Abstract
In many hematologic malignancies, the adoptive transfer of chimeric antigen receptor (CAR) T cells has demonstrated notable success; nevertheless, further improvements are necessary to optimize treatment efficacy. Current CAR-T therapies are particularly discouraging for solid tumor treatment. The immunosuppressive microenvironment of tumors affects CAR-T cells, limiting the treatment’s effectiveness and safety. Therefore, enhancing CAR-T cell infiltration capacity and resolving the immunosuppressive responses within the tumor microenvironment could boost the anti-tumor effect. Specific strategies include structurally altering CAR-T cells combined with targeted therapy, radiotherapy, or chemotherapy. Overall, monitoring the tumor microenvironment and the status of CAR-T cells is beneficial in further investigating the viability of such strategies and advancing CAR-T cell therapy.
Similar content being viewed by others
Background
CAR (chimeric antigen receptor)-T cell therapy is a cell-based treatment method in which T lymphocytes are genetically engineered to express antibodies with specificity for tumor-associated antigens [1]. By synthesizing corresponding antibodies, immune cells are directed to the location of tumor cells. This approach has demonstrated efficacy in treating relapsed/refractory B-cell leukemia and lymphoma, particularly in targeting CD19, which has proven effective in cases of acute lymphoblastic leukemia [2]. CAR-T cell therapy exhibits high specificity, potent cytotoxicity, and long-lasting efficacy and can control tumor progression in patients for whom conventional treatments have failed [3, 4]. Despite these benefits, numerous challenges persist. For example, tumor heterogeneity [5] and antigen escape [6, 7] lead to immune evasion and difficult-to-treat relapses. Moreover, the on-target off-tumor toxicity is a major limitation. Additionally, physical and cytokine barriers can hinder CAR-T infiltration into tumors, while immune-suppressive molecules and cells within the tumor microenvironment (TME) [5] can cause metabolic competition [8], resulting in insufficient energy for T cells and subsequent T cell exhaustion. Indeed, the complex and heterogeneous TME impacts the activation and function of infiltrating effector T cells, compromising their persistence, proliferation, and potency [9]. These limitations result in < 50% of patients with B-cell malignancies that receive CAR-T cell therapy experiencing sustained disease control [10,11,12]. While CAR-T cells exhibit signs of activity in solid tumors, the durability degree remains unknown.
In our review, we found that the crux of the disparity in efficacy between solid tumors and hematologic malignancies may lie in the solid tumor’s intact and functional TME. Hence, a better understanding of the TME’s composition and function can guide the identification of areas of concern, facilitating further research.
TME composition and function
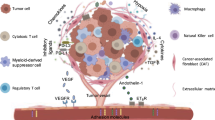
In the process of tumorigenesis and development, various substances, including tumor cells, endothelial cells, cancer-associated fibroblasts (CAFs), immune cells, extracellular matrix, cell-secreted cytokines, chemokines, blood vessels, and lymphatics, form a relatively organized microstructure alongside the tumor. The proliferation and infiltration of tumor cells, recruitment of immune cells, growth of blood vessels and lymphatics, and release of various cytokines and inflammatory factors create an extremely conducive environment for tumor growth known as the TME. The TME resembles a battleground for promoting and suppressing tumor immunity. The process of tumor immunity has been previously reviewed [13], and the main process is summarized in Fig. 1. A review of mantle cell lymphoma (MCL) summarizes the role of the TME in tumor expansion and resistance mechanisms [14]. Further exploration is required to determine whether and how the intensity of anti-tumor immunity can be modulated in “cold tumors” that have already formed an immunosuppressive TME (ITME).
In the early stages of tumor formation, adaptive immune cells migrate to the site of the tumor. However, the incidence of non-virus-related cancers has not significantly increased in HIV patients [15], and the incidence of cancer does not necessarily increase with the decrease of adaptive immune cells in transgenic mouse models [16, 17]. Thus, adaptive immune cells recruited early into the tumor may undergo a “betrayal,” gradually transitioning into an immunosuppressive phenotype and upregulating immunosuppressive genes in the adverse TME influenced by dysregulated cytokines and chemokines [18, 19]. Maintaining the correct anti-tumor phenotype of immune cells and their enrichment in the TME is crucial to overcoming this issue.
Aberrant epigenetic modifications can reshape the TME from an anti-tumor to an immunosuppressive environment [20]. This is also a primary mechanism by which the TME incites defection and influences the behavior of infiltrating cells such as fibroblasts, endothelial cells, lymphocytes, and tumor cells [21]. For CAFs, a process of maturation requires abnormal epigenetic changes, including histone modifications and DNA methylation [22]. The TME can also reshape the composition and function of immune cells by interfering with DNA methylation [23], enriching immunosuppressive cell phenotypes within the tumor [24], and contributing to the scarcity of normal immune cells within the TME [25]. The genetic phenotype of macrophages is the most plastic [26], maintaining an M2 phenotype (alternatively activated macrophages) in offspring after undergoing various epigenetic changes [27]. The TME can control the differentiation of T cells into pre-cancerous (regulatory T cells) or anti-cancer (CD8+ T cells) subtypes by regulating the DNA and RNA methylation of immune cells [28,29,30]. Additionally, the lack of glucose and amino acids, along with increased reactive oxygen species (ROS), can affect the DNA methylation and histone modification of CD8+ T cells within the TME [31].
The tumor microenvironment (TME) resembles a battleground for promoting and suppressing tumor immunity. The process of tumor immunity includes: (1) tumor cell death and release of cell-associated antigens; (2) dendritic cells capturing and presenting antigens via the major histocompatibility complex (MHC) complex after processing; (3) activation of T cells by various cytokines; (4) recruitment of T cells to the tumor site via chemokines such as CX3CL1 (C-X-C motif chemokine), CXCL9, etc.; (5) infiltration of activated T cells into the tumor location; (6) recognition of corresponding tumor cells by activated T cells; (7) killing of tumor cells by T cells through secretion of granular enzymes, perforins, etc. Throughout this process, tumor cells develop various mechanisms to counteract immunity, such as a) reducing expression of highly immunogenic antigens; b) inducing dysfunction of dendritic cells and inhibiting their antigen-presenting ability through secretion of immunosuppressive factors (e.g., (IL)-6, IL-10); c) inhibiting T cell infiltration into the tumor; d) regulating the function of immunosuppressive cells like M2 macrophages and myeloid-derived suppressor cells (MDSCs); e) activating immunosuppressive signaling pathways and enhancing immunosuppressive metabolism, e.g., creating an acidic environment induced by anaerobic glycolysis
Interactions between CAR-T cells and the TME
Although CAR-T therapy holds immense promise, it faces numerous challenges, including tumor heterogeneity [5] and antigen escape [6, 7], leading to immune evasion and difficult-to-treat relapses. Physical and cytokine barriers hinder CAR-T infiltration into tumors to target immune-suppressive molecules and cells within the TME [5]. Moreover, metabolic competition, including oxygen [32], glucose [32, 33], and amino acids [34], results in insufficient energy for T cells, leading to T cell exhaustion. The complex and heterogeneous TME impacts the activation and function of infiltrating effector T cells, compromising their persistence, proliferation, and potency. Therefore, a comprehensive understanding of the interaction between the TME and CAR-T cells is essential. Here, we analyze potential mechanisms from the perspective of the TME.
The delivery of CAR-T cells in solid tumors is impeded by several variables. The TME plays a crucial role in tumor development and possesses the ability to resist internal and external changes, such as developing resistance to treatments [35]. One of the main causes of treatment resistance is the increased interstitial fluid pressure (IFP) and inadequate blood supply to the tumor, which restricts the delivery and distribution of drugs within the tumor mass [36]. Additionally, the ineffectiveness of vascular endothelial cells prevents immune cells from migrating from tumor blood vessels into the tumor tissue [37]. Moreover, the TME is characterized by the presence of a large number of immunosuppressive cells, including tumor-associated macrophages (TAMs) and regulatory T cells (Tregs); the protective niches formed within the TME can shield tumor cells from the effects of therapy [38].
TAMs are the most abundant immune cells infiltrating human tumors, with their accumulation associated with poor prognosis for multiple tumor types. TAMs can maintain cancer progression by secreting growth factors that stimulate tumor cell proliferation, proteolytic enzymes that promote matrix remodeling and metastasis, pro-angiogenic factors that support angiogenesis, and ROS and nitric oxide (NO) that induce genetic instability in tumor cells [39]. TAMs can also cause T cell metabolic starvation by releasing immunosuppressive prostaglandins, interleukin (IL)-10 and transforming growth factor (TGF)-β, and amino acid-depleting enzymes (e.g., arginase 1 or indoleamine 2,3-dioxygenase (IDO)). Additionally, TAMs inhibit T-cell-mediated anti-tumor immunity by expressing immune checkpoint ligands, such as PD-L1 (programmed death-ligand 1), PD-L2, B7-H4, or VISTA (V-domain Ig suppressor of T cell activation). Furthermore, TAMs can promote the recruitment and immunosuppressive activity of Tregs [39] while preventing CD8+ T cells from entering the tumor by creating long-lasting physical interactions with them that block T-cell-mediated anti-tumor immune responses [40].
Indeed, there is clear preclinical evidence that TAMs can mediate resistance to immunotherapy, including adoptive cell transfer therapy. For example, in a mouse model of isogenic melanoma, depletion of TAMs by CSF-1R inhibitors improved the efficacy of adoptive metastatic tumor-specific T cells [40]. The superior antitumor activity of the combination therapy was associated with reduced macrophages within the tumor, promoting the expansion, intratumoral accumulation, and increased function of adoptive metastatic T cells.
MDSCs play a supporting role in tumor progression, and their accumulation is associated with poor clinical outcomes in cancer patients [41]. The defining feature of MDSCs is their powerful ability to suppress immune responses, with T cells being their primary targets. The immunosuppression mechanism induced by MDSCs is the same as that of TAMs, including the production of NO and ROS, elimination of key nutrition factors required for T cell proliferation (e.g., arginine, cysteine, or tryptophan), production of IL-10 and TGF-β, induction of Tregs [42], and impeding a disintegrin and the metalloproteinase-17 (ADAM17) responsible for L-selectin-ectodomain cleavage [43]. MDSCs have also been shown to limit the effects of CAR-T cell therapy. For instance, in a clinical trial of third-generation CD19 CAR-T cell therapy, low levels of MDSCs were associated with responses in patients with lymphoma and leukemia [44]. By using CAR-T cells that target various antigens, MDSCs can inhibit the proliferative activity and cytotoxicity of CAR-T [45].
Tregs are crucial for maintaining immune tolerance to autoantigens but can also inhibit anti-tumor immunity [46]; their presence in tumors is associated with poor prognosis in various cancers. Specifically, Tregs inhibit the cytotoxicity of antigen-specific CD8+ T cells via competitive consumption of IL-2, secretion of immunosuppressive factors (e.g., IL-10 or TGF-β), CTLA-4 mediated inhibition of antigen-presenting cells (APCs), prevention of optimal T cell activation, and lysing effector cells through Granzyme B and/or perforin release [46]. In the TME, Tregs can inhibit the antitumor efficacy of adoptive tumor-targeting effector T cells [47]. Moreover, in a first-in-human study of epidermal growth factor receptor variant III (EGFRvIII)-specific CAR for glioblastoma, analysis of tumor specimens from patients undergoing surgery after treatment showed an influx of immunosuppressive Treg cells, which may have limited the antitumor effects of CAR-T cell therapy [48]. In addition, the importance of this effector in predicting the balance of Tregs in immunotherapy response has been highlighted [49].
The characteristics of CAR-T cell exhaustion closely resemble those of T cell exhaustion, including a continuous decline in the ability to lyse tumor cells, release cytokines, and undergo self-cloning. Specifically, this manifests as upregulated expression of inhibitory receptors (programmed cell death protein 1 (PD1), cytotoxic T-lymphocyte associated protein 4 (CTLA-4)) [50], reduced secretion of cytokines (IL-2, TNF), and diminished reactivity to tumors (epigenetic modifications) [51]. Within the TME, adverse conditions can exacerbate T cell exhaustion. One study found that CAR-T products associated with short reactions were characterized by insufficient proliferative and functional capacity [52]. These characteristics are typical of T cell exhaustion and terminal differentiation. Hence, the effectiveness of CAR-T products may depend on population size and the cell’s ability to proliferate, a trait intrinsically associated with early T-cell differentiation.
CAR-T cell exhaustion is often a continuous process that gradually transforms from precursor exhausted T cells (Tpex) to terminal exhausted T cells (Tex term) (Fig. 2). Similar to other types of Tex cells, Tpex cells are epigenetically locked into an exhausted state [53]. Tex term are derived from precursor depletion T cells [54] and are characterized by high expression of eomesodermin (EOMES), thymocyte selection-associated high mobility group box protein (TOX), CD69, and checkpoint inhibitors (e.g., PD-1, lymphocyte activating gene-3 (LAG-3), or T cell immunoglobulin domain and mucin domain 3 (TIM-3)) and low expression of T-box expressed in T cell (T-bet) [55]. In the progression to advanced dysfunction, traditional effector properties, including the ability to produce IL-2, tumor necrosis factor (TNF), and interferon (IFN)-γ, gradually disappear [56, 57]. The inhibitory receptors expressed by Tex are also known as checkpoint inhibitors (i.e., PD-1, LAG-3, 2B4, and CTLA-4). Tex cells are also characterized by highly active TCR-responsive transcription factors, including TOX, B-cell activated transcription factor (BATF), interferon regulatory factor 4 (IRF4), and nuclear factor of active T cell (NFAT) [58] (Fig. 2). The phenotype and transcription profile of Tex has recently been extended to demonstrate their fundamental differences with ordinary T cells based on different epigenetic landscapes [59].
