Abstract
Tertiary lymphoid structures (TLS) are clusters of immune cells that resemble and function similarly to secondary lymphoid organs (SLOs). While TLS is generally associated with an anti-tumour immune response in most cancer types, it has also been observed to act as a pro-tumour immune response. The heterogeneity of TLS function is largely determined by the composition of tumour-infiltrating lymphocytes (TILs) and the balance of cell subsets within the tumour-associated TLS (TA-TLS). TA-TLS of varying maturity, density, and location may have opposing effects on tumour immunity. Higher maturity and/or higher density TLS are often associated with favorable clinical outcomes and immunotherapeutic response, mainly due to crosstalk between different proportions of immune cell subpopulations in TA-TLS. Therefore, TLS can be used as a marker to predict the efficacy of immunotherapy in immune checkpoint blockade (ICB). Developing efficient imaging and induction methods to study TA-TLS is crucial for enhancing anti-tumour immunity. The integration of imaging techniques with biological materials, including nanoprobes and hydrogels, alongside artificial intelligence (AI), enables non-invasive in vivo visualization of TLS. In this review, we explore the dynamic interactions among T and B cell subpopulations of varying phenotypes that contribute to the structural and functional diversity of TLS, examining both existing and emerging techniques for TLS imaging and induction, focusing on cancer immunotherapies and biomaterials. We also highlight novel therapeutic approaches of TLS that are being explored with the aim of increasing ICB treatment efficacy and predicting prognosis.
Similar content being viewed by others
Introduction
Effective anti-tumour immunity necessitates the presence of tumour-infiltrating lymphocytes (TILs). This has been particularly evidenced by the identification of tertiary lymphoid structures (TLS), representing well-organized clusters of TILs that elicit delayed immune responses [1]. TLS, immune cell clusters akin to secondary lymphoid organs (SLOs), emerge postnatally within non-lymphoid tissues, typically absent under normal physiological conditions, and predominantly manifest in chronic inflammatory conditions, including cancer, chronic infections, and autoimmune diseases [1, 2]. The context of chronic inflammation is strongly associated with tumour development. Analyses of lymphocyte infiltration and the spatial aggregation of TLS within the tumour microenvironment (TME) of patients with colorectal cancer (CRC), non-small-cell lung cancer (NSCLC), breast cancer (BC), and melanoma indicate that a patient’s prognosis correlates with a higher degree of lymphocyte infiltration, density, and maturation [3,4,5,6]. Evaluating the maturity of TLS across different tumour types is critical for establishing TLS as a reliable prognostic tool in oncology. In addition, inducing a higher density and maturity of TLS in cancer patients contributes to the enhancement of the body’s anti-tumour immunity. Furthermore, promoting a greater density and maturity of TLS in cancer patients significantly enhances the body’s anti-tumour immune response.
In recent decades, immunotherapy targeting immune checkpoints—specifically programmed cell death protein 1 (PD-1), and its ligands programmed death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)—has emerged as a pivotal strategy against various solid tumours. Nevertheless, immune checkpoint blockade (ICB) faces challenges, including clinical application limitations and safety concerns, potentially resulting in inflammatory side effects or immune-related adverse events (irAEs) [7, 8]. Intratumoral TLS has gained recognition as a biomarker capable of predicting and potentially enhancing ICB therapy efficacy [9, 10]. Analyses of intratumoral TLS have demonstrated a positive correlation with clinical benefits in patients, irrespective of PD-L1 expression status, facilitating new markers’ use in ICB therapy patient selection [11]. The distinct features of nanoparticles in multimodal and molecular imaging enable them to be utilized for extremely delicate non-invasive multimodal imaging of biological occurrences in vivo [12]. Developing more efficient, non-invasive techniques for visualizing and inducing TLS represents a novel research direction [13].
In this review, we outline the tissue and cellular structure of TLS and explore the pathways and molecular mechanisms involved in TLS formation and maturation. The dual effects of immune cell crosstalk within TLS on anti-tumour immunity are described, and the potential of TLS as a cancer biomarker is highlighted. Finally, the current induction strategies for TLS are summarized, and the dilemmas and solutions for its future development are discussed.
Development and characterization of mature TLS
Based on the impact of mature TLS on tumour prognosis and treatment, it is important to understand the mechanisms of immune cell crosstalk within the internal tissue and cellular structure of mature TLS in the subsequent anti-tumour immune response. It is also essential to understand the important mechanisms of action during TLS formation and maturation.
Composition of mature TLS
The mature tertiary lymphoid structure is an internal region found in lymphoid follicles formed by CD20 + B cells that are surrounded by CD3 + T cells. This structure is similar to a secondary lymphoid organ (SLO). It comprises mainly of T cells, B cells, follicular dendritic cells (FDCs), dendritic cells (DCs), stromal cells, macrophages, endothelial cells, and high endothelial venules (HEVs). Presently, the T-cell region within the TLS has been thoroughly researched, being recognized as a biomarker for clinical outcome prediction [14, 15]. Characterization of TLS composition in 39 intrahepatic cholangiocarcinoma (iCCA) samples using multiplex immunohistochemistry (mIHC) revealed that the spatial distribution and abundance of TLS were significantly correlated with prognosis, and T follicular helper (Tfh) cells and regulatory T (Treg) cells may play a key role in determining the functional localization of spatially distinct TLS [16]. The function of B cells in tumour immunity has emerged as a focal point of recent research [9, 17, 18]. By employing single-cell technologies, immunohistochemistry (IHC) and immunofluorescence (IF) analyses, distinctive subpopulations of B cells have been discerned in human tumours, identified by phenotypic markers for which a general consensus has been reached, and numerous phenotypes have been found to play disparate roles in tumour prognosis [19]. However, it is important to note that the function of B cells within TLS is affected by the degree of TLS maturation. In order to achieve a complete anti-tumour immune response, immunoglobulin class switching and affinity maturation are necessary. Ayana T Ruffin and colleagues demonstrated that in human papillomavirus (HPV)-induced head and neck squamous cell carcinoma (HNSCC), tumour-infiltrating B-cell (TIL-B) transcriptional profiles, as well as spatial organization of TLS immune cells compatible with germinal centers (GCs), bear a significant association with a favorable prognosis in patients [20]. The induction of TLS in a mouse model of HPV HNSCC was found to bolster the response to PD-1 blockade therapy, which was weakened by CD20 + B cell elimination. These studies have shown that improving TIL-B cell responses could serve as a complementary approach to T cell-mediated immunotherapy. There is potential for new ideas and research directions regarding the roles of T and B cells in TLS in combating tumours. The focus is on exploring potential mechanisms of immune cell crosstalk in mature TLS.
Formation and maturation of TLS
TLS and SLO share similarities, making it possible to study TLS by exploring the developmental processes of SLO. Nevertheless, the cellular components and molecular pathways involved in the initiation and developmental processes of TLS vary due to different tissue origins and disease states, and their intrinsic functional properties differ from those of SLO (Fig. 1a; Table 1) [21]. The formation of TLS depends on intricate ligand-receptor interactions between different cell types, including innate lymphocytes (ILCs), stromal cells, endothelial cells and B cells. CD3-CD4-/+ CXCR5IL-7Rα + hi ILCs, also known as lymphoid tissue inducing cells (LTi), are an essential component of SLO development [22]. Stromal fibroblasts, characterized by the expression of podoplanin and fibroblast activation protein-α (FAP), have been shown to be associated with the formation of TLS in both mouse and human models [23, 24]. The secretion of IL-7 and CXCL13 by stromal fibroblasts recruits and induces LTi cell proliferation and expansion, contributing to the development of SLOs. Subsequent secretion of lymphotoxin α1β2 (LTα1β2) by LTi cells induces maturation of lymphoid tissue-forming cells (LTo), leading to up-regulation of CXCL13, CCL19 and CCL21, which promotes the infiltration of B and T cells into the TLS [9, 25, 26]. Additionally, HEVs are involved in the process of TLS maturation by promoting intratumoral lymphocyte aggregation and infiltration, creating an environment conducive to further TLS maturation [27]. Following TLS maturation, activated immune cells transit from TLS to the tumour bed through HEVs, thereby exerting potent anti-tumour immunity against cancer cells [28].
TLS formation modelling. (a) The initial process of TLS. Local accumulation of pro-inflammatory molecules and chemokines can recruit lymphoid tissue-inducing (LTi) cells to sites of inflammation. LTi cells interact with local lymphoid tissue organizer (LTo) cells to initiate expression of various cytokines (IL-7, IL-17 and RANKL) and cytokine receptors (IL-7R, IL-17R and RANK) and TLS development. Many immune cells can replace LTi cells, such as T helper 17 (Th17) cells, CD8 + T cells, B cells, M1-polarized macrophages, innate lymphoid cell-3(ILC3) and natural killer cells. Similarly, cancer-associated fibroblast (CAF), vascular smooth muscle cells (VAMC) and adipocyte can replace LTo cells. Through the binding of lymphotoxin α1β2 (LTα1β2) to LTβ receptor (LTβR), this signaling pathway promotes the secretion of vascular endothelial growth factor A (VEGFA) and vascular endothelial growth factor C (VEGFC) to promote HEV development. It can also promote the secretion of adhesion molecules used to recruit immune cells, such as vascular adhesion molecule 1 (VCAM 1), intercellular adhesion molecule 1 (ICAM1), and mucosal vascular addressin cell adhesion molecule 1 (MADCAM 1) as well as chemokines (CCL19, CCL21, CXCL12, and CXCL13). These chemokines induce LTα1β2 expression on lymphocytes, recruit lymphocytes from nearby HEVs into TLS and form T-cell and B-cell zones. (b) The maturity stage of TLS. Maturation of TLS undergoes three stages of evolution. From loose lymphoid aggregates to primary follicles of T and B cells with macrophages and DCs, to mature polarized structures containing GCs, host cells, HEVs, and lymph vessels (LVs). (c) The location of TLS in the body. The location of TLS in the human body includes stromal, peritumoral and intratumoral. IL-7, interleukin-7; RANK, NF-κB receptor activator; RANKL, NF-κB ligand receptor activator; CCL19, C-C motif chemokine 19; CXCL12, C-X-C motif chemokine 12
Mature TLSs are typically associated with positive anti-tumour immune responses in cancer. However, the role of TLS in the cancer immune landscape varies, with not all TLS actively contributing to the immune response against cancer. This variability may be attributed to differences in TLS density and maturity, which influence their structural composition and cellular makeup. The maturation of TLS is a multi-stage process, characterized by distinct structural and functional phases (Fig. 1b). Immature TLS, also known as early TLS (E-TLS), represent the initial stage, consisting of loosely aggregated T cells, B cells, and stromal cells. These early structures act as a foundation, recruiting additional immune cells and evolving into more organized forms in response to chemokines and cytokines (e.g. CXCL13, CXCL12, TNF-α, IL-17) secreted by stromal or lymphocyte cells [25, 46]. The resulting primary follicular-like TLS (PFL-TLS) feature T-cell-surrounded B-cell clusters with follicular DCs but lack GCs [25]. The transition to secondary follicle-like TLS (SFL-TLS) marks the final maturation stage, distinguished primarily by the emergence of GC activity. Mature TLS also exhibit a different positional relationship to the tumour (Fig. 1c). Despite these classifications, the efficacy of E-TLS in inducing anti-tumour responses remains unproven, and the presence of TA-TLS does not guarantee a beneficial immune response. Current evidence suggests that only TLS with GCs are functional in combating tumours [47]. Research further elucidates this complexity. Pathological and gene expression profiling of 127 early liver lesions revealed that 24% of the samples exhibited E-TLS and that these were clearly associated with immunosuppression and elevated expression of immunodeficient genes, which could not prevent progression to hepatocellular carcinoma (HCC) and appeared to favor immune evasion [48]. In another study involving 273 HCC patients, findings indicated a correlation between the risk of early tumour relapse and the presence, as well as the level of maturity, of TLS within the tumours [49]. Notably, the presence of mature TLS within tumours significantly improved the overall survival rates of patients with perihepatic cholangiocarcinoma. This outcome starkly contrasts with the effects observed in the presence of E-TLS or PFL-TLS, underscoring the critical role of TLS maturity in patient prognosis [50].
