Abstract
Background
The Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae) bacterial isolates that produce extended-spectrum β-lactamases (ESBLs) contribute to global life-threatening infections. This study conducted a systematic review and meta-analysis on the global prevalence of ESBLs in co-existing E. coli and K. pneumoniae isolated from humans, animals and the environment.
Methods
The systematic review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) [ID no: CRD42023394360]. This study was carried out following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. One hundred and twenty-six eligible studies published on co-existing antibiotic resistance in E. coli and K. pneumoniae between 1990 and 2022 were included.
Results
The pooled prevalence of ESBL-producing E. coli and K. pneumoniae was 33.0% and 32.7% for humans, 33.5% and 19.4% for animals, 56.9% and 24.2% for environment, 26.8% and 6.7% for animals/environment, respectively. Furthermore, the three types of resistance genes that encode ESBLs, namely blaSHVblaCTX−M,blaOXA, and blaTEM, were all detected in humans, animals and the environment.
Conclusions
The concept of “One-Health” surveillance is critical to tracking the source of antimicrobial resistance and preventing its spread. The emerging state and national surveillance systems should include bacteria containing ESBLs. A well-planned, -implemented, and -researched alternative treatment for antimicrobial drug resistance needs to be formulated.
Similar content being viewed by others
Background
The extended-spectrum β-lactamase (ESBL) produced by Enterobacteriaceae is a common source of antimicrobial resistance (AMR) in both animals and humans [1]. These ESBL-producing bacteria are often associated with multidrug resistance (MDR) against other antibiotic classes [2]. They develop resistance to third- and fourth-generation cephalosporins, as well as aztreonam, which is among the last medications available to treat infections caused by Escherichia coli and Klebsiella pneumoniae [1, 3,4,5]. However, they are not resistant to carbapenems or cephamycin [4].
Escherichia coli and K. pneumoniae are two examples of ESBL-producing Enterobacteriaceae (ESBL-E) that were the first to be linked to infections in the medical context [6, 7]. Escherichia coli and K. pneumoniae can cause a wide range of serious illnesses, which are becoming increasingly difficult to treat due to the development of antibiotic resistance [8]. Carbapenem is an antibiotic used as a last option to treat beta-lactamase Enterobacteriaceae transfer [9]. The spread of E. coli and K. pneumoniae extended spectrum cephalosporinase in animal populations, the environment, and the community proves that such bacterial strain are transmitted and persist outside of clinical settings [8, 10].
In the Enterobacteriaceae family of commensal or pathogenic bacteria, ESBLs are crucial antibiotic resistance determinants that are passed on through horizontal gene transfer [9]. Among several ESBL gene variants, the most prevalent and clinically relevant are blaTEM, blaSHV (sulphydryl variable) and blaCTX−M, (cefotaxime-hydrolyzing β-lactamase) [4]. There are several families of ESBLs, including the blaTEMs-type ESBLs and the blaSHVs-type ESBLs, with variants that differ only by a few amino acid substitutions [4]. There are some unique characteristics in each of the ESBL families. The blaTEM−1, blaTEM−2 and their ESBL derivatives are usually carried by transposons like Tn1, Tn2, or Tn3 [4, 11]. An association has been found between the spread of blaCTX−M-producing enzymes and E. coli belonging to the ST131 clonal group [12]. It has been reported that ST131 isolates contain several blaCTX−M types, including blaCTX−M−15 [13]. The empirical treatment of ESBL-producing Enterobacteriaceae infections has become increasingly difficult [14].
