Abstract
Extrachromosomal circular DNA (eccDNA) refers to a type of circular DNA that originate from but are likely independent of chromosomes. Due to technological advancements, eccDNAs have recently emerged as multifunctional molecules with numerous characteristics. The unique topological structure and genetic characteristics of eccDNAs shed new light on the monitoring, early diagnosis, treatment, and prediction of cancer. EccDNAs are commonly observed in both normal and cancer cells and function via different mechanisms in the stress response to exogenous and endogenous stimuli, aging, and carcinogenesis and in drug resistance during cancer treatment. The structural diversity of eccDNAs contributes to the function and numerical diversity of eccDNAs and thereby endows eccDNAs with powerful roles in evolution and in cancer initiation and progression by driving genetic plasticity and heterogeneity from extrachromosomal sites, which has been an ignored function in evolution in recent decades. EccDNAs show great potential in cancer, and we summarize the features, biogenesis, evaluated functions, functional mechanisms, related methods, and clinical utility of eccDNAs with a focus on their role in evolution and cancer.
Similar content being viewed by others
Introduction
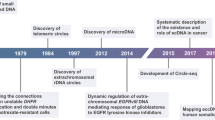
Extrachromosomal circular DNA (eccDNA) refers to circular DNA that originate from chromosomes, but once generated, these DNAs are likely independent of chromosomal DNA. EccDNA residing in nuclei was first discovered by Alix Bassel and Yasuo Hoota in 1964 [1] and was referred to as double minutes (DMs). EccDNA was first identified by karyotyping as DNA that exists independent of the chromosomes in cancer cells [2] but has emerged as a new type of molecule because of their numerous characteristics and functions identified due to technological advancements, particularly next-generation sequencing and bioinformatics [3,4,5]. EccDNAs are sufficiently long to harbor their own origins of replication, are able to encode amino acids, and have been observed in tumor tissues, where they most frequently harbor oncogenes or genes related to drug resistance in cancer therapy [6,7,8,9]. Other functions of eccDNAs, such as functions in aging [10,11,12] and heterogeneity [13], as well as putative roles, including dosage compensation, have gradually been discovered. The genotype diversity and genomic plasticity within cancer cells are known to be affected by genome instability and genome alterations [14]. EccDNA can rapidly remodel the genome through its diversity, including structural, functional, and numerical diversity, which efficiently drives the evolution of cancer and life [6, 8, 9]. In this review, we discuss the characteristics, biogenesis, methodology, and mechanisms of eccDNA functions with a focus on their role in evolution and cancer.
Molecular structure of eccDNA
Size of eccDNA
The size of eccDNAs varies widely, ranging from dozens of bases to hundreds of thousands of bases; in addition, the majority are smaller than 1,000 bp [15, 16], and 99% of eccDNAs are shorter than 25 kb. More than 50% of eccDNAs originate from genic or pseudogenic regions [16]. Most of the eccDNAs found in normal cells are shorter and usually less than 500 bp [17,18,19,20]. However, the genetic content of eccDNAs shows marked differences among leukocytes from the same individual [16]. Fetal-derived eccDNAs are shorter than maternal eccDNAs [21], and fetal eccDNAs in plasma are relatively hypomethylated compared with maternal eccDNAs [22]. EccDNAs from tumors have smaller sizes and prefer end coordinates from those derived from normal cells [23,24,25]. In summary, the sizes and levels of eccDNAs exhibit lifestyle-, life-stage-, tissue-, and disease-specific features, and these findings shed new light on tracing the origin of eccDNAs [16, 26,27,28] and advancing cancer diagnosis and treatment methods.
Distribution of eccDNA
The vast majority of eccDNA originates from repetitive sequences [17, 29,30,31,32]. EccDNAs with a length less than 500 bp are called small polydisperse circular DNA (spcDNA) [17, 29,30,31,32], and a few spcDNA molecules are hybridized to unique sequences [29, 31,32,33]. The eccDNA distribution is not restricted to particular areas in the genome [34]. EccDNAs originate from tens of thousands of unique sites in the genome and are enriched in specific areas (called hotspots), including untranslated areas and regions with a high GC content; however, in transcriptionally activated chromatin, eccDNAs prefer to reside in circularization hotspots, but this finding appears to be controversial (Fig. 1A) [15].
Origins and types of eccDNA. A EccDNA is enriched in specific areas (hotspots), including untranslated (3’-UTR and 5’-UTR) areas, regions with a high GC content, and transcriptionally active chromatin. B EccDNA types based on the genetic content [34]
Gene-rich chromosomes contribute to more eccDNAs per megabase, e.g., gene-rich chromosomes 17 and 19 contribute to a higher average frequency of eccDNAs per megabase than other chromosomes; moreover, the ratio of eccDNAs per megabase to coding genes is positively correlated [16]. For example, the most transcribed protein-coding gene in muscle, titin (TTN), has the greatest amount of eccDNAs [16]. Noncoding gene areas show a lower correlation with the eccDNA frequency, which suggests that the transcription or other features of coding genes affect the frequency of eccDNA formation. A substantial portion of eccDNA sequences originate from helitrons (a class of mobile elements known to transpose via a circular intermediate) [35], cut-and-paste transposons, and exons.
Genetic features and category of eccDNA
Genomic DNA flanking 80% of circular microDNA has direct repeats of 2–15 bases [15]. Approximately 60% of eccDNAs arise from unique sequences, and > 90% of genomic sites harbor direct repeats of several bases. The junction of these repeats creates a unique paired-end sequence, which is useful for the recognition of eccDNA through a bioinformatic analysis of sequence data [36]. The distribution of the eccDNA sequences in chromosomes is distant from each other in prostate and ovarian cancer cell lines, which indicates that the sites of eccDNA formation depend on the cell lineage [37]. EccDNAs have been categorized into the following 4 types according to their size and sequence: spcDNA, telomeric circle, microDNA, and eccDNA [3]. Based on their genomic origin and genetic content, we propose that eccDNAs can also be categorized into the following 7 types: full gene eccDNA, exon eccDNA, intron eccDNA, repeat eccDNA, repeat-intergenic eccDNA, intergenic eccDNA, transposable element (TE) eccDNA, and promoter/enhancer eccDNA (Fig. 1B). TE eccDNA and promoter/enhancer eccDNA can be reinserted into other types of eccDNAs to generate larger eccDNAs called function-enhanced eccDNAs because eccDNAs are able to gradually become enlarged through assembly from smaller circular elements [38,39,40]. These factors form the structural diversity of eccDNAs and serve as the genetic basis of their functional and numerical diversity.
