Abstract
Introduction
A growing number of publications report variation in the distribution of cardiometabolic risk factors (CMRFs) at different geographic scales. A review of these variations may help inform policy and health service organisation.
Aim
To review studies reporting variation in the geographic distribution of CMRFs and its association with various proxy measures of area-level socioeconomic disadvantage (ASED) among the adult ( ≥ 18 years) population across the world.
Methods
A systematic search for published articles was conducted in four databases (MEDLINE (Ovid), PubMed, Scopus and Web of Science) considering the interdisciplinary nature of the review question. Population-based cross-sectional and cohort studies on geographic variations of one or more biological proxies of CMRFs with/without an analysed contextual association with ASED were included. Two independent reviewers screened the studies and PRISMA guidelines were followed in the study selection and reporting.
Result
A total of 265 studies were retrieved and screened, resulting in 24 eligible studies. The review revealed reports of variation in the distribution of CMRFs, at varying geographic scales, in multiple countries. In addition, consistent associations between ASED and higher prevalence of CMRFs were demonstrated. The reports were mainly from industrialised nations and small area geographic units were frequently used.
Conclusion
Geographic variation in cardiometabolic risk exists across multiple spatial scales and is positively associated with ASED. This association is independent of individual-level factors and provides an imperative for area-based approaches to informing policy and health service organisation. The study protocol is registered in International prospective register of systematic reviews (Register No: CRD42018115294) PROSPERO 2018.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) associated metabolic risk factors represent major global public health concerns. CVD is the leading cause of human death, accounting for 17.7 million (31%) of the 56.4 million total deaths reported worldwide in 2015 [1]. Coronary heart disease (7.4 million) and stroke (6.7 million) were responsible for the greatest mortality within CVD and have remained the leading cause for mortality for the last 15 years [2]. CVD and its associated metabolic risk factors are listed in the top 15 causes of disability adjusted life years (DALY) globally [3]. In keeping with historical trends, deaths due to CVD are projected to increase steeply and reach more than 23.6 million annually by 2030 [4].
An important way to control CVD is by focussing on reducing associated metabolic risk factors. In low resource settings, vulnerable and disadvantaged groups are more likely to be exposed to unhealthy products and practices and develop metabolic risk factors for the development of CVD [5]. Cardiometabolic risk factors (CMRFs) such as diabetes mellitus (DM), hyperlipidaemia, high body mass index (BMI), and chronic kidney disease (CKD) can predispose and worsen CVD. Individual level approaches to prevent and control these risk factors have demonstrated limited success as evidenced by its increasing rates [6,7,8]. Thus it is important, in addition, to discern the contextual associations of development of these risk factors to assist in mitigating this global epidemic.
Geographic inequalities in the distribution of CMRFs at varying scales are reported in multiple studies from different countries in association with area-level socioeconomic disadvantage (ASED). Reviewing the area level distribution patterns and associated area level disadvantages reported in these studies may deepen our understanding of the higher prevalence of CMRFs in some geographic areas. Most recent relevant reviews in this area have broadly covered the influence of physical, social and service environment characteristics on CVD risk [9,10,11,12]. However, the potentially important influence of ASED is critically under-examined. Systematic synthesis of evidence regarding this globally reported variation and association can inform policy development and healthcare service planning to detail area level approaches, in addition to individual level measures, to prevent and control CMRFs effectively.
Therefore, the questions attempted to answer in this review are: Is there any geographic variation in the distribution of CMRFs among the adult population (aged 18 years and above) across the world, and is this variation associated with ASED? The studies expected to include were epidemiological or population based cross sectional and/or cohort studies.
Methods
A review protocol was developed and registered in International prospective register of systematic reviews, PROSPERO 2018 (Register No: CRD42018115294) Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018115294.
Four databases; MEDLINE (Ovid), PubMed, Scopus and Web of science databases were chosen for the search, considering the breadth of fields they cover and the interdisciplinary nature of the review question. Also, hand-searches of related articles served as ‘other sources’ of studies. The database search strategy commenced with two general search domains [1]: studies on CMRFs in singular and composite forms; and [2] geographic and spatial health studies. An intersectional retrieval of studies from both these domains yielded a narrower list of studies on geographic variation in CMRFs. A third domain [3] studies addressing area-level measures of socioeconomic disadvantages were further intersected with the retrieved studies to create a focal list of studies addressing geographic association of CMRFs with ASED. This approach maximised the number of potentially eligible studies identified compared to using single domain searches. Figure 1 conceptualizes the major search domains and their intersections used in the review.