Intercellular interaction in TLS promotes anti-tumour immunity
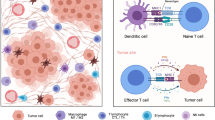
Previous studies have highlighted that elevated densities of TILs are associated with favorable clinical outcomes across a wide array of solid tumours, underscoring the therapeutic promise of TIL-based approaches [51, 52]. Within the milieu of TIL-positive tumours, mature TLS emerge as pivotal arenas for intricate immune cell interactions [53]. These interactions within an active TLS microenvironment not only facilitate the (re)activation of initial or memory immune cells but also promote the expansion of effector cells, a process that surpasses the capabilities of TILs navigating the tumour bed without directional cues. Despite these beneficial dynamics, the accumulation of regulatory cells within TLS may dampen immune responses, leading to the potential inactivation or dormancy of TLS [54]. Nonetheless, when immune cells within TLS work collaboratively, particularly when B cells produce tumour-specific antibodies and cytotoxic T lymphocytes are activated, they orchestrate a potent anti-tumour immunity, thereby significantly improving the patient’s prognosis (Fig. 2) [19].
The roles of TLS in tumour immunity. (a) Anti-tumour effects of immune cell crosstalk in TLS. In the germinal centre (GC) of TLS, GC B cells differentiate into memory B cells and plasma cells (PCs) after receiving antigen delivery from FDCs and interacting with T follicular helper cells (Tfh). In the T-cell compartment, differentiated B cells and dendritic cells (DCs) present antigenic peptides to effector T cells, promoting infiltration of effector T cells into the tumour bed. Plasma cells follow the trajectory of fibroblasts into the tumour bed and produce anti-tumour IgG antibodies. In tumours, memory B cells can destroy tumour cells by expressing tumour necrosis factor-associated apoptosis-inducing ligand (TRAIL) or releasing granzyme B. Fc receptors on natural killer (NK) cells and macrophages can bind to a constant region of anti-tumour antibodies and destroy tumour cells through antibody-dependent cell-mediated cytotoxicity (ADCC) or antibody-dependent cell-mediated phagocytosis (ADCP). (b) Tumour-promoting effect of immune cell crosstalk in TLS. IgA PCs are associated with the secretion of suppressor cytokines, which generate an immunosuppressive environment favouring the emergence of M2 macrophages, myeloid-derived suppressor cells (MDSC), regulatory T (Treg) cells, and B (Breg) cells, and suppressing the function of effector T cells. Th2 cells produce IL-4 and IL-13, respectively, which promote tumour entry into the circulation by increasing epidermal growth factor expression and indirectly inhibit effector T cell function by increasing TGF-β production by MDSC. Macrophages upregulate PD-L1 to support local immunosuppression after ADCP. Complement component 1q (C1q) produced by macrophages binds to IgG-covered tumour cells, activating the classical complement cascade reaction and promoting tumour growth and angiogenesis. B Cells also produce VEGF, which promotes angiogenesis
Antigen presentation and differentiation of B cells
The multifaceted role of B cells as antigen-presenting cells in TLS
As antigen-presenting cells (APCs), B cells have the capacity to activate and proliferate into plasma cells (PCs) by specifically interacting with cognate antigens through B cell receptors (BCRs), initiating signaling cascades that lead to antigen internalization [55]. Within TLS, B cells can present internalized and processed antigens in association with major histocompatibility complex (MHC) II molecules to CD4 + T cells via the BCR, triggering their activation [52]. In ovarian cancer, B cells demonstrate the capability to present antigenic peptides to CD8 + T cells via MHC I molecules, providing evidence that antigen presentation by MHC I may be of significant importance [56]. Rita Cabrita et al. uncovered that individual B cells within TLS express high levels of both MHC class I and class II molecules, suggesting the proficiency of TLS B cells in antigen presentation [57]. Furthermore, Sheng Hong et al. utilized RNA phage Qβ-derived virus-like particles as a model antigen to show that antigen-specific B cells act as potent APCs, capable of activating naïve CD4 + T cells and encouraging their differentiation into T follicular helper (Tfh) cells [58]. Studies on NSCLC have revealed that tumour-infiltrating B cells present antigens and activate CD4 + T cells in certain NSCLC patients. The activation or depletion of TIL-B phenotypes correlates with CD4 + T cell phenotypes and is associated with improved clinical outcomes in NSCLC patients, showcasing elevated TME activation and enhanced TIL-B antigen presentation [59].
Differentiation of B cells into plasma cells for antibody production
Recent studies have demonstrated a significant correlation between the high density of TIL-B and PCs within TLS and improved clinical outcomes across various cancer types [60,61,62]. These PCs can generate tumour-specific antibodies that attach to tumour cells. These antibodies can block the activity of specific target proteins on tumour cells, trigger the complement system, and enhance both antibody-dependent cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) [63]. Although the exact mechanism by which PCs and their antibodies in TLS contribute to cancer inhibition is not fully comprehended, a notable presence of B cells and PCs capable of producing IgG and IgA in TLS associated with ovarian cancer, melanoma, lung adenocarcinoma (LUAD), and NSCLC has been observed and found to be beneficial in improving the overall survival (OS) of patients [64,65,66,67]. This observation underscores their pivotal role in augmenting anti-tumour immunity. Mucosa-associated lymphoid tissue with active GCs was identified in 56% of lymphocyte-infiltrating tumours in surgical specimens of pancreatic ductal adenocarcinoma (PDAC). Additionally, restricted areas exhibited the B-cell lymphoma 6 (BCL6), CD21, and Ki67 expression, indicative of somatic hypermutagenesis and affinity maturation of GC B cells. The study found a rise in the proportion of IgG-expressing B cells in both intratumoral and peripheral blood of TLS patients. Analysing the BCR sequences of intratumoral TLS led to the identification of IgG1 subclass heavy chain as the predominant type, indicating that IgG1 class switching is favored in B cells within TLS during antigen recognition [68]. Additional investigations into PDAC have revealed that PDAC not only harbors mature TLS, enhancing T cell functionality and containing a diverse array of tumour-reactive T cells, but also facilitates the differentiation of B cells into PCs within these structures. This process is attributed to the orchestrated interactions among TGF-β signaling, CXCL13 expression by activated T cells, and the supportive role of TGF-β-producing fibroblasts, which collectively promote B cell activation and the sophisticated development of TLS [69].
In NSCLC, Claire and colleagues examined the level of differentiation of B cells located within the tumour using IHC. They found that all B cells present in the tumour had a stage of differentiation that was aligned with their in situ organization in TLS, as revealed by comparison with SLOs. IgG and IgA antibodiesagainst tumour antigens including LAGE-1, MAGEA1, MAGEC2, TP53, NY-ESO-1, and other MAGE antigens were discovered in the supernatants of NSCLC patient B-cells via enzyme-linked immunosorbent assay (ELISA) analysis, indicating the possibility of TLS as an active location for humoral immune responses [70]. In clear cell renal cell carcinoma (RCC), all stages of B-cell maturation leading to PC formation were identified by the researchers. The PC-related gene MZB1, along with the immunoglobulin genes IGHG1 and IGHA1, displayed high expression levels in both the TLS region and the tumour region away from the TLS [71]. This is a strong indication that in TLS, partially antibody-secreting PCs are generated after in situ antigen-driven B-cell activation, and have the ability to migrate to distant sites. Specific labelling of PCs and fibroblasts shows that the former align with fibroblast trajectories and embed within fibroblast networks, locally and further afield within the TLS region [71]. This data suggests that PCs can spread distally along fibroblast paths within the tumour bed (Fig. 2a).
In ovarian cancer, TLS is often surrounded by densely infiltrating PCs, who produce antibodies within the tumour. Their ability to target tumour-associated antigens (TAAs) has also been demonstrated. Although Iglesia and colleagues found that B-cell profiles were inversely associated with OS in glioblastoma and renal cancer via assessing several published B-cell/PC profiles for 11 cancer types, they also stated that bioinformatics methods still have limitations because different B-cell characteristics and other immune cell characteristics usually produce different prognostic outcomes in the same tumour type [72].
T cells exert both cytotoxic and helper functions
During the anti-tumour immune response, APCs capture tumour antigens from tumour tissue and migrate to draining lymph nodes (DLNs), where they present these antigens to T-cells, triggering T-cell activation [73]. TA-TLS form in close proximity to malignant cells within the TME, facilitating a more direct and efficient T-cell activation compared to distant DLNs [74]. APCs can rapidly migrate into the TLS and present antigenic peptides to T cells, and the amount of tumour antigen in the TLS can exceed that in the tumour-draining lymph node (TDLN) [75]. Importantly, a study by Miao He et al. demonstrated that TLS maturation in NSCLC is linked to a lower risk of postoperative recurrence in patients, whereas tumour-invasive TDLN reduces the prognostic relevance of TLS [76].
Within the B-cell zone of TLS, GC B cells are closely correlated with total TILs, which are marked by Tfh TIL, T helper 1-directed CD4 TIL, TIL-B antibody secretion and long-term survival, indicating the existence of mature TLS [54, 77]. The principal accessory cell of TIL-B is closely linked to Tfh TIL, identified by expression of CXCR5, a marker known for its role in anti-tumour immune responses within TLS across various tumour types [78]. It’s crucial to note that Tfh cells, typically located within and adjacent to B-cell follicles, are indispensable for B-cell development and survival. They synergistically promote B-cell activation and differentiation by facilitating interactions between inducible co-stimulatory molecules such as ICOS:ICOS ligands (ICOSL) and CD40:CD40 ligand (CD40L), along with cytokines including IL-10 and IL-21. These interactions subsequently enhance the expression of Bcl6, thereby stabilizing the Tfh cell phenotype [10, 79, 80]. This mechanism aligns with observations from a CRC mouse model, where the introduction of Helicobacter hepaticus (Hhep) led to an increase in TLS numbers around the tumour, with the CD4 + T-cell response driven by Tfh cells playing a pivotal role. The absence of Tfh cells, however, resulted in the disappearance of TLS and diminished immune cell infiltration [81]. Thus, functional Tfh cells within TLS are key to effectively activating GC B-cells and facilitating the production of immunoglobulin antibodies, while also inducing the expression of IFN-γ and GZMB in activated CD8 + T-cells, enabling their activation and egress from the TLS [54].
Recently, researchers discovered two lymphocyte subsets that are age-dependent - CD153PD-1CD4 senescence-associated T (SAT) cells and CD30T-bet age-associated B-cells (ABCs) - in a mouse model of induced senescence for renal injury TLS. In this model, CD153 or CD30 gene deletion impairs the functional induction of SAT cells and reduces the number of ABC and GC B cells, thereby affecting TLS formation. This study also verified the presence of Tfh cells with similar SAT gene expression and CD153/CD30 signaling pathway in humans [82]. In addition, in mouse spleen age-associated CD4 + T cell subpopulations were also observed in the spleen of mice, including exhaustive cells (with gene expression similar to SAT cells), cytotoxic cells, and Treg cells [83]. This suggests that age-related changes in immune cell function may have broader systemic effects beyond renal injury, perhaps affecting cancer immunity.