The role of immunosuppressive cells and cytokine systems, such as the modulation of CD8+ TIL exhaustion-related transcriptional characteristics by Tregs expressing IL-10 and IL-35 in the TME, contribute to T cell exhaustion [60]. Unique, persistent, antigen-specific interactions between TAMs and CD8+ T cells further drive T cell exhaustion, with this response accelerated under hypoxic conditions [61], aligning with the hypoxic environment of the TME.
Hypoxia and acidic pH conditions are hallmarks of the TME, playing crucial roles in tumor development. Acidic pH arises from the anaerobic glycolysis of membrane proteins such as ATPases, leading to the secretion of protons (H+) and lactate. Tumor cells undergo metabolic reprogramming, engaging in anaerobic respiration even in the presence of oxygen [62] and metabolizing glutamine to produce lactate [63]. The acidic environment promotes the expression of vascular endothelial growth factor A (VEGFA) and IL-8, among other angiogenic factors, serving as key players in cancer cell migration and invasion. Additionally, hypoxia is a common feature among many TMEs, where cells located at the center of tumors experience hypoxia due to their distance from existing blood vessels, leading to nutrient deprivation. Consequently, tumor cells upregulate hypoxia-induced angiogenic factors, such as VEGF, to overcome proliferation limitations. However, neoangiogenesis in tumors presents certain deficiencies compared to normal vasculature, with tumor vascular endothelial cells creating gaps that cause vascular leakage and non-laminar flow, resulting in discontinuous perfusion, blood clotting, and localized tissue edema. Despite tumor cells adapting to the hypoxic environment, the antitumor cells within the tumor fail to adapt.
T cell subsets are divided into naive T cells (Tn), effector T cells (Tef), central memory (Tcm), effector memory (Tem), etc. [64]. T cell activation and metabolic reprogramming occur simultaneously. Tn metabolism relies primarily on oxidative phosphorylation (OXPHOS) and fatty acid oxidation (FAO) to provide energy. Upon encountering an antigen, the T cell receptor (TCR) and CD28 synergistically activate the PI3K–AKT–mTOR pathway, effectively activating transcription factors (e.g., HIF-1α, c-MYC) to upregulate the expression of glucose transporter type 1 (GLUT1) and promote glycolysis [65]. However, T cells have different metabolic requirements depending on their function. The metabolism of Tn cells relies predominantly on OXPHOS and FAO for energy. Upon encountering an antigen, Tn cells are activated and differentiate into Tef cells. During this process, glycolysis is enhanced, while mitochondrial metabolism and OXPHOS are weakened. Hence, Tef cells require more glucose and oxygen for survival than Tn. Importantly, within the TME, tumor cells also use glycolysis as the main source of energy, making it difficult for Tef cells to gain an advantage in nutritional competition, which is only further compounded by the hypoxic environment in the TME. Thus, given that CAR-T cells closely resemble Tef cells, the low available energy supply contributes to the unsatisfactory anti-tumor activity of CAR-T cells. Furthermore, the secretion of various hypoxia-inducible factors further drives these cells into a state of incompetence or exhaustion.
The process of T cell recognition and destruction of tumor cells is fraught with obstacles. PD1, a type I transmembrane protein belonging to the immunoglobulin superfamily, is a member of the CD28/CTLA-4 immune checkpoint expansion family. It binds to two ligands, PD-L1 and PD-L2, which downregulate the immune system and promote self-tolerance to prevent autoimmune responses, achieving immune escape through immune downregulation and self-tolerance. Its expression is diverse across subtypes [66]. Cancer cells, including tumor-associated myeloid cells, often overexpress PD-L1, which interacts with the PD1 receptor on adaptive immune cells, inhibiting immune surveillance [67]. Tumor escape mechanisms include downregulation of receptors, low antigen expression, and expression of checkpoint ligands such as PD-L1, collectively reducing infiltration, persistence, and functionality of immune cells within the tumor [68]. Moreover, current studies have confirmed the existence of many immune checkpoints related to T cell exhaustion and impaired cytotoxicity (LAG-3 [69], TIM-3 [70], T-bet, CD47, etc.), many of which have been reviewed elsewhere [71].
In summarizing the mechanisms of interaction between CAR-T cells and the TME during therapy, we recognize a close relationship between CAR-T cell efficacy and the immune microenvironment [72]. To address these immune microenvironmental challenges, we review optimization strategies to enhance CAR-T efficacy, reduce tumor recurrence, and mitigate potential adverse effects during application.
Structural improvements of CAR-T
CAR-T cell efficacy against cancers is determined by the structure of the CAR and its impact on the TME [73, 74]. The CAR construct design, particularly the selection and optimization of intracellular signaling domains (ICDs), is an adjustable element that can improve CAR-T cell persistence and lead to greater on-target cytotoxicity [75]. Calibration of CAR activation can alleviate tumor-induced CAR-T cell exhaustion. Mutations in the two distal CD3ζ ITAMs (immunoreceptor tyrosine activation motif) in CAR-T cells create highly functional T cells with naive/memory gene signatures capable of inducing sustained tumor control [76]. CAR modification has considerable potential, as reflected in their differentiation ability, cytotoxic effects, infiltration ability, persistence, and exhaustion ability. Many non-specific immune cells are involved in this process, as are myriad other components of the TME. The structural enhancement of CAR-T cells based on these parameters can boost their antitumor potential (Fig. 3).
Enhancement strategy for the CAR-T framework. The structure of CAR-T cells is modified by blocking immune checkpoints, targeting immunosuppressive TAMs, reshaping the cytokine system, and regulating gene expression. Blocking the immune checkpoints primarily involves the CD47 and PD1 systems. Expressing scFv (single-chain variable fragments) of PD-1-TREM2 (triggering receptors expressed by myeloid cells) or converting scFv into the VH single domain (heavy chain only) can enhance the ability to block the PD1 immune checkpoint. The CD47 system blocks the CD47-SIRPα (signal regulatory protein α) pathway primarily through SIRPα-Fc expression. Remodeling cytokine systems include the expression of chemokine ligands, receptors, and pro-inflammatory factors producing 4/15ICR (IL-4 and IL-15 inverted cytokine receptor), 4/21ICR (IL-4 and IL-21 inverted cytokine receptor), and TGF-β (transforming growth factor-β) traps to alter the immunosuppressive factors IL-4 and TGF-β. Targeting immune cells is achieved by expressing the scFv of FRβ, TREM2, TR2 (tumor necrosis factor-related apoptosis-induced ligand-receptor 2), CD123, CD24, the Lg (laminin G-like) domain of GAS6 (growth arrest-specific protein 6), and SIRPα-Fc. Gene regulation includes the knockout of DNMT3A (DNA methyltransferase 3α) and overexpression of RUNX-3 (RUNX family transcription factor 3), FOXO1 (forkhead box protein O1), and c-JUN to promote the production of poorly differentiated T cells; overexpression of T-bet (T-box transcription factor) promotes the production of Th1 (T helper) pro-inflammatory cells
Targeting immune checkpoints
Many clinical studies and preclinical models have demonstrated that inhibiting the PD-1 immunosuppressive pathway can improve the function of CAR-expressing genetically modified T cells, which improves tumor eradication [77, 78]. CAR-T cells are engineered to produce antagonistic anti-PD-1 scFv (single-chain variable fragments) that act in a paracrine and autocrine manner. This causes the CAR-T cells and tumor-specific T cells to undergo a reduction in apoptosis. Moreover, given that the secretion of scFv is limited to tumors, toxicity associated with systemic checkpoint inhibition can be limited [79, 80]. The folding of scFv influences the affinity and specificity of CAR toward its target antigen. Single-domain antibody fragments (VHH) can be used as suitable antigen recognition domains in CAR-T cells; alternatively, the anti-PD-L1 single-domain antibodies isolated from the VNAR (semi-synthetic shark antigen receptor variable region) phage library are smaller, easier to express, and more stable [81, 82]. In contrast, TREM2 (triggering receptor expressed by myeloid cell-2) decreases the number of MDSCs and TAMs, effectively enhancing the effect of anti-PD-1 immunotherapy with a prolonged half-life [83, 84]. However, these T cells suffer from reduced survival and cytotoxicity as the dysfunction caused by disrupted PD1 signaling is accompanied by faster maturation and upregulation of the exhaustion marker TIGIT (T-cell immunoreceptor with Ig and ITIM domains) in CAR-T cells, leading to cell death progression and functional failure. Therefore, PD1 downregulation as a method to increase the quality of therapeutic CAR-T cells requires further optimization [85].
Human leukocyte antigen-G (HLA-G)—an immune checkpoint that converts effector cells into Tregs—slows T cell differentiation and proliferation and causes the expansion of MDSCs, which is of great value for tumor immune escape [86]. Even in the face of repeated stimulation, anti-HLA-G CAR-T cells develop into long-term memory effector cells that are lethal against HLA-G (+) tumor cells [87]. However, Altvater et al. showed that HLA-G and HLA-E act primarily on myeloid bystander cells with limited impact on the function of antigen-specific T cells. These results did not support the combination of CAR-T cell therapy with HLA-G or HLA-E checkpoint blockade [88]. Furthermore, the immune checkpoint CD47 blockade enhances the phagocytosis of tumor cells by bone marrow cells, promotes antigen-presenting cell activity, and activates adaptive immune responses [89]. Moreover, CAR-T cells secreting a CD47 blocker SIRPα (signal regulatory protein α)-Fc fusion protein (SIRF–CAR-T) significantly reduced tumor load, significantly prolonged survival, induced central Tcm (memory T cells) in several tumor models with equal immune potential, reduced bone MDSCs, and increased CD11c+ dendritic cells (DCs) and M1 macrophages in tumors [90]. Hence, CD47 blockade synergizes with CAR-T cells to enhance innate and adaptive immune responses. Nevertheless, additional immune CPB (checkpoint blocking) methods continue to be evaluated, including those targeting LAG-3 [69], TIM3 [70], TIGIT [91], and CTLA-4 [92, 93] (Table 1). Given that it addresses two essential elements of a potent immune attack—the existence and efficient durability of immune cells—combination immunotherapy with CAR-T cells and CPB may represent the next major development in immunotherapy [94].
Targeting cytokine systems
Chemokine systems
One of the obstacles for CAR-T cells is insufficient transport to tumor lesions, and the mismatch of chemokines and homologous receptors on tumor and T cells is a cause of insufficient homing. Thus, promoting the binding of suitable chemokines to their receptors, including IL-8 and its receptors (CXCR1, CXCR2, and CX3CR1-CX3CL1), can enhance the transport of T cells within tumors, recruit more immune cells, and trigger the desired anti-tumor response [95–97]. One such response is the increased expression of chemokine receptors. Only memory B cells and specific myeloid cells, notably neutrophils and basophils, reportedly express CXCR2, while human peripheral T cells and tumor-infiltrating T cells in liver cancer do not [98]. Without altering cytotoxicity, CXCR2 expression in CAR-T cells markedly accelerates tumor-specific accumulation and in vivo transport [99, 100]. For example, combining IL-2 and GM-CSF (granulocyte-macrophage colony-stimulating factor) promotes the expression of CXCR3 (C-X-C motif chemokine receptor), which is a biomarker sensitive to PD-1 blocking [101]. IL-2 induces CXCR3 expression in CAR-T cells by activating the PI3K–AKT (phosphatidylinositol 3-kinase/protein kinase B) pathway [102]. Furthermore, GM-CSF acts as an immunomodulator of APCs and induces CXCR3 expression in CAR-T cells by activating ERK1/2 (extracellular regulated protein kinases) rather than p38–MAPK (mitogen-activated protein kinase) signaling [102, 103]. Hence, the high degree of similarity in terms of IL-2 and GM-CSF activation simplifies their combination by avoiding additional toxicity.
Many CAFs can secrete stromal cell-derived factor 1α (SDF-1α), a CXCR4 ligand. Mechanistically, impaired activation of STAT3 (signal transduction and transcriptional activator 3) signaling in CXCR4–CAR-T cells inhibits the secretion of SDF-1α by CAFs via the NF-κB (nuclear factor κB) pathway. Ultimately, inhibiting MDSC migration to the tumor through the STAT3–NF-κB–SDF-1α axis results in greater CXCR4–CAR-T cell infiltration [104].
CXCL9 is a major ligand of CXCR3 that controls T-cell migration and prevents tumor angiogenesis [105, 106]. CAR-T cells secreting human IL-7 and CCL19 (7 × 19) exhibit enhanced expansion and migration in vitro and significantly reverse the TME by reducing the infiltration of polymorphonuclear MDSCs and Tregs and recruiting more DCs. The recruited DCs interact with CD4+ T cells, promoting their differentiation into memory subgroup-like T cells and downregulating the expression of depletion markers (including PD1 and TIGIT) by T cells [107, 108]. Additionally, Feng et al. reported that this type of CAR-T cell provides a strategy for overcoming the low survival rate, poor durability, and TME inhibition [109]. In fact, a patient diagnosed with advanced liver cancer achieved total eradication of the tumor following intratumoral injection of 7 × 19 CAR-T cells for 30 days [110]. Collectively, these findings support the practicality and therapeutic application of chemokine ligands and their receptor-modified CARs.