Biogenesis and replication of eccDNA
Outline
EccDNAs may arise via many different pathways with different mechanisms. Some models of eccDNA formation, including the breakage-fusion-bridge (BFB) cycle, chromothripsis, episome model, translocation-deletion-amplification model, and ‘lost-and-found’ event of DNA [3, 21, 36, 37], have been proposed, and DNA breakage, DNA recombination, and DNA rearrangement play critical roles in these models [36, 41,42,43]. These models of eccDNA formation work collectively, but the details remain elusive. DNA replication, DNA transcription processes, and other events are also regarded as potential mechanisms [36]. Overall, eccDNA is generated from various processes, but further research related to DNA metabolism is needed.
Breakage-fusion-bridge (BFB) cycle
Most eccDNA molecules include or are adjacent to short direct repeats, and 72.4% (human) and 8.7% (pigeon) of 30,000 unique eccDNAs, as characterized by eccDNA sequencing, are derived from repetitive elements [34]. In addition, specialized eccDNAs have also been noted to arise from repetitive genomic sequences, such as telomeric DNA or rDNA [36]. Furthermore, several researchers have found that some nonrepetitive spcDNA sequences are flanked on both ends by, on average, 9–11 bp of direct repeats [17, 31, 32, 44]. These findings suggest that DNA repair pathways, such as homologous recombination and microhomology-mediated end joining between two short repeats, may generate DNA circles.
Repetitive DNA sequences can be excised by homologous recombination to generate larger eccDNAs [45, 46]. The formation of eccDNAs smaller than < 2 kb is considered a consequence of intramolecular recombination or rearrangement between adjacent repeats (telomeric, centromeric, and satellites) mediated by a double-strand break [15] and enlarged through the BFB cycle [3]. The mechanism of the BFB cycle is illustrated in Fig. 2A.
Mechanisms of eccDNA biogenesis. A A dicentric anaphase bridge forms due to the loss of telomeres, and the telomere-free bridge is elongated by a repetitive cycle of replication, broken into random fragments under stress, and looped out to form eccDNA. B Chromothripsis, a type of severe DNA damage caused by exogenous stress, forms single- or double-strand breakages. With the DNA repair system, most of the DNA fragments are removed by different mechanisms, but some of the fragments are ligated and circularized into eccDNAs. C The translocation-deletion-amplification mechanism, which is frequently triggered by exogenous stimuli, is repaired or removed by the DNA repair system, but the retained or cleaved DNA fragments may generate eccDNAs. D Circular DNAs are generated during the DNA synthesis process in a DNA slippage and R-loop manner. Circular DNAs are produced by cleavage and ligation and can be enlarged by the integration of other DNA components, such as TEs and enhancers/promoters. BFB, breakage-fusion-bridge
Chromothripsis
Chromothripsis results from severe DNA damage induced by exogenous stimuli. The circularization of DNA fragments from chromosome breakage generates eccDNAs (Fig. 2B). EccDNA is derived from both coding and noncoding genomic regions and is present in normal physiological states [47]. However, some researchers have speculated that chromothripsis is one of the mechanisms of eccDNA formation. The chemotherapeutic-induced apoptosis of lymphoblastoid cells significantly promotes eccDNA production [41], but eccDNAs are not byproducts of apoptosis-driven fragmentation [16]. It is well known that most carcinogens are mutagens that lead to DNA damage. The accumulation of eccDNA has been found after exposure to the carcinogen 7,1-dimethylbenz[a]anthracene and the DNA replication inhibitor hydroxyurea [17]. Cycloheximide, an inhibitor of protein synthesis, also induces 70-fold elevation of eccDNAs in murine cells [17]. Human cells with a specific defect in the DNA repair pathway produce an increased number of eccDNA molecules [30]. The deletion of MSH3, a gene involved in the DNA mismatch repair pathway, decreases the eccDNA levels to 80% [37]. The CTC1/STN1/TEN1 protein complex, which is known to participate in the maintenance of telomeres, has been shown to contribute to t-circle formation [48]. Prokaryotic cells with SGS1, a protein involved in DNA repair, have increased eccDNA levels [49]. EccDNA can be generated by CRISPR/CAS9 technology, which supports the notion that the DNA repair pathway participates in the formation of eccDNAs [43]. These findings suggest that DNA damage is involved in the biogenesis of eccDNAs, but the underlying pathways and mechanisms need further investigation.
Translocation-deletion-amplification mechanism
The translocation-deletion-amplification mechanism, which is frequently triggered by exogenous stimuli, may be repaired or removed by the DNA repair system. The retained or cleaved DNA fragments may generate eccDNAs (Fig. 2C). Gene amplification adjacent to a breakage point is more frequent, as supported by the amplification of MYC-containing eccDNA [50].
Episome model
A research group recently showed that MYC-containing DMs in leukemia cases arise by excision and amplification, and this finding supports the eccDNA formation model called the episome model [38] (Fig. 2D). This amplification is mediated by small circular DNAs, which are referred to as episomes. An episome is likely an unrecognized type of structural variation in the genome, but findings from other research studies have noted a possible role of circular DNAs in the movement of TEs [51]. The frequency of these elements in cancer cells is higher than that in normal cells, which indicates that these elements might contribute to the instability of the genome. The consistency between eccDNAs generated through recombination events within the genome and their recombination junctions supports the possibility that retrotransposable elements may move around the genome through DNA circularization [52]. EccDNAs from DNA circularization result in gene copy number variations when circles contain genes and origins of replication [53], which contributes to additional gain of novel functions. For example, a previous study revealed the eccDNA-based amplification and transmission of herbicide resistance in the crop weed Amaranthus palmeri [54]. Most retrotransposable elements move through a copy-and-paste or cut-and-paste mechanism, and this movement is likely to have a functional impact on the genomic context [51]. For example, transposon-like sequences, called telomere-bearing elements (TBEs), in Oxytricha are excised as eccDNA molecules during rearrangement, which results from rejoining to regenerate the target [55] (Fig. 2D). The accumulation of eccDNAs in normal cells from GC-rich and transcriptionally activated regions of the genome suggests that R-loop generation and repair may be involved in the formation of eccDNA [37].
Role of DNA replication in eccDNA biogenesis
A review has proposed that replication slippage during DNA replication is also involved in the formation of eccDNA [56]. Replication slippage is commonly mediated by direct repeats in the genome but cleaved by the DNA repair system, which ultimately generates DNA circles. Because this notion is partially supported by little evidence, further investigation is needed.
Collectively, these studies suggest that the formation of eccDNA is dependent on the sequence, organization, replication, and damage repair of DNA. However, some researchers argue that the formation of eccDNAs may occur in the absence of any repetitive DNA elements, probably using a microhomology sequence of 8 nucleotides flanking the amplified sequence [36].