The review included epidemiological or population-based cross-sectional and cohort studies on: geographic variation of one or more biological proxies of CMRFs, with/without an analysed contextual association with ASED. Obesity, diabetes mellitus (DM), hyperlipidaemia, and indices of low kidney function were the included biological proxies of CMRFs. Hypertension is included only when reported with other biological proxies of CMRFs, but not independently considering its limited summation into an overall cardiometabolic risk in an individual. Studies involving type 1 DM and gestational DM were excluded as they were out of scope for the current review pertaining to the geographic or area based contexts of the CMRFs. Studies measuring area-level characteristics other than ASED were also excluded.
All search outcomes were limited to: human studies; adult population (≥ 18 years); and availability in English language. The initial search included studies from year 1995; and latter it was modified to 01/01/2001 due to minimal publications on the review topic between the years 1995–2000. The search was last updated on 30/11/2018. Adopted search strategy in Ovid MEDLINE, and search result URLs of remaining databases are available in Additional file 1.
All retrieved studies were screened by two independent reviewers (RT and RW) in three stages to reduce the risk of bias. In stage 1, articles from all databases were combined and screened to remove duplicates. Titles and abstracts of remaining articles were screened for eligibility in stage 2. The final stage of study selection was done after full text reading of the remaining studies. Qualities of the individual studies were assessed using the STROBE checklist for cohort, case–control and cross-sectional studies (www.strobe-statement.org). The second coder repeated all three stages in parallel, and selected studies were matched at the conclusion of each stage and any differences were resolved by consensus and arbitration. Other review team members (AD, DJM and XF) served as additional reviewers when required.
Data extraction and coding of the chosen studies were carried out using two pilot-tested templates for consistency. Template 1 focused on the geographic variation in CMRFs and was used to extract information on author, year, nation, study design, sample size and characteristics, geographic unit of reporting, studied CMRFs, and the study outcome. Data on behavioural risk factors were not extracted as these were not included in the current review. Template 2 addressed the association of ASED and cardiometabolic risk prevalence, and extracted additional data on the reported proxies of ASED and its association status. An additional template was used for thematic mapping of the data in included studies for further qualitative syntheses. Study origin, representation, nature of problem, ecological context, and evidence strength were the mapped themes.
The two independent review authors extracted and coded the data, and any discrepancies were resolved through discussions between the authors. Summary measures used in this review are descriptive and based on the frequency of relevant studies to its denominator. Endnote software was used to keep track of the bibliographic details of the studies throughout the selection and data extraction process.
Results
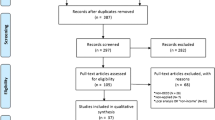
A total of 265 individual studies were retrieved from four electronic databases (n = 251) and hand searches of reference lists (n = 14). Studies from electronic data bases included 91 Ovid Medline, 80 PubMed, 58 Scopus, and 22 Web of science publications.
Figure 2 shows the screening process as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses PRISMA guidelines (www.prisma-statement.org) [13]. Stage 1 screening combined studies from all sources and removed the duplicates (n = 99). Duplicates in removed order: Ovid Medline (n = 0); PubMed (n = 80); Scopus (n = 10); Web of science (n = 3); and hand-searches (n = 6). After removing duplicates, 166 studies were forwarded for stage 2 screening. Stage 2 screening excluded 130 studies based on title and abstract screens, forwarding 36 studies for the full text screen. Studies excluded in stage 2 mainly addressed genetic, cellular, instrumental or pharmacological research regarding CMRFs. Studies on type 1 DM, paediatric or juvenile DM and gestational DM were also excluded at this stage as per the exclusions stated. Stage 3 screening carefully considered the whole full text of articles and 12 records were excluded with reason (list available in Additional file 2) leaving 24 studies for the systematic synthesis. PRISMA 2009 guidelines are followed in reporting the review and the checklist available in Additional file 3.
The review is structured into three sections. Screened research articles retrieved through ‘AND’ intersections of search domain 1 and 2 (n = 8) are reviewed in “Introduction” section: Geographic variation in the prevalence of cardiometabolic risk factors. Screened articles retrieved by intersecting domains 1 and 3 (n = 16) are reviewed in “Methods” section: Area level deprivation and cardiometabolic risk prevalence. Overall synthesis based on the total reviewed studies (n = 24) is presented in “Results” section: Overall synthesis of the studies.