Interleukin systems
Armored CAR-T cells can genetically modify CAR constructs using various inflammatory cytokines or ligands to promote the engagement of host innate and adaptive immune cells and improve the ITME [111, 112]. IL-12 can induce the release of IFN-γ from natural killer (NK) cells and CD4+ and CD8+ T cells and promote a decrease in Treg abundance and activation of the bone marrow chamber, ultimately remodeling the TME and achieving a lasting anti-tumor response [113,114,115]. Armored CAR-T cells capable of constitutively secreting IL-12 show the consumption of TAM and resist endogenous PD-L1-induced inhibition, improving survival [116]. Additionally, for CAR-T cells to produce IFN-γ, which is required to destroy tumor cells, NF-κB is activated by IL-12 signal transduction [117, 118]. The autocrine effect of reversible nanoscale IL-12 limits the risk of tumor exudation and systemic toxicity, such as treatment-related death and unstable blood flow [117, 119]. It also significantly promotes the secretion of CCL2 (C-C motif chemokine receptor), CCL5, and CXCL10 to resist the inhibition of tumor blood vessels and recruit more CD8+ CAR-T cells [120, 121]. In summary, IL-12 and CAR-T cell therapies have value in enhancing anti-tumor ability (Fig. 4).
IL-15 encourages the immunometabolic adaptation of CAR-T cells by inducing the proliferation of CD8+ T, NK, and B cells in tumors and decreasing immunosuppressive factors, including IL-10, arginase-1, TGF-β, and MDSCs [122, 123]. Brog et al. reported that activation of endogenous anti-tumor immunity is the key mechanism affecting the efficacy of engineered super2 (IL-2 superkine) + IL-33 CAR-T cells in various solid tumor models. This can prevent antigen loss, change the leukocyte ratio in the TME, and activate a wide range of endogenous innate and acquired immune cells through the joint action of Super2 and IL-33. Thus, this cell-induced response does not rely on a single type of immune cell but compensates for the loss of individual cell subsets through widespread immunological activation [124].
Effect of IL-12 on the TME. IL-12 reduces the number of Tregs and TAMs in the TME, while the increase of chemokine ligands can resist the inhibition of tumor blood vessels and promote T-cell infiltration. Simultaneously, the heightened release of IFN-γ, facilitated by IL-12 through the stimulation of NF-κB, is vital in eliminating cancerous cells
Immunosuppressive cytokine treatment plays an important role. IL-4 is released by tumor cells in the TME and can induce antigen-dependent proliferation of CAR-T cells, the key regulator of T cell depletion [125]. 4/21ICR (inverted cytokine receptor) is the reverse cytokine receptor of IL-4 and IL-21 and binds the extracellular domain of the IL-4 receptor to the intracellular region of the IL-21 receptor. Under the stimulation of IL-4, 4/21ICR activates the signal transducer and activator of the transcription (STAT)3 pathway, promotes Th17-like polarization and tumor-targeted killing, and enhances this effect in the IL-4(+) TME [126]. A technique was developed to transform IL-4R (receptor) inhibition signals into IL-15R activation signals using IL-15R as a transmembrane and intracellular domain. This resulted in stronger activation, degranulation, cytokine release, and cytotoxic cells in IL-4-rich tumors, and optimized expansion ability and persistence, while the number of poorly differentiated T cells increased [127]. ICRs can improve the efficiency of T cells in the ITME by protecting CAR-T cells from the immunosuppressive effects of IL-4 and enhancing the function of CAR-T cells in the presence of IL-4. Therefore, 4/21ICR and 4/15ICR CAR T-cell therapies effectively treat IL-4-rich solid tumors. In addition, TGF-β is a well-known immunosuppressive cytokine in the TME. The extracellular domain of TGFRII acts as a TGF-β trap, causing microglia to differentiate into pro-inflammatory and anti-tumor phenotypes, weakening the immunosuppressive effect of TGF-β [128]. Furthermore, blocking the TGF-β signal transduction pathway can decrease PD-1 expression, lessen acquired immune cell depletion, and induce long-term activation of these cells [129,130,131,132]. In summary, cytokines are used to activate immunity or convert immunosuppressive signals into activation signals to improve the ITME.
The transformation of multiple cytokines may achieve better results than modified therapy alone. For instance, a combination of multiple factors has successfully promoted the expansion of CD4+ memory T cells [133]. In the fourth generation of Nectin4 (nectin cell adhesion molecule-4)-7 × 19 CAR-T cells, the proportion of Tscm (stem cell-like memory T cell) subsets in CD8+ T cells increased significantly, possibly due to the difference in armored cytokines [134, 135]. In addition, the early use of receptor engineering to transmit activation, co-stimulation, and cytokine support signals can induce T cells to respond to unique expression patterns in tumors. For example, the receptors of TGF-β and IL-4 were modified to make cells resistant to TGF-β and IL-4, achieving an optimal balance between CD4+ and CD8+ cells with good persistence [136]. Recently, the transient and high transfection rates of RNA modification were shown to produce safer and more effective CAR-T cells [137, 138]. Hence, combining RNA and receptor engineering may produce unique results. RNA engineering has been used to knock down the expression of immunosuppressive molecular receptors and immune checkpoints, enhancing the homing ability and endurance of CAR-T cells [139]. In summary, cytotoxicity can be enhanced, and the TME can be improved through combined receptor and mRNA engineering. The mechanisms by which distinct cytokine combinations affect T cell phenotype expression vary, as do the manifestations of distinct T cell phenotypes [140, 141]. Therefore, further research is required to develop a strategy that permits CAR-T cells to effectively and durably suppress tumors.
Targeting immunosuppressive cells
Various immune cells play significant roles in CAR-T cell therapy (Fig. 5). In particular, targeting TAMs, MDSCs, and Tregs may yield good benefits [142,143,144]. The FRβ, a folate receptor, can target TAMs and MDSCs. Thus, restoring the TME involves FRβ-CAR-T cells specifically depleting immunosuppressive TAM subsets [145]. Furthermore, GAS6 (growth arrest-specific protein 6)-CAR-T cells can eliminate AXL-positive tumor-associated macrophages due to the expression of receptor tyrosine kinases by TAMs [146, 147]. Moreover, blocking CD47/SIRPα and CD24 SIGLEC-10 (sialic acid binding Ig like lectin 10) signal transduction can effectively promote tumor cell phagocytosis by macrophages, suggesting that dual blockade could facilitate the transition of macrophages from M2 to M1 (classically activated macrophages) and regulate macrophage-related immune surveillance [148]. However, exclusively blocking macrophages is also an effective strategy for regulating the TME. Indeed, CD24–CAR-T cells polarize macrophages to an M1-like phenotype, reversing the immunosuppression caused by abundant M2-like macrophages in the TME. Additionally, released IFN-γ and TNFα can further enhance this polarization [149, 150]. Furthermore, SIRF–CAR-T cells significantly improve the transformation to an M1-like phenotype by inhibiting CD47/SIRPα signaling [90].
Measures to reshape the immunosuppressive cell composition in the tumor microenvironment (TME). (A) The CD47–SIRPα and CD24–SIGLEC pathways can be blocked by CAR-T cells, polarizing macrophages toward the M1 phenotype. TNF-α and IFN-γ secretion can be stimulated by blocking the CD24– SIGLEC-10 pathway, facilitating the differentiation of M1-like cells. (B) Binding targets on tumor-associated macrophages (TAMs) to single-chain Fv fragments (scFv) expressing agonistic TR2 (tumor necrosis factor-related apoptosis-induced ligand-receptor 2) antibody, FRβ, TREM2, CD123, or the Lg (laminin G-like) domain of GAS-6 can facilitate TAM elimination. (C) MDSCs can be depleted by scFv-expressing TREM2 and TR2 antibodies
TREM2+ myeloid cells exhibit a potent inhibitory effect on T cell proliferation in vitro [84], while TREM2+ TAMs display M2-like immunosuppressive behavior [151]. Hence, the specific targeting of TREM2 reduces the proportion of TAMs and MDSCs [83]. Similarly, TR2 (tumor necrosis factor-related apoptosis-induced ligand-receptor 2) is a receptor expressed on MDSCs that activates apoptosis when bound to its soluble ligand TRAIL (tumor necrosis factor-related apoptosis-induced ligand). Thus, an scFv encoding an agonistic TR2 antibody can induce apoptosis not only in MDSCs but also in TAMs sensitive to TR2 [152, 153]. Moreover, anti-CD123 CAR-T cells detect and eliminate TAMs, overriding immunosuppression, even though M-MDSCs and M2 cells express CD123 [154, 155]. Although NKG2DLs (natural killer group 2 member D ligands) are expressed at low levels in M-MDSCs and tumor cells, not M2 cells, CAR-T cells that dual-target CD123 and NKG2DLs selectively target immunosuppressive cells (M-MDSCs and M2 cells) while exerting anti-tumor effects [155].
CD74 knockout may help boost T cell infiltration and accumulation in tumors by specifically decreasing Treg infiltration in the TME, not normal tissues [156]. However, tyrosine-protein kinase LCK (lymphocyte-specific tyrosine kinase)-deficient CARs might be a more effective treatment strategy for solid tumors resistant to Treg infiltration [157]. Certain interleukins are also essential for altering the immune cell milieu, including the activation of additional DCs, B cells, NK cells, and other immune cells that support anti-tumor activities, while supporting the elimination of immunosuppressive cells [118, 124, 158].
Additionally, pretreatment of the TME with FRβ-specific CAR-T cells can enhance the effectiveness of other types of CAR-T cells [145]. This requires an intimate understanding of optimal timing for sequential therapy and the time required to induce anti-tumor immune response in the TME. Liu et al. combined two types of CAR-T cells, with a sequential interval between the two effects of 7 days; the outcome proved effective [159]. Therefore, we emphasize that in the mixing of two different CAR-T cells, the dose combination and interval time of the two consecutive medications should be explored to achieve maximum benefit.
Gene expression regulation
The regulation of gene expression greatly affects immune cell function. Depletion of CAR-T cells can occur through gene regulation of the T cell’s multifunctional developmental potential [160,161,162]. In a preclinical model, DNMT3A (DNA methyltransferase 3α) knockout prevented the methylation of several transcriptional regulators that maintain human T-cell stem cell-like differentiation [163]. In addition, c-Jun (the activator protein-1 complex) and FOXO1(Forkhead box protein O1) transcription factors can be upregulated to reduce T-cell depletion [164,165,166]. Furthermore, RUNX3 (RUNX family transcription factor 3) regulates T-cell immunity by retaining CAR-T cells’ low differentiation and boosting their in vivo persistence. Zhu et al. designed RUNX3-overexpressing (OE) CAR T cells to improve their phenotype and reduce the release of cytokines while maintaining function [167, 168]. In phase I clinical investigations, RUNX3 was overexpressed in CAR-T cells, leading to the invasion of CD8 + T cells into the cancer microenvironment with good anti-tumor efficacy and controlled safety. In this trial, the objective response rate (ORR) was 16.7%, and the disease control rate (DCR) was 50% [169]. Indeed, enhanced anti-tumor activity may play a key role in memory CD8+ T cells, such as Trm (tissue-resident memory T cells) subsets, and upregulate CCL3 and CCL20 to promote CD8+ T cell recruitment [170, 171]. In contrast to RUNX3, the primary regulatory factor, T-bet, decreases Trm abundance and increases effector cells [172, 173]. Specifically, T-bet induces Th1 phenotypes in CD4+ T cells, creating a potent pro-inflammatory response that can increase CAR expression [174]. Thus, by activating the Th1 phenotype of CD4+ T cells, gene modulation can boost cytotoxicity and enhance the production of memory-like T cells.
Combination therapy
The limitations of single CAR-T cell therapy can be overcome via combination with other anti-tumor therapies, including chemotherapy, radiotherapy, and targeted therapy. These combined therapies can improve the CAR-T cell therapeutic environment and promote the infiltration of CAR-T cells into tumor tissues [94, 175].
Combination with targeted therapy
It can reverse the immunosuppression of bone marrow monocytes in the TME and aid in limiting tumor recurrence when taken in concert with specific monoclonal antibodies [176] (Table 2). When CAR-T therapy and PD1 inhibitors are used together, the upregulation of PD-L1 expression induced by TME pressure can be addressed, improving CAR-T cell-mediated cytotoxicity [177]. One such example is nivolumab, which can prolong CAR-T cytotoxicity, notably extending survival by up to 60 days in vitro [178]. Similarly, after CAR-T cell therapy, the administration of pembrolizumab is well-tolerated in patients with recurrent or refractory B-cell lymphoma, with the best ORR reaching 33%. Within these respondents, T-cell depletion decreased while CAR-T cell activation and proliferation increased [179]. Furthermore, given that high S1PR3 expression positively correlates with resistance to PD-1-based immunotherapy [180], Gao et al. sought to improve anti-PD-1 therapy effects by pharmacologically inhibiting S1PR3 (sphingosine 1-phosphate).
Different target antigens on CAR-T cells combined with PD1 inhibitors can boost the anti-tumor effect. Hence, they can be employed in broad therapy as they demonstrate neither selectivity for the target antigen nor specificity for distinct malignancies. Higher levels of PD1 expression are associated with the CAR-T cells’ increased ability to eliminate malignant cells [181]. Tumor development can be markedly slowed by a combined therapy that prescreens high-expressing PD1 CAR-T cells (Tcm subsets) and suppresses PD1 [181, 182]. Additionally, it can help determine when to add checkpoint inhibitors. Moreover, CAR-T cell therapy with PD-L1 (rather than PD1) inhibitors, such as atezolizumab and avelumab, can increase M1-like subset production and decrease M2-like subsets through IFN-γ signal transduction [183].