Role of eccDNA replication in eccDNA biogenesis
Whether eccDNA replication is independent of cell proliferation remains elusive. EccDNAs appear to undergo extrachromosomal replication via a rolling circle mechanism [56]. An estimate, probably an underestimate, of the eccDNA number has been quantified by electron micrographs (EMs) using preparations from defined numbers of cells, and the results suggest the existence of at least 125 ~ 200 DNA circles per DT40 cell [37]. The contribution of DNA replication to eccDNA production, however, is controversial; some studies have found that the eccDNA levels increase when ongoing replication is blocked by replication inhibitors [17], whereas other studies have found that eccDNA can be formed in the absence of any DNA replication [45]. However, we speculate that replication is independent of mitosis, which helps cells survive under challenging conditions, such as hazardous chemical exposure or nutrient deficiency, and is thus not suitable for mitosis.
Function and mechanism of eccDNA
EccDNAs play important roles in genetic variation, evolution, genomic instability, genomic plasticity, drug resistance, environmental adaption, mutation, and tumorigenesis. Some identified functions of eccDNA have been summarized in a previous review [36]. We updated and reorganized the recently identified roles of eccDNA (Fig. 3). Genetic variation is the fundamental change of other aspects. Gene amplification is a type of genetic variation whose role has been validated in various biological processes, and the reintegration of eccDNAs provides an efficient pathway for gene amplification [36, 43]. EccDNA may contribute to variations in the genome content through evolution [44]. In both human tumor cell lines and yeast, the significant correlation between the appearance of eccDNAs and the selective growth and/or resistance advantage of cells indicates that eccDNAs influence the phenotype of cells. In yeast, eccDNAs participate in gene amplification [57] and thereby facilitate adaptation to nutrient-limiting environments [42, 58, 59], specific amino acid limitation [60], aging [10,11,12], and senescence caused by rDNA circles [61]. Similarly, in plants, eccDNA plays a role in transmissible herbicide resistance [54], and 49 of the 59 genes encoded by the eccDNA replicon are transcriptionally active when replicon-containing glyphosate-resistant plants are exposed to glyphosate [62]. In human tumor cells, eccDNA may drive increases in the oncogene copy number to yield a high level of oncogene products [38, 63]. Some researchers have speculated that eccDNA may contribute to the expression of different isoforms of a gene by interfering with or promoting the transcription of specific exons [15]. The theoretical functions of eccDNA include the expression of regulatory RNAs that sponge transcription factors, as has been partially validated [64], and this finding suggests that one of the underlying mechanisms through which eccDNA drives gene expression involves genetic variation, such as gene amplification and rearrangement. Moreover, eccDNA-containing oncogenes show significantly higher amplification through the mechanism of eccDNA formation than through chromosomal amplification [65], but this mechanism is not the only function of eccDNA in its biological roles. A recent study found that small artificial eccDNAs suppress gene expression by producing short regulatory RNAs [64]. Collectively, the published studies show that the role and mechanism of eccDNAs are dependent on the gene content they contain and the structures of the elements. EccDNA provides genetic plasticity and heterogeneity driven by extra chromosomes, which remains an ignored power of evolution in recent decades.
Based on the defined characteristics of the genetic structure of eccDNA, we propose that the diversity of eccDNAs, including the structure, number, and function of diversity, is an important origin driving the evolution of the genome and life (Fig. 4). To ensure a better understanding, we consider all the eccDNAs in cells or organisms to be adaptive toolkits that may respond to any possible stress caused by the environment. Each eccDNA plays a unique role in coping with the stimulus immediately or after its evolution because loosened chromatin facilitates eccDNA-encoding gene products more efficiently. Exposure to environmental stimuli results in the generation of various eccDNAs that form the original diversity of eccDNA. Although most eccDNAs initially have no adaptive advantages, eccDNAs eventually have the chance to obtain the genomic architecture and are prone to gain adaptive advantages to facilitate the evolvability of cells exposed to varied levels of environmental stimuli or nutrients, which results in the formation of countless eccDNAs from many alternations in DNA [60]. Once functional eccDNA is generated in a somatic cell, the structural diversity and amount are maintained even though many nonfunctional eccDNAs are present in cells. Moreover, eccDNAs generally separate randomly during mitosis due to the absence of a centromere [8] and replicate independent of cell mitosis [66], which causes significant heterogeneity in complement eccDNAs across the population and thereby results in substantial phenotypic plasticity [8]. Gene copy number amplification is increased much faster via the accumulation of eccDNA rather than chromosomal DNA [65]. Population heterogeneity can be selected according to the dosage of eccDNAs, not only by an increased dosage [8, 67] but also by a decreased dosage, due to the ease of eccDNA loss during cell division [68,69,70].
Diversity of eccDNA and its roles in evolution. A Structural diversity of eccDNA. Different types of eccDNA have different genetic contents, which constitute the eccDNA structural diversity. B Functional diversity of eccDNA. The eccDNA structural diversity and unique topological structure contribute to the multiple functions of eccDNA by potentially driving the expression of coding RNAs, noncoding RNAs, and other RNAs. C Numerical diversity of eccDNA as well as the role and mechanism of eccDNA in evolution (modified based on Verhaak et al. [71]). Cells with eccDNA functional diversity formed from different pathways triggered by exogenous stimuli have an enhanced opportunity to generate eccDNA numerical diversity, which is attributed to cell survival advantages in environmental adaptation and evolution. The cycle number represents the copy number of eccDNA
EccDNAs derived from gene-rich chromosome regions may influence the genotype of somatic cells through the alteration of gene copy numbers and the transcription of full-length genes, truncated genes, or regulatory RNAs (Fig. 4B) [16]. Moreover, the eccDNA diversity identified in a previous study supports a model in which any part of the human genome can contribute to eccDNA [16]. These findings have also been validated by the previous finding that the oncogene MET in glioblastoma cells is amplified on eccDNA molecules, as observed by fluorescent in situ hybridization (FISH) [8].
EccDNAs are transcriptionally activated and contribute to phenotypic variation by driving full-length and/or truncated gene expression, and during this course, regulatory RNAs may also be transcribed [16]. The underlying mechanism may include loosened chromatin, variations in the topological DNA structure, epigenetic changes, and the reintegration of promoters, enhancers, and TEs. EccDNAs can be categorized into several types, including full-gene eccDNA, exon eccDNA, intron eccDNA, repeat eccDNA, and intergenic eccDNA, depending on their sequence contents, which can be transcribed into all defined transcripts, including mRNA, microRNA, long noncoding RNA, and circular RNA.