The presence of ESBL-producing E. coli and K. pneumoniae in humans, animals, and the environment poses a public health threat. In addition, it is crucial to maintain current data on ESBL-producing E. coli and K. pneumoniae in health systems to minimize the consequences of ESBL-producing bacteria. A few systematic reviews have been published on ESBL-producing E. coli in animals, humans, and the environment in Bangladesh and South America [15, 16], as well as ESBL-producing E. coli and K. pneumoniae in the United States of America [17]. However, there is limited information on comprehensive data available to estimate the global prevalence of co-existing ESBL-producing E. coli and K. pneumoniae in animals, humans, and the environment. The current study is a systematic review and meta-analysis aimed at providing a comprehensive prevalence of co-existing ESBL-producing E. coli and K. pneumoniae, in animals, humans, and the environment based on available data published globally.
Materials and methods
Protocol registration
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration no: CRD42023394360.
Study design and systematic review protocol
Using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [18], data extraction, screening, and analysis involved searching database systems for potentially pertinent articles, assessing their relevance, and evaluating whether they were acceptable for review.
Search strategy
This study used several database systems, including ScienceDirect, PubMed, Google Scholar, and Scopus to search for articles published in English from 1 to 1990 until 28 November 2022. Literature searches were conducted using keywords comprising “ESBL, extended-spectrum beta-lactamase,” “extended-spectrum β-lactamase,” “Human,” “Animal,” “Environment,” “Escherichia coli or Klebsiella pneumoniae,” and “E. coli or K. pneumoniae.” The last search took place on 28 November 2022.
Study selection
Journal article titles and abstracts were reviewed for suitability for the inclusion and exclusion criteria by three authors namely TR, TM, and OT. Two reviewers independently analysed all the studies from the search by title, abstract, and selected full texts, and a third reviewer arbitrated for any divergence. Full-text journal articles published in English were included, while reviews, conference abstracts, and book chapters were excluded. Journal articles were selected for full-text review if they investigated the co-existence of ESBL-producing E. coli and K. pneumoniae only and reported the number of isolates and the positive number of ESBL isolates from animals, humans, and the environment.
Inclusion and exclusion criteria
Criteria for inclusion of studies were: (i) studies investigating the prevalence of ESBL-producing E. coli and K. pneumoniae in animals, humans, and the environment. The following exclusion criteria was used: (ii) no total number of isolates, (iii) no abstracts, reviews, experiments, and book chapters, and (iv) an unclear ESBL identification method.
Data extraction
The following data were extracted and summarized by two authors independently (TR and TM) from the final selected studies: (1) author name, (2) year of publication, (3) location, (4) year of publication, (5) type of samples, (6) ESBL identification methods, (7) positive samples of E. coli and K. pneumoniae isolates from animals, humans and the environment, (8) total number of ESBL positive E. coli and K. pneumoniae isolates, and (9) genes encoded for antibiotic resistance (ESBL). The recorded data were compiled in Microsoft Excel for further analysis.
Quality assessment of included studies
A critical appraisal tool developed by the Joanna Briggs Institute (JBI) was used to assess each study’s bias risk [19]. There are nine questions in this JBI instrument, which are: (1) Was the sample frame appropriate to address the target population? (2) Were study participants recruited in an appropriate way? (3) Was the sample size adequate? (4) Were the study subjects and setting described in detail? (5) Was data analysis conducted with sufficient coverage of the identified sample? (6) Were valid methods used for the identification of the condition? (7) Was the condition measured in a standard, reliable way for all participants? (8) Was there appropriate statistical analysis? (9) Was the response rate adequate, and, if not, was the low response rate managed appropriately? Each answer was scored 0 or 1 based on whether the answer was “yes” or “no”. NA was used to indicate that the question was not relevant to the study. Studies with scores of 7–8 indicated a low risk of bias, scores of 5–6 indicated a moderate risk of bias, and scores less than 5 indicated a high risk of bias. Only articles with low bias risks were included in this study.