Several other targeted medications can increase CAR-T cell effectiveness through different mechanisms [184, 185] (Table 2). Olaparib, a poly (ADP-ribose) polymerase inhibitor (PARPi), can diminish the expression of SDF1α generated by CAF to prevent angiogenesis and impede MDSC migration [186,187,188]. Furthermore, by activating the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway, Olaparib can promote the penetration of CD8+ CAR-T cells in the TME [189]. Additionally, LEN (lenalidomide-bind to the protein cereblon) polarizes CAR-T cells toward Th1 and CD8 early differentiation types, such as Tn (naive T cell) or Tcm, markedly decreasing the tumor load and extending survival time [190, 191]. It also reduces PD1 and TIM3 expression to minimize CAR-T cell exhaustion and downregulates VEGFC (vascular endothelial growth factor) produced by TAMs to promote CAR-T cell penetration [190, 192, 193]. Tcm differentiation can be also encouraged by TOPK (T-LAK cell-originated protein kinase) and AKT inhibitors [194,195,196]. Among these, AKT inhibitors rely on FOXO1 expression to be independent of the mTOR/S6 (mammalian target of rapamycin) pathway, whereas TOPK inhibitors depend on the inhibition of mTOR signaling [196, 197]. Considering these diverse mechanisms, combining targeted therapy exhibits a discernible impact on augmenting T cells, representing a promising avenue for enhancing CAR-T cell immunotherapy (Fig. 6).
Impact of CAR-T combination treatment on the tumor microenvironment (TME) and CAR-T cells. (a–d) Effects of the combined treatment of targeted drugs and CAR-T on the TME and CAR-T cells. (e) Chemotherapy or radiotherapy coupled with CAR-T enhances CAR-T cell penetration into tumors and reshapes the ITME into an immune-activated TME.
Combination with chemotherapy and radiotherapy
A large amount of data shows that lymphocyte depletion can induce cytokine regulation and promote tumor-specific T-cell responses, demonstrating a potential for improving adoptive metastatic cell therapy [198]. Murad et al. recently discovered that pretreating CAR-T cells with cyclophosphamide (Cy) markedly alters the TME, creates pro-inflammatory myeloid and T cell features in tumors, and increases the recruitment of APCs and endogenous and adoptive metastatic T cells [199]. Low-dose carboplatin can encourage the early and persistent invasion of CAR-T cells while also lessening the tumor burden. TME modifications might be crucial for both tumor response and CAR-T cell invasion [200]. Immunogenic cell death induced by Ox (oxaliplatin) and Cy can activate TAMs to express T cell-recruiting chemokines. CXCR3, CXCR6, and CCR5 (C-C motif chemokine receptors) are at least partially responsible for early infiltration, whereas CXCR3 is responsible for the height of peak infiltration. Furthermore, Ox/Cy can increase tumor susceptibility to PD-L1 and enhance CAR-T cell-mediated tumor suppression and longevity [201]. As Ox and Cy are not often used together, various delivery schedules must be examined to achieve sufficient lymphocyte infiltration, facilitating TME modification and enhancing CAR-T cell responsiveness. In conclusion, by expressing the chemokine system, chemotherapy can assist T-cell penetration into tumors and enhance the ITME (Fig. 6).
Several studies have confirmed the need for RT (radiotherapy) before immunotherapy. RT induces immune cell infiltration and remodeling of the TME, and pretreatment improves T cell transport to tumor sites by enhancing immunogenicity [202]. A more potent and long-lasting immune response and a comprehensive anti-tumor response can be achieved by intravenously injecting CAR-T cells following local RT as they can rapidly multiply and extravasate across the entire tumor matrix, whereas a longer-lasting immune response may be related to improved immune escape [203, 204]. Tumor-related infiltrating molecules, including ICAM-1 (intracellular adhesion molecule-1), may be upregulated in response to increased infiltration [205]. Significantly, in the examination of a double-tumor mouse model, not only were the tumor cells on the exposed side eliminated, but the killing capability of CAR-T on the non-irradiated side of the tumor was boosted, confirming the distant effect of CAR-T therapy [205]. However, based on significant preclinical and clinical data, RT might cause tumor-infiltrating MDSCs to become activated and proliferate while decreasing T-cell infiltration [206, 207]. Subsequent research demonstrated that in conjunction with CXCR2 blockage, it could successfully boost the infiltration of CAR-T cells and greatly inhibit MDSC trafficking to the tumor [208]. In addition to improving blood circulation and the therapeutic index of solid tumors, mild hyperthermia can decrease the dense tumor structure and IFP while attracting CAR-T cells along with endogenous immune cells [209]. Physical factor therapy, such as photothermal therapy or radiotherapy, may enhance the intratumor invasive capacity of CAR-T cells through activation independently of the chemokine system.
We observed the production of reactive bone marrow cells during chemotherapy and RT, causing an anti-immune response [206, 210]. Furthermore, reactive bone marrow cells facilitate metastasis [211]. This emphasizes the importance of suppressing these bone marrow cells and reducing the risk of adverse effects. Controlling the dosage of RT and chemotherapy to reduce the toxicity of adoptive cell transfer treatment and the build-up of myelosuppressive cells is critical [200, 208, 212].
Blood components for leukapheresis
In addition to the TME, recent findings indicate that favorable CAR-T expansion is associated with naive T cells, such as CXCR3+ monocytes discovered during apheresis before therapy. A longitudinal study of post-treatment samples revealed that the CXCR3+ monocyte population was decreased, with a loss of CAR-T persistence and reduced abundance, while the number of CXCR3– classical monocytes increased [153]. Additionally, as indicators of poor CAR-T therapy outcomes, higher levels of circulating Tregs and MDSCs frequently result in non-response [154, 155]. Indeed, the myeloid environment prior to CAR-T delivery can effectively predict and regulate the patient’s CAR-T cell proliferation and response. A favorable immune state with high IL-12 and TRAIL before infusion is conducive to CAR-T responses [155]. Moreover, CAR-T gene expression profiles indicate that genes involved in cell activation, signal transduction, inflammation, and cytokine/chemokine release are downregulated at this time [121]. Systemic immune dysregulation negatively impacts CAR-T cells [156, 157]. Patients with high tumor burden have higher levels of immune dysregulation, with increased serum inflammatory markers and tumor IFN signaling [158]. Expression of IFN signaling is associated with a lack of durable responses, which may be due to insufficient expansion of CAR-T cells [158]. Immune dysregulation is also critical to relapse. During relapse following CAR-T treatment, the ratio of monocytes/macrophages increases considerably, whereas the ratio of T cells decreases [157]. Macrophages can also produce high levels of IL-6, further exacerbating immune disorders and even causing cytokine release syndrome (CRS) [159]. Thus, patients with tumors have a poor response to CAR-T cells due to their systemic immune dysregulation. CAR-T efficacy is particularly affected by a patient’s blood composition pre- and post-CAR-T infusion. Hence, efforts must be made to improve these variables to improve CAR-T cell therapy outcomes.
Conclusions and outlooks
Prediction and monitoring
The functionality of T-cells and the immune status can be assessed through the visualization of CAR-T cells, therapeutic monitoring, and endogenous cell imaging. These methods help determine whether CAR-T cells penetrate the tumor site and provide insights into their limited survival, proliferation, and duration within the TME.
First, assessing the cytotoxicity of targeted CAR-T cells in normal tissues could be helpful based on the expression of adenovirus target antigens in normal mouse tissues, particularly in different CAR-T cell designs with TME regulation [213]. Recently, Harari-Steinfeld et al. studied a new type of artificial acellular target particles (aaTPs) based on biomaterials. The simple, cheap, modular, and precisely controllable synthesis of aaTPs makes it possible to develop standardized CAR-T cell detection in vitro, offering key advantages over traditional detection methods using target cell lines [214]. High-throughput screening systems and three-dimensional suspension plates can screen in vitro tumor models effectively killed by specific CAR from the reconstructed TME and analyze the cytotoxic effect of CAR-T on solid tumors combined with high-throughput image cytometry [215,216,217]. In addition to producing dynamic results that vary with time and dose, this study will aid the progression of an in vitro model for CAR-T therapy. However, the diverse secretion spectrum of different cytokines during CAR-T therapy can be shown using a digital nano-plasma microarray immunosensor and can be used to analyze the CRS during this procedure [218] to offer guidelines for the potential clinical development of safer, more efficient immunotherapy, as well as to design a range of CAR architectures. Given that inhibitory immune checkpoint expression does not always correspond to a weakened anti-tumor immune response, single-cell sequencing (SCS) is an invaluable tool to discover novel inhibitory immune checkpoints and determine the timing of CPB application for individual patients, which can support individualized treatment [219,220,221].
Second, the SCS is critical in the surveillance of CAR-T cells. Dynamic identification of cellular states in vivo is reportedly enhanced by single-cell RNA sequencing (scRNA-seq) for repeated longitudinal analyses of cells [133]. Additionally, the CAR-T cell homing function can be easily checked as fluorescent or radioactive labeling of CAR-T cells can immediately identify the T cell location when cytotoxicity occurs [222]. Similar to its functional value, intravital microscopy imaging has demonstrated CAR-T cell homing along with T cell-mediated death in real-time within the tumor matrix [203]. The accurate homing capacity of CAR-T cells can optimize their therapeutic effects, providing a wide range of opportunities for precise navigation and cancer immunotherapy [223,224,225]. With these newly developed monitoring techniques, it is no longer insurmountable to grasp the changes in CAR-T cells and the TME in a timely and accurate manner during the CAR-T treatment of tumors.
Conclusion and perspectives
The TME is considered the key factor affecting the efficacy of CAR-T cells; therefore, reshaping the ITME into a widely immune-activated microenvironment is of great significance for its subsequent development. Enhancing the structure of CAR-T or combining it with other immunotherapies fosters the interaction with the TME to improve the anti-tumor effect. On the one hand, it can stimulate host immune cells and trigger the immune response of non-T cell bystanders; on the other hand, it can more precisely infiltrate tumors. Nonetheless, dynamic monitoring of the TME and CAR-T cell state can help evaluate the safety and effectiveness of armed CAR-T cells and identify the ideal moment to combine them with other immunotherapies. Multi-antigen targeting can reduce recurrence caused by antigen loss, whereas a single target with high expression in tumor tissue or low expression in healthy cells can increase the specificity of CAR-T cells. Since targeting numerous antigens typically results in reduced specificity, there may be contradictions between the two schemes. As a result, more cancer patients will benefit from accurate medical treatment if the choice of the target antigen is balanced according to each patient’s unique circumstances.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- 4/15ICR:
-
IL-4, IL-15 inverted cytokine receptor
- 4/21ICR:
-
IL-4, IL-21 inverted cytokine receptor
- 7 × 19:
-
IL-7 and CCL19
- aaTPs:
-
Acellular artificial target particles
- ACT:
-
Adoptive Cell Transfer Therapy
- AKT:
-
Protein Kinase B
- APC:
-
Antigen-presenting cells
- BATF:
-
B-cell activated transcription factor
- CAFs:
-
Cancer-associated fibroblasts
- CAR:
-
Chimeric antigen receptor
- CCL:
-
C-C motif chemokine
- CCR:
-
C-C motif chemokine receptor
- cGAS-STING:
-
Cyclic GMP–AMP synthase-stimulator of interferon genes
- c-Jun:
-
The activator protein-1 complex
- CSF-1R:
-
Colony stimulating factor 1 receptor
- CXCR:
-
C-X-C motif chemokine receptor
- CXCL:
-
C-X-C motif chemokine
- CPB:
-
Checkpoint blocking
- CR:
-
Control rate
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen 4
- Cy:
-
Cyclophosphamide
- DC:
-
Dendritic cells
- DNMT3A:
-
DNA methyltransferase 3α
- EGFRvIII:
-
Epidermal growth factor receptor variant III
- EOMES:
-
Eomesodermin
- ERK:
-
Extracellular regulated protein kinases
- FAO:
-
Fatty acid oxidation
- FOXO1:
-
Forkhead box protein O1
- FRβ:
-
Folate receptor β
- Gas6:
-
Growth arrest-specific protein 6
- GLUT1:
-
Glucose transporter type 1
- GM-CSF:
-
Granulocyte/macrophage colony-stimulating factor
- HLA-G/E:
-
Human leucocyte antigen-G/E
- HTS:
-
High-throughput screening
- ICAM-1:
-
Intracellular adhesion molecule-1
- ICDs:
-
Intracellular signaling domains
- IDO:
-
Indoleamine 2,3-dioxygenase
- IFN:
-
Interferon
- IFP:
-
interstitial fluid pressure
- IL:
-
Interleukin
- ITAMs:
-
Immunoreceptor tyrosine activation motif
- ITME:
-
Immune-suppressive tumor microenvironments
- LAG-3:
-
Lymphocyte activating gene-3
- Lck:
-
Lymphocyte-specific tyrosine kinase
- Lg:
-
Laminin G-like
- LEN:
-
Lenalidomide
- M1-like:
-
Classical activated macrophages
- M2-like:
-
Alternatively activated macrophages
- MAPK:
-
Mitogen-activated protein kinase
- MDSCs:
-
Myeloid-derived suppressor cells
- MHC:
-
Major histocompatibility complex
- mTOR:
-
Mammalian target of rapamycin
- Nectin4:
-
Nectin cell adhesion molecule-4
- NFAT:
-
Nuclear factor of active T cell
- NF-κB:
-
Nuclear factor ΚB
- NKG2DLs:
-
Natural killer group 2 member D ligands
- ORR:
-
Objective response rate
- Ox:
-
Oxaliplatin
- OXPHOS:
-
Oxidative phosphorylation
- PARPi:
-
Poly (ADP- ribose) polymerase inhibitor
- PD1:
-
Programmed cell death protein-1
- PD-L1:
-
Programmed cell death ligand 1
- PKM2:
-
Pyruvate kinase isozyme typeM2
- PI3K:
-
Phosphatidylinositol 3-kinase
- RR:
-
Response rate
- RT:
-
Radiotherapy
- RUNX3:
-
RUNX Family Transcription Factor 3
- ROS:
-
Reactive oxygen species
- S1PR3:
-
Sphingosine 1-phosphate receptor 3
- scFv:
-
Single-chain variable fragments
- SDF-1α:
-
Stromal cell-derived factor 1α
- Siglec-10:
-
Sialic Acid Binding Ig Like Lectin 10
- SIRPα:
-
Signal regulatory protein α
- STAT3:
-
Signal transduction and transcriptional activator 3
- Super2:
-
IL-2 superkine
- TAMs:
-
Tumor-associated macrophages
- T-bet:
-
T-box transcription factor
- Tcm:
-
Central memory T cells
- Tef:
-
Effector T cells
- Tem:
-
Effector memory
- Texterm :
-
Terminal exhausted T cells
- TGF-β:
-
Transforming growth factor-β
- Th:
-
T helper cells
- TIGIT:
-
T-cell immunoreceptor with Ig and ITIM domains
- Tim3:
-
T cell immunoglobulin domain and mucin domain-3
- TLR-7a:
-
Toll-like receptor 7 agonists
- TME:
-
Tumor microenvironment
- Tn:
-
Naive T cell
- TOPK:
-
T-LAK cell-originated protein kinase
- TOX:
-
Thymocyte selection-associated high mobility cell box
- Tpex:
-
Precursor exhausted T cells
- TR2:
-
Tumor necrosis factor-related apoptosis-induced ligand-receptor 2
- TRAIL:
-
Tumor necrosis factor-related apoptosis-induced ligand
- Tregs:
-
Regulatory T cells
- TREM2:
-
Triggering receptors expressed by myeloid cells 2
- Trm:
-
Tissue-resident memory T cells
- Tscm:
-
Stem cell-like memory T cells
- VEGF:
-
Vascular endothelial growth factor
- VH :
-
Heavy chain
- VISTA:
-
V-domain immunoglobulin suppressor of T cell activation
- VNAR :
-
Semi-synthetic shark antigen receptor variable region
References
Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nature reviews. Clin Oncol. 2016;13(1):25–40.