Geographic variation in the prevalence of cardiometabolic risk factors
Table 1 summarizes the eight studies reviewed under this section [13,14,15,16,17,18,19,20]. Geographic variation in the prevalence of one or more CMRFs is reported in each of these studies. Most of the studies (7/8) reported hyperglycaemia as an important biomarker displaying geographic variation in cardiometabolic risk [13,14,15,16,17,18, 20,] followed by dyslipidaemia (4/8), body mass index (4/8), blood pressure (BP) (3/8) and reduced glomerular filtration rate (GFR) (1/8).
All studies reported geographic variation in the prevalence of CMRFs, regardless of the geographic unit of analysis used [13,14,15,16,17,18,19,20]. Most of these studies were from Europe (4/8), predominantly from Western Europe (3/8) [15, 16, 18, 19]. These reports were from UK, [19] Spain, [18] France, [16] and Luxembourg [15]. In the UK, geographic variation in the prevalence of risk factors such as obesity, smoking, diabetes, hypertension and high cholesterol were reported across four main regions: South England: Midlands: and Wales: Scotland: and North England [19]. A higher prevalence of CMRFs was reported in southern Spain (Andalusia), which was found in close association with sedentary lifestyle and markers of socioeconomic disadvantage [18]. Variation in the distribution of diabetes, high BMI (≥ 25 kg/m2), abdominal obesity, hypertension, high cholesterol and low glomerular filtration rate were reported at both canton and municipality levels in Luxemburg, Western Europe [15].
BMI and resting heart rate were reported to have greater geographic variation among matched cohorts in France and Australia [16].
Other reports in this section were from Oceania (2/8), East Asia (2/8) and North America 1/8)—sourced from Australia, China, South Korea and US [13, 14, 16, 17, 20]. A geographic variation of 42% was reported in the odds of being diagnosed with DM among adults in Sydney, Australia [14]. In another Australian metropolitan based cohort, glycated haemoglobin (HbA1c) was reported to have geographic variation among matched cohorts in Australia and France [16]. In China, significant variation in the regional prevalence of diabetes was reported after adjusting for age, sex and urban/rural socioeconomic circumstances [17]. Geographic clustering of cardiometabolic risk factors were reported at administrative district level in South Korea [13]. The presence of a ‘diabetic belt’ with higher prevalence of diagnosed diabetes (> 11.0%) was reported in the United States, consisting of 644 counties in its 15 mostly southern states [20]. Though the risk profiles and parameters varied, all these studies consistently reported geographic variation in its CMRFs.
The geographic scales of area-based units reported in all these studies ranged from large regions [17,18,19] within countries to smaller jurisdictional administration units [13,14,15,16, 20] and trended towards smaller geographic areas over time. Easily accessible pre-existing geographic units and boundaries were used in these studies but most weren’t explicit on the spatial extention and average population within their geographic units. Three studies had relied only on self-reports on anthropometric, behavioural, biochemical, physiological and diagnostic categories of data, risking for recall bias and misclassifications [13, 14, 20].
Area level deprivation and cardiometabolic risk prevalence
Table 2 summarises the 16 studies reviewed under this section [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Reported studies were mainly from Europe (7/16) and North America (7/16), followed by Oceania (1/16) and South America (1/16). Studies from Europe were predominantly reported from the western region and sourced from UK, Germany, Czech Republic and France. Reports from North America were mainly from USA (6/7) and Canada (1/7). There was only one study from Oceania, sourced from Australia [26]. Most of these studies were sourced from industrialised nations, except one study from Brazil, [21] a developing nation in South America.
All studies reported associations of higher prevalence of CMRFs with greater ASED [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Various measures of the biological proxies of CMRFs reported includ biochemical, anthropometric, physiologic, behavioural and diagnostic categories of data. Census sourced data on ASED were used in most of these studies (12/16), whereas other survey sourced data were used in the remaining studies (4/16) to construct summary scores or indices on ASED. The categories of measures used to calculate ASED in these studies were area-level proportions of: median income; education; occupation; housing; transport; dependent population; social class; social capital; environment; security; family structure; disability; internet access; and insurance coverage. A minimum of one category of these measures were used in all the studies [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
The samples characteristics and variables considered were notably heterogeneous across studies. The sampling frame of most (7/16) of these studies were population based lists, however service provider (4/16) and employee (3/16) lists were also used. Two studies had used a combination of both population lists and service provider given lists [27, 30]. Though subjects in all studies used adult age limits (≥ 18 years), divergent age groups were sampled across all of the studies. Also gender [34, 35] and race [22, 23] specific sampling were used in two studies each. Heterogeneity of these sample characteristics makes a comparison and further quantitative synthesis difficult.
