large inter-individual variability exists in the risk of adverse effects in patients on statins. Muscle toxicity is a relatively common adverse effect, occurring in 1–5 % of cases [31, 32]. Severe muscle toxicity is rare but represents a significant source of mortality [33]. This toxicity sometime appears to be dose-dependent, with higher levels of statins conferring higher overall risk. Moreover, clinicians should take into consideration a series of other potential risk factors (i.e., female, advanced age, diabetes mellitus, hypothyroidism and vitamin D deficiency) [34]. In our research, by different treatment protocol, three studies with myopathy are included. This was the first meta-analysis on the relationship between the polymorphism and statin-associated muscle toxicity. The four models of polymorphism were all associated with the risk of muscle toxicity when patients were treated with statins for more than 5 months. Thus, the treatment duration may influence the safety of statin treatment. Given that limited studies were included, the results should be interpreted with caution, and further larger studies should be performed.
Statins reduce the risk of major vascular events in a linear fashion, with a 20 % risk reduction for every 1 mmol/L decrease in low-density-lipoprotein cholesterol(LDL-C) [35]. Although an association exists between the ABCB1 C3435T polymorphism and cholesterol-lowering therapy, more attention has been paid to the interaction between SNPs and other factors. Numerous basic pharmacogenomics studies have revealed that candidate genes, such as CETP, HMGCR, SLCO1B1, ABCB1, and PCSK9,and their polymorphisms may represent pharmacogenomics biomarkers for predicting statin treatment outcomes [8]. One study evaluated the effect of the ABCB1 haplotypes (1236C>T, 2677G>A/T and 3435C>T) on TC and LDL-C responses to simvastatin and demonstrated a reduction in the T–non-G–T haplotype frequency in patients with myalgia compared with the non-ADR group (P = 03) [10]. Thus, various SNPs coexisting in the same or different genes might be related to cholesterol-lowering therapy while on statin therapy, and attention should be paid to gene-gene interactions, including SLCO1B1 or PCSK9 with ABCB1 C3435T.
Both genetic and environmental factors influence the lipid-lowering effect of statins. For example, smokers and patients with hypertension exhibit smaller decreases in LDL-C in response to statin treatment [36]. Furthermore, some studies have demonstrated that statins may be less effective in HIV-infected individuals, possibly as a result of their inherent viral resistance to statins or to highly active antiretroviral therapy [37]. Furthermore, drugs, such as amiodarone [38], and inflammation [39] may lead to a poor response to statin due to the decline in LDL-R protein [40]. In this meta-analysis, owing to the small population size as well as the inconsistent stratification in environmental exposures, further detection of the gene-environment interaction could not be performed. Therefore, more sophisticated gene-gene and gene-environment interactions should be included in a future analysis to obtain a more comprehensive understanding.
If a patient is highly resistant or intolerant to statin treatment, especially for those with genetic susceptibility (e.g., those with certain ABCB1 genotypes), there are several other treatment possibilities. One such drug is ezetimibe, which is a selective inhibitor of dietary and biliary cholesterol absorption at the brush border of the intestine acting that targetsNPC1L1 [41]. The study IMPROVE-IT evaluated the potential effect of ezetimibe on major cardiovascular (CV) events and reported a clear benefit from combination treatment with simvastatin and ezetimibe in patients with acute coronary syndrome and low LDL-C [42]. Furthermore, researchers have recently begun to focus on another type of lipid-lowering drug based on PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibition by a monoclonal antibody, such as alirocumab and evolocumab; phase III clinical trials have recently been initiated. Two recent studies demonstrated that alirocumab or evolocumab, when added to statin therapy, significantly reduced LDL-C levels and reduced the incidence of cardiovascular events [43, 44]. PCSK9 inhibition may represent an option for patients who are statin-intolerant or whose LDL‑C level remains high despite maximal statin therapy with less adverse effects [45]. The above treatment options may be well suited for patients who respond poorly to statins owing to their ABCB1 genotypes and provide fresh insight into the lipid-lowering treatment; however, substantial trails should be arranged for further assessment.
Although considerable efforts were put forth in our study design, there were some inherent limitations. First, the number of studies included is limited, especially with respect to the information on the risk of myopathy in patients treated with statin. Thus, the conclusion should be interpreted with caution. Moreover, some detailed information such as the age and sex of the patients were not available in some studies, and the studies primarily focused on patients in Latin America, all of which further limit our assessment. Furthermore, gene-gene or gene-environment interactions, can influence the clinical characteristics, and genotyping methods may affect the results. The above elements can be controlled more effectively through a separate analysis of distinct variables to which we did not have access. And we also need to promote the “clinical” value of the report widely since limited hospital has carried out the genotyping methods.