Montironi C, Muñoz-Pinedo C, Eldering E. Hematopoietic versus solid cancers and T cell dysfunction: looking for similarities and distinctions. Cancers (Basel). 2021;13(2):284.
Frey NV, Porter DL. CAR T-cells merge into the fast lane of cancer care. Am J Hematol. 2016;91(1):146–50.
Jena B, Moyes JS, Huls H, Cooper LJN. Driving CAR-Based T-Cell therapy to Success. Curr Hematol Malig Rep. 2014;9(1):50–6.
Zhang Z, Wang T, Wang X, Zhang Y, Song S, Ma C. Improving the ability of CAR-T cells to hit solid tumors: challenges and strategies. Pharmacol Res. 2022;175:106036.
Majzner RG, Heitzeneder S, Mackall CL. Harnessing the Immunotherapy Revolution for the treatment of Childhood Cancers. Cancer Cell. 2017;31(4):476–85.
Majzner RG, Mackall CL. Tumor Antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8(10):1219–26.
Mangal JL, Handlos JL, Esrafili A, Inamdar S, Mcmillian S, Wankhede M, Gottardi R, Acharya AP. Engineering Metabolism of Chimeric Antigen Receptor (CAR) cells for developing efficient immunotherapies. Cancers (Basel). 2021;13(5):1123.
Frigault MJ, Lee J, Basil MC, Carpenito C, Motohashi S, Scholler J, Kawalekar OU, Guedan S, Mcgettigan SE, Posey AJ, et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res. 2015;3(4):356–67.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52.
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, Dahiya S, Lunning M, Lekakis L, Reagan P, et al. Standard-of-care Axicabtagene Ciloleucel for relapsed or refractory large B-Cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119–28.
Schultz LM, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, Stefanski HE, Talano JA, Moskop A, Margossian SP, et al. Disease Burden affects outcomes in Pediatric and Young Adult B-Cell Lymphoblastic Leukemia after Commercial Tisagenlecleucel: a Pediatric Real-World chimeric Antigen receptor Consortium Report. J Clin Oncol. 2022;40(9):945–55.
Fu T, Dai LJ, Wu SY, Xiao Y, Ma D, Jiang YZ, Shao ZM. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. 2021;14(1):98.
Saleh K, Cheminant M, Chiron D, Burroni B, Ribrag V, Sarkozy C. Tumor Microenvironment and Immunotherapy-based approaches in Mantle Cell Lymphoma. Cancers (Basel). 2022;14(13):3229.
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet (London England). 2007;370:59–67.
Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–6.
Ciampricotti M, Vrijland K, Hau C, Pemovska T, Doornebal CW, Speksnijder EN, Wartha K, Jonkers J, de Visser KE. Development of metastatic HER2 + breast cancer is independent of the adaptive immune system. J Pathol. 2011;224(1):56–66.
Mascaux C, Angelova M, Vasaturo A, Beane J, Hijazi K, Anthoine G, Buttard B, Rothe F, Willard-Gallo K, Haller A, et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature. 2019;571(7766):570–5.
Pylaeva E, Korschunow G, Spyra I, Bordbari S, Siakaeva E, Ozel I, Domnich M, Squire A, Hasenberg A, Thangavelu K, et al. During early stages of cancer, neutrophils initiate anti-tumor immune responses in tumor-draining lymph nodes. Cell Rep. 2022;40(7):111171.
Pan X, Zheng L. Epigenetics in modulating immune functions of stromal and immune cells in the tumor microenvironment. Cell Mol Immunol. 2020;17(9):940–53.
Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357(6348).
Kehrberg RJ, Bhyravbhatla N, Batra SK, Kumar S. Epigenetic regulation of cancer-associated fibroblast heterogeneity. Biochim Biophys Acta Rev Cancer. 2023;1878(3):188901.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50.
Davidov V, Jensen G, Mai S, Chen S, Pan P. Analyzing one cell at a TIME: analysis of myeloid cell contributions in the Tumor Immune Microenvironment. Front Immunol. 2020;11:1842.
Zhong F, Lin Y, Zhao L, Yang C, Ye Y, Shen Z. Reshaping the tumour immune microenvironment in solid tumours via tumour cell and immune cell DNA methylation: from mechanisms to therapeutics. Br J Cancer. 2023;129(1):24–37.
Zhang Y, He M, Wang Y, Liao A. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. 2017;8:120.
Tymoszuk P, Evens H, Marzola V, Wachowicz K, Wasmer MH, Datta S, Muller-Holzner E, Fiegl H, Bock G, van Rooijen N, et al. In situ proliferation contributes to accumulation of tumor-associated macrophages in spontaneous mammary tumors. Eur J Immunol. 2014;44(8):2247–62.
Lio CJ, Rao A. TET enzymes and 5hmC in adaptive and innate Immune systems. Front Immunol. 2019;10:210.
Morales-Nebreda L, Mclafferty FS, Singer BD. DNA methylation as a transcriptional regulator of the immune system. Transl Res. 2019;204:1–18.
Calle-Fabregat CDL, Morante-Palacios O, Ballestar E. Understanding the relevance of DNA methylation changes in Immune differentiation and disease. Genes (Basel). 2020;11(1):110.
Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–80.
Martinez M, Moon EK. CAR T Cells for Solid Tumors: new strategies for finding, infiltrating, and surviving in the Tumor Microenvironment. Front Immunol. 2019;10:128.
Hope HC, Salmond RJ. Targeting the tumor microenvironment and T cell metabolism for effective cancer immunotherapy. Eur J Immunol. 2019;49(8):1147–52.
Newick K, O’Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68(1):139–52.
Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25(4):198–213.
Nia HT, Munn LL, Jain RK. Physical traits of cancer. Science. 2020;370(6516).
Huinen ZR, Huijbers E, van Beijnum JR, Nowak-Sliwinska P, Griffioen AW. Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol. 2021;18(8):527–40.
Weissleder R, Pittet MJ. The expanding landscape of inflammatory cells affecting cancer therapy. Nat Biomed Eng. 2020;4(5):489–98.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416.
Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guerin M, Biton J, Ouakrim H, et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci U S A. 2018;115(17):E4041–50.
Zhang S, Ma X, Zhu C, Liu L, Wang G, Yuan X. The role of myeloid-derived suppressor cells in patients with solid tumors: a Meta-analysis. PLoS ONE. 2016;11(10):e0164514.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74.
Yang Y, Li C, Liu T, Dai X, Bazhin AV. Myeloid-derived suppressor cells in tumors: from mechanisms to Antigen specificity and Microenvironmental Regulation. Front Immunol. 2020;11:1371.
Enblad G, Karlsson H, Gammelgard G, Wenthe J, Lovgren T, Amini RM, Wikstrom KI, Essand M, Savoldo B, Hallbook H, et al. A phase I/IIa trial using CD19-Targeted third-generation CAR T cells for Lymphoma and Leukemia. Clin Cancer Res. 2018;24(24):6185–94.
Di S, Zhou M, Pan Z, Sun R, Chen M, Jiang H, Shi B, Luo H, Li Z. Combined adjuvant of poly I:C improves Antitumor effects of CAR-T cells. Front Oncol. 2019;9:241.
Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–71.
June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117(6):1466–76.
O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9.
Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–45.
Xia A, Zhang Y, Xu J, Yin T, Lu XJ. T cell dysfunction in Cancer Immunity and Immunotherapy. Front Immunol. 2019;10:1719.
Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8 + T cell exhaustion versus memory. Immunity. 2012;37(6):1130–44.
Kirouac DC, Zmurchok C, Deyati A, Sicherman J, Bond C, Zandstra PW. Deconvolution of clinical variance in CAR-T cell pharmacology and response. Nat Biotechnol. 2023;41(11):1606–17.
Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354(6316):1160–5.
van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20(4):218–32.
Dolina JS, Van Braeckel-Budimir N, Thomas GD, Salek-Ardakani S. CD8(+) T cell exhaustion in Cancer. Front Immunol. 2021;12:715234.
Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994–1004.
Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–99.
Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60.
Zander R, Kasmani MY, Chen Y, Topchyan P, Shen J, Zheng S, Burns R, Ingram J, Cui C, Joshi N, et al. Tfh-cell-derived interleukin 21 sustains effector CD8(+) T cell responses during chronic viral infection. Immunity. 2022;55(3):475–93.
Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, et al. Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20(6):724–35.
Kersten K, Hu KH, Combes AJ, Samad B, Harwin T, Ray A, Rao AA, Cai E, Marchuk K, Artichoker J, et al. Spatiotemporal co-dependency between macrophages and exhausted CD8(+) T cells in cancer. Cancer Cell. 2022;40(6):624–38.
Callao V, Montoya E. Toxohormone-like factor from microorganisms with impaired respiration. Science. 1961;134(3495):2041–2.
Cohen AS, Geng L, Zhao P, Fu A, Schulte ML, Graves-Deal R, Washington MK, Berlin J, Coffey RJ, Manning HC. Combined blockade of EGFR and glutamine metabolism in preclinical models of colorectal cancer. Transl Oncol. 2020;13(10):100828.
Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–7.
Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, Mccormick LL, Fitzgerald P, Chi H, Munger J, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–82.
Krishnan C, Warnke RA, Arber DA, Natkunam Y. PD-1 expression in T-cell lymphomas and reactive lymphoid entities: potential overlap in staining patterns between lymphoma and viral lymphadenitis. Am J Surg Pathol. 2010;34(2):178–89.
Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, Goswami S, Allison JP. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021;11(4):838–57.
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–23.
Zhang Y, Zhang X, Cheng C, Mu W, Liu X, Li N, Wei X, Liu X, Xia C, Wang H. CRISPR-Cas9 mediated LAG-3 disruption in CAR-T cells. Front Med. 2017;11(4):554–62.
Jafarzadeh L, Masoumi E, Mirzaei HR, Alishah K, Fallah-Mehrjardi K, Khakpoor-Koosheh M, Rostamian H, Noorbakhsh F, Hadjati J. Targeted knockdown of Tim3 by short hairpin RNAs improves the function of anti-mesothelin CAR T cells. Mol Immunol. 2021;139:1–9.
Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, Saldmann A, Gey A, Oudard S, Tartour E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2(2):e000213.
Zhao K, Ren C, Tang D, Zhao L, Chen X, Wang Y, Xu K. The altering cellular components and function in tumor microenvironment during remissive and relapsed stages of anti-CD19 CAR T-cell treated lymphoma mice. Front Immunol. 2023;14:1101769.