Data synthesis and data analysis
Comprehensive Meta-Analysis Software v.4.0 (https://www.meta-analysis.com/) was used to conduct the meta-analysis of the prevalence of ESBL-producing E. coli and K. pneumoniae in humans, animals, and the environment. The pooled prevalence of E. coli and K. pneumoniae isolates was measured and subgroup analysis was performed according to continent, country, year of publication, settings, ESBL-resistant genes, and samples collected. Random-effects models were used to generate forest plots showing the study-specific effect sizes with a 95% confidence interval (CI) for the pooled prevalence (PP). The I2 statistic was used to measure heterogeneity among studies. A value close to 0% indicates no heterogeneity, whereas a value close to 25%, 50%, and 75% corresponds to low, moderate, and high heterogeneity, respectively. The p-values correspond to the heterogeneities between studies from a Chi-squared test of the null hypothesis that there is no heterogeneity.
Publication bias
Publication bias was measured using funnel plots to test for symmetry and this was further complemented using the Beg and Mazumdar rank correlation test and Egger’s regression test. For all the tests, a p-value of < 0.05 was considered statistically significant.
Results
Search and screening results
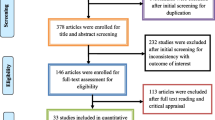
A total of 2854 studies were retrieved after the initial search was conducted across four databases, including 688 from PubMed, 925 from ScienceDirect, 980 from Google Scholar, and 261 from Scopus (Fig. 1). The abstracts of 1161 articles were screened based on the inclusion and exclusion criteria. A total of 526 studies were initially considered eligible and were thus subjected to full-text evaluation. After full-text examination, 126 animals (n = 17) were eligible for inclusion [humans (n = 101), environment (n = 5), animals and environment (n = 3)].
Characteristics of eligible studies
All included studies were published from 1990 to 2022, with most of the studies having been conducted between 2000 and 2022. Out of four continents, Asia had the highest number of published studies (n = 80), followed by Africa (n = 37), Europe (n = 11), and the least was from North America (n = 8). The number of confirmed ESBL-producing E. coli and K. pneumoniae isolates in animals, human, and the environment ranged from 1 to 4706 globally. Numerous studies recorded the expression of antibiotic-resistant genes for the class of beta-lactams, including blaOXA 20 (16%), blaCTX−M 65(52%), blaTEM 58 (46%), blaSHV 65 (52%), blaCMY, 2 (2%), blaNDM 2 (2%), blaPER 2 (2%), and blaVEB, 2 (2%). However, six genes (blaDHA, blaFOX, blaMOX, blaKPC, blaVIM, and blaampC) were recorded once by different studies.
Meta-analysis results on overall prevalence
Pooled prevalence estimates (PPE) of ESBL-producing E. coli and K. pneumoniae in animals, humans, and the environment, as well as a summary of the subgroup analysis, are shown in Tables 1 and 2, and 3. A total of 79,497 were confirmed as E. coli, while 37,998 were confirmed as K. pneumoniae. Only 18,923 and 8502 were confirmed as ESBL-producing E. coli and K. pneumoniae, respectively.
Prevalence of ESBL in humans
The global prevalence of co-existing ESBL-producing E. coli and K. pneumoniae in humans was investigated in 101 studies (Supplementary Table S1; Fig. 2), of which the pooled prevalence was 33.0% (0.330; 95% CI: 0.282–0.381) for E. coli and 32.7% (0.327; 95% CI: 0.286–0.371) for K. pneumoniae, ranging from 0.6 to 99.9%. The mean effect size is 0.330 with a 95% confidence interval of 0.282 to 0.381 for E. coli (Fig. 3A); the prediction interval (0.282 to 0.381) reflects the endpoints of this distribution. While the mean effect size is 0,327 with a 95% confidence interval of 0.286 to 0.371 for K. pneumoniae (Fig. 3B), the prediction interval shows that there were some populations where the effect was as low as 0.286 and others where it was as high as 0.371. According to the study year, ESBL-producing E. coli was more prevalent in the 2021–2022 year interval with a PPE of 53% (95% CI: 41.1–65.5%), while ESBL-producing K. pneumoniae were more prevalent in the 2000–2010 period with PPE of 39.7% (95% CI: 31.8–48.1). The period of year interval 1990–2000 had the lowest PPE for ESBL-producing E. coli and K. pneumoniae with 10.9% (95% CI: 04.4–24.5) and 25.4% (95% CI: 7.1–60.0), respectively.