The samples were mostly accessed from existing study cohorts, laboratory databases, national surveys and audit lists. The sample size of studies ranged from 342 adults to a maximum of 91,776 adults, mostly larger in size. Census administration units were the most commonly used neighbourhood proxy, followed by other administrative units and electoral wards. Pre-existing geographic boundaries were mostly adopted to define the spatial unit, but their spatial extents of the unit of analyses were not stated in most of the studies.
Overall synthesis of the studies
Significant features of the included studies were identified to aid synthesis of the findings. These features were the origin of the study, its representativeness, nature of the CMRFs studied, the ecological context and the strength of evidence presented. These features were then formulated into five themes, mapping the related data for further analyses (Table 3).
We had plotted all the included studies to identify their global region of origin and the economic nature of the source country. Most of the studies published were from Europe (11/24), closely followed by America (9/12), (two studies were cross national, hence counted under both the nations and corresponding regions). Fewer publications were found from Oceania (3/24), and Asia (2/24). However no identified studies were from Africa. Studies from developing nations were fewer (3/24) compared with studies from industrialised nations (21/24). This emphasises a gap in related publications from Asia–pacific and African regions, especially from nations of developing and underdeveloped economies. The global representativeness of this review is hence limited, and the review findings may be more generalizable to industrialised nations.
The target populations for included studies are shown in Table 3. The sample frame of most of the studies were population based lists (13/24 studies), however service providers’ lists (5/24) and employees lists (3/24) were also used. Both population and service providers’ lists were used in three studies (3/24). All the population based studies used a random sampling technique to ensure the population representativeness. However, the response rates varied (15–90.5%) in these studies. Two studies had a response rate < 50%, suggesting a risk of responder bias despite a probability sampling method being employed [29, 36].
Ecological contexts of the included studies were analysed by extracting area level characteristics (Table 3). Area level units used in these studies extended from small areas (10/24), to medium areas (9/24) and large areas (5/24). Small area units were mostly based on census, administrative or zip code area with an average ~ 1000 residing population. Medium area units had an average ~ 5000 population and the large area units were mostly regions, provinces and districts. ASED gradients were based on area level measures of ranged from 1 to 7 measures, however single measures of income or overcrowding as an indirect proxy of ASED raised concerns regarding their comprehensiveness in comparison to aggregate measures of ASED.
The nature of CMRFs and the strength of evidence in relation to associations with outcomes were mapped by extracting data on the categories of CMRFs measured, the source of data and the mode of analyses (Table 3). Biological proxy categories of CMRFs were mostly biochemical (18/24), followed by anthropometric (18/24), physiologic (15/24), and diagnostic (4/24) in nature. Self-reported data on these categories of CMRFs had the highest risk for misclassification due to reporting bias or errors. Studies which adopted a combined mode of both statistical and spatial analyses provided a better ecological context of CMRFs than with statistical analyses alone.
Discussion
ASED was repeatedly demonstrated to be associated with higher cardiometabolic risk. Higher ASED was consistently reported to have an association with cardiovascular risk; whereas lower ASED was associated with reduced cardiovascular risk. Such associations were often demonstrated independently of individual level characteristics such as socioeconomic status, education and duration of exposure to area. Type 2 diabetes and high body mass index (BMI) were reported to be more prevalent in disadvantaged areas. Related studies report that the type of neighbourhood food outlets [37,38,39], poor physical activity resources [39], individual perception of area level features [40] residential density and service availability [41] were all explanatory variables associated with cardiometabolic risk prevalence among people living in disadvantaged neighbourhoods.