In summary, the theoretical and defined functions of eccDNA as well as their mechanisms include aging, signaling communication, drug resistance, sponging of transcription factors, environmental adaption via adaptive DNAs, stimulation of immunofunction, gene regulation by expressing regulatory RNAs, contribution to heterogeneity through diversity, and gene dosage compensation [57]. More functions should be revealed via further investigations (Fig. 4), and the evaluated functions related to cancer and drugs are summarized in Table 1.
Methodology for eccDNA research
Previously, the de novo discovery of eccDNA was performed by EMs, Giemsa staining of metaphase chromosomes (karyotype), or two-dimensional (2D) gel electrophoresis, but these methods provide little information on eccDNA sequences. EccDNA was first detected and characterized by EMs with the generation of eccDNA-specific libraries [83]. The approximate size of eccDNAs was clearly identified by EMs, but data on the eccDNA number were rarely obtained [84]. Moreover, finding sequence information for eccDNAs is difficult [56]. Based on nucleotide pairing, other techniques, such as Southern blotting, inverse PCR, and FISH, provide evidence only about specific eccDNA elements. FISH, EMs, and Giemsa staining of metaphase chromosomes can provide localization information in cells. CsCl gradient purification and EM imaging approaches have been applied to investigate eccDNAs from several other organisms [15, 85,86,87,88,89,90,91]. Another research group verified the existence of DMs in human cancer cells by karyotype preparations and CsCl gradient purification [92, 93]. Fluorescence microscopy has been used to quantify changes in large eccDNAs and DMs between cancerous and normal cells [65]. An image analysis software package combined with fluorescence imaging has been used to quantify eccDNA copies [65]. Neutral–neutral 2D gel electrophoresis has been widely used for the identification and characterization of DNA replication forks [94, 95]. Several micrograms of genomic DNA is sufficient for these analyses, and pretreatment with restriction enzymes or exonucleases and the use of low-molecular-weight fractions of genomic DNA [96, 97] can improve the separation and resolution of eccDNA [98, 99]. In addition, 2D gel electrophoresis has been used for the measurement and characterization of eccDNAs [100] and has expanded the exploration of smaller eccDNAs [101]. However, most eccDNAs detected by 2D gels exist in the form of open circles. EccDNA detection using a 2D gel is insensitive, particularly for eccDNA with a supercoiled structure, and 2D gels are unable to discriminate dispersed repeats within a population of eccDNAs [56].
The sequence information of all types of known eccDNAs in a population of cells remains limited, and the technical limitations of the above-mentioned methods hinder advancements to clarifying aspects of eccDNAs [56]. Thus, a high-throughput sequencing technique with pretreatment and bioinformatics algorithms addressing these issues in eccDNA analysis has been recently developed. Pretreatment enhances the efficiency of the separation of linear and circular DNA [102, 103]. Bowtie 2 is a computational tool based on next-generation sequencing with bioinformatics algorithms for eccDNA identification and downstream analyses. Bowtie 2 (version 2.2.25) aligns the paired-end reads to the nematode (ce10) or human (hg38) reference genomes [104]. Picard deduplicates the mapped reads, and SAMtools sorts and indexes the unique reads. A separate positioning approach using both unique and repeated k-mer sequences is used to process the sequences that cannot be uniquely mapped [105]. The repetitive elements found in eccDNA fractions can be divided into unique chromosomal, focal, dispersed, and intrachromosomal repeats based on Dfam databases [106].
Advances in sequencing technologies have allowed the genome-scale identification and mapping of eccDNAs that range in size from 0.1 kb to 2 kb from human cell lines and mice [15, 37]. The commonly used cloning techniques with high resolution in analyzing eccDNAs are sufficiently sensitive under various conditions, which makes them less representative with respect to the sequence content and organization of eccDNA [56].
Due to the low abundance of eccDNA, the purification of supercoiled circles on cesium chloride ethidium bromide (CsCl-EtBr) density gradients requires a large amount of biomaterials and has a low yield of open circles, which is the primary form of eccDNA [56]. A recent study used an approach based on CsCl-EtBr followed by tagmentation and high-throughput sequencing [47], and this approach overcomes the reduced yield of eccDNAs obtained by enrichment using exonuclease. Researchers have developed DNA topology-dependent approaches for enrichment and characterization, and this approach provides a comprehensive and robust profile of eccDNAs [47]. The high-throughput sequencing of exonuclease-resistant, rolling circle-amplified sequences followed by a paired-end computational method to identify junctional sequences allows the characterization of eccDNA sequences at base pair resolution [15]. Using these methods, however, the number of longer eccDNAs may be underestimated because smaller circles are amplified at a higher rate than larger circles even though EM suggests that most of the circles are small [37]. Digestion with Msp I requires a CCGG recognition site, which limits the proportion of eccDNAs that can be measured [21]. Furthermore, this approach might theoretically be biased toward larger eccDNA molecules due to a greater chance of containing such recognition sites. Tagmentation is more sensitive than the Msp I digestion method, particularly for shorter eccDNAs. On average, only 19.76% of the eccDNA molecules identified by the tagmentation approach have at least one CCGG motif. Thus, only approximately one-fifth of eccDNAs identified by the tagmentation approach are potentially detectable using the Msp I method [21]. Methods based on exonuclease III digestion can quantify covalently closed circular DNA [26]. We should note that eccDNAs are often unrecognized or lost by whole-genome studies relying on existing methods. The use of appropriate methods for eccDNA research is necessary for obtaining accurate results. Finally, we summarize the goals, advantages, and limitations of the methods that have been applied in eccDNA research (Table 2) and organize this information into a flowchart (Fig. 5).