Follicular regulatory T (Tfr) cells and B cells interact in TLS to promote tumour progression
In the cancer immunity cycle, the balanced ratio of effector T cells to follicular regulatory T cells (Tfr) is critical, determining the outcome of cancer immunity. Tfr cells, characterized by the CD25 + CXCR5 + GARP + FOXP3 + phenotype and a demethylated forkhead box protein 3 (FOXP3) gene, undergo differentiation through multiple signaling pathways, primarily regulated by FOXP3 and Bcl-6 [50, 84]. The presence of Bcl-6 in Tfr cells, a key transcription factor also found in Tfh cells, suggests a functional link between Tfr and Tfh cells. Recent studies demonstrate that activation of IL-2, STAT3, and STAT5 pathways facilitates the conversion of memory Tfh cells into functional Tfr cells, targeting the FOXP3 and BCL6 genes [85]. Tfr cells produce high levels of TGF-β, which significantly inhibit Tfh cell expansion, GC activation of self-reactive B cells, and autoantibody production [86, 87]. However, mouse studies have demonstrated that IL-21 impedes Tfr cell development by inhibiting protein kinase B (Akt) phosphorylation and diminishing TGF-β and Foxp3 expression [85]. Consequently, the ratio of functional Tfh to Tfr cells dictates the varied impacts on tumour immunity [88]. In BC, Tfr cells have been observed to inhibit Tfh cell function within TLS [54]. Another study identified a significant presence of tumour-infiltrating regulatory T (Ti-Treg) cells around BC tumours, and these Ti-Treg cells were associated with recurrence and death in patients [89]. In NSCLC patients, Tregs migrating within TLS exhibited poor prognostic value, indicating that these TLS-associated Treg actively suppress the immune functions of certain cells such as DCs, T and/or B cells, mirroring findings in lung tumour models [90]. A negative correlation was observed in NSCLC between TLS-B cell density and DPP4 gene expression, a receptor implicated in TCR-mediated T-cell co-activation and Treg-mediated immunosuppression on TIL CD4 + T cells [67]. In early-stage lung cancer, an enrichment and infiltration of Tfr cells within intratumoral TLS were noted, with Tfr cells showing a negative correlation to CD8 + T cells [88].
The ultimate effect of B cells within a tumour is influenced in many ways, within the TLS, by their own phenotype, secreted cytokines, the type of antibody produced by differentiation into PCs, the presence and activation of Treg and activated macrophages [91, 92]. The presence of PD-1 in activated B cells inhibits BCR-mediated signalling, affecting self-maturation and antibody production. In turn, anti-PD-L1 antibodies acting on PD-L1 + Breg cells can impair their inhibitory effect on CD8 + and CD4 + T cells [93]. Although both IgG- and IgA-secreting PCs have been observed in RCC, only a small fraction of IgA is present, and effective anti-tumour activity is mainly associated with IgG [94]. IgA production may be associated with immunosuppressive TGF-β, as well as the secretion of inhibitory cytokines PD-L1 and IL-10 [52, 95]. This suggests that they are usually associated with a poor prognosis (Fig. 2b) [96]. Additionally, a high proportion of intratumoral IgA was observed to be associated with poor prognosis in LUAD [65]. Breg subsets, a subtype of B cells, can induce Treg cell differentiation and are associated with poor clinical outcomes in cancer [97]. Cytokines such as IL-10, IL-35, and TGF-β produced by Breg cells may indirectly inhibit the anti-tumour immune response on the one hand, and on the other hand, may also impair the effector function of T cells, tilting the macrophage towards an immunosuppressive phenotype [1]. This ultimately leads to an inefficient anti-tumour immune response. The presence of Breg cells in E-TLS-like lymphoid aggregates containing Treg cells was observed in BC, and their coexistence resulted in shorter metastasis-free survival compared to Treg cells alone [98].
Tumour-associated myeloid cells have been reported to be associated with poor prognosis. Tumour-associated macrophage-expressing PD-L1 can directly or indirectly suppress T cell responses by recruiting Tregs [99]. Additionally, macrophage-produced indoleamine 2,3-dioxygenase (IDO) has an inhibitory effect on immune cells [100]. M2-polarized macrophages are associated with poor cancer prognosis in BC, liposarcoma, and gastrointestinal stromal tumours [101,102,103]. The analysis of TLS in patients with colorectal liver metastases revealed that the density of Treg cells, M2 macrophages, and Tfh cells in intratumoral TLS was significantly higher than that in peritumoral TLS and positively correlated with poor survival [104]. In a rat model of lymphatic malformation (LM), M2 polarized macrophages may accumulate in infected LM via TLS and contribute to disease progression by secreting VEGF [105].
TLS as biomarkers for tumour prognosis
Numerous studies have demonstrated a correlation between TLS and heightened objective response rates, indicating that TLS is a promising marker for immunotherapy. In light of TLS’s contribution to tumour immunity, researchers have increasingly explored the nature of TLS’s existence and its genetic signature to develop precise and dependable predictive biomarkers for cancer patients’ prognosis and treatment (Table 2).
The number, density and location of TLS affect tumour prognosis
The presence of TLS in solid tumours is generally associated with a favorable prognosis due to indicative effective immune infiltration. The significantly lower number of TLS in patients with stage III NSCLC compared to those with stage II suggests that tumour cells evade the immune response by dysregulating specific chemokine pathways to promote TLS formation during cancer progression [113, 114]. Moreover, lung cancer patients with chronic obstructive pulmonary disease (COPD) exhibited fewer B cells and TLS, correlating with earlier mortality [115]. Analysis in triple-negative breast cancer (TNBC) revealed that high-density PCs were associated with increased TLS counts and a better prognosis than in TNBC with low-density PCs [116].
Further investigations have shown that both peritumoral and intratumoral TLS are present in mice and humans, occasionally extending into normal tissues distant from the tumour [91]. It is currently unclear whether the difference in spatial location between intratumoral and peritumoral TLS holds predictive and/or prognostic value. The evidence available suggests that intratumoral TLS may have better prognostic significance, although this conclusion has yet to be confirmed by extensive analyses [117]. In BC, IHC analysis revealed that the presence of TLS adjacent to or distal from the tumour border correlated with a worse prognosis compared to those without peritumoral TLS, with an increase in the density of peritumoral TLS further exacerbating the prognosis [118]. Another study on BC revealed that TILs were observed at nearly all metastatic sites, whereas TLS were only identified in the liver and lungs, with their presence being almost negligible in brain metastatic lesions [119]. This study implies that the existence and structure of TA-TLS are connected to the TME and anatomical position, and that TLS tissue at diverse spatial levels is created by varied mechanisms that require further exploration in regards to their immunological properties and implications for patient survival and response to treatment.
TLS detection and quantitative analysis
Current conventional methods for detecting TLS include hematoxylin and eosin (H&E) staining, IF, mIHC, and spatial transcriptomics [120]. These techniques rely on the examination of tumour samples from specific regions, hence the unavailability of surgically obtained samples due to inoperable conditions limits their effectiveness in TLS detection. The selection of specific circulating markers and/or the combined development and application of visual imaging characterization techniques may enhance the accuracy and specificity of TLS detection. Research in this area is ongoing (Fig. 3).
Strategies for quantitative analysis of TLS. TLS as a predictive marker for cancer detection and immune response can be detected and quantified by H&E staining, mIHC, Laser Capture Microdissection (LCM), spatial transcriptomics, and flow cytometry. The application of nanomaterials and artificial intelligence (AI) has led to advances in non-invasive imaging with nanoprobe-based CT and/or imaging. Imaging information can be further analyzed by AI algorithms to improve the efficiency and specificity of TLS detection
Quantitative assessment methods for TA-TLS can currently be divided into two main categories: genomic and histological analyses. However, histological analysis is prone to subjective interpretations, leading to potential bias. Given these disadvantages, histological analysis may not suffice for the efficient and accurate future assessment of TLS compared to genomic approaches [121]. A unique expression signature of nine genes (CD79B, CD1D, CCR6, LAT, SKAP1, CETP, EIF1AY, RBP5, PTGDS) was initially identified through differential gene expression analysis in melanoma, predicting prognosis and response to immunotherapy [57]. Similarly, in a cohort of 515 LUAD patients, these nine transcriptome-wide genes accurately predicted the presence of TLS, demonstrating that TLS characteristics serve as an independent positive prognostic factor for LUAD patients [122]. To quantify TLS, researchers calculated a TLS feature score based on the average expression levels of these nine genes. After determining the optimal threshold for high and low TLS feature scores using X-tile software, they correlated these scores with overall survival rates in patients. Patients with higher TLS feature score expression levels were categorized into a high TLS group, indicating a stronger presence of TLS within the tumour [123]. Not coincidentally, differential gene expression analysis of endometrial carcinoma (EC) showed that TLS was associated with CD8 + T-cell infiltration and L1CAM overexpression, which was only present in morphologically mature TLS with GCs [124]. As previously mentioned, stromal cells are a key factor in the formation of TLS. IFN induces the expression of chemokines including CXCL13 and CXCR5 in lung fibroblasts, thereby promoting the formation of TLS [109]. The expression of CXCL13, associated with tumoural TLS, serves as a potential biomarker for TLS presence in bladder cancer and may predict patient responsiveness to ICI in advanced stages of the disease [108].
Imaging histology, as a non-invasive imaging method, has shown great potential in the diagnosis and treatment of tumours by combining learning models with digital pathology [125]. It has been reported that in patients with NSCLC and intrahepatic cholangiocarcinoma (ICC), it is possible to combine the imaging features of computed tomography (CT) or magnetic resonance imaging (MRI) with clinical information for tumour diagnosis, efficacy evaluation, and prediction of molecular markers [126, 127]. Emerging research suggests the potential of combining these imaging features with advanced learning models and digital pathology for enhanced TLS visualization. A recent study on LUAD revealed that lung nodules with a more solid portion were associated with a high TLS density by CT scanning [128]. Preoperative CT imaging histograms were utilized to predict TLS status and recurrence-free survival in ICC patients, proving more effective than standalone imaging histograms or clinical models in assessing intratumoral TLS status [129]. Researchers developed a graphical whole-slide imaging (WSI) method for assessing lymphocyte aggregation, enabling differentiation between TLS and other infiltrates by analyzing CT imaging characteristics [130]. It is crucial to note that CT imaging primarily reveals higher-density lymphocyte aggregates, rather than the distinctive structures of TLS. While imaging results align with histopathological findings of TLS locations, they offer correlative predictions rather than definitive insights. Furthermore, exploratory technologies, including the deployment of nanoprobes for identifying TLS-specific markers in imaging scans, signal significant advancements in the realm of immunological imaging. Li et al. identified CT imaging characteristics indicative of TLS, enabling preoperative, non-invasive TLS prediction in HCC [131]. In a study employing needle optical coherence tomography (OCT) for the assessment of LNs, researchers considered the region of rapid signal decay to be the GC and minimized the imaging probe to look at B cells and the GC in the internal nodes, which theoretically allows imaging of the TLS [132]. Although promising, these innovative techniques remain in the experimental phase and necessitate further validation before being deemed fully developed.