Related systematic reviews published in this area of research investigate associations for different geographically distributed factors with CVD. Chaix (2009) reviewed the associations between neighbourhood social environments and CHD, and proposed a theoretical model of a mediating mechanism focussing on the social interactional environment [10]. Consistent associations of obesity or hypertension with lower levels of area socioeconomic status, urbanization, street intersection, accessibility to supermarkets, social cohesion, service availability and residential density; and higher levels of noise pollution and density of convenience stores, were reviewed and reported by Leal [11]. Frequent inverse associations of the common indices of ASED with childhood obesity were reported in the UK [9]. Consistent associations between socio economic disadvantage and central adiposity was reported by Slopen [12]. All these reviews report important methodological inadequacies and the need for further research in this area, which support the findings of the current review.
Recent advances in geographic information systems (GIS) and analytical approaches were utilised in the studies reporting geographic variation in CMRFs. These studies have demonstrated advances in various analytical tools and the potential for plotting area level risk parameters. Geocoding and mapping of existing large population based datasets has become feasible with newer computational tools through linking location data; such as map co-ordinates, addresses or postcodes [42]. These tools have the capacity to visually display area based factors, in contrast with traditional table and graph methods, and this has the potential to enhance impact on subsequent area level health care policy development and resource allocation [43,44,45]. In addition, systematic quantitative analyses are possible with these spatial tools which create opportunities to investigate the role of environmental factors in explaining any geographic aggregations beyond random effects [46].
National estimates of CVD have limited utility in informing prevention and management of CVD within discrete communities. The disease patterns at smaller areas may significantly differ from national and regional prevalence reports, thus small area analysis is important in order to understand local patterns and requirements [47]. Small-area level analyses also have the potential to reveal area level contexts and dependencies of CMRFs and such analyses can highlight areas for targeted preventive interventions.
CVD and its associated CMRFs continue to evolve as a major global health threat. It is the highest cause of mortality and the highest absorber of health care expenditure in many developed nations [6, 48, 49]. Once diagnosed, the ongoing costs of care and productivity loss due to consequent disability and premature death creates a large economic burden not only to the individual and family, but to the nation—especially when half the people dying are found to be in their prime productive years [50]. Thus, CVD and its associated metabolic risk factors emerge as a threat not only to human health and life, but to the sustainable development and economies of nations. Hence, improving public health program effectiveness in reducing CVD must be a research priority.
Limitations
Firstly, the cross sectional nature of the reviewed studies precluded causative interpretations. Second, the global representativeness of the review is limited mainly due to publication gaps from Asia–pacific and African regions of the World. Third, the scope of our review excluded examination of behavioural, dietary and activity related risk factors and also other area level characteristics to focus only on the biological proxies of CMRFs. Fourth, methodological heterogeneity within the retrieved studies prohibited a meta-analytical synthesis of the findings. The sample characteristics, geographical scales and the CMRFs’ risk profiles varied substantially across the studies impeding any further quantitative synthesis.
Recommendations and future directions
Finding geographic variation in CMRFs (if any) and its association with ASED may assist in understanding the contexts of risk. Such studies have the potential to inform contextual planning of interventions for prevention and management of cardiometabolic risk. However, most of the studies in this review do not report the spatial extents of their units of analysis. This is important as associations are likely to be different at different levels of aggregation, and limits the ability to assess the likelihood of spatial scale effects in these studies [22, 23] known as the Modifiable Areal Unit Problem [51, 52]. When data are aggregated to larger geographic units, small-area anomalies may be diluted or smoothed over [22]. Using smaller rather than larger area scales can help to reduce the likelihood of missing important small area anomalies [53]. Similarly, supplementing individual level data along with area level data could minimise group effects due to area level aggregation of data [53]. Leveraging both individual- and area-level data provides a more complete picture to inform planning, policy and practice [46, 53]. Future research directions should include hierarchical multilevel analyses to yield comprehensive picture of the contextual aspects of risk factors, to help aid both individual and area-level better preventive initiatives.
Conclusion
Cardiometabolic risk distribution varied significantly across different geographic scales reported in multiple studies. In addition, there is strong evidence that area-level disadvantage is significantly associated with CMRFs, irrespective of individual-level characteristics. This review highlights the need for area-based preventive approaches in addition to individual-level approaches to prevent and control CMRFs and their consequent CVD outcomes.