Conclusion
In summary, this meta-analysis indicated that decreases in LDL-C as well as TC levels upon statin treatment were associated with the ABCB1 C3435T homozygous variation, and people with the CC+CT genotype were more likely to exhibit elevated serum HDL-C. In addition, statin treatment for more than 5 months increased the risk of muscle toxicity. However, additional studies are needed to address an advanced empirical approach, and standardized stratification in further tasks is warranted to validate our findings.
References
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–52.
Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58.
Sever PS, Poulter NR, Dahlof B, Wedel H, Collins R, Beevers G, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28:1151–7.
Gotto Jr AM. Review of primary and secondary prevention trials with lovastatin, pravastatin, and simvastatin. Am J Cardiol. 2005;96:34F–8F.
Bercovich D, Friedlander Y, Korem S, Houminer A, Hoffman A, Kleinberg L, et al. The association of common SNPs and haplotypes in the CETP and MDR1 genes with lipids response to fluvastatin in familial hypercholesterolemia. Atherosclerosis. 2006;185:97–107.
Klein I, Sarkadi B, Varadi A. An inventory of the human ABC proteins. Biochim Biophys Acta. 1999;1461:237–62.
Fojo A, Lebo R, Shimizu N, Chin JE, Roninson IB, Merlino GT, et al. Localization of multidrug resistance-associated DNA sequences to human chromosome 7. Somat Cell Mol Genet. 1986;12:415–20.
Kitzmiller JP, Binkley PF, Pandey SR, Suhy AM, Baldassarre D, Hartmann K. Statin pharmacogenomics: pursuing biomarkers for predicting clinical outcomes. Discov Med. 2013;16:45–51.
Kajinami K, Brousseau ME, Ordovas JM, Schaefer EJ. Polymorphisms in the multidrug resistance-1 (MDR1) gene influence the response to atorvastatin treatment in a gender-specific manner. Am J Cardiol. 2004;93:1046–50.
Fiegenbaum M, da Silveira FR, Van der Sand CR, Van der Sand LC, Ferreira ME, Pires RC, et al. The role of common variants of ABCB1, CYP3A4, and CYP3A5 genes in lipid-lowering efficacy and safety of simvastatin treatment. Clin Pharmacol Ther. 2005;78:551–8.
Salacka A, Binczak-Kuleta A, Kaczmarczyk M, Hornowska I, Safranow K, Clark JS. Possible association of ABCB1:c.3435T>C polymorphism with high-density-lipoprotein-cholesterol response to statin treatment--a pilot study. Bosn J Basic Med Sci. 2014;14:144–9.
Rosales A, Alvear M, Cuevas A, Saavedra N, Zambrano T, Salazar LA. Identification of pharmacogenetic predictors of lipid-lowering response to atorvastatin in Chilean subjects with hypercholesterolemia. Clin Chim Acta. 2012;413:495–501.
Rodrigues AC, Rebecchi IM, Bertolami MC, Faludi AA, Hirata MH, Hirata RD. High baseline serum total and LDL cholesterol levels are associated with MDR1 haplotypes in Brazilian hypercholesterolemic individuals of European descent. Braz J Med Biol Res. 2005;38:1389–97.
Shabana MF, Mishriki AA, Issac MS, Bakhoum SW. Do MDR1 and SLCO1B1 polymorphisms influence the therapeutic response to atorvastatin? A study on a cohort of Egyptian patients with hypercholesterolemia. Mol Diagn Ther. 2013;17:299–309.
Hoenig MR, Walker PJ, Gurnsey C, Beadle K, Johnson L. The C3435T polymorphism in ABCB1 influences atorvastatin efficacy and muscle symptoms in a high-risk vascular cohort. J Clin Lipidol. 2011;5:91–6.
Ferrari M, Guasti L, Maresca A, Mirabile M, Contini S, Grandi AM, et al. Association between statin-induced creatine kinase elevation and genetic polymorphisms in SLCO1B1, ABCB1 and ABCG2. Eur J Clin Pharmacol. 2014;70:539–47.
Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med. 2009;150:858–68.
Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–3.
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–7.
Kreisberg RA, Oberman A. Clinical review 141: lipids and atherosclerosis: lessons learned from randomized controlled trials of lipid lowering and other relevant studies. J Clin Endocrinol Metab. 2002;87:423–37.
Tanigawara Y. Role of P-glycoprotein in drug disposition. Ther Drug Monit. 2000;22:137–40.
Su J, Xu J, Li X, Zhang H, Hu J, Fang R, et al. ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: a meta-analysis. PLoS One. 2012;7:e46366.