Advancement in Artificial Intelligence (AI) technology has introduced innovative means to conduct deep learning on graphical data. These techniques can be combined with image features to facilitate a novel method of recognizing TLS. HookNet, a segmentation model for histopathological WSIs, can accurately identify GC-containing TLS by leveraging recognition of previously identified TLS [133]. This methodology has already been implemented in the detection of lung cancer. Additionally, TESLA, a machine learning framework for annotating tissue at the pixel level in spatial transcriptomics, enables the direct annotation of immune and tumour cells in histological images and can further detect TLS in TME [134]. The future integration of AI with genomics, spatial genomics, and immuno-scoring could enable the development of robust TLS quantification methods. These methods have the potential to be applied across a wide range of cancer types, ensuring stability in gene-level quantification.
Preclinical induction of TLS and prospects for its application
The presence of TLS and B cells across a range of tumour types, including carcinomas, melanomas, soft tissue sarcomas, and RCC, is predictive of a positive response to ICB therapy, indicating the potential for ICB to assume an enhanced role in the treatment of tumours rich in B cells and TLS [11, 17, 57, 135, 136]. Furthermore, plasmacytoid B-cell profiles and TLS-associated B-cell profiles have been found to have a positive correlation with ICB responses in melanoma and LUAD [107, 137,138,139]. To improve clinical outcomes post-ICB, therapies supporting TLS induction and activating B cells are necessary and require further research.
In mouse models of cancer and in patients with cancer, cytokines and chemokines, chemotherapy, radiotherapy, cancer vaccines and ICB treatments have demonstrated the ability to induce intra-tumour formation of TLS and increase the infiltration of immune cells (Table 3). In pancreatic cancer, targeted LIGHT localizes to the tumour vasculature via vascular-targeting peptide (VTP), inducing vascular normalization and the formation of HEV and TLS through the self-amplifying cycle of pancreatic cancer. The mechanism may be that LIGHT stimulates macrophages to express the inflammatory cytokines CXCL13, CXCL21, TNF, and IL-6, which recruits T-cells and thus promotes TLS formation [140, 141]. Low doses of STING agonists enhance T-cell and DC infiltration, foster an inflammatory TME, and stimulate DC maturation, which leads to the local secretion of CCL19, CCL21, LTα, LTβ, and LIGHT, thereby promoting vascular normalization and TLS formation [142]. In a trial of 39 patients with resected PDAC treated with a GM-CSF vaccine, 33 exhibited TLS aggregates within two weeks of vaccination. And inhibition of the Treg signalling pathway and enhancement of Th17 signalling within these TLS aggregates were found to be associated with increased survival [143]. Analysis of TLS in pancreatic cancer patients undergoing preoperative neoadjuvant chemoradiotherapy (NAC) through IHC revealed altered TME compositions, increased immune cell proportions, and favorable prognoses [144]. However, a study on lung squamous cell carcinoma (LSCC) patients indicated that corticosteroid use during chemotherapy impacted GC and TLS formation [47]. It is crucial to acknowledge that definitive evidence supporting the capacity of certain immunotherapies to initiate TLS in cancer is presently insufficient, necessitating further empirical research. Specifically, although radiotherapy has been observed to trigger the formation of intratumoral TLS, this phenomenon primarily results from the immediate depletion of local immune cells caused by low-dose radiation, accompanied by a diminution in the size of pre-existing TLS, which typically recuperates within a fortnight [145, 146]. Hence, induction of TLS by radiotherapy is currently not supported by sufficient evidence. Importantly, conventional cancer immunotherapies exhibit limitations, such as varying systemic toxicities and heterogeneous therapeutic outcomes, depending on the type of immunotherapy employed [7, 147]. Recent advancements in nanotechnology and biomaterials have unveiled significant potential for innovation in cancer immunotherapy (Fig. 4) [148].
Methods and prospects for inducing TLS in mice and cancer patients in vivo The emergence of TLS has been induced in the past in mice or cancer patients by means of cytokines and chemokines, chemotherapy, radiotherapy, cancer vaccines and immunotherapy. Recently lysosomal viruses (OV) have also raised possibilities in TLS induction. In addition to this, biomaterials show considerable promise for TLS induction. Collagen matrices, hydrogels, nanomaterials, three-dimensional (3D) scaffolds, and organoids all have the potential to serve as new induction modalities
Collagen matrix
The formation of collagen in mammalian connective tissue requires intricate intra- and extracellular processes. Its bioactivity arises from its complex structure, which has facilitated its recent biosynthetic applications [157]. In mice, the creation of TLS was initially achieved by implanting artificial collagen matrices derived from a thymic stromal cell line expressing LTα, producing functional TLS [158]. Subsequent implantation of these structures into severely combined immunodeficient mice resulted in a robust secondary immune response and production of high levels of high-affinity IgG antibodies [159]. Similarly, collagen sponge scaffolds containing a combination of chemokines CCL19, CCL21, CXCL12, CXCL13 and soluble RANKL as well as LTα1β2 were implanted into mouse kidneys, and TLS were successfully constructed containing isolated B and T cell regions as well as HEVs [160].
Hydrogel
Hydrogels are unique entities consisting of a crosslinked polymer mesh in three dimensions, capable of extending the duration of drug effectiveness owing to their favourable biological characteristics like biocompatibility, water retention, and bioadhesive qualities, rendering them an exceptional therapeutic vehicle [161]. According to a study by Alberto Purwada et al., hydrogels with stromal cells expressing BAFF and IL-4 enhanced GC responses, facilitating antibody class switching events [162]. Recently, researchers have constructed an interferon gene activator hydrogel that initiates and activates the stimulator of interferon genes (STING) and TLR-9 pathway to enhance TLS formation [163].
Nanomaterials
As transport carriers, nanomaterials can transport biologically active combinatorial loads to specific sites in the body in a controlled manner and through their high specificity, thereby effectively reducing dose and off-target toxicity [164]. The combination of nanomedicine and immunotherapy has been effective in enhancing anti-tumour immune responses and improving the safety of therapeutic responses [165]. In a study simulating a mouse model of nasopharyngeal carcinoma, researchers developed a nanovaccine composed of Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) and tannic acid, combined with Mn2 + and cytosine-phosphate-guanine (CpG) as dual adjuvants. This formulation synergistically activates DCs, B cells, and T cells through the TLR-9 and STING signaling pathways. Additionally, it enhances the secretion of CCL19/CCL21, CXCL10, and CXCL13 in the TME through the activation of the LT-α and LT-β pathways, which together promote the recruitment of peripheral immune cells, thereby promoting the formation of TLS, enhancing local immune response and delaying tumour growth [166]. The ‘Nano-sapper,’ a biomaterial comprising an anti-fibrotic core of phosphate-modified α-mangostin and a plasmid encoding the immune-boosting cytokine LIGHT, has been demonstrated to promote TLS formation and impede tumour growth. This biomaterial achieves its effects by reversing the abnormal activation of fibroblasts, diminishing collagen deposition, normalizing the vasculature within tumours, and enhancing the local expression of chemokines by lymphocytes. These actions lead to increased infiltration of CTLs, the induction of TLS within tumours, the remodeling of the TME, and an improved efficacy of ICB therapy [167].
3D technology and organoid
Three-dimensional (3D) scaffolds, fabricated via 3D printing technology, offer a superior delivery mechanism for cancer vaccines over hydrogel scaffolds, attributed to their outstanding porous structure and biosafety. Studies have demonstrated that these 3D scaffolds, when loaded with immunomodulators, can recruit and activate numerous immune cells, particularly APCs and T cells, mimicking a lymphoid organ’s function. This process facilitates the development of an “artificial tertiary lymphoid structure,” proven to inhibit tumour growth effectively [168].
Human organoids have enormous potential for biomedical applications in organ development and disease onset, preclinical drug development, and regenerative medicine [169]. Organoids are essentially multicellular clusters formed in vitro by 3D cell culture techniques, giving us a possible model in terms of TLS induction [170]. Küçükköse E and colleagues developed a humanized mouse model for spontaneous multi-organ tumour metastasis using patient-derived organoids. The study found that ICB therapy successfully eradicated liver metastases without affecting peritoneal metastases, which, contrary to the shrinking primary tumour, increased after therapy cessation, with B-cell infiltration and TLS formation observed at the primary tumour and liver metastasis sites but not at peritoneal metastases, potentially explaining the variability in ICB treatment outcomes [171].
Nonetheless, the clinical application of organoid model construction is limited due to its low success rate, difficulty in blood vessel formation, large repeatability and variation, and difficulty in cost control [172]. Recent studies combining organoids with 3D bioprinting and organ vaccines have opened up new possibilities for precision research in cancer modelling and have addressed some of the dilemmas and limitations encountered in organoid research. 3D bioprinting and organ microarrays can generate or mimic the vasculature system to supply nutrients to organoids, allowing for greater growth and development [172, 173]. Goyal et al. also found that T and B lymphocytes isolated non-invasively from human peripheral blood can spontaneously assemble into ectopic lymphoid follicles in a dual-channel organ-on-chip microfluidic control system [174]. These have inspired new ideas for TLS induction strategies.
Through the elaboration of conventional cancer immunotherapy as well as methods developed on the basis of nanotechnology and biomaterials to induce TLS, it can be concluded that this mechanism of TLS induction may be formed through a network mechanism in which multiple cells, multiple chemokines and multiple signalling pathways act together. Although the intermediate effects produced by the different induction strategies are not identical, they all seem to attenuate the immunosuppressive effects in TME while boosting the function of immune cells, thus activating the signalling cascade pathways for TLS production.
Conclusion and perspectives
The spatial structure of TLS (presence of GCs), its cellular composition (high-density TIL infiltration and differences in immune cell phenotypes), and its relationship to tumour location (peri-tumour or intra-tumour) affect the prognosis of tumour patients [3, 4, 25, 48, 175]. Notably, the dual role of TLS in tumour immunity—termed the “double-edged sword” effect—may hinge more on the proportions of various immune cell subpopulations within the TLS, particularly given the observed variability in T and/or B cell phenotypes. Among T cell subsets, CD4 + T follicular helper (Tfh) cells, expressing high levels of CXCR5 and Bcl6, along with CD8 + T cells, are the primary contributors to anti-tumour immunity among T cell subsets. But the precise mechanism underlying the cytotoxic effect of TLS-modified CD8 + T cells on tumour cells remains unclear. Tfh cells facilitate B cell recruitment through the high expression of co-stimulatory receptors (ICOS and CD153), thereby slowing tumour progression. Tfr cells, characterised as CD25CXCR5GARPFOXP3, inhibit Tfh cell activity with the involvement of TGF-β. Despite the lack of consensus on markers for Breg cells, their secretion of immunosuppressive cytokines such as IL-10, IL-35, and TGF-β can inhibit effector T cell activity and promote the expansion of Treg cells, thereby facilitating tumour growth. The characterization of B cell subpopulations through RNA sequencing (RNA-seq) or specific antibodies revealed that, with the exception of Breg cells, all B cell subpopulations generally correlate with positive clinical outcomes. The in situ activation of GC-B cells within TLS for differentiation and antibody production further underscores the significance of B cells within the cancer immune cycle. The phenotype of different immune cell subpopulations is largely dependent on cytokines with different immune properties in the TME, as well as on the influence of cell signalling pathways, which are not yet fully understood.
As a novel specific biomarker to influence and determine the prognosis of cancer patients, the future development of TLS involves two main aspects. Although biomarkers of TLS have been widely studied, there is no established uniform standard for quantification of TLS in different patients and different cancers. First, we need to focus on the difficulties that the heterogeneity of TLS poses for the quantification and assessment of different cancer types. The combined application of specific TLS markers, imaging technologies and AI in histopathology may open up a new path for TLS detection and quantitative analysis.