Abbreviations
- AU:
-
Australia
- ASED:
-
area-level socioeconomic disadvantage
- BP:
-
blood pressure
- BMI:
-
body mass index
- BS:
-
blood sugar
- CD:
-
census collection district
- CHD:
-
coronary heart disease
- CMRFs:
-
cardiometabolic risk factors
- CKD:
-
chronic kidney disease
- CVD:
-
cardiovascular disease
- CVH:
-
cardiovascular health
- DM:
-
diabetes mellitus
- eGFR:
-
estimated Glomerular filtration rate
- FBG:
-
fasting blood glucose
- FG:
-
fasting glucose
- FI:
-
fasting insulin
- FPG:
-
fasting plasma glucose
- GFR:
-
glomerular filtration rate
- GR:
-
Germany
- HbA1c:
-
glycated haemoglobin
- HDL:
-
high density lipoprotein
- HR:
-
heart rate
- HT:
-
hypertension
- IR:
-
insulin resistance
- IRS:
-
insulin resistance syndrome
- IRIS:
-
ilôts regroupés pour l’information statistique
- LDL:
-
low density lipoprotein
- LGA:
-
local government area
- POA:
-
postal area
- PRISMA:
-
preferred reporting items for systematic reviews and meta-analyses
- SES:
-
socioeconomic status
- SLA:
-
statistical local area
- TC:
-
total cholesterol
- TCR:
-
total cardiometabolic risk
- T2DM:
-
type 2 diabetes mellitus
- TG:
-
triglycerides
- TRIRIS:
-
groups of around three IRIS areas
- WC:
-
waist circumference
References
WHO. Cardiovascular diseases (CVDs): Key facts [Internet]. 2016. http://www.who.int/mediacentre/factsheets/fs317/en/.
Schutzer SE, Fraser-Liggett CM, Casjens SR, Qiu WG, Dunn JJ, Mongodin EF, et al. Whole-genome sequences of thirteen isolates of Borrelia burgdorferi. J Bacteriol. 2011;193(4):1018–20.
Murray CJL, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369(5):448–57.
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603.
World Health Organisation. WHO | The top 10 causes of death [Internet]. World Health Organization; 2017 http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed 2018 Mar 10.
Cannon CP. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin Cornerstone. 2008;9(2):24–41.
Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51(15):1512–24.
Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008.
El-Sayed AM, Scarborough P, Galea S. Socioeconomic inequalities in childhood obesity in the United Kingdom: a systematic review of the literature. Obes Facts. 2012;5:671–92.
Chaix B. Geographic life environments and coronary heart disease: a literature review, theoretical contributions, methodological updates, and a research agenda. Annu Rev Public Health. 2009;30(1):81–105. https://doi.org/10.1146/annurev.publhealth.031308.100158.
Leal C, Chaix B. The influence of geographic life environments on cardiometabolic risk factors: a systematic review, a methodological assessment and a research agenda. Obes Rev. 2011;12(3):217–30.
Slopen N, Goodman E, Koenen KC, Kubzansky LD. Socioeconomic and other social stressors and biomarkers of cardiometabolic risk in youth: a systematic review of less studied risk factors. PLoS ONE. 2013;8(5):e64418.
Oh WS, Yoon S, Noh J, Sohn J, Kim C, Heo J. Geographical variations and influential factors in prevalence of cardiometabolic diseases in South Korea. PLoS ONE. 2018;13(10):e0205005.
Astell-Burt T, Feng X, Kolt GS, McLean M, Maberly G. Understanding geographical inequities in diabetes: multilevel evidence from 114,755 adults in Sydney, Australia. Diabetes Res Clin Pract. 2014;106(3):e68–73.
Alkerwi A, Bahi IE, Stranges S, Beissel J, Delagardelle C, Noppe S, et al. Geographic variations in cardiometabolic risk factors in luxembourg. Int J Environ Res Public Health. 2017;14(6):16.
Paquet C, Chaix B, Howard NJ, Coffee NT, Adams RJ, Taylor AW, et al. Geographic clustering of cardiometabolic risk factors in metropolitan centres in France and Australia. Int J Environ Res Public Health. 2016;13(5):21.
Zhou M, Astell-Burt T, Bi Y, Feng X, Jiang Y, Li Y, et al. Geographical variation in diabetes prevalence and detection in china: multilevel spatial analysis of 98,058 adults. Diabetes Care. 2015;38(1):72–81.
Valdes S, Garcia-Torres F, Maldonado-Araque C, Goday A, Calle-Pascual A, Soriguer F, et al. Prevalence of obesity, diabetes and other cardiovascular risk factors in Andalusia (Southern Spain). Comparison with national prevalence data. The Diabetes study. Rev Esp Cardiol. 2014;67(6):442–8.