Roselli E, Faramand R, Davila ML. Insight into next-generation CAR therapeutics: designing CAR T cells to improve clinical outcomes. J Clin Invest. 2021;131(2):e142030.
Haydar D, Ibañez-Vega J, Crawford JC, Chou CH, Guy CS, Meehl M, Yi Z, Perry S, Laxton J, Cunningham T, et al. CAR T-cell design-dependent remodeling of the Brain Tumor Immune Microenvironment modulates tumor-associated macrophages and anti-glioma activity. Cancer Res Commun. 2023;3(12):2430–46.
Moreno-Cortes E, Franco-Fuquen P, Garcia-Robledo JE, Forero J, Booth N, Castro JE. ICOS and OX40 tandem co-stimulation enhances CAR T-cell cytotoxicity and promotes T-cell persistence phenotype. Front Oncol. 2023;13:1200914.
Schoutrop E, Poiret T, El-Serafi I, Zhao Y, He R, Moter A, Henriksson J, Hassan M, Magalhaes I, Mattsson J. Tuned activation of MSLN-CAR T cells induces superior antitumor responses in ovarian cancer models. J Immunother Cancer. 2023;11(2):e005691.
Harrasser M, Gohil SH, Lau H, Della PM, Muczynski V, Patel D, Miranda E, Grigoriadis K, Grigoriadis A, Granger D, et al. Inducible localized delivery of an anti-PD-1 scFv enhances anti-tumor activity of ROR1 CAR-T cells in TNBC. Breast Cancer Res. 2022;24(1):39.
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126(8):3130–44.
Nakajima M, Sakoda Y, Adachi K, Nagano H, Tamada K. Improved survival of chimeric antigen receptor-engineered T (CAR-T) and tumor-specific T cells caused by anti-programmed cell death protein 1 single-chain variable fragment-producing CAR-T cells. Cancer Sci. 2019;110(10):3079–88.
Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847–56.
Li D, English H, Hong J, Liang T, Merlino G, Day CP, Ho M. A novel PD-L1-targeted shark V(NAR) single-domain-based CAR-T cell strategy for treating breast cancer and liver cancer. Mol Ther Oncolytics. 2022;24(849 – 63.
Xie YJ, Dougan M, Jailkhani N, Ingram J, Fang T, Kummer L, Momin N, Pishesha N, Rickelt S, Hynes RO, et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc Natl Acad Sci U S A. 2019;116(16):7624–31.
Chen J, Zhu T, Jiang G, Zeng Q, Li Z, Huang X. Target delivery of a PD-1-TREM2 scFv by CAR-T cells enhances anti-tumor efficacy in colorectal cancer. Mol Cancer. 2023;22(1):131.
Molgora M, Esaulova E, Vermi W, Hou J, Chen Y, Luo J, Brioschi S, Bugatti M, Omodei AS, Ricci B, et al. TREM2 modulation remodels the Tumor Myeloid Landscape enhancing Anti-PD-1 immunotherapy. Cell. 2020;182(4):886–900.
Kalinin RS, Ukrainskaya VM, Chumakov SP, Moysenovich AM, Tereshchuk VM, Volkov DV, Pershin DS, Maksimov EG, Zhang H, Maschan MA, et al. Engineered removal of PD-1 from the surface of CD19 CAR-T cells results in increased activation and diminished survival. Front Mol Biosci. 2021;8:745286.
Carosella ED, Rouas-Freiss N, Tronik-Le Roux D, Moreau P, Lemaoult J. HLA-G: an Immune Checkpoint Molecule. Adv Immunol. 2015;127:33–144.
Anna F, Bole-Richard E, Lemaoult J, Escande M, Lecomte M, Certoux JM, Souque P, Garnache F, Adotevi O, Langlade-Demoyen P, et al. First immunotherapeutic CAR-T cells against the immune checkpoint protein HLA-G. J Immunother Cancer. 2021;9(3):e001998.
Altvater B, Kailayangiri S, Pérez LL, Urban K, Greune L, Flügge M, Meltzer J, Farwick N, König S, Görlich D, et al. HLA-G and HLA-E Immune checkpoints are widely expressed in Ewing Sarcoma but have limited functional impact on the Effector functions of Antigen-Specific CAR T cells. Cancers (Basel). 2021;13(12):2857.
Qiu Y, Liao P, Wang H, Chen J, Hu Y, Hu R, Zhang H, Li Z, Cao M, Yang Y, et al. Enhanced tumor immunotherapy by polyfunctional CD19-CAR T cells engineered to secrete anti-CD47 single-chain variable fragment. Int J Biol Sci. 2023;19(15):4948–66.
Chen H, Yang Y, Deng Y, Wei F, Zhao Q, Liu Y, Liu Z, Yu B, Huang Z. Delivery of CD47 blocker SIRPα-Fc by CAR-T cells enhances antitumor efficacy. J Immunother Cancer. 2022;10(2):e003737.
Yang F, Zhang F, Ji F, Chen J, Li J, Chen Z, Hu Z, Guo Z. Self-delivery of TIGIT-blocking scFv enhances CAR-T immunotherapy in solid tumors. Front Immunol. 2023;14:1175920.
Prinz LF, Riet T, Neureuther DF, Lennartz S, Chrobok D, Hübbe H, Uhl G, Riet N, Hofmann P, Hösel M, et al. An anti-CD19/CTLA-4 switch improves efficacy and selectivity of CAR T cells targeting CD80/86-upregulated DLBCL. Cell reports. Medicine. 2024;5(2):101421.
Zhou X, Cao H, Fang S, Chow RD, Tang K, Majety M, Bai M, Dong MB, Renauer PA, Shang X, et al. CTLA-4 tail fusion enhances CAR-T antitumor immunity. Nat Immunol. 2023;24(9):1499–510.
Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36(5):471–82.
Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, Dyson KA, Grippin AJ, Deleyrolle LP, Zhang W, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. 2019;10(1):4016.
Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74(24):7168–74.
Trinh T, Adams WA, Calescibetta A, Tu N, Dalton R, So T, Wei M, Ward G, Kostenko E, Christiansen S, et al. CX3CR1 deficiency-induced TIL tumor restriction as a novel addition for CAR-T design in solid malignancies. iScience. 2023;26(4):106443.
Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944–71.
Liu G, Rui W, Zheng H, Huang D, Yu F, Zhang Y, Dong J, Zhao X, Lin X. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur J Immunol. 2020;50(5):712–24.
Dai Z, Lin X, Wang X, Zou X, Yan Y, Wang R, Chen Y, Tasiheng Y, Ma M, Wang X, et al. Ectopic CXCR2 expression cells improve the anti-tumor efficiency of CAR-T cells and remodel the immune microenvironment of pancreatic ductal adenocarcinoma. Cancer Immunol Immunother. 2024;73(4):61.
Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, Freeman GJ, Boland GM, Luster AD. Intratumoral Activity of the CXCR3 chemokine system is required for the efficacy of Anti-PD-1 therapy. Immunity. 2019;50(6):1498–512.
Liu L, Cheng Y, Zhang F, Chen J, Tian P, Shi W, Zhou F, Yang M, Zhou M, Liu B. IL-2/GM-CSF enhances CXCR3 expression in CAR-T cells via the PI3K/AKT and ERK1/2 pathways. J Cancer Res Clin Oncol. 2023;149(9):5547–57.
Jinquan T, Quan S, Jacobi HH, Jing C, Millner A, Jensen B, Madsen HO, Ryder LP, Svejgaard A, Malling HJ, et al. CXC chemokine receptor 3 expression on CD34(+) hematopoietic progenitors from human cord blood induced by granulocyte-macrophage colony-stimulating factor: chemotaxis and adhesion induced by its ligands, interferon gamma-inducible protein 10 and monokine induced by interferon gamma. Blood. 2000;96(4):1230–8.
Sun R, Sun Y, Wu C, Liu Y, Zhou M, Dong Y, Du G, Luo H, Shi B, Jiang H, et al. CXCR4-modified CAR-T cells suppresses MDSCs recruitment via STAT3/NF-κB/SDF-1α axis to enhance efficacy against pancreatic cancer. Mol Ther. 2023;31(11):3193–209.
Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26(3):311–27.
Tian Y, Wen C, Zhang Z, Liu Y, Li F, Zhao Q, Yao C, Ni K, Yang S, Zhang Y. CXCL9-modified CAR T cells improve immune cell infiltration and antitumor efficacy. Cancer Immunol Immunother. 2022;71(11):2663–75.
Goto S, Sakoda Y, Adachi K, Sekido Y, Yano S, Eto M, Tamada K. Enhanced anti-tumor efficacy of IL-7/CCL19-producing human CAR-T cells in orthotopic and patient-derived xenograft tumor models. Cancer Immunol Immunother. 2021;70(9):2503–15.
Lu LL, Xiao SX, Lin ZY, Bai JJ, Li W, Song ZQ, Zhou YH, Lu B, Wu WZ. GPC3-IL7-CCL19-CAR-T primes immune microenvironment reconstitution for hepatocellular carcinoma therapy. Cell Biol Toxicol. 2023;39(6):3101–19.
Feng DD, Chen XH, Guo JJ, Wang KK, Zhang XM, Gao JM. [Preliminary study of the fourth-generation CAR-T cells targeting CS1 in the treatment of refractory and recurrent multiple myeloma]. Zhonghua Zhong Liu Za Zhi. 2021;43(6):657–65.
Pang N, Shi J, Qin L, Chen A, Tang Y, Yang H, Huang Y, Wu Q, Li X, He B, et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14(1):118.
Alizadeh D, Wong RA, Gholamin S, Maker M, Aftabizadeh M, Yang X, Pecoraro JR, Jeppson JD, Wang D, Aguilar B, et al. IFNγ is critical for CAR T cell-mediated myeloid activation and induction of endogenous immunity. Cancer Discov. 2021;11(9):2248–65.
Hawkins ER, D’Souza RR, Klampatsa A. Armored CAR T-Cells: the next chapter in T-Cell Cancer immunotherapy. Biologics. 2021;15:95–105.
Berraondo P, Etxeberria I, Ponz-Sarvise M, Melero I. Revisiting Interleukin-12 as a Cancer Immunotherapy Agent. Clin Cancer Res. 2018;24(12):2716–8.
Agliardi G, Liuzzi AR, Hotblack A, De Feo D, Núñez N, Stowe CL, Friebel E, Nannini F, Rindlisbacher L, Roberts TA, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun. 2021;12(1):444.
Hu J, Sun C, Bernatchez C, Xia X, Hwu P, Dotti G, Li S. T-cell Homing Therapy for Reducing Regulatory T Cells and preserving effector T-cell function in large solid tumors. Clin Cancer Res. 2018;24(12):2920–34.
Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep. 2017;7(1):10541.
Jun LE, Cullen C, Murad JP, Gumber D, Park AK, Yang J, Stern LA, Adkins LN, Dhapola G, Gittins B, et al. Antigen-dependent IL-12 signaling in CAR T cells promotes regional to systemic disease targeting. bioRxiv. 2023;14(1):4737.
Zhu Y, Wang K, Yue L, Zuo D, Sheng J, Lan S, Zhao Z, Dong S, Hu S, Xin C et al. Mesothelin CAR-T cells expressing tumor-targeted immunocytokine IL-12 yield durable efficacy and fewer side effects. Pharmacol Res. 2024;107186.
Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, Nahvi AV, Ngo LT, Sherry RM, Phan GQ, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res. 2015;21(10):2278–88.
Luo Y, Chen Z, Sun M, Li B, Pan F, Ma A, Liao J, Yin T, Tang X, Huang G, et al. IL-12 nanochaperone-engineered CAR T cell for robust tumor-immunotherapy. Biomaterials. 2022;281:121341.
Zhang PF, Wang C, Zhang L, Li Q. Reversing chemokine/chemokine receptor mismatch to enhance the antitumor efficacy of CAR-T cells. Immunotherapy. 2022;14(6):459–73.
Zannikou M, Duffy JT, Levine RN, Seblani M, Liu Q, Presser A, Arrieta VA, Chen CJ, Sonabend AM, Horbinski CM, et al. IL15 modification enables CAR T cells to act as a dual targeting agent against tumor cells and myeloid-derived suppressor cells in GBM. J Immunother Cancer. 2023;11(2):e006239.
Goldberg L, Haas ER, Urak R, Vyas V, Pathak KV, Garcia-Mansfield K, Pirrotte P, Singhal J, Figarola JL, Aldoss I, et al. Immunometabolic adaptation of CD19-Targeted CAR T cells in the Central Nervous System Microenvironment of patients promotes Memory Development. Cancer Res. 2024;84(7):1048–64.
Brog RA, Ferry SL, Schiebout CT, Messier CM, Cook WJ, Abdullah L, Zou J, Kumar P, Sentman CL, Frost HR, et al. Superkine IL-2 and IL-33 armored CAR T cells reshape the Tumor Microenvironment and reduce growth of multiple solid tumors. Cancer Immunol Res. 2022;10(8):962–77.
Stewart CM, Cox MJ, Sakemura RL, Ogbodo EJ, Can I, Manriquez-Roman C, Yun K, Sirpilla O, Girsch JH, Huynh T, et al. Identification of IL-4 as a Key Regulator of CAR T-Cell exhaustion using Functional Genomics and correlates of the Zuma-1 clinical trial. Blood. 2022;140:4536–7.
Wang Y, Jiang H, Luo H, Sun Y, Shi B, Sun R, Li Z. An IL-4/21 inverted cytokine receptor improving CAR-T cell potency in immunosuppressive solid-Tumor Microenvironment. Front Immunol. 2019;10:1691.