Flowchart for eccDNA research. The schematic comprehensively details eccDNA research, and some of the steps are not essential. In their research, researchers can select some of the steps based on the strengths and limitations of the methods listed in Table 1. Karyotyping and EMs, which are classic techniques in most labs, can qualitatively identify eccDNAs. Deep sequencing shows numerous strengths but is expensive. The process of eccDNA structure validation, which can be achieved by an integrated approach combining FISH and inverse PCR, is critical for studying eccDNA. Functional and mechanistic assays include transcriptomic, proteomic, and other approaches
Perspective and challenges
EccDNAs contribute to several roles, including cell genotype, heterogeneity, and adaptation to environmental factors, such as lifestyle, nutrition, and stress. Therefore, the characteristics of tumor-specific eccDNAs in tumor and matched specimens may be helpful for the diagnosis and prognosis of these diseases. Recent studies have shown that the size distributions [107], end locations [108], end motifs [109], and epigenetic features [22] of eccDNAs are helpful for tracing their origin. EccDNAs, including those larger than 2 kb [110], can be released as extracellular free DNAs from healthy and unhealthy cells into biological fluid under different circumstances and may serve as novel biomarkers to shed new insights for the early detection of cancer, the monitoring of responses to drug treatment, and cancer survival [110,111,112,113,114]. We summarize the potential clinical utility of eccDNA in cancer in Table 3 based on differences in the copies, sequences, structure, and function of eccDNAs. However, the identification of specific eccDNAs with different functional roles requires additional investigation, which depends on novel tools and method innovation. More attention should be given to the functions and relative mechanisms of eccDNAs as well as the associated methodology because the discovery of and further research on eccDNAs are challenges of sequencing technology, which still cannot discriminate eccDNA from chromosomal DNA.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- eccDNA:
-
Extrachromosomal circular DNA
- DMs:
-
Double minutes
- spcDNA:
-
Small polydisperse circular DNA
- cfDNA:
-
Cell-free DNA
- UTR:
-
Untranslated region
- BFB:
-
Breakage-fusion bridge
- TE:
-
Transposable element
- TBEs:
-
Telomere-bearing elements
- FISH:
-
Fluorescent in situ hybridization
- EMs:
-
Electron micrographs
References
Hota Y, Bassel A. Molecular size and circularity of DNA in cells of mammals and higher plants. Proc Natl Acad Sci U S A. 1965;53:356–62.
Kucheria K. Double minute chromatin bodies in a sub-ependymal glioma. Br J Cancer. 1968;22:696–7.
Liao Z, Jiang W, Ye L, Li T, Yu X, Liu L. Classification of extrachromosomal circular DNA with a focus on the role of extrachromosomal DNA (ecDNA) in tumor heterogeneity and progression. Biochim Biophys Acta Rev Cancer. 2020;1874:188392.
Moller HD. Circle-Seq: isolation and sequencing of chromosome-derived circular DNA elements in cells. Methods Mol Biol. 2020;2119:165–81.
Kumar P, Kiran S, Saha S, Su Z, Paulsen T, Chatrath A, Shibata Y, Shibata E, Dutta A. ATAC-seq identifies thousands of extrachromosomal circular DNA in cancer and cell lines. Sci Adv. 2020;6:a2489.
Koche RP, Rodriguez-Fos E, Helmsauer K, Burkert M, MacArthur IC, Maag J, Chamorro R, Munoz-Perez N, Puiggros M, Dorado GH, et al. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma. Nat Genet. 2020;52:29–34.
Wu S, Turner KM, Nguyen N, Raviram R, Erb M, Santini J, Luebeck J, Rajkumar U, Diao Y, Li B, et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature. 2019;575:699–703.
DeCarvalho AC, Kim H, Poisson LM, Winn ME, Mueller C, Cherba D, Koeman J, Seth S, Protopopov A, Felicella M, et al. Discordant inheritance of chromosomal and extrachromosomal DNA elements contributes to dynamic disease evolution in glioblastoma. Nat Genet. 2018;50:708–17.
Morton AR, Dogan-Artun N, Faber ZJ, MacLeod G, Bartels CF, Piazza MS, Allan KC, Mack SC, Wang X, Gimple RC, et al. Functional enhancers shape extrachromosomal oncogene amplifications. Cell. 2019;179:1330–41.
Hull RM, Houseley J. The adaptive potential of circular DNA accumulation in ageing cells. Curr Genet. 2020;66:889–94.
Qiu GH, Zheng X, Fu M, Huang C, Yang X. The decreased exclusion of nuclear eccDNA: from molecular and subcellular levels to human aging and age-related diseases. Ageing Res Rev. 2021;67:101306.
Hull RM, King M, Pizza G, Krueger F, Vergara X, Houseley J. Transcription-induced formation of extrachromosomal DNA during yeast ageing. PLoS Biol. 2019;17:e3000471.
Tandon I, Pal R, Pal JK, Sharma NK. Extrachromosomal circular DNAs: an extra piece of evidence to depict tumor heterogeneity. Future Sci OA. 2019;5:O390.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Shibata Y, Kumar P, Layer R, Willcox S, Gagan JR, Griffith JD, Dutta A. Extrachromosomal microDNAs and chromosomal microdeletions in normal tissues. Science. 2012;336:82–6.
Moller HD, Mohiyuddin M, Prada-Luengo I, Sailani MR, Halling JF, Plomgaard P, Maretty L, Hansen AJ, Snyder MP, Pilegaard H, et al. Circular DNA elements of chromosomal origin are common in healthy human somatic tissue. Nat Commun. 2018;9:1069.
Sunnerhagen P, Sjoberg RM, Karlsson AL, Lundh L, Bjursell G. Molecular cloning and characterization of small polydisperse circular DNA from mouse 3T6 cells. Nucleic Acids Res. 1986;14:7823–38.
Oda T, Omura S, Yamamoto S, Nishida S, Hirata S. Circular DNA’s from HeLa cell nuclei and mitochondria. Acta Med Okayama. 1970;24:405–15.
Motejlek K, Assum G, Krone W, Kleinschmidt AK. The size of small polydisperse circular DNA (spcDNA) in angiofibroma-derived cell cultures from patients with tuberous sclerosis (TSC) differs from that in fibroblasts. Hum Genet. 1991;87:6–10.
Neidlinger C, Assum G, Krone W, Dietrich C, Hochsattel R, Klotz G. Increased amounts of small polydisperse circular DNA (spcDNA) in angiofibroma-derived cell cultures from patients with tuberous sclerosis (TS). Hum Genet. 1988;79:286–8.
Sin S, Jiang P, Deng J, Ji L, Cheng SH, Dutta A, Leung TY, Chan K, Chiu R, Lo Y. Identification and characterization of extrachromosomal circular DNA in maternal plasma. Proc Natl Acad Sci U S A. 2020;117:1658–65.
Sin S, Ji L, Deng J, Jiang P, Cheng SH, Heung M, Lau C, Leung TY, Chan K, Chiu R, Lo Y. Characteristics of fetal extrachromosomal circular DNA in maternal plasma: methylation status and clearance. Clin Chem. 2021;67(5):788–96.
Jiang P, Lo Y. The long and short of circulating cell-free DNA and the ins and outs of molecular diagnostics. Trends Genet. 2016;32:360–71.
Jiang P, Chan CW, Chan KC, Cheng SH, Wong J, Wong VW, Wong GL, Chan SL, Mok TS, Chan HL, et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc Natl Acad Sci U S A. 2015;112:E1317–25.