Second, we need to effectively induce the occurrence and maturation of TLS in tumours. Traditional induction methods through injection of cytokines or chemokines, radiotherapy, chemotherapy, ICB therapy and cancer vaccines have successfully induced TLS in mouse models or humans, but novel biomaterials such as collagen mechanisms, hydrogels, nanomaterials as well as the development of organoid and 3D technologies have reinvigorated TLS induction and enhanced the induction efficiency. The possibility of using complex technologies, including novel biomaterials, to generate inducible TLS, is being investigated with very promising results in preclinical models [176]. Nevertheless, the translation of these technological tools into the clinic still needs to address issues of tissue safety, cost control and selection of appropriate humanised in vivo models. This is essential to guide further precision and efficiency of TLS in immunotherapy and prognosis.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- 3D:
-
three-dimensional
- ABCs:
-
Age-associated B-cells
- ADCC:
-
Antibody-dependent cytotoxicity
- ADCP:
-
Antibody-dependent cellular phagocytosis
- AI:
-
Artificial Intelligence
- APCs:
-
Antigen-presenting cells
- BC:
-
Breast cancer
- Bcl6:
-
B-cell lymphoma 6
- BCRs:
-
B cell receptors
- Breg:
-
Regulatory B cells
- C1q:
-
Complement component 1q
- CAF:
-
Cancer-associated fibroblast
- CCL:
-
C-C motif chemokine
- COPD:
-
Chronic obstructive pulmonary disease
- CpG:
-
Cytosine-phosphate-guanine
- CRC:
-
Colorectal cancer
- CT:
-
Computed tomography
- CTLA-4:
-
Cytotoxic T - Lymphocyte Antigen 4
- CXCL:
-
C-C motif chemokine
- DCs:
-
Dendritic cells
- DLNs:
-
Draining lymph nodes
- EBNA1:
-
EBV nuclear antigen 1
- EBV:
-
Epstein-Barr virus
- EC:
-
Endometrial carcinoma
- ELISA:
-
Enzyme-linked immunosorbent assay
- E-TLS:
-
Early TLS
- FAP:
-
Fibroblast activation protein
- FDCs:
-
Follicular dendritic cells
- F-IHC:
-
Fluorescence immunohistochemistry
- FOXP3:
-
Forkhead box protein 3
- GC:
-
Germinal centers
- H&E:
-
Hematoxylin and eosin staining
- HCC:
-
Hepatocellular carcinoma
- HEVs:
-
High endothelial venules
- Hhep:
-
Helicobacter hepaticus
- HNSCC:
-
head and neck squamous cell carcinoma
- HPV:
-
Human papillomavirus
- ICAM1:
-
Intercellular adhesion molecule 1
- ICB:
-
Immune checkpoint blockade
- ICC:
-
Intrahepatic cholangiocarcinoma
- iCCA:
-
Intrahepatic cholangiocarcinoma
- ICOS:
-
Inducible co-stimulatory molecules
- ICOSL:
-
Inducible co-stimulatory molecular ligands
- IDO:
-
Indoleamine 2,3-dioxygenase
- IF:
-
Immunofluorescence
- IFN:
-
Interferon
- IHC:
-
Immunohistochemistry
- IL:
-
Interleukin
- ILCs:
-
Innate lymphocytes
- ILFs:
-
Isolated lymphoid follicle
- IrAE:
-
Immune-related adverse events
- LCM:
-
Laser Capture Microdissection
- LM:
-
Lymphatic malformation
- LNs:
-
Lymph nodes
- LSCC:
-
Lung squamous cell carcinoma
- LTi:
-
Lymphoid tissue inducing cells
- LTo:
-
Lymphoid tissue-forming cells
- LTα1β2:
-
Lymphotoxinα1β2
- LTβR:
-
LTβ receptor
- LUAD:
-
Lung adenocarcinoma
- LVs:
-
Lymph vessel
- MADCAM 1:
-
Mucosal vascular addressin cell adhesion molecule 1
- MDSC:
-
Myeloid-derived suppressor cells
- MHC:
-
Major histocompatibility complex
- MIHC:
-
Multiplex immunohistochemistry
- MRI:
-
Magnetic resonance imaging
- NALT:
-
Nasal associated lymphoid tissue
- NKT:
-
Natural killer T cells
- NSCLC:
-
Non-small-cell lung cancer
- OCT:
-
Optical coherence tomography
- OS:
-
Overall survival
- OV:
-
Lysosomal viruses
- PCs:
-
Plasma cells
- PD-1:
-
Programmed cell death protein 1
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PD-L1:
-
Programmed cell death ligand 1
- PFL-TLS:
-
Primary follicular-like TLS
- RANK:
-
NF-κB receptor activator
- RANKL:
-
NF-κB ligand receptor activator
- RCC:
-
Renal cell carcinoma
- RNA-seq:
-
RNA sequencing
- SAT:
-
Senescence-associated T-cells
- SCID:
-
Severely combined immunodeficient
- SFL-TLS:
-
Secondary follicle-like TLS
- SLOs:
-
Secondary lymphoid organs
- STING:
-
Stimulator of interferon genes
- TAAs:
-
Tumour-associated antigens
- TA-TLS:
-
Tumour-associated tertiary lymphoid structure
- TCRs:
-
T cell receptors
- TDLN:
-
Tumour-draining lymph node
- Tfh:
-
T follicular helper cells
- Tfr:
-
Follicular regulatory T cells
- TGF:
-
Transforming growth factor
- Th:
-
T helper cells
- TIL-B:
-
Tumour-infiltrating B-cell
- TILs:
-
Tumour-infiltrating lymphocytes
- Ti-Treg:
-
Tumour-infiltrating regulatory T
- TLR9:
-
Toll-like receptor 9
- MyD88:
-
Myeloid differentiation factor 88
- TLSs:
-
Tertiary lymphoid structures
- TME:
-
Tumour microenvironment
- TNBC:
-
Triple-negative breast cancer
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cell
- VAMC:
-
Vascular smooth muscle cells
- VCAM 1:
-
Vascular adhesion molecule 1
- VEGF:
-
Vascular endothelial growth factor
- VTP:
-
Vascular targeting peptide
- WSI:
-
Whole-slide image
References
Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol, 18:842–59.
Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol, 14:447–62.
Posch F, Silina K, Leibl S, Mündlein A, Moch H, Siebenhüner A, Samaras P, Riedl J, Stotz M, Szkandera J et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology, 7:e1378844.
Sun X, Liu W, Sun L, Mo H, Feng Y, Wu X, Li C, Chen C, Li J, Xin Y et al. Maturation and abundance of tertiary lymphoid structures are associate d with the efficacy of neoadjuvant chemoimmunotherapy in resectable no n-small cell lung cancer. J Immunother Cancer, 10:e005531.
Wang Q, Sun K, Liu R, Song Y, Lv Y, Bi P, Yang F, Li S, Zhao J, Li X et al. Single-cell transcriptome sequencing of B-cell heterogeneity and terti ary lymphoid structure predicts breast cancer prognosis and neoadjuvan t therapy efficacy. Clin Translational Med, 13:e1346.
Maibach F, Sadozai H, Seyed Jafari SM, Hunger RE, Schenk M. Tumor-infiltrating lymphocytes and their prognostic value in cutaneous melanoma. Front Immunol, 11:2105.
Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. Cancer J Clin, 70:86–104.
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D et al. Management of immunotherapy-related toxicities, Version 1.2019. J Natl Compr Cancer Network: JNCCN, 17:255–89.
Lauss M, Donia M, Svane IM, Jönsson G. B cells and tertiary lymphoid structures: friends or foes in Cancer Im munotherapy? Clin cancer Research: Official J Am Associa Tion Cancer Res, 28:1751–8.
Sato Y, Silina K, van den Broek M, Hirahara K, Yanagita M. The roles of tertiary lymphoid structures in chronic diseases. Nat Rev Nephrol, 19:525–37.
Vanhersecke L, Brunet M, Guégan J-P, Rey C, Bougouin A, Cousin S, Moulec SL, Besse B, Loriot Y, Larroquette M et al. Mature tertiary lymphoid structures predict immune checkpoint inhibito r efficacy in solid tumors independently of PD-L1 expression. Nat cancer, 2:794–802.
Fan D, Cao Y, Cao M, Wang Y, Cao Y, Gong T. Nanomedicine in cancer therapy. Signal Transduct Target Therapy, 8:293.
Li H, Lin W-P, Zhang Z-N, Sun Z-J. Tailoring biomaterials for monitoring and evoking tertiary lymphoid St ructures. Acta Biomater, 172:1–15.
Cottrell TR, Taube JM. PD-L1 and emerging biomarkers in Immune Checkpoint Blockade Therapy. Cancer J (Sudbury Mass), 24:41–6.
Chen P-L, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K et al. Analysis of Immune signatures in Longitudinal Tumor samples yields ins ight into biomarkers of response and mechanisms of resistance to Immun E Checkpoint Blockade. Cancer Discov, 6:827–37.
Ding G-Y, Ma J-Q, Yun J-P, Chen X, Ling Y, Zhang S, Shi J-Y, Chang Y-Q, Ji Y, Wang X-Y et al. Distribution and density of tertiary lymphoid structures predict clini cal outcome in intrahepatic cholangiocarcinoma. J Hepatol, 76:608–18.
Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G et al. B cells and tertiary lymphoid structures promote immunotherapy respons e. Nature, 577:549–55.
Sautès-Fridman C, Verneau J, Sun C-M, Moreira M, Chen TW-W, Meylan M, Petitprez F, Fridman WH. Tertiary lymphoid structures and B cells: clinical impact and therapeu tic modulation in cancer. Semin Immunol, 48:101406.
Fridman WH, Meylan M, Petitprez F, Sun C-M, Italiano A, Sautès-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour imm une contexture and clinical outcome. Nat Reviews Clin Oncol, 19:441–57.
Ruffin AT, Cillo AR, Tabib T, Liu A, Onkar S, Kunning SR, Lampenfeld C, Atiya HI, Abecassis I, Kürten CHL et al. B cell signatures and tertiary lymphoid structures contribute to outco me in head and neck squamous cell carcinoma. Nat Commun, 12:3349.
Marinkovic T, Marinkovic D. Biological mechanisms of ectopic lymphoid structure formation and thei r pathophysiological significance. Int Rev Immunol, 40:255–67.
Ware MB, Wolfarth AA, Goon JB, Ezeanya UI, Dhar S, Ferrando-Martinez S, Lee BH. The role of Interleukin-7 in the formation of Tertiary Lymphoid Struct ures and their prognostic value in gastrointestinal cancers. J Immunotherapy Precision Oncol, 5:105–17.
Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, Roulois D, Turner J, Sylvestre M, Asam S et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure f ormation and local pathology. Proceedings of the National Academy of Sciences of the United States o f America, 116:13490–13497.
Collison J. Immunofibroblasts are the cornerstone of TLS formation in pSS. Nat Rev Rheumatol, 15:513.
Dieudé M, Kaci I, Hébert M-J. The impact of programmed cell death on the formation of Tertiary Lymph oid structures. Front Immunol, 12:696311.
Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL Jr. Immune Cell Infiltration and Tertiary Lymphoid structures as Determina nts of Antitumor Immunity. J Immunol (Baltimore Md. 1950;200:432–42.
Hua Y, Vella G, Rambow F, Allen E, Antoranz Martinez A, Duhamel M, Takeda A, Jalkanen S, Junius S, Smeets A et al. Cancer immunotherapies transition endothelial cells into HEVs that gen erate TCF1 < sup>+ T lymphocyte niches through a feed-forward loop. Cancer Cell, 40:1600–e16181610.