Zhou Y, Farooq MA, Ajmal I, He C, Gao Y, Guo D, Duan Y, Jiang W. Co-expression of IL-4/IL-15-based inverted cytokine receptor in CAR-T cells overcomes IL-4 signaling in immunosuppressive pancreatic tumor microenvironment. Biomed Pharmacother. 2023;168:115740.
Li Y, Wu H, Chen G, Wei X, Wang C, Zhou S, Huang A, Zhang Z, Zhan C, Wu Y, et al. Arming Anti-EGFRvIII CAR-T with TGFβ trap improves Antitumor Efficacy in Glioma Mouse models. Front Oncol. 2020;10:1117.
Stüber T, Monjezi R, Wallstabe L, Kühnemundt J, Nietzer SL, Dandekar G, Wöckel A, Einsele H, Wischhusen J, Hudecek M. Inhibition of TGF-β-receptor signaling augments the antitumor function of ROR1-specific CAR T-cells against triple-negative breast cancer. J Immunother Cancer. 2020;8(1):e000676.
Liang S, Zheng R, Zuo B, Li J, Wang Y, Han Y, Dong H, Zhao X, Zhang Y, Wang P, et al. SMAD7 expression in CAR-T cells improves persistence and safety for solid tumors. Cell Mol Immunol. 2024;21(3):213–26.
Zanvit P, van Dyk D, Fazenbaker C, Mcglinchey K, Luo W, Pezold JM, Meekin J, Chang CY, Carrasco RA, Breen S et al. Antitumor activity of AZD0754, a dnTGFβRII-armored, STEAP2-targeted CAR-T cell therapy, in prostate cancer. J Clin Invest. 2023;133(22).
Tran TM, Chand TB, Nurmukhambetova S, Lee JJ, Hu P, Tran NQ, Steimle B, Dash P, Schneider D. Armored TGFβRIIDN ROR1-CAR T cells reject solid tumors and resist suppression by constitutively-expressed and treatment-induced TGFβ1. J Immunother Cancer. 2024;12(4).
Lu X, Lofgren SM, Zhao Y, Mazur PK. Multiplexed transcriptomic profiling of the fate of human CAR T cells in vivo via genetic barcoding with shielded small nucleotides. Nat Biomed Eng. 2023;7(9):1170–87.
Li F, Zhao S, Wei C, Hu Y, Xu T, Xin X, Zhu T, Shang L, Ke S, Zhou J, et al. Development of Nectin4/FAP-targeted CAR-T cells secreting IL-7, CCL19, and IL-12 for malignant solid tumors. Front Immunol. 2022;13:958082.
Raeber ME, Zurbuchen Y, Impellizzieri D, Boyman O. The role of cytokines in T-cell memory in health and disease. Immunol Rev. 2018;283(1):176–93.
Sukumaran S, Watanabe N, Bajgain P, Raja K, Mohammed S, Fisher WE, Brenner MK, Leen AM, Vera JF. Enhancing the potency and specificity of Engineered T cells for Cancer Treatment. Cancer Discov. 2018;8(8):972–87.
Beck JD, Reidenbach D, Salomon N, Sahin U, Türeci Ö, Vormehr M, Kranz LM. MRNA therapeutics in cancer immunotherapy. Mol Cancer. 2021;20(1):69.
Meister H, Look T, Roth P, Pascolo S, Sahin U, Lee S, Hale BD, Snijder B, Regli L, Ravi VM, et al. Multifunctional mRNA-Based CAR T cells display promising Antitumor Activity against Glioblastoma. Clin Cancer Res. 2022;28(21):4747–56.
Qiao Y, Chen J, Wang X, Yan S, Tan J, Xia B, Chen Y, Lin K, Zou F, Liu B, et al. Enhancement of CAR-T cell activity against cholangiocarcinoma by simultaneous knockdown of six inhibitory membrane proteins. Cancer Commun (Lond). 2023;43(7):788–807.
López-Cantillo G, Urueña C, Camacho BA, Ramírez-Segura C. CAR-T cell performance: how to improve their persistence? Front Immunol. 2022;13:878209.
Turtle CJ, Hanafi L, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, et al. CD19 CAR-T cells of defined CD4+:CD8 + composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38.
Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76.
Tumino N, Weber G, Besi F, Del BF, Bertaina V, Paci P, Quatrini L, Antonucci L, Sinibaldi M, Quintarelli C, et al. Polymorphonuclear myeloid-derived suppressor cells impair the anti-tumor efficacy of GD2.CAR T-cells in patients with neuroblastoma. J Hematol Oncol. 2021;14(1):191.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18.
Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O’Connor RS, Minutolo NG, Casado-Medrano V, Lopez G, Matsuyama T, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021;12(1):877.
Fan J, Yu Y, Yan L, Yuan Y, Sun B, Yang D, Liu N, Guo J, Zhang J, Zhao X. GAS6-based CAR-T cells exhibit potent antitumor activity against pancreatic cancer. J Hematol Oncol. 2023;16(1):77.
Myers KV, Amend SR, Pienta KJ. Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer. 2019;18(1):94.
Yang Y, Wu H, Yang Y, Kang Y, He R, Zhou B, Guo H, Zhang J, Li J, Ge C, et al. Dual blockade of CD47 and CD24 signaling using a novel bispecific antibody fusion protein enhances macrophage immunotherapy. Mol Therapy Oncolytics. 2023;31:100747.
Sun F, Cheng Y, Wanchai V, Guo W, Mery D, Xu H, Gai D, Siegel E, Bailey C, Ashby C, et al. Bispecific BCMA/CD24 CAR-T cells control multiple myeloma growth. Nat Commun. 2024;15(1):615.
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal LJ, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–6.
Zhang H, Liu Z, Wen H, Guo Y, Xu F, Zhu Q, Yuan W, Luo R, Lu C, Liu R, et al. Immunosuppressive TREM2(+) macrophages are associated with undesirable prognosis and responses to anti-PD-1 immunotherapy in non-small cell lung cancer. Cancer Immunol Immunotherapy: CII. 2022;71(10):2511–22.
Nalawade SA, Shafer P, Bajgain P, Mckenna MK, Ali A, Kelly L, Joubert J, Gottschalk S, Watanabe N, Leen A et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J Immunother Cancer. 2021;9(11).
Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein N, et al. Selective targeting of myeloid-derived suppressor cells in Cancer patients using DS-8273a, an Agonistic TRAIL-R2 antibody. Clin cancer Research: Official J Am Association Cancer Res. 2017;23(12):2942–50.
Ruella M, Klichinsky M, Kenderian SS, Shestova O, Ziober A, Kraft DO, Feldman M, Wasik MA, June CH, Gill S. Overcoming the immunosuppressive Tumor Microenvironment of Hodgkin Lymphoma Using Chimeric Antigen Receptor T Cells. Cancer Discov. 2017;7(10):1154–67.
Jin X, Xie D, Sun R, Lu W, Xiao X, Yu Y, Meng J, Zhao M. CAR-T cells dual-target CD123 and NKG2DLs to eradicate AML cells and selectively target immunosuppressive cells. Oncoimmunology. 2023;12(1):2248826.
Bonnin E, Rodrigo Riestra M, Marziali F, Mena Osuna R, Denizeau J, Maurin M, Saez JJ, Jouve M, Bonté P, Richer W, et al. CD74 supports accumulation and function of regulatory T cells in tumors. Nat Commun. 2024;15(1):3749.
Suryadevara CM, Desai R, Farber SH, Choi BD, Swartz AM, Shen SH, Gedeon PC, Snyder DJ, Herndon JEN, Healy P, et al. Preventing lck activation in CAR T cells confers Treg Resistance but requires 4-1BB signaling for them to persist and Treat Solid Tumors in Nonlymphodepleted hosts. Clin cancer Research: Official J Am Association Cancer Res. 2019;25(1):358–68.
Lanitis E, Rota G, Kosti P, Ronet C, Spill A, Seijo B, Romero P, Dangaj D, Coukos G, Irving M. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of IL-15 coexpression. J Exp Med. 2021;218(2).
Liu Y, Sun Y, Wang P, Li S, Dong Y, Zhou M, Shi B, Jiang H, Sun R, Li Z. FAP-targeted CAR-T suppresses MDSCs recruitment to improve the antitumor efficacy of claudin18.2-targeted CAR-T against pancreatic cancer. J Transl Med. 2023;21(1):255.
Seo H, González-Avalos E, Zhang W, Ramchandani P, Yang C, Lio CJ, Rao A, Hogan PG. BATF and IRF4 cooperate to counter exhaustion in tumor-infiltrating CAR T cells. Nat Immunol. 2021;22(8):983–95.
Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19(11):665–74.
Chen Z, Arai E, Khan O, Zhang Z, Ngiow SF, He Y, Huang H, Manne S, Cao Z, Baxter AE, et al. In vivo CD8(+) T cell CRISPR screening reveals control by Fli1 in infection and cancer. Cell. 2021;184(5):1262–80.
Prinzing B, Zebley CC, Petersen CT, Fan Y, Anido AA, Yi Z, Nguyen P, Houke H, Bell M, Haydar D, et al. Deleting DNMT3a in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 2021;13(620):eabh0272.
Chan JD, Scheffler CM, Munoz I, Sek K, Lee JN, Huang YK, Yap KM, Saw N, Li J, Chen A et al. FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy. Nature. 2024.
Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, Anbunathan H, Lattin J, Jones R, Tieu V, et al. C-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576(7786):293–300.
Heitzeneder S, Bosse KR, Zhu Z, Zhelev D, Majzner RG, Radosevich MT, Dhingra S, Sotillo E, Buongervino S, Pascual-Pasto G, et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell. 2022;40(1):53–69.
Zhu X, Li W, Gao J, Shen J, Xu Y, Zhang C, Qian C. RUNX3 improves CAR-T cell phenotype and reduces cytokine release while maintaining CAR-T function. Med Oncol. 2023;40(3):89.
Wang Y, Zhang H, Du G, Luo H, Su J, Sun Y, Zhou M, Shi B, Li H, Jiang H, et al. Enforced expression of Runx3 improved CAR-T cell potency in solid tumor via enhancing resistance to activation-induced cell death. Mol Ther. 2023;31(3):701–14.
Fu Q, Zheng Y, Fang W, Zhao Q, Zhao P, Liu L, Zhai Y, Tong Z, Zhang H, Lin M, et al. RUNX-3-expressing CAR T cells targeting glypican-3 in patients with heavily pretreated advanced hepatocellular carcinoma: a phase I trial. EClinicalMedicine. 2023;63:102175.
Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, Tihy I, Tartour E. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6(1):87.
Song Q, Shang J, Zhang C, Chen J, Zhang L, Wu X. Transcription factor RUNX3 promotes CD8(+) T cell recruitment by CCL3 and CCL20 in lung adenocarcinoma immune microenvironment. J Cell Biochem. 2020;121(5–6):3208–20.
Zens KD, Chen JK, Guyer RS, Wu FL, Cvetkovski F, Miron M, Farber DL. Reduced generation of lung tissue-resident memory T cells during infancy. J Exp Med. 2017;214(10):2915–32.
Connors TJ, Baird JS, Yopes MC, Zens KD, Pethe K, Ravindranath TM, Ho SH, Farber DL. Developmental Regulation of Effector and Resident Memory T Cell Generation during Pediatric viral respiratory tract infection. J Immunol. 2018;201(2):432–9.
Gacerez AT, Sentman CL. T-bet promotes potent antitumor activity of CD4(+) CAR T cells. Cancer Gene Ther. 2018;25(5–6):117–28.
Wang AX, Ong XJ, D’Souza C, Neeson PJ, Zhu JJ. Combining chemotherapy with CAR-T cell therapy in treating solid tumors. Front Immunol. 2023;14:1140541.
Dacek MM, Kurtz KG, Wallisch P, Pierre SA, Khayat S, Bourne CM, Gardner TJ, Vogt KC, Aquino N, Younes A, et al. Potentiating antibody-dependent killing of cancers with CAR T cells secreting CD47-SIRPα checkpoint blocker. Blood. 2023;141(16):2003–15.
Ou Z, Dou X, Tang N, Liu G. Pressure increases PD-L1 expression in a549 lung adenocarcinoma cells and causes resistance to anti-ROR1 CAR T cell-mediated cytotoxicity. Sci Rep. 2022;12(1):6919.
Zhang G, Zhao Y, Liu Z, Liu W, Wu H, Wang X, Chen Z. GD2 CAR-T cells in combination with Nivolumab exhibit enhanced antitumor efficacy. Transl Oncol. 2023;32:101663.
Chong EA, Alanio C, Svoboda J, Nasta SD, Landsburg DJ, Lacey SF, Ruella M, Bhattacharyya S, Wherry EJ, Schuster SJ. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood. 2022;139(7):1026–38.
Gao G, Liao W, Shu P, Ma Q, He X, Zhang B, Qin D, Wang Y. Targeting sphingosine 1-phosphate receptor 3 inhibits T-cell exhaustion and regulates recruitment of proinflammatory macrophages to improve antitumor efficacy of CAR-T cells against solid tumor. J Immunother Cancer. 2023;11(8):e006343.
Toews K, Grunewald L, Schwiebert S, Klaus A, Winkler A, Ali S, Zirngibl F, Astrahantseff K, Wagner DL, Henssen AG, et al. Central memory phenotype drives success of checkpoint inhibition in combination with CAR T cells. Mol Carcinog. 2020;59(7):724–35.