Jiang P, Sun K, Tong YK, Cheng SH, Cheng T, Heung M, Wong J, Wong V, Chan H, Chan K, et al. Preferred end coordinates and somatic variants as signatures of circulating tumor DNA associated with hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2018;115:E10925–33.
Gaubatz JW, Flores SC. Purification of eucaryotic extrachromosomal circular DNAs using exonuclease III. Anal Biochem. 1990;184:305–10.
Cohen S, Menut S, Mechali M. Regulated formation of extrachromosomal circular DNA molecules during development in Xenopus laevis. Mol Cell Biol. 1999;19:6682–9.
Cohen S, Yacobi K, Segal D. Extrachromosomal circular DNA of tandemly repeated genomic sequences in Drosophila. Genome Res. 2003;13:1133–45.
Bertelsen AH, Humayun MZ, Karfopoulos SG, Rush MG. Molecular characterization of small polydisperse circular deoxyribonucleic acid from an African green monkey cell line. Biochemistry-Us. 1982;21:2076–85.
Motejlek K, Schindler D, Assum G, Krone W. Increased amount and contour length distribution of small polydisperse circular DNA (spcDNA) in Fanconi anemia. Mutat Res. 1993;293:205–14.
Stanfield SW, Helinski DR. Cloning and characterization of small circular DNA from Chinese hamster ovary cells. Mol Cell Biol. 1984;4:173–80.
Stanfield SW, Lengyel JA. Small circular DNA of Drosophila melanogaster: chromosomal homology and kinetic complexity. Proc Natl Acad Sci U S A. 1979;76:6142–6.
van Loon N, Miller D, Murnane JP. Formation of extrachromosomal circular DNA in HeLa cells by nonhomologous recombination. Nucleic Acids Res. 1994;22:2447–52.
Moller HD, Ramos-Madrigal J, Prada-Luengo I, Gilbert M, Regenberg B. Near-random distribution of chromosome-derived circular DNA in the condensed genome of pigeons and the larger, more repeat-rich human genome. Genome Biol Evol. 2020;12:3762–77.
Kapitonov VV, Jurka J. Helitrons on a roll: eukaryotic rolling-circle transposons. Trends Genet. 2007;23:521–9.
Paulsen T, Kumar P, Koseoglu MM, Dutta A. Discoveries of extrachromosomal circles of DNA in normal and tumor cells. Trends Genet. 2018;34:270–8.
Dillon LW, Kumar P, Shibata Y, Wang YH, Willcox S, Griffith JD, Pommier Y, Takeda S, Dutta A. Production of extrachromosomal microDNAs is linked to mismatch repair pathways and transcriptional activity. Cell Rep. 2015;11:1749–59.
Storlazzi CT, Lonoce A, Guastadisegni MC, Trombetta D, D’Addabbo P, Daniele G, L’Abbate A, Macchia G, Surace C, Kok K, et al. Gene amplification as double minutes or homogeneously staining regions in solid tumors: origin and structure. Genome Res. 2010;20:1198–206.
Carroll SM, DeRose ML, Gaudray P, Moore CM, Needham-Vandevanter DR, Von Hoff DD, Wahl GM. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol. 1988;8:1525–33.
Helmsauer K, Valieva ME, Ali S, Chamorro GR, Schopflin R, Roefzaad C, Bei Y, Dorado GH, Rodriguez-Fos E, Puiggros M, et al. Enhancer hijacking determines extrachromosomal circular MYCN amplicon architecture in neuroblastoma. Nat Commun. 2020;11:5823.
Mehanna P, Gagne V, Lajoie M, Spinella JF, St-Onge P, Sinnett D, Brukner I, Krajinovic M. Characterization of the microDNA through the response to chemotherapeutics in lymphoblastoid cell lines. PLoS One. 2017;12:e184365.
Moller HD, Parsons L, Jorgensen TS, Botstein D, Regenberg B. Extrachromosomal circular DNA is common in yeast. Proc Natl Acad Sci U S A. 2015;112:E3114–22.
Shoshani O, Brunner SF, Yaeger R, Ly P, Nechemia-Arbely Y, Kim DH, Fang R, Castillon GA, Yu M, Li J, et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature. 2021;591:137–41.
Huang Y, Ding W, Zhang M, Han J, Jing Y, Yao W, Hasterok R, Wang Z, Wang K. The formation and evolution of centromeric satellite repeats in Saccharum species. Plant J. 2021;106(3):616–29.
Cohen S, Mechali M. A novel cell-free system reveals a mechanism of circular DNA formation from tandem repeats. Nucleic Acids Res. 2001;29:2542–8.
Cohen S, Agmon N, Sobol O, Segal D. Extrachromosomal circles of satellite repeats and 5S ribosomal DNA in human cells. Mob DNA. 2010;1:11.
Shoura MJ, Gabdank I, Hansen L, Merker J, Gotlib J, Levene SD, Fire AZ. Intricate and cell type-specific populations of endogenous circular DNA (eccDNA) in Caenorhabditis elegans and Homo sapiens. G3 (Bethesda). 2017;7:3295–303.
Huang C, Jia P, Chastain M, Shiva O, Chai W. The human CTC1/STN1/TEN1 complex regulates telomere maintenance in ALT cancer cells. Exp Cell Res. 2017;355:95–104.
Sinclair DA, Guarente L. Extrachromosomal rDNA circles–a cause of aging in yeast. Cell. 1997;91:1033–42.
Van Roy N, Vandesompele J, Menten B, Nilsson H, De Smet E, Rocchi M, De Paepe A, Pahlman S, Speleman F. Translocation-excision-deletion-amplification mechanism leading to nonsyntenic coamplification of MYC and ATBF1. Genes Chromosomes Cancer. 2006;45:107–17.
Mourier T. Potential movement of transposable elements through DNA circularization. Curr Genet. 2016;62:697–700.
Moller HD, Larsen CE, Parsons L, Hansen AJ, Regenberg B, Mourier T. Formation of extrachromosomal circular DNA from long terminal repeats of retrotransposons in Saccharomyces cerevisiae. G3 (Bethesda). 2015;6:453–62.
Mourier T. Transposable elements and circular DNAs. Mob Genet Elements. 2016;6:e1240748.
Koo DH, Molin WT, Saski CA, Jiang J, Putta K, Jugulam M, Friebe B, Gill BS. Extrachromosomal circular DNA-based amplification and transmission of herbicide resistance in crop weed Amaranthus palmeri. Proc Natl Acad Sci U S A. 2018;115:3332–7.
Williams K, Doak TG, Herrick G. Developmental precise excision of Oxytricha trifallax telomere-bearing elements and formation of circles closed by a copy of the flanking target duplication. Embo J. 1993;12:4593–601.