Vella G, Hua Y, Bergers G. High endothelial venules in cancer: regulation, function, and therapeu tic implication. Cancer Cell, 41:527–45.
Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol, 26:627–50.
Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean M-C. Tertiary lymphoid structures in cancers: Prognostic Value, Regulation, and manipulation for therapeutic intervention. Front Immunol, 7:407.
Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol, 33:297–305.
Manzo A, Bombardieri M, Humby F, Pitzalis C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthriti s: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev, 233:267–85.
Thaunat O, Patey N, Caligiuri G, Gautreau C, Mamani-Matsuda M, Mekki Y, Dieu-Nosjean M-C, Eberl G, Ecochard R, Michel J-B, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol (Baltimore Md. 1950;185:717–28.
Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in Cancer: drivers of Antitumor Immunity, Immunosuppression, or Bystander sentinels in Disease? Front Immunol, 8:1830.
Gago da Graça C, van Baarsen LGM, Mebius RE. Tertiary lymphoid structures: diversity in their development, Composit ion, and role. J Immunol (Baltimore Md. 1950;206:273–81.
Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Investig, 124:953–9.
Sato Y, Mii A, Hamazaki Y, Fujita H, Nakata H, Masuda K, Nishiyama S, Shibuya S, Haga H, Ogawa O et al. Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tis sues in the kidney. JCI Insight, 1:e87680.
Denton AE, Carr EJ, Magiera LP, Watts AJB, Fearon DT. Embryonic FAP < sup>+ lymphoid tissue organizer cells generate the reticular network of adult lymph nodes. J Exp Med, 216:2242–52.
Ruddle NH. Basics of Inducible Lymphoid organs. Curr Top Microbiol Immunol, 426:1–19.
van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Höpken UE, Lipp M, Niederreither K et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol, 10:1193–9.
Sato Y, Boor P, Fukuma S, Klinkhammer BM, Haga H, Ogawa O, Floege J, Yanagita M. Developmental stages of tertiary lymphoid tissue reflect local injury and inflammation in mouse and human kidneys. Kidney Int, 98:448–63.
Sato Y, Tamura M, Yanagita M. Tertiary lymphoid tissues: a regional hub for kidney inflammation. Nephrology, dialysis, transplantation: official publication of the Eu ropean Dialysis and Transplant Association - European Renal Associatio n, 38:26–33.
Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G et al. Lymphatic neoangiogenesis in human kidney transplants is associated Wi th immunologically active lymphocytic infiltrates. J Am Soc Nephrology: JASN, 15:603–12.
Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, Spencer J, Pitzalis C. Ectopic lymphoid structures support ongoing production of class-switch ed autoantibodies in rheumatoid synovium. PLoS Med, 6:e1.
Le Pottier L, Devauchelle V, Fautrel A, Daridon C, Saraux A, Youinou P, Pers J-O. Ectopic germinal centers are rare in Sjogren’s syndrome salivary gland s and do not exclude autoreactive B cells. J Immunol (Baltimore Md. 1950;182:3540–7.
McDonald KG, McDonough JS, Dieckgraefe BK, Newberry RD. Dendritic cells produce CXCL13 and participate in the development of m urine small intestine lymphoid tissues. Am J Pathol, 176:2367–77.
Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P, Curioni-Fontecedro A et al. Germinal centers determine the prognostic relevance of Tertiary Lympho id structures and are impaired by corticosteroids in Lung squamous cel l carcinoma. Cancer Res, 78:1308–20.
Meylan M, Petitprez F, Lacroix L, Di Tommaso L, Roncalli M, Bougoüin A, Laurent A, Amaddeo G, Sommacale D, Regnault H et al. Early hepatic lesions display immature Tertiary lymphoid structures an d show elevated expression of Immune Inhibitory and Immunosuppressive molecules. Clin cancer Research: Official J Am Associa Tion Cancer Res, 26:4381–9.
Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, Luciani A, Amaddeo G, Derman J, Charpy C et al. Intra-tumoral tertiary lymphoid structures are associated with a low r isk of early recurrence of hepatocellular carcinoma. J Hepatol, 70:58–65.
Zhang F-P, Zhu K, Zhu T-F, Liu C-Q, Zhang H-H, Xu L-B, Xiao G, Liu C. Intra-tumoral secondary follicle-like tertiary lymphoid structures are Associated with a Superior prognosis of overall survival of Perihilar Cholangiocarcinoma. Cancers, 14:6107.
Liu D, Heij LR, Czigany Z, Dahl E, Lang SA, Ulmer TF, Luedde T, Neumann UP, Bednarsch J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Experimental Clin cancer Research: CR, 41:127.
Laumont CM, Nelson BH. B cells in the tumor microenvironment: multi-faceted organizers, regul ators, and effectors of anti-tumor immunity. Cancer Cell, 41:466–89.
Garaud S, Dieu-Nosjean M-C, Willard-Gallo K. T follicular helper and B cell crosstalk in tertiary lymphoid structur es and cancer immunotherapy. Nat Commun, 13:2259.
Noël G, Fontsa ML, Garaud S, De Silva P, de Wind A, Van den Eynden GG, Salgado R, Boisson A, Locy H, Thomas N et al. Functional Th1-oriented T follicular helper cells that infiltrate huma n breast cancer promote effective adaptive immunity. J Clin Investig, 131:e139905.
Rastogi I, Jeon D, Moseman JE, Muralidhar A, Potluri HK, McNeel DG. Role of B cells as antigen presenting cells. Front Immunol, 13:954936.
Hladíková K, Koucký V, Bouček J, Laco J, Grega M, Hodek M, Zábrodský M, Vošmik M, Rozkošová K, Vošmiková H et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squ amous cell carcinoma via cell-to-cell interactions with CD8 < sup>+ T cells. J Immunother Cancer, 7:261.
Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J et al. Tertiary lymphoid structures improve immunotherapy and survival in mel anoma. Nature, 577:561–5.
Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z, Wang W, Zhou X, Zhang F, Ge Q et al. B cells are the Dominant Antigen-presenting cells that activate naive CD4 < sup>+ T cells upon immunization with a virus-derived Nanopar ticle Antigen. Immunity, 49:695–e708694.
Bruno TC, Ebner PJ, Moore BL, Squalls OG, Waugh KA, Eruslanov EB, Singhal S, Mitchell JD, Franklin WA, Merrick DT et al. Antigen-presenting Intratumoral B cells affect CD4 < sup>+ TIL phe notypes in Non-small Cell Lung Cancer patients. Cancer Immunol Res, 5:898–907.
Garg K, Maurer M, Griss J, Brüggen M-C, Wolf IH, Wagner C, Willi N, Mertz KD, Wagner SN. Tumor-associated B cells in cutaneous primary melanoma and improved cl inical outcome. Hum Pathol, 54:157–64.
Liu X, Wu S, Yang Y, Zhao M, Zhu G, Hou Z. The prognostic landscape of tumor-infiltrating immune cell and immunom odulators in lung cancer. Biomed Pharmacotherapy = Biomedecine Pharmacotherapie, 95:55–61.
Hennequin A, Derangère V, Boidot R, Apetoh L, Vincent J, Orry D, Fraisse J, Causeret S, Martin F, Arnould L et al. Tumor infiltration by tbet + effector T cells and CD20 + B cells is asso ciated with survival in gastric cancer patients. Oncoimmunology, 5:e1054598.
Wouters MCA, Nelson BH. Prognostic significance of Tumor-infiltrating B cells and plasma cells in Human Cancer. Clin cancer Research: Official J Am Associa Tion Cancer Res, 24:6125–35.
Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are Associated with Tertiary lymphoid structures, cytolytic T-Cell responses, and Superior Prognosis in Ovar Ian Cancer. Clin cancer Research: Official J Am Associa Tion Cancer Res, 22:3005–15.
Isaeva OI, Sharonov GV, Serebrovskaya EO, Turchaninova MA, Zaretsky AR, Shugay M, Chudakov DM. Intratumoral immunoglobulin isotypes predict survival in lung adenocar cinoma subtypes. J Immunother Cancer, 7:279.
Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melano ma. Cancer Res, 72:1070–80.
Germain C, Devi-Marulkar P, Knockaert S, Biton J, Kaplon H, Letaïef L, Goc J, Seguin-Givelet A, Gossot D, Girard N et al. Tertiary Lymphoid Structure-B Cells Narrow Regulatory T Cells Impact i n lung Cancer patients. Front Immunol, 12:626776.
Gunderson J, Rajamanickam A, Bui V, Bernard C, Pucilowska B, Ballesteros-Merino J, Schmidt C, McCarty M, Philips K, Piening M. B, : Germinal center reactions in tertiary lymphoid structures associate Wi th neoantigen burden, humoral immunity and long-term survivorship in p ancreatic cancer. Oncoimmunology, 10:1900635.
Kinker GS, Vitiello GAF, Diniz AB, Cabral-Piccin MP, Pereira PHB, Carvalho MLR, Ferreira WAS, Chaves AS, Rondinelli A, Gusmão AF et al. Mature tertiary lymphoid structures are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Gut, 72:1927–41.
Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med, 189:832–44.
Meylan M, Petitprez F, Becht E, Bougoüin A, Pupier G, Calvez A, Giglioli I, Verkarre V, Lacroix G, Verneau J et al. Tertiary lymphoid structures generate and propagate anti-tumor antibod y-producing plasma cells in renal cell cancer. Immunity, 55:527–e541525.
Iglesia MD, Parker JS, Hoadley KA, Serody JS, Perou CM, Vincent BG. Genomic analysis of Immune Cell infiltrates across 11 Tumor types. J Natl Cancer Inst, 108:djw144.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity, 39:1–10.
Tang H, Zhu M, Qiao J, Fu Y-X. Lymphotoxin signalling in tertiary lymphoid structures and immunothera py. Cell Mol Immunol, 14:809–18.
Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes ignorance to solid tum ors via crosspresentation by bone marrow-derived stromal cells. Immunity, 17:737–47.
He M, He Q, Cai X, Liu J, Deng H, Li F, Zhong R, Lu Y, Peng H, Wu X et al. Intratumoral tertiary lymphoid structure (TLS) maturation is influence d by draining lymph nodes of lung cancer. J Immunother Cancer, 11:e005539.
Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, Van den Eynden G, Naveaux C, Lodewyckx J-N, Boisson A, Duvillier H et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight, 5:e129641.
Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G et al. CD4+ follicular helper T cell infiltration predicts breast cancer surv ival. J Clin Investig, 123:2873–92.
Crotty S. T Follicular Helper Cell Biology: a decade of Discovery and diseases. Immunity, 50:1132–48.
Chaurio RA, Anadon CM, Lee Costich T, Payne KK, Biswas S, Harro CM, Moran C, Ortiz AC, Cortina C, Rigolizzo KE et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes tfh cell differentiation and formation of intra-tumoral tertiary lymphoid struc tures. Immunity, 55:115–e128119.
Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, Bruno TC, Vignali DAA, Hand TW. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity, 54:2812–e28242814.
Sato Y, Oguchi A, Fukushima Y, Masuda K, Toriu N, Taniguchi K, Yoshikawa T, Cui X, Kondo M, Hosoi T et al. CD153/CD30 signaling promotes age-dependent tertiary lymphoid tissue e xpansion and kidney injury. J Clin Investig, 132:e146071.
Elyahu Y, Hekselman I, Eizenberg-Magar I, Berner O, Strominger I, Schiller M, Mittal K, Nemirovsky A, Eremenko E, Vital A et al. Aging promotes reorganization of the CD4 T cell landscape toward extre me regulatory and effector phenotypes. Sci Adv, 5:eaaw8330.