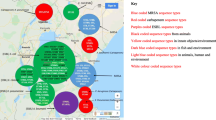
World map showing number of articles from different countries that reported the ESBL-producing E. coli and K. pneumoniae in humans (https://www.mapchart.net/world.html (accessed on 10 March 2023)
According to settings, hospitals had a high PPE for both ESBL-producing E. coli and K. pneumoniae, with 49% (95% CI: 41.1–57.4) and 45.0% (95% CI: 38.8–53.4), respectively. The lowest PPE for ESBL-producing E. coli was observed in tertiary hospitals with 37.6%, and 35.1% for ESBL-producing K. pneumoniae. In military hospitals, E. coli had 42.3% and K. pneumoniae had 19.9% PPE. Furthermore, for the type of samples screened, ESBL-producing E. coli and K. pneumoniae were more prevalent in faecal samples with 52.7% (95% CI: 29.3–74.9) and 37.7% (95% CI: 28.7–47.7) for urine. For various specimens, E. coli had 34.2% PPE and K. pneumoniae had 28.8% PPE.
At continent level, Africa reported the highest PPE at 44.3% (95% CI: 32.2–57.2) and 32.8% (95% CI: 24.6–42.2) for ESBL-producing E. coli and K. pneumoniae. Lastly, the PPE at country level indicates that Thailand registered the highest PPE at 52.1% for ESBL-producing E. coli, while the USA had a PPE of 55.7% for ESBL-producing K. pneumoniae.
Prevalence of ESBL in animals
The characteristics of all eligible animal studies included in this review were presented in Supplementary Table S2. Using a random-effect model, the pooled prevalence of ESBL-producing E. coli and K. pneumoniae was estimated at 33.5% (95% CI: 0.131–0.361) for E. coli and 19.4% (95% CI: 0.097–0.349) with high heterogeneity (I2: 97.364% and I2: 95.120%, respectively), with a mean effect size of 0.417 and 0.194 for E. coli and K. pneumoniae, respectively.
In terms of regions, the majority of the studies have performed in Africa (n = 9) (Fig. 4). For ESBL-producing E. coli, the highest estimate of 49.6% (95% CI: 28.2–71.1) was observed from Africa, and pooled prevalence was estimated at 49.6% (95% CI: 28.2–71.1) for ESBL-producing K. pneumoniae. However, Europe and North America were not included in the meta-analysis due to their low number of studies (fewer than three studies).
World map showing number of articles from different countries that reported the ESBL-producing E. coli and K. pneumoniae in animals (https://www.mapchart.net/world.html (accessed on 10 March 2023)
When analysing prevalence data in subgroups categorized by the countries, studies from Nigeria had a PPE of 33.4% (95% CI: 3.5–87.2) for ESBL-producing E. coli, while ESBL-producing K. pneumoniae had the lowest PPE at 11.9% (95% CI: 8.0–17.5). Furthermore, chicken sample analysis revealed a PPE of 48.0% (95% CI: 14.0–80.0) for ESBL-producing E. coli and 43.5% (15.0–77.0) for ESBL-producing K. pneumoniae observed from n = 287 and n = 82, respectively. Regarding the prevalence of ESBL-producing E. coli and K. pneumoniae according to study year, ESBL-producing E. coli was more prevalent in the 2011–2020 period with a PPE of 75% (95% CI: 41.5–62.1), while ESBL-producing K. pneumoniae showed a PPE of 23.7% (95% CI: 7.6–53.9) in the 2001–2010 period. Figure 5 shows a forest plot of individual point estimates for the combined prevalence estimates of (A) ESBL-producing E. coli and (B) K. pneumoniae in animals.