Lawlor DA, Bedford C, Taylor M, Ebrahim S. Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women’s Heart and Health Study. J Epidemiol Commun Health. 2003;57(2):134–40.
Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40(4):434–9.
Barber S, Diez Roux AV, Cardoso L, Santos S, Toste V, James S, et al. At the intersection of place, race, and health in Brazil: residential segregation and cardio-metabolic risk factors in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Soc Sci Med. 2018;199:67–76.
Keita AD, Judd SE, Howard VJ, Carson AP, Ard JD, Fernandez JR. Associations of neighborhood area level deprivation with the metabolic syndrome and inflammation among middle- and older-age adults. BMC Public Health. 2014;14:1319.
Clark CR, Ommerborn MJ, Hickson DMA, Grooms KN, Sims M, Taylor HA, et al. Neighborhood disadvantage, neighborhood safety and cardiometabolic risk factors in African Americans: biosocial associations in the Jackson Heart Study. PLoS ONE. 2013;8(5):e63254.
Cox M, Boyle PJ, Davey PG, Feng Z, Morris AD. Locality deprivation and Type 2 diabetes incidence: a local test of relative inequalities. Soc Sci Med. 2007;65(9):1953–64.
Gabert R, Thomson B, Gakidou E, Roth G. Identifying high-risk neighborhoods using electronic medical records: a population-based approach for targeting diabetes prevention and treatment interventions. PLoS ONE. 2016;11(7):e0159227.
Bonney A, Mayne DJ, Jones BD, Bott L, Andersen SE, Caputi P, et al. Area-level socioeconomic gradients in overweight and obesity in a community-derived cohort of health service users—a cross-sectional study. PLoS ONE. 2015;10(8):e0137261.
Unger E, Diez-Roux AV, Lloyd-Jones DM, Mujahid MS, Nettleton JA, Bertoni A, et al. Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7(4):524–31.
Mujahid MS, Diez Roux AV, Borrell LN, Nieto FJ. Cross-sectional and longitudinal associations of BMI with socioeconomic characteristics. Obes Res. 2005;13(8):1412–21.
Maier W, Scheidt-Nave C, Holle R, Kroll LE, Lampert T, Du Y, et al. Area level deprivation is an independent determinant of prevalent type 2 diabetes and obesity at the national level in Germany. Results from the National Telephone Health Interview Surveys “German Health Update” GEDA 2009 and 2010. PLoS ONE. 2014;9(2):e89661.
Roux AVD, Jacobs DR, Kiefe CI. Neighborhood characteristics and components of the insulin resistance syndrome in young adults the coronary artery risk development in young adults (CARDIA) study. Diabetes Care. 2002;25(11):1976–82.
Cubbin C, Sundquist K, Ahlén H, Johansson S-E, Winkleby MA, Sundquist J. Neighborhood deprivation and cardiovascular disease risk factors: protective and harmful effects. Scand J Public Health. 2006;34(3):228–37.
Dragano N, Bobak M, Wege N, Peasey A, Verde PE, Kubinova R, et al. Neighbourhood socioeconomic status and cardiovascular risk factors: a multilevel analysis of nine cities in the Czech Republic and Germany. BMC Public Health. 2007;7(1):1.
Silhol R, Zins M, Chauvin P, Chaix B. Investigating the spatial variability in incidence of coronary heart disease in the Gazel cohort: the impact of area socioeconomic position and mediating role of risk factors. J Epidemiol Commun Health. 2011;65(2):137–43.
Lawlor DA, Davey Smith G, Patel R, Ebrahim S. Life-course socioeconomic position, area deprivation, and coronary heart disease: findings from the British Women’s Heart and Health Study. Am J Public Health. 2005;95(1):91–7.
Andersen AF, Carson C, Watt HC, Lawlor DA, Avlund K, Ebrahim S. Life-course socio-economic position, area deprivation and Type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabet Med. 2008;25(12):1462–8.
Naimi AI, Paquet C, Gauvin L, Daniel M. Associations between area-level unemployment, body mass index, and risk factors for cardiovascular disease in an urban area. Int J Environ Res Public Health. 2009;6(12):3082–96.
Astell-Burt T, Feng X. Geographic inequity in healthy food environment and type 2 diabetes: Can we please turn off the tap? Med J Aust. 2015;203:246–8.