Fonseca VR, Ribeiro F, Graca L. T follicular regulatory (tfr) cells: dissecting the complexity of tfr- cell compartments. Immunol Rev, 288:112–27.
Hao H, Nakayamada S, Yamagata K, Ohkubo N, Iwata S, Inoue Y, Zhang M, Zhang T, Kanda Satoh Y, Shan Y et al. Conversion of T Follicular Helper Cells to T Follicular Regulatory Cel ls by Interleukin-2 through transcriptional regulation in systemic lup us Erythematosus. Arthritis Rheumatol (Hoboken NJ), 73:132–42.
McCarron MJ, Marie JC. TGF-β prevents T follicular helper cell accumulation and B cell autore activity. J Clin Investig, 124:4375–86.
Clement RL, Daccache J, Mohammed MT, Diallo A, Blazar BR, Kuchroo VK, Lovitch SB, Sharpe AH, Sage PT. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat Immunol, 20:1360–71.
Wang J, Jiang D, Zheng X, Li W, Zhao T, Wang D, Yu H, Sun D, Li Z, Zhang J et al. Tertiary lymphoid structure and decreased CD8 < sup>+ T cell infil tration in minimally invasive adenocarcinoma. iScience, 25:103883.
Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activa ted in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res, 69:2000–9.
Devi-Marulkar P, Fastenackels S, Karapentiantz P, Goc J, Germain C, Kaplon H, Knockaert S, Olive D, Panouillot M, Validire P et al. Regulatory T cells infiltrate the tumor-induced tertiary lymphoïd stru ctures and are associated with poor clinical outcome in NSCLC. Commun Biology, 5:1416.
Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer, 19:307–25.
Sautès-Fridman C, Dimberg A, Verma V. Editorial: tertiary lymphoid structures: from Basic Biology to Transla tional impact in Cancer. Front Immunol, 13:870862.
Sun X, Zhang T, Li M, Yin L, Xue J. Immunosuppressive B cells expressing PD-1/PD-L1 in solid tumors: a min i review. QJM: Monthly J Association Physicians, 115:507–12.
Sl NJJT, Gt N. B: Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology, 10:1900508.
Shalapour S, Lin X-J, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T et al. Inflammation-induced IgA + cells dismantle anti-liver cancer immunity. Nature, 551:340–5.
Shalapour S, Karin M. The neglected brothers come of age: B cells and cancer. Semin Immunol, 52:101479.
Michaud D, Steward CR, Mirlekar B, Pylayeva-Gupta Y. Regulatory B cells in cancer. Immunol Rev, 299:74–92.
Ishigami E, Sakakibara M, Sakakibara J, Masuda T, Fujimoto H, Hayama S, Nagashima T, Sangai T, Nakagawa A, Nakatani Y, Otsuka M. Coexistence of regulatory B cells and regulatory T cells in tumor-infi ltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast cancer (Tokyo Japan), 26:180–9.
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol, 20:7–24.
Yiong CS, Lin TP, Lim VY, Toh TB, Yang VS. Biomarkers for immune checkpoint inhibition in sarcomas - are we close to clinical implementation? Biomark Res, 11:75.
DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol, 19:369–82.
Resag A, Toffanin G, Benešová I, Müller L, Potkrajcic V, Ozaniak A, Lischke R, Bartunkova J, Rosato A, Jöhrens K et al. The Immune contexture of Liposarcoma and its clinical implications. Cancers, 14:4578.
Roulleaux Dugage M, Jones RL, Trent J, Champiat S, Dumont S. Beyond the driver mutation: immunotherapies in gastrointestinal stroma l tumors. Front Immunol, 12:715727.
Zhang C, Wang X-Y, Zuo J-L, Wang X-F, Feng X-W, Zhang B, Li Y-T, Yi C-H, Zhang P, Ma X-C et al. Localization and density of tertiary lymphoid structures associate wit h molecular subtype and clinical outcome in colorectal cancer liver me tastases. J Immunother Cancer, 11:e006425.
Zhang W, He KF, Yang JG, Ren JG, Sun YF, Zhao JH, Zhao YF. Infiltration of M2-polarized macrophages in infected lymphatic malform ations: possible role in disease progression. Br J Dermatol, 175:102–12.
Nie Y, Fan H, Li J, Lei X, Zhang T, Wang Y, Mao Z, Tao K, Song W. Tertiary lymphoid structures: Associated multiple immune cells and ana lysis their formation in hepatocellular carcinoma. FASEB Journal: Official Publication Federation Am Soc Ieties Experimental Biology, 36:e22586.
Griss J, Bauer W, Wagner C, Simon M, Chen M, Grabmeier-Pfistershammer K, Maurer-Granofszky M, Roka F, Penz T, Bock C et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun, 10:4186.
Groeneveld CS, Fontugne J, Cabel L, Bernard-Pierrot I, Radvanyi F, Allory Y, de Reyniès A. Tertiary lymphoid structures marker CXCL13 is associated with better s urvival for patients with advanced-stage bladder cancer treated with i mmunotherapy. Eur J cancer (Oxford England: 1990), 148:181–9.
Denton AE, Innocentin S, Carr EJ, Bradford BM, Lafouresse F, Mabbott NA, Mörbe U, Ludewig B, Groom JR, Good-Jacobson KL, Linterman MA. Type I interferon induces CXCL13 to support ectopic germinal center fo rmation. J Exp Med, 216:621–37.
Gomez-Nguyen A, Gupta N, Sanaka H, Gruszka D, Pizarro A, DiMartino L, Basson A, Menghini P, Osme A, DeSalvo C et al. Chronic stress induces colonic tertiary lymphoid organ formation and p rotection against secondary injury through IL-23/IL-22 signaling. Proceedings of the National Academy of Sciences of the United States o f America, 119:e2208160119.
Masuda T, Tanaka N, Takamatsu K, Hakozaki K, Takahashi R, Anno T, Kufukihara R, Shojo K, Mikami S, Shinojima T et al. Unique characteristics of tertiary lymphoid structures in kidney clear cell carcinoma: prognostic outcome and comparison with bladder cancer. J Immunother Cancer, 10:e003883.
Fleig S, Kapanadze T, Bernier-Latmani J, Lill JK, Wyss T, Gamrekelashvili J, Kijas D, Liu B, Hüsing AM, Bovay E et al. Loss of vascular endothelial notch signaling promotes spontaneous form ation of tertiary lymphoid structures. Nat Commun, 13:2022.
Rakaee M, Kilvaer TK, Jamaly S, Berg T, Paulsen E-E, Berglund M, Richardsen E, Andersen S, Al-Saad S, Poehl M et al. Tertiary lymphoid structure score: a promising approach to refine the TNM staging in resected non-small cell lung cancer. Br J Cancer, 124:1680–9.
Pimenta EM, Barnes BJ. Role of Tertiary lymphoid structures (TLS) in Anti-tumor Immunity: Pot Ential Tumor-Induced Cytokines/Chemokines that regulate TLS formation in epithelial-derived cancers. Cancers, 6:969–97.
Tang J, Ramis-Cabrer D, Curull V, Wang X, Mateu-Jiménez M, Pijuan L, Duran X, Qin L, Rodríguez-Fuster A, Aguiló R, Barreiro E. B cells and tertiary lymphoid structures influence survival in lung ca ncer patients with resectable tumors. Cancers, 12:2644.
Seow DYB, Yeong JPS, Lim JX, Chia N, Lim JCT, Ong CCH, Tan PH, Iqbal J. Tertiary lymphoid structures and associated plasma cells play an impor tant role in the biology of triple-negative breast cancers. Breast Cancer Res Treat, 180:369–77.
Rodriguez AB, Engelhard VH. Insights into Tumor-Associated Tertiary lymphoid structures: Novel Tar gets for Antitumor Immunity and Cancer Immunotherapy. Cancer Immunol Res, 8:1338–45.
Rodriguez AB, Peske JD, Woods AN, Leick KM, Mauldin IS, Meneveau MO, Young SJ, Lindsay RS, Melssen MM, Cyranowski S et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors v ia cancer-associated fibroblasts. Cell Rep, 36:109422.
Lee M, Heo S-H, Song IH, Rajayi H, Park HS, Park IA, Kim Y-A, Lee H, Gong G, Lee HJ. Presence of tertiary lymphoid structures determines the level of tumor -infiltrating lymphocytes in primary breast cancer and metastasis. Mod Pathology: Official J United States Canadi Acad Pathol Inc, 32:70–80.
Munoz-Erazo L, Rhodes JL, Marion VC, Kemp RA. Tertiary lymphoid structures in cancer - considerations for patient pr ognosis. Cell Mol Immunol, 17:570–5.
Yang M, Che Y, Li K, Fang Z, Li S, Wang M, Zhang Y, Xu Z, Luo L, Wu C et al. Detection and quantitative analysis of tumor-associated tertiary lymph oid structures. J Zhejiang Univ Sci B, 24:779–95.
Feng H, Yang F, Qiao L, Zhou K, Wang J, Zhang J, Tian T, Du Y, Shangguan H. Prognostic significance of Gene Signature of Tertiary Lymphoid Structu res in patients with lung adenocarcinoma. Front Oncol, 11:693234.
Wang L, Gong S, Pang L, Zhang S, Zhang X, He W. Genomic properties and clinical outcomes associated with tertiary lymp hoid structures in patients with breast cancer. Sci Rep, 13:13542.
Horeweg N, Workel HH, Loiero D, Church DN, Vermij L, Léon-Castillo A, Krog RT, de Boer SM, Nout RA, Powell ME et al. Tertiary lymphoid structures critical for prognosis in endometrial can cer patients. Nat Commun, 13:1373.
Xu Y, Su G-H, Ma D, Xiao Y, Shao Z-M, Jiang Y-Z. Technological advances in cancer immunity: from immunogenomics to sing le-cell analysis and artificial intelligence. Signal Transduct Target Therapy, 6:312.
Vaidya P, Bera K, Patil PD, Gupta A, Jain P, Alilou M, Khorrami M, Velcheti V, Madabhushi A. Novel, non-invasive imaging approach to identify patients with advance d non-small cell lung cancer at risk of hyperprogressive disease with immune checkpoint blockade. J Immunother Cancer, 8:e001343.
Zhang J, Wu Z, Zhang X, Liu S, Zhao J, Yuan F, Shi Y, Song B. Machine learning: an approach to preoperatively predict PD-1/PD-L1 exp ression and outcome in intrahepatic cholangiocarcinoma using MRI bioma rkers. ESMO open, 5:e000910.
Ren F, Xie M, Gao J, Wu C, Xu Y, Zang X, Ma X, Deng H, Song J, Huang A et al. Tertiary lymphoid structures in lung adenocarcinoma: characteristics a nd related factors. Cancer Med, 11:2969–77.
Xu Y, Li Z, Yang Y, Li L, Zhou Y, Ouyang J, Huang Z, Wang S, Xie L, Ye F et al. A CT-based radiomics approach to predict intra-tumoral tertiary lympho id structures and recurrence of intrahepatic cholangiocarcinoma. Insights into Imaging, 14:173.
Schaadt NS, Schönmeyer R, Forestier G, Brieu N, Braubach P, Nekolla K, Meyer-Hermann M, Feuerhake F. Graph-based description of tertiary lymphoid organs at single-cell lev El. PLoS Comput Biol, 16:e1007385.