Cohen S, Segal D. Extrachromosomal circular DNA in eukaryotes: possible involvement in the plasticity of tandem repeats. Cytogenet Genome Res. 2009;124:327–38.
Mansisidor A, Molinar TJ, Srivastava P, Dartis DD, Pino DA, Blitzblau HG, Klein H, Hochwagen A. Genomic copy-number loss is rescued by self-limiting production of DNA circles. Mol Cell. 2018;72:583–93.
Lehman MK, Nuxoll AS, Yamada KJ, Kielian T, Carson SD, Fey PD. Protease-mediated growth of Staphylococcus aureus on host proteins is opp3 dependent. Mbio. 2019;10(2):e02553-18.
Camenzind T, Lehmann A, Ahland J, Rumpel S, Rillig MC. Trait-based approaches reveal fungal adaptations to nutrient-limiting conditions. Environ Microbiol. 2020;22:3548–60.
Gresham D, Usaite R, Germann SM, Lisby M, Botstein D, Regenberg B. Adaptation to diverse nitrogen-limited environments by deletion or extrachromosomal element formation of the GAP1 locus. Proc Natl Acad Sci U S A. 2010;107:18551–6.
Storci G, Bacalini MG, Bonifazi F, Garagnani P, De Carolis S, Salvioli S, Olivieri F, Bonafe M. Ribosomal DNA instability: an evolutionary conserved fuel for inflammaging. Ageing Res Rev. 2020;58:101018.
Molin WT, Yaguchi A, Blenner M, Saski CA. The EccDNA replicon: a heritable, extranuclear vehicle that enables gene amplification and glyphosate resistance in Amaranthus palmeri. Plant Cell. 2020;32:2132–40.
Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–84.
Paulsen T, Shibata Y, Kumar P, Dillon L, Dutta A. Small extrachromosomal circular DNAs, microDNA, produce short regulatory RNAs that suppress gene expression independent of canonical promoters. Nucleic Acids Res. 2019;47:4586–96.
Turner KM, Deshpande V, Beyter D, Koga T, Rusert J, Lee C, Li B, Arden K, Ren B, Nathanson DA, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature. 2017;543:122–5.
Podobnik V, Smolic V. Peripheral uveitis caused by Toxocara larvae. Lijec Vjesn. 1990;112:312–4.
Ubeda JM, Raymond F, Mukherjee A, Plourde M, Gingras H, Roy G, Lapointe A, Leprohon P, Papadopoulou B, Corbeil J, Ouellette M. Genome-wide stochastic adaptive DNA amplification at direct and inverted DNA repeats in the parasite Leishmania. PLoS Biol. 2014;12:e1001868.
Beverley SM, Coderre JA, Santi DV, Schimke RT. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984;38:431–9.
Haber DA, Schimke RT. Unstable amplification of an altered dihydrofolate reductase gene associated with double-minute chromosomes. Cell. 1981;26:355–62.
Nathanson DA, Gini B, Mottahedeh J, Visnyei K, Koga T, Gomez G, Eskin A, Hwang K, Wang J, Masui K, et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science. 2014;343:72–6.
Verhaak R, Bafna V, Mischel PS. Extrachromosomal oncogene amplification in tumour pathogenesis and evolution. Nat Rev Cancer. 2019;19:283–8.
Zhou YH, Chen Y, Hu Y, Yu L, Tran K, Giedzinski E, Ru N, Gau A, Pan F, Qiao J, et al. The role of EGFR double minutes in modulating the response of malignant gliomas to radiotherapy. Oncotarget. 2017;8:80853–68.
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77.
Valent A, Benard J, Clausse B, Barrois M, Valteau-Couanet D, Terrier-Lacombe MJ, Spengler B, Bernheim A. In vivo elimination of acentric double minutes containing amplified MYCN from neuroblastoma tumor cells through the formation of micronuclei. Am J Pathol. 2001;158:1579–84.
Ruiz-Herrera A, Smirnova A, Khoriauli L, Nergadze SG, Mondello C, Giulotto E. Gene amplification in human cells knocked down for RAD54. Genome Integr. 2011;2:5.
Hahn P, Nevaldine B, Morgan WF. X-ray induction of methotrexate resistance due to dhfr gene amplification. Somat Cell Mol Genet. 1990;16:413–23.
Von Hoff DD, Waddelow T, Forseth B, Davidson K, Scott J, Wahl G. Hydroxyurea accelerates loss of extrachromosomally amplified genes from tumor cells. Cancer Res. 1991;51:6273–9.
Cai M, Zhang H, Hou L, Gao W, Song Y, Cui X, Li C, Guan R, Ma J, Wang X, et al. Inhibiting homologous recombination decreases extrachromosomal amplification but has no effect on intrachromosomal amplification in methotrexate-resistant colon cancer cells. Int J Cancer. 2019;144:1037–48.
Morales C, Garcia MJ, Ribas M, Miro R, Munoz M, Caldas C, Peinado MA. Dihydrofolate reductase amplification and sensitization to methotrexate of methotrexate-resistant colon cancer cells. Mol Cancer Ther. 2009;8:424–32.
Jia X, Guan R, Cui X, Zhu J, Liu P, Zhang L, Wang D, Zhang Y, Dong K, Wu J, et al. Molecular structure and evolution mechanism of two populations of double minutes in human colorectal cancer cells. J Cell Mol Med. 2020;24:14205–16.
Von Hoff DD, McGill JR, Forseth BJ, Davidson KK, Bradley TP, Van Devanter DR, Wahl GM. Elimination of extrachromosomally amplified MYC genes from human tumor cells reduces their tumorigenicity. Proc Natl Acad Sci U S A. 1992;89:8165–9.
Eckhardt SG, Dai A, Davidson KK, Forseth BJ, Wahl GM, Von Hoff DD. Induction of differentiation in HL60 cells by the reduction of extrachromosomally amplified c-myc. Proc Natl Acad Sci U S A. 1994;91:6674–8.
Kanoh T, Saigo K, Yamagishi M. Neutrophils with ring-shaped nuclei in chronic neutrophilic leukemia. Am J Clin Pathol. 1986;86:748–51.
Kunisada T, Yamagishi H. Rapid microscale procedure for visualizing intracellular plasmid DNA by electron microscopy. Plasmid. 1983;9:8–16.
Agsteribbe E, Kroon AM, van Bruggen EF. Circular DNA from mitochondria of Neurospora crassa. Biochim Biophys Acta. 1972;269:299–303.
Billheimer FE, Avers CJ. Nuclear and mitochondrial DNA from wild-type and petite yeast: circularity, length, and buoyant density. Proc Natl Acad Sci U S A. 1969;64:739–46.