Prevalence of ESBL from the environment
For the environment, studies on the co-existing ESBL-producing E. coli and K. pneumoniae were conducted in five countries (Nigeria, India, Tunisia, Nepal, and Thailand) (Supplementary Table S3). The PPE for isolates from the environment were 56.9% (95% CI: 0.316–0.790), predicted interval (0.031 to 0.982) for E. coli and 24.2% (0.242; 95% CI: 0.082–0.534), predicted interval (0.003 to 0.973) for K. pneumoniae. The PPE of ESBL-producing E. coli in Asia was 47.9% (95% CI: 30.2–66.1), while ESBL-producing K. pneumoniae gave the highest estimate at 28.8% (95% CI: 4.2–78.6). The prevalence of ESBL-producing E. coli from 2011 to 2022 was 56.9% 95% CI: 31.6–79.0), while ESBL-producing K. pneumoniae had the lowest estimate at 24.2% 95% CI: 8.2–53.4).
Prevalence of ESBL from both animals and the environment
The E. coli and K. pneumoniae were screened from 625 isolates of both animals and the environment, 96 of which were ESBL-producing E. coli, and 33 were ESBL-producing K. pneumoniae (Supplementary Table S4). The overall PPE for ESBL-producing E. coli was 26.8% based on three studies (95% CI: 4.9–72.3). The ESBL-producing K. pneumoniae had a PPE of 6.7% (95% CI: 1.5–25.5) (Table 4).
Subgroup analysis by ESBL-resistant genes
We employed a random-effects model to analyze subgroups of ESBL genes evaluated in three or more studies. The blaSHVblaCTX−M,blaOXA and blaTEM genes were included in the meta-analysis for animals, humans, and the environment. As a result of the small number of studies, blaNDM, blaKPC, blaCMY, blaOXA, blaVEB, blaPER, blaGES, blaMOX, blaAmpC, and blaPSE were not included in the meta-analysis.
Pooled prevalence rate in humans
The overall incidence rate of ESBL-producing E. coli genes was 35.9% (26.0–47.1), 32.3% (22.8–43.5), 31.7% (23.0–42.0), and 2.6% (8.3–42.6), for the blaCTX−M,blaTEM,blaSHV and blaOXA, respectively. The PPE according to ESBL-producing K. pneumoniae resistance genes was 35.9% (26.0–47.1), 32.3% (22.8–43.5), 31.7% (23.0–42.0) and 2.6% (8.3–42.6), for the blaCTXM,blaTEM,blaSHV and blaOXA, respectively.
Pooled prevalence rate in animals
ESBL-producing E. coli and K. pneumoniae in animals consisted of the blaSHV,blaCTXM,blaOXA, and blaTEM genes. In total, four genes or gene groups were analyzed. The PPE was observed on ESBL-producing E. coli for the blaSHV,blaCTXM,blaOXA and blaTEM at 29.8% (17.0–46.8), 27.5% (14.8–45.3), 20.4% (4.7–56.9) and 19.1% (6.2–45.8), respectively. For ESBL-producing K. pneumoniae prevalence estimates are close to 29.7% (11.3–47.8), 26.5% (12.7–47.1), 21.3% (9.3–41.7) and 18.2% (6.9–40.1), blaOXA,blaTEM,blaSHV and blaCTXM respectively.
Pooled prevalence rate in the environment
The PPE for the blaCTXM gene was estimated to be 51.4% (25.8–76.2) and 22.6% (6.5–55.2) for ESBL-producing E. coli and K. pneumoniae, respectively. For studies on the blaSHV gene, PPE was estimated to be 53.2% (12.8–89.8) for ESBL-producing E. coli and 32.2% (8.2–71.5) for ESBL-producing K. pneumoniae.