Li P, Liang Y, Zeng B, Yang G, Zhu C, Zhao K, Xu Z, Wang G, Han C, Ye H et al. Preoperative prediction of intra-tumoral tertiary lymphoid structures based on CT in hepatocellular cancer. Eur J Radiol, 151:110309.
Shostak E, Hariri LP, Cheng GZ, Adams DC, Suter MJ. Needle-based Optical Coherence Tomography to Guide Transbronchial Lymp h Node Biopsy. J Bronchol Interventional Pulmonol, 25:189–97.
van Rijthoven M, Balkenhol M, Siliņa K, van der Laak J, Ciompi F. HookNet: multi-resolution convolutional neural networks for semantic s egmentation in histopathology whole-slide images. Med Image Anal, 68:101890.
Hu J, Coleman K, Zhang D, Lee EB, Kadara H, Wang L, Li M. Deciphering tumor ecosystems at super resolution from spatial transcri ptomics with TESLA. Cell Syst, 14:404–e417404.
Liu Z, Meng X, Tang X, Zou W, He Y. Intratumoral tertiary lymphoid structures promote patient survival and immunotherapy response in head neck squamous cell carcinoma. Cancer Immunol Immunotherapy: CII, 72:1505–21.
Petitprez F, de Reyniès A, Keung EZ, Chen TW-W, Sun C-M, Calderaro J, Jeng Y-M, Hsiao L-P, Lacroix L, Bougoüin A et al. B cells are associated with survival and immunotherapy response in sar coma. Nature, 577:556–60.
Budczies J, Kirchner M, Kluck K, Kazdal D, Glade J, Allgäuer M, Kriegsmann M, Heußel C-P, Herth FJ, Winter H et al. A gene expression signature associated with B cells predicts benefit f rom immune checkpoint blockade in lung adenocarcinoma. Oncoimmunology, 10:1860586.
Hao D, Han G, Sinjab A, Gomez-Bolanos LI, Lazcano R, Serrano A, Hernandez SD, Dai E, Cao X, Hu J et al. The single-cell Immunogenomic Landscape of B and plasma cells in early -stage lung adenocarcinoma. Cancer Discov, 12:2626–45.
Patil NS, Nabet BY, Müller S, Koeppen H, Zou W, Giltnane J, Au-Yeung A, Srivats S, Cheng JH, Takahashi C et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-sm all cell lung cancer. Cancer Cell, 40:289–e300284.
Johansson-Percival A, He B, Li Z-J, Kjellén A, Russell K, Li J, Larma I, Ganss R. De novo induction of intratumoral lymphoid structures and vessel norma lization enhances immunotherapy in resistant tumors. Nat Immunol, 18:1207–17.
Johansson-Percival A, He B, Ganss R. Immunomodulation of Tumor vessels: it takes two to Tango. Trends Immunol, 39:801–14.
Chelvanambi M, Fecek RJ, Taylor JL, Storkus WJ. STING agonist-based treatment promotes vascular normalization and tert iary lymphoid structure formation in the therapeutic melanoma microenv ironment. J Immunother Cancer, 9:e001906.
Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogen ic foci of immune regulation. Cancer Immunol Res, 2:616–31.
Kuwabara S, Tsuchikawa T, Nakamura T, Hatanaka Y, Hatanaka KC, Sasaki K, Ono M, Umemoto K, Suzuki T, Sato O et al. Prognostic relevance of tertiary lymphoid organs following neoadjuvant chemoradiotherapy in pancreatic ductal adenocarcinoma. Cancer Sci, 110:1853–62.
Boivin G, Kalambaden P, Faget J, Rusakiewicz S, Montay-Gruel P, Meylan E, Bourhis J, Lesec G, Vozenin M-C. Cellular Composition and Contribution of Tertiary lymphoid structures to Tumor Immune Infiltration and Modulation by Radiation Therapy. Front Oncol, 8:256.
Wang D, Huang L, Qian D, Cao Y, Wu X, Xu P, Ming L, Tang J, Huang Z, Yin Y, Zhou L. Low-dose radiotherapy promotes the formation of tertiary lymphoid stru ctures in lung adenocarcinoma. Front Immunol, 14:1334408.
Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the practitioner. J Clin Oncology: Official J Am Societ Y Clin Oncol, 33:2092–9.
Chen W, Li C, Jiang X. Advanced Biomaterials with intrinsic Immunomodulation effects for Canc Er Immunotherapy. Small Methods, 7:e2201404.
Delvecchio FR, Fincham REA, Spear S, Clear A, Roy-Luzarraga M, Balkwill FR, Gribben JG, Bombardieri M, Hodivala-Dilke K, Capasso M, Kocher HM. Pancreatic Cancer chemotherapy is potentiated by induction of Tertiary lymphoid structures in mice. Cell Mol Gastroenterol Hepatol, 12:1543–65.
GeurtsvanKessel CH, Willart MAM, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus ADME, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid struc tures in the lung of influenza virus-infected mice. J Exp Med, 206:2339–49.
van Hooren L, Vaccaro A, Ramachandran M, Vazaios K, Libard S, van de Walle T, Georganaki M, Huang H, Pietilä I, Lau J et al. Agonistic CD40 therapy induces tertiary lymphoid structures but impair s responses to checkpoint blockade in glioma. Nat Commun, 12:4127.
Morcrette G, Hirsch TZ, Badour E, Pilet J, Caruso S, Calderaro J, Martin Y, Imbeaud S, Letouzé E, Rebouissou S et al. <i > APC germline hepatoblastomas demonstrate cisplatin-induced intr atumor tertiary lymphoid structures</i >. Oncoimmunology, 8:e1583547.
Benzerdjeb N, Dartigues P, Kepenekian V, Valmary-Degano S, Mery E, Avérous G, Chevallier A, Laverriere M-H, Villa I, Harou O et al. Tertiary lymphoid structures in epithelioid malignant peritoneal mesot helioma are associated with neoadjuvant chemotherapy, but not with pro gnosis. Virchows Archiv: Int J Pathol, 479:765–72.
Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A, Cremer I, Goc J, Régnard J-F, Dieu-Nosjean M-C, Damotte D. Immune contexture and histological response after neoadjuvant chemothe rapy predict clinical outcome of lung cancer patients. Oncoimmunology, 5:e1255394.
van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, van Montfoort ML, van Rooijen C, Peters D, Broeks A et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urot helial cancer: the NABUCCO trial. Nat Med, 26:1839–44.
Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell r esponses that localize in mucosal lesions. Sci Transl Med, 6:221ra213.
Liu X, Zheng C, Luo X, Wang X, Jiang H. Recent advances of collagen-based biomaterials: multi-hierarchical str ucture, modification and biomedical applications. Mater Sci Eng C Mater Biol Applicati ons, 99:1509–22.
Suematsu S, Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat Biotechnol, 22:1539–45.
Okamoto N, Chihara R, Shimizu C, Nishimoto S, Watanabe T. Artificial lymph nodes induce potent secondary immune responses in Nai ve and immunodeficient mice. J Clin Investig, 117:997–1007.
Kobayashi Y, Watanabe T. Gel-trapped lymphorganogenic chemokines trigger Artificial Tertiary ly mphoid organs and Mount Adaptive Immune responses in vivo. Front Immunol, 7:316.
Bashir S, Hina M, Iqbal J, Rajpar AH, Mujtaba MA, Alghamdi NA, Wageh S, Ramesh K, Ramesh S. Fundamental concepts of hydrogels: synthesis, Properties, and their ap plications. Polymers, 12:2702.
Purwada A, Singh A. Immuno-engineered organoids for regulating the kinetics of B-cell deve lopment and antibody production. Nat Protoc, 12:168–82.
Jin X-K, Liang J-L, Zhang S-M, Ji P, Huang Q-X, Qin Y-T, Deng X-C, Liu C-J, Zhang X-Z. Engineering metal-based hydrogel-mediated tertiary lymphoid structure formation < i > via activation of the STING pathway for enhanced immu notherapy. Mater Horiz, 10:4365–79.
Cheung AS, Mooney DJ. Engineered materials for Cancer Immunotherapy. Nano Today, 10:511–31.
Peng S, Xiao F, Chen M, Gao H. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Imm unotherapy. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 9:e2103836.
Wen Z, Liu H, Qiao D, Chen H, Li L, Yang Z, Zhu C, Zeng Z, Chen Y, Liu L. Nanovaccines fostering Tertiary Lymphoid structure to Attack Mimicry N asopharyngeal carcinoma. ACS Nano, 17:7194–206.
Huang Y, Chen Y, Zhou S, Chen L, Wang J, Pei Y, Xu M, Feng J, Jiang T, Liang K et al. Dual-mechanism based CTLs infiltration enhancement initiated by Nano-s apper potentiates immunotherapy against immune-excluded tumors. Nat Commun, 11:622.
Zhang Y, Xu J, Fei Z, Dai H, Fan Q, Yang Q, Chen Y, Wang B, Wang C. 3D Printing Scaffold Vaccine for Antitumor Immunity. Adv Mater (Deerfield Beach Fla), 33:e2106768.
Bock C, Boutros M, Camp JG, Clarke L, Clevers H, Knoblich JA, Liberali P, Regev A, Rios AC, Stegle O et al. The Organoid Cell Atlas. Nat Biotechnol, 39:13–7.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organo id technologies. Sci (New York NY), 345:1247125.
Küçükköse E, Heesters BA, Villaudy J, Verheem A, Cercel M, van Hal S, Boj SF, Borel Rinkes IHM, Punt CJA, Roodhart JML et al. Modeling resistance of colorectal peritoneal metastases to immune chec kpoint blockade in humanized mice. J Immunother Cancer, 10:e005345.
Qu J, Kalyani FS, Liu L, Cheng T, Chen L. Tumor organoids: synergistic applications, current challenges, and fut ure prospects in cancer therapy. Cancer Commun (London England), 41:1331–53.
Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG, Feinberg AW. 3D bioprinting of collagen to rebuild components of the human heart. Sci (New York NY), 365:482–7.
Goyal G, Prabhala P, Mahajan G, Bausk B, Gilboa T, Xie L, Zhai Y, Lazarovits R, Mansour A, Kim MS et al. Ectopic Lymphoid Follicle Formation and Human Seasonal Influenza Vacci nation Responses Recapitulated in an Organ-on-a-Chip. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 9:e2103241.
Sofopoulos M, Fortis SP, Vaxevanis CK, Sotiriadou NN, Arnogiannaki N, Ardavanis A, Vlachodimitropoulos D, Perez SA, Baxevanis CN. The prognostic significance of peritumoral tertiary lymphoid structure s in breast cancer. Cancer Immunol Immunotherapy: CII, 68:1733–45.
Aoyama S, Nakagawa R, Mulé JJ, Mailloux AW. Inducible tertiary lymphoid structures: Promise and challenges for Tra nslating a New Class of Immunotherapy. Front Immunol, 12:675538.
Acknowledgements
Not applicable.
Funding
This study was supported by the Major Science and Technology projects of Henan Province (Grant No. 221100310100).
Author information
Authors and Affiliations
Contributions
YQR, YYZ and ZQL provided direction and guidance throughout the preparation of this manuscript. MJX and YQR wrote and edited the manuscript. YQR reviewed and made significant revisions to the manuscript. XWH, and ZQL revised and edited the manuscript. YHB, STL, ANZ, HX, SYW collected and prepared the related papers. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y., Xu, M., Ren, Y. et al. Tertiary lymphoid structural heterogeneity determines tumour immunity and prospects for clinical application. Mol Cancer 23, 75 (2024). https://doi.org/10.1186/s12943-024-01980-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-024-01980-6