Garrigues A, Escargueil AE, Orlowski S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc Natl Acad Sci U S A. 2002;99:10347–52.
Tous M, Ribas V, Ferre N, Escola-Gil JC, Blanco-Vaca F, Alonso-Villaverde C, et al. Turpentine-induced inflammation reduces the hepatic expression of the multiple drug resistance gene, the plasma cholesterol concentration and the development of atherosclerosis in apolipoprotein E deficient mice. Biochim Biophys Acta. 2005;1733:192–8.
Jeannesson E, Siest G, Bastien B, Albertini L, Aslanidis C, Schmitz G, et al. Association of ABCB1 gene polymorphisms with plasma lipid and apolipoprotein concentrations in the STANISLAS cohort. Clin Chim Acta. 2009;403:198–202.
Rebecchi IM, Rodrigues AC, Arazi SS, Genvigir FD, Willrich MA, Hirata MH, et al. ABCB1 and ABCC1 expression in peripheral mononuclear cells is influenced by gene polymorphisms and atorvastatin treatment. Biochem Pharmacol. 2009;77:66–75.
Li Q, Hong J, Wu J, Huang ZX, Li QJ, Yin RX, et al. The role of common variants of ABCB1 and CYP7A1 genes in serum lipid levels and lipid-lowering efficacy of statin treatment: a meta-analysis. J Clin Lipidol. 2014;8:618–29.
Aydin MU, Aygul N, Altunkeser BB, Unlu A, Taner A. Comparative effects of high-dose atorvastatin versus moderate-dose rosuvastatin on lipid parameters, oxidized-LDL and inflammatory markers in ST elevation myocardial infarction. Atherosclerosis. 2015;239:439–43.
Feng Q, Wilke RA, Baye TM. Individualized risk for statin-induced myopathy: current knowledge, emerging challenges and potential solutions. Pharmacogenomics. 2012;13:579–94.
Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–90.
Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116:408–16.
Magni P, Macchi C, Morlotti B, Sirtori CR, Ruscica M. Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur J Intern Med. 2015;26:82–8.
Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–63.
Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, Shiboski S, et al. Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the Cholesterol and Pharmacogenetics (CAP) study. Am J Cardiol. 2006;97:843–50.
Boccara F, Simon T, Lacombe K, Cohen A, Laloux B, Bozec E, et al. Influence of pravastatin on carotid artery structure and function in dyslipidemic HIV-infected patients receiving antiretroviral therapy. AIDS. 2006;20:2395–8.
Al-Sarraf A, Li M, Frohlich J. Statin resistant dyslipidemia in a patient treated with amiodarone. BMJ Case Rep. 2011;2011. doi:10.1136/bcr.08.2011.4620.
Chen Y, Ku H, Zhao L, Wheeler DC, Li LC, Li Q, et al. Inflammatory stress induces statin resistance by disrupting 3-hydroxy-3-methylglutaryl-CoA reductase feedback regulation. Arterioscler Thromb Vasc Biol. 2014;34:365–76.
Reiner Z. Resistance and intolerance to statins. Nutr Metab Cardiovasc Dis. 2014;24:1057–66.
Reiner Z. Combined therapy in the treatment of dyslipidemia. Fundam Clin Pharmacol. 2010;24:19–28.
Blazing MA, Giugliano RP, Cannon CP, Musliner TA, Tershakovec AM, White JA, et al. Evaluating cardiovascular event reduction with ezetimibe as an adjunct to simvastatin in 18,144 patients after acute coronary syndromes: final baseline characteristics of the IMPROVE-IT study population. Am Heart J. 2014;168:205–12. e201.
Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015.
Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015.
Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol. 2014;11:563–75.
Acknowledgments
We are very grateful to Professor Yi Hu for helping with the design and writing. We also thank all the participants in this study.
Funding
The research was supported by grants from: Scientific Research Fund of Zhejiang Provincial Education Department (Y201224146).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
JS, HX, and JY conceived of the study, and participated in its design and coordination. QY, JY, LJ, and SY performed the experiments. JS, YZ, and QY performed the statistical analysis. JS, YZ, HX, JZ, YZ, and YL contributed reagents, materials, and analysis tools. JS and HX drafted the manuscript. All authors read and approved the final manuscript.
Jia Su and Hongyu Xu contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Su, J., Xu, H., Yang, J. et al. ABCB1 C3435T polymorphism and the lipid-lowering response in hypercholesterolemic patients on statins: a meta-analysis. Lipids Health Dis 14, 122 (2015). https://doi.org/10.1186/s12944-015-0114-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-015-0114-2