Buongiorno-Nardelli M, Amaldi F, Lava-Sanchez PA. Electron microscope analysis of amplifying ribosomal DNA from Xenopus laevis. Exp Cell Res. 1976;98:95–103.
Ono T, Ozeki Y, Okubo S, Inoki S. Characterization of nuclear and satellite DNA from trypanosomes. Biken J. 1971;14:203–15.
Smith CA, Vinograd J. Small polydisperse circular DNA of HeLa cells. J Mol Biol. 1972;69:163–78.
Stanfield S, Helinski DR. Small circular DNA in Drosophila melanogaster. Cell. 1976;9:333–45.
Wong FY, Wildman SG. Simple procedure for isolation of satellite DNA’s from tobacco leaves in high yield and demonstration of minicircles. Biochim Biophys Acta. 1972;259:5–12.
Cox D, Yuncken C, Spriggs AI. Minute chromatin bodies in malignant tumours of childhood. Lancet. 1965;1:55–8.
Radloff R, Bauer W, Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967;57:1514–21.
Dijkwel PA, Hamlin JL. Mapping replication origins by neutral/neutral two-dimensional gel electrophoresis. Methods. 1997;13:235–45.
Friedman KL, Brewer BJ. Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol. 1995;262:613–27.
Cohen S, Lavi S. Induction of circles of heterogeneous sizes in carcinogen-treated cells: two-dimensional gel analysis of circular DNA molecules. Mol Cell Biol. 1996;16:2002–14.
Regev A, Cohen S, Cohen E, Bar-Am I, Lavi S. Telomeric repeats on small polydisperse circular DNA (spcDNA) and genomic instability. Oncogene. 1998;17:3455–61.
Navratilova A, Koblizkova A, Macas J. Survey of extrachromosomal circular DNA derived from plant satellite repeats. BMC Plant Biol. 2008;8:90.
Zellinger B, Akimcheva S, Puizina J, Schirato M, Riha K. Ku suppresses formation of telomeric circles and alternative telomere lengthening in Arabidopsis. Mol Cell. 2007;27:163–9.
Joly JR, Winn WC. Legionella pneumophila subgroups, monoclonal antibody reactivity, and strain virulence in Burlington, Vermont. J Infect Dis. 1988;158:1412.
Schneider SS, Hiemstra JL, Zehnbauer BA, Taillon-Miller P, Le Paslier DL, Vogelstein B, Brodeur GM. Isolation and structural analysis of a 1.2-megabase N-myc amplicon from a human neuroblastoma. Mol Cell Biol. 1992;12:5563–70.
Palas KM, Kushner SR. Biochemical and physical characterization of exonuclease V from Escherichia coli. Comparison of the catalytic activities of the RecBC and RecBCD enzymes. J Biol Chem. 1990;265:3447–54.
Grossman LI, Watson R, Vinograd J. Restricted uptake of ethidium bromide and propidium diiodide by denatured closed circular DNA in buoyant cesium chloride. J Mol Biol. 1974;86:271–83.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9.
Li W, Freudenberg J, Miramontes P. Diminishing return for increased Mappability with longer sequencing reads: implications of the k-mer distributions in the human genome. BMC Bioinformatics. 2014;15:2.
Hubley R, Finn RD, Clements J, Eddy SR, Jones TA, Bao W, Smit AF, Wheeler TJ. The Dfam database of repetitive DNA families. Nucleic Acids Res. 2016;44:D81–9.
Lo YM, Chan KC, Sun H, Chen EZ, Jiang P, Lun FM, Zheng YW, Leung TY, Lau TK, Cantor CR, Chiu RW. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med. 2010;2:61r–91r.
Sun K, Jiang P, Wong A, Cheng Y, Cheng SH, Zhang H, Chan K, Leung TY, Chiu R, Lo Y. Size-tagged preferred ends in maternal plasma DNA shed light on the production mechanism and show utility in noninvasive prenatal testing. Proc Natl Acad Sci U S A. 2018;115:E5106–14.
Serpas L, Chan R, Jiang P, Ni M, Sun K, Rashidfarrokhi A, Soni C, Sisirak V, Lee WS, Cheng SH, et al. Dnase1l3 deletion causes aberrations in length and end-motif frequencies in plasma DNA. Proc Natl Acad Sci U S A. 2019;116:641–9.
Kumar P, Dillon LW, Shibata Y, Jazaeri AA, Jones DR, Dutta A. Normal and cancerous tissues release extrachromosomal circular DNA (eccDNA) into the circulation. Mol Cancer Res. 2017;15:1197–205.
Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell. 2014;54:716–27.
Cai ZX, Chen G, Zeng YY, Dong XQ, Lin MJ, Huang XH, Zhang D, Liu XL, Liu JF. Circulating tumor DNA profiling reveals clonal evolution and real-time disease progression in advanced hepatocellular carcinoma. Int J Cancer. 2017;141:977–85.
Zhu J, Zhang F, Du M, Zhang P, Fu S, Wang L. Molecular characterization of cell-free eccDNAs in human plasma. Sci Rep. 2017;7:10968.
Khatami F, Larijani B, Tavangar SM. The presence of tumor extrachomosomal circular DNA (ecDNA) as a component of liquid biopsy in blood. Med Hypotheses. 2018;114:5–7.
Meng X, Qi X, Guo H, Cai M, Li C, Zhu J, Chen F, Guo H, Li J, Zhao Y, et al. Novel role for non-homologous end joining in the formation of double minutes in methotrexate-resistant colon cancer cells. J Med Genet. 2015;52:135–44.
Acknowledgements
We thank the investigators whose studies have not yet been cited.
Funding
This work was supported by the National Natural Science Foundation of China (81202231), Natural Science Foundation of Guangdong Province (2018A0303130240, 2020A15150120, and 2020A1515110614), Medical Scientific Research Funding of Guangdong Province, China (A2018225 and A2020189), Scientific Research Funding of Guangdong Medical University (B2017021, B2019027), and Discipline Construction Project of Guangdong Medical University (4SG21021G).
Author information
Authors and Affiliations
Contributions
Xiaoxuan Ling designed and conceptualized the study and wrote the article. Yali Han, Jinxue Meng, Bohuan Zhong, Jialong Chen, and He Zhang collected the documents and reviewed the manuscript. Jiheng Qin and Jing Pang illustrated the images. Linhua Liu designed and conceptualized the study and wrote and revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ling, X., Han, Y., Meng, J. et al. Small extrachromosomal circular DNA (eccDNA): major functions in evolution and cancer. Mol Cancer 20, 113 (2021). https://doi.org/10.1186/s12943-021-01413-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-021-01413-8