Sailer CJ, Hong Y, Dahal A, Ryan AT, Mir S, Gerber SA, Reagan PM, Kim M. PD-1(hi) CAR-T cells provide superior protection against solid tumors. Front Immunol. 2023;14:1187850.
Yamaguchi Y, Gibson J, Ou K, Lopez LS, Ng RH, Leggett N, Jonsson VD, Zarif JC, Lee PP, Wang X, et al. PD-L1 blockade restores CAR T cell activity through IFN-γ-regulation of CD163 + M2 macrophages. J Immunother Cancer. 2022;10(6):e004400.
Liu M, Wang X, Li Z, Zhang R, Mu J, Jiang Y, Deng Q, Sun L. Synergistic effect of ibrutinib and CD19 CAR-T cells on Raji cells in vivo and in vitro. Cancer Sci. 2020;111(11):4051–60.
Huang Y, Qin Y, He Y, Qiu D, Zheng Y, Wei J, Zhang L, Yang DH, Li Y. Advances in molecular targeted drugs in combination with CAR-T cell therapy for hematologic malignancies. Drug Resist Updat. 2024;74:101082.
Guan G, Zhang Y, Lu Y, Liu L, Shi D, Wen Y, Yang L, Ma Q, Liu T, Zhu X, et al. The HIF-1α/CXCR4 pathway supports hypoxia-induced metastasis of human osteosarcoma cells. Cancer Lett. 2015;357(1):254–64.
Jiang K, Li J, Zhang J, Wang L, Zhang Q, Ge J, Guo Y, Wang B, Huang Y, Yang T, et al. SDF-1/CXCR4 axis facilitates myeloid-derived suppressor cells accumulation in osteosarcoma microenvironment and blunts the response to anti-PD-1 therapy. Int Immunopharmacol. 2019;75:105818.
Sun R, Luo H, Su J, Di S, Zhou M, Shi B, Sun Y, Du G, Zhang H, Jiang H, et al. Olaparib suppresses MDSC Recruitment via SDF1α/CXCR4 Axis to improve the anti-tumor efficacy of CAR-T cells on breast Cancer in mice. Mol Ther. 2021;29(1):60–74.
Ji F, Zhang F, Zhang M, Long K, Xia M, Lu F, Li E, Chen J, Li J, Chen Z, et al. Targeting the DNA damage response enhances CD70 CAR-T cell therapy for renal carcinoma by activating the cGAS-STING pathway. J Hematol Oncol. 2021;14(1):152.
Jin Z, Xiang R, Qing K, Li D, Liu Z, Li X, Zhu H, Zhang Y, Wang L, Xue K, et al. Lenalidomide overcomes the resistance to third-generation CD19-CAR-T cell therapy in preclinical models of diffuse large B-cell lymphoma. Cell Oncol (Dordr). 2023;46(4):1143–57.
Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong K, Bradner JE, Kaelin WGJ. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–9.
Tettamanti S, Rotiroti MC, Giordano AG, Arcangeli S, Zhang R, Banerjee P, Galletti G, Mcmanus S, Mazza M, Nicolini F, et al. Lenalidomide enhances CD23.CAR T cell therapy in chronic lymphocytic leukemia. Leuk Lymphoma. 2022;63(7):1566–79.
Zhang Q, Dong Y, Zhai Z, Tao L, Tao Q. Lenalidomide treatment of isolated central nervous system relapse in acute lymphoblastic leukemia after HSCT and CAR-T cell therapy. Acta Haematol. 2024.
Mehra V, Agliardi G, Dias Alves Pinto J, Shafat MS, Garai AC, Green L, Hotblack A, Arce Vargas F, Peggs KS, van der Waart AB et al. AKT inhibition generates potent polyfunctional clinical grade AUTO1 CAR T-cells, enhancing function and survival. J Immunother Cancer. 2023;11(9).
Zhang Q, Ding J, Sun S, Liu H, Lu M, Wei X, Gao X, Zhang X, Fu Q, Zheng J. Akt inhibition at the initial stage of CAR-T preparation enhances the CAR-positive expression rate, memory phenotype and in vivo efficacy. Am J Cancer Res. 2019;9(11):2379–96.
Zhang Q, Zheng F, Chen Y, Liang CL, Liu H, Qiu F, Liu Y, Huang H, Lu W, Dai Z. The TOPK inhibitor HI-TOPK-032 enhances CAR T-cell therapy of hepatocellular carcinoma by upregulating memory T cells. Cancer Immunol Res. 2024.
Klebanoff CA, Crompton JG, Leonardi AJ, Yamamoto TN, Chandran SS, Eil RL, Sukumar M, Vodnala SK, Hu J, Ji Y et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI insight. 2017;2(23).
Scurr M, Pembroke T, Bloom A, Roberts D, Thomson A, Smart K, Bridgeman H, Adams R, Brewster A, Jones R, et al. Low-dose Cyclophosphamide induces antitumor T-Cell responses, which associate with survival in metastatic colorectal Cancer. Clin cancer Research: Official J Am Association Cancer Res. 2017;23(22):6771–80.
Murad JP, Tilakawardane D, Park AK, Lopez LS, Young CA, Gibson J, Yamaguchi Y, Lee HJ, Kennewick KT, Gittins BJ, et al. Pre-conditioning modifies the TME to enhance solid tumor CAR T cell efficacy and endogenous protective immunity. Mol Ther. 2021;29(7):2335–49.
Porter LH, Zhu JJ, Lister NL, Harrison SG, Keerthikumar S, Goode DL, Urban RQ, Byrne DJ, Azad A, Vela I, et al. Low-dose carboplatin modifies the tumor microenvironment to augment CAR T cell efficacy in human prostate cancer models. Nat Commun. 2023;14(1):5346.
Srivastava S, Furlan SN, Jaeger-Ruckstuhl CA, Sarvothama M, Berger C, Smythe KS, Garrison SM, Specht JM, Lee SM, Amezquita RA, et al. Immunogenic Chemotherapy Enhances Recruitment of CAR-T Cells to lung tumors and improves Antitumor Efficacy when combined with checkpoint blockade. Cancer Cell. 2021;39(2):193–208.
Spary LK, Al-Taei S, Salimu J, Cook AD, Ager A, Watson HA, Clayton A, Staffurth J, Mason MD, Tabi Z. Enhancement of T cell responses as a result of synergy between lower doses of radiation and T cell stimulation. J Immunol. 2014;192(7):3101–10.
Murty S, Haile ST, Beinat C, Aalipour A, Alam IS, Murty T, Shaffer TM, Patel CB, Graves EE, Mackall CL, et al. Intravital imaging reveals synergistic effect of CAR T-cells and radiation therapy in a preclinical immunocompetent glioblastoma model. Oncoimmunology. 2020;9(1):1757360.
Liang T, Song Y, Gu L, Wang Y, Ma W. Insight into the progress in CAR-T cell therapy and combination with other therapies for Glioblastoma. Int J Gen Med. 2023;16:4121–41.
Wang T, Zhang K, You F, Ma R, Yang N, Tian S, An G, Yang L. Preconditioning of radiotherapy enhances efficacy of B7-H3-CAR-T in treating solid tumor models. Life Sci. 2023;331:122024.
Jiménez-Cortegana C, Galassi C, Klapp V, Gabrilovich DI, Galluzzi L. Myeloid-derived suppressor cells and Radiotherapy. Cancer Immunol Res. 2022;10(5):545–57.
Yang X, Lu Y, Hang J, Zhang J, Zhang T, Huo Y, Liu J, Lai S, Luo D, Wang L, et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the Radioresistance of Pancreatic Cancer. Cancer Immunol Res. 2020;8(11):1440–51.
Zhang B, Hu M, Ma Q, Li K, Li X, He X, Shu P, Chen Y, Gao G, Qin D, et al. Optimized CAR-T therapy based on spatiotemporal changes and chemotactic mechanisms of MDSCs induced by hypofractionated radiotherapy. Mol Ther. 2023;31(7):2105–19.
Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C, Ogunnaike EA, Ligler FS, Dotti G, Gu Z. Photothermal Therapy promotes Tumor Infiltration and Antitumor Activity of CAR T cells. Adv Mater. 2019;31(23):e1900192.
Innamarato P, Kodumudi K, Asby S, Schachner B, Hall M, Mackay A, Wiener D, Beatty M, Nagle L, Creelan BC, et al. Reactive myelopoiesis triggered by Lymphodepleting Chemotherapy Limits the Efficacy of Adoptive T Cell Therapy. Mol Ther. 2020;28(10):2252–70.
Wu C, Zhong Q, Shrestha R, Wang J, Hu X, Li H, Rouchka EC, Yan J, Ding C. Reactive myelopoiesis and FX-expressing macrophages triggered by chemotherapy promote cancer lung metastasis. JCI Insight. 2023;8(9).
Owen K, Ghaly R, Shohdy KS, Thistlethwaite F. Lymphodepleting chemotherapy practices and effect on safety and efficacy outcomes in patients with solid tumours undergoing T cell receptor-engineered T cell (TCR-T) therapy: a systematic review and meta-analysis. Cancer Immunol Immunother. 2023;72(4):805–14.
Liao Q, Liu Z, Zhu C, He H, Feng M, Jiang L, Ding X, Sun R, Zhang X, Xu J. Rapid generation of a mouse model for evaluating on-target normal tissue toxicity of human CAR-T cells using replication-defective recombinant adenovirus. J Adv Res. 2023;47(163 – 71.
Harari-Steinfeld R, Abhinav AV, Cuevas L, Marincola F, Roh KH. Standardized in-vitro evaluation of CAR-T cells using acellular artificial target particles. Front Immunol. 2022;13:994532.
Chen Z, Han S, Sanny A, Chan DL, van Noort D, Lim W, Tan AH, Park S. 3D hanging spheroid plate for high-throughput CAR T cell cytotoxicity assay. J Nanobiotechnol. 2022;20(1):30.
Chen Z, Han S, Kim S, Lee C, Sanny A, Tan AH, Park S. A 3D hanging spheroid-filter plate for high-throughput drug testing and CAR T cell cytotoxicity assay. Analyst. 2024;149(2):475–81.
Zurowski D, Patel S, Hui D, Ka M, Hernandez C, Love AC, Lin B, Moore A, Chan LL. High-throughput method to analyze the cytotoxicity of CAR-T cells in a 3D tumor spheroid model using image cytometry. SLAS Discov. 2023;28(3):65–72.
Ma B, Liu X, Zhang Z, Ma C, Chand R, Patwardhan S, Wang C, Thamphiwatana SD, Chen P, Chen W. A digital nanoplasmonic microarray immunosensor for multiplexed cytokine monitoring during CAR T-cell therapy from a leukemia tumor microenvironment model. Biosens Bioelectron. 2023;230:115247.
Abdoli SM, Hemmat N, Khaze SV, Derakhshani A, Baradaran F, Brunetti O, Fasano R, Bernardini R, Silvestris N, Baradaran B. A systematic review on PD-1 blockade and PD-1 gene-editing of CAR-T cells for Glioma Therapy: from deciphering to Personalized Medicine. Front Immunol. 2021;12:788211.
Bassez A, Vos H, Van Dyck L, Floris G, Arijs I, Desmedt C, Boeckx B, Vanden Bempt M, Nevelsteen I, Lambein K, et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat Med. 2021;27(5):820–32.
Deng W, Ma Y, Su Z, Liu Y, Liang P, Huang C, Liu X, Shao J, Zhang Y, Zhang K et al. Single-cell RNA-sequencing analyses identify heterogeneity of CD8(+) T cell subpopulations and novel therapy targets in melanoma. Mol Ther Oncolytics. 2021;20(105 – 18.
Zurowski D, Patel S, Hui D, Ka M, Hernandez C, Love AC, Lin B, Moore A, Chan LL. High-throughput method to analyze the cytotoxicity of CAR-T cells in a 3D tumor spheroid model using image cytometry. SLAS Discovery: Adv life Sci R D. 2023;28(3):65–72.
Kolahi AH, Imanpour A, Rezaee H, Ezzatifar F, Zarei-Behjani Z, Rostami M, Azami M, Behestizadeh N, Rezaei N. Mesenchymal stromal cells and CAR-T cells in regenerative medicine: the homing procedure and their effective parameters. Eur J Haematol. 2023;112(2):153–73.
Safarzadeh KP, Safarzadeh KP, Rahbarizadeh F. Addressing the obstacles of CAR T cell migration in solid tumors: wishing a heavy traffic. Crit Rev Biotechnol. 2022;42(7):1079–98.
Tang X, Yang Y, Zheng M, Yin T, Huang G, Lai Z, Zhang B, Chen Z, Xu T, Ma T, et al. Magnetic-acoustic sequentially actuated CAR T cell microrobots for Precision Navigation and in situ Antitumor Immunoactivation. Adv Mater. 2023;35(18):e2211509.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YHL and SJW contributed to conception and manuscript design.DHY supervised the manuscript. XTX, ZXY and QSL drafted the manuscript. ZYL, LW and JWD collected the related references. XTX, ZXY, YHL, WSJ and DHY participated in the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, X., Yang, Z., Lu, Q. et al. Reshaping the tumor immune microenvironment to improve CAR-T cell-based cancer immunotherapy. Mol Cancer 23, 175 (2024). https://doi.org/10.1186/s12943-024-02079-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-024-02079-8