Millstein RA, Yeh H-C, Brancati FL, Batts-Turner M, Gary TL. Food availability, neighborhood socioeconomic status, and dietary patterns among blacks with type 2 diabetes mellitus. Medscape J Med. 2009;11(1):15.
Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the multi-ethnic study of atherosclerosis (mesa). JAMA Intern Med. 2015;175(8):1311–20. https://doi.org/10.1001/jamainternmed.2015.2691.
Baldock K, Paquet C, Howard N, Coffee N, Hugo G, Taylor A, et al. Associations between resident perceptions of the local residential environment and metabolic syndrome. J Environ Public Health. 2012;2012:589409.
Chaix B. Geographic life environments and coronary heart disease: a literature review, theoretical contributions, methodological updates, and a research agenda. Annu Rev Public Health. 2009;30:81–105.
Stevens CD, Schriger DL, Raffetto B, Davis AC, Zingmond D, Roby DH. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff. 2014;33(8):1383–90.
Angier H, Likumahuwa S, Finnegan S, Vakarcs T, Nelson C, Bazemore A, et al. Using geographic information systems (GIS) to identify communities in need of health insurance outreach: an OCHIN practice-based research network (PBRN) report. J Am Board Fam Med. 2014;27(6):804–10. https://doi.org/10.3122/jabfm.2014.06.140029.
Auchincloss AH, Gebreab SY, Mair C, Diez Roux AV. A review of spatial methods in epidemiology. Ann Rev Public Health. 2012;33:107–22.
Bazemore A, Phillips RL, Miyoshi T. Harnessing geographic information systems (GIS) to enable community-oriented primary care. J Am Board Fam Med. 2010;23(1):22–31. https://doi.org/10.3122/jabfm.2010.01.090097.
Elliott P, Wartenberg D. Spatial epidemiology: current approaches and future challenges. Environ Health Perspect. 2004;112:998–1006.
Occelli F, Deram A, Genin M, Noel C, Cuny D, Glowacki F, et al. Mapping end-stage renal disease (ESRD): spatial variations on small area level in northern France, and association with deprivation. PLoS ONE. 2014;9(11):e110132.
World Health Organization; World Heart Federation and World Stroke Organization. Global Atlas on cardiovascular disease prevention and control. Glob Atlas Cardiovasc Dis Prev Control. 2011;155. https://www.cabdirect.org/cabdirect/abstract/20123402600%0Afile:///C:/Users/USER/Downloads/9789241564373_eng(2).pdf.
World Health Organization. WHO | Noncommunicable diseases. WHO. WHO; 2017. http://www.who.int/mediacentre/factsheets/fs355/en/. Accessed 2018 Mar 10.
Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Reddy Bloom L, Fathima S, et al. The global economic burden of noncommunicable diseases. World Econ Forum. 2011;1–46. http://ideas.repec.org/p/gdm/wpaper/8712.html.
Openshaw S. Modifiable areal unit problem. Concepts Tech Mod Geogr. 1989;38:169–74.
Openshaw S, Taylor PJ. A million or so correlation coefficients, three experiments on the modifiable areal unit problem. In: Wrigley N, editor. Statistical applications in the spatial science. London: Pion; 1979. p. 127–44. https://trove.nla.gov.au/work/10094088
Wakefield, Jonathan HL. Spatial aggregation and the ecological fallacy. Handbook of spatial statistics. 2010; pp. 541–558. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4209486/.
Authors’ contributions
Review conception and design: RT, AB, DM, and XF. Search strategy and literature search: RT. Study coding, selection and data extraction: RT, RW. Review and interpretation: RT, AB, DM. Drafting of manuscript: RT, AB, DM. Critical revision: RT, AB, DM. Final revision: RT, AB, DM, and XF
Acknowledgements
This review has been conducted with the support of the Australian Government Research Training Program Scholarship.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1.
The search strategy and results URLs.
Additional file 2.
List of excluded full text studies with reason.
Additional file 3.
PRISMA 2009 systematic review content checklist.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Toms, R., Bonney, A., Mayne, D.J. et al. Geographic and area-level socioeconomic variation in cardiometabolic risk factor distribution: a systematic review of the literature. Int J Health Geogr 18, 1 (2019). https://doi.org/10.1186/s12942-018-0165-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12942-018-0165-5