Risk of publication bias of included studies
Publication bias was measured using funnel plots to test for symmetry, and this was further complemented using the Begg and Mazumdar rank correlation test and Egger’s regression test. The funnel plot was used to analyze the publication bias. Figure 6 displays a practically symmetrical distribution of all included studies on both sides of the funnel plot, indicating a relatively low potential for publication bias (p-value < 0.02595) for faecal samples from humans (ESBL-producing E. coli and K. pneumoniae).
Discussion
In this review of data published between January 1, 1990 and November 28, 2022, key findings have been made regarding the burden of co-existing E. coli and K. pneumoniae positively expressing ESBL which provide useful information for health professionals in a variety of fields. There are some big differences between animals, humans and the environment in the prevalence of ESBL positive E. coli and K. pneumoniae. We therefore chose to examine the prevalence of ESBL positive co-existing E. coli and K. pneumoniae from each source and do a subgroup analysis. This study analyzed data on ESBL-producing co-existing E. coli and K. pneumoniae isolated from human, animal, and environmental samples worldwide. Many articles were excluded from our systematic literature review because they only reported the prevalence E. coli or K. pneumoniae separately. However, the aim of this study was to include only studies that reported both co-existing E. coli and K. pneumoniae.
ESBL of E. coli and K. pneumoniae isolated from humans
Escherichia coli and K. pneumoniae species are the most common cause of infections, particularly in countries with underdeveloped healthcare systems [20, 21]. The ESBL-producing E. coli and K. pneumoniae strains have been identified as prominent multidrug-resistant pathogens linked to hospital-acquired infections [16, 20, 21]. They are also known to cause several common infections in children, including gastroenteritis, urinary tract infections, septicaemia, and neonatal meningitis [16, 22]. It is also known that K. pneumoniae is one of the most common bacteria that causes of opportunistic healthcare-associated infections, which are made worse by its ability to produce ESBL enzymes [23, 24].
The PPE based on 17,513 ESBL positive E. coli and 8165 ESBL positive K. pneumoniae was 33% and 32.7% respectively, from 101 studies in humans. This is comparatively higher than similarly reported PPE from a review conducted in Bangladesh, where PPE of ESBL-producing E. coli was 17% [16], and in South America, where PPE was 2.2% [15]. The study conducted in Nepal on ESBL-producing K. pneumoniae had a lower PPE of 5% for ESBL-producing K. pneumoniae [24]. The variation could be due to the result of a difference in the time of the study, also be due to differences in geographical properties, sample categories, and types, as well as sample size and identification methods.
Furthermore, this study found that E. coli had a high PPE of 52.7% on stool samples from 243 positive ESBL-producing E. coli, while K. pneumoniae had a high PPE of 37.7% on urine samples. This is not surprising, as K. pneumoniae is one of the bacteria that cause urinary tract infections [25, 26]. However, most of the stool samples containing the stx-positive gene of E. coli were typical diarrheal samples [27].
ESBL of E. coli and K. pneumoniae isolated from animals
In this study, 872 ESBL-producing E. coli isolates from 17 studies and 324 ESBL-producing K. pneumoniae from 16 studies were subjected to meta-analysis. The E. coli and K. pneumoniae PPE from this study are 33.5% and 19.4%, respectively. Our results are comparable to a previous systematic review study by Islam et al. [16] that reported prevalence of 22% for for ESBL-producing E. coli in animals. However, PEE in our study was higher than the one obtained in India, where PPE of ESBL-producing E. coli and K. pneumoniae was 9% and 10%, respectively [28]. The variation could be the result of a difference in the time of the study. The other reason could be the methodology used to detect the number of published articles included in the current study.
The subgroup analysis at continental level showed that the PPE was higher for ESBL-producing E. coli in Africa and Asia, with 49.6% and 44.5%, respectively. Our findings were consistent with a previous study that reported ESBL in E. coli isolated from environments in Bangladesh at 39% [16]. The PPE from chicken samples was 48.0% for E. coli and 43.5% for K. pneumoniae. Other animals such as cattle, goats, birds, dogs, cats, camels, fish, and horses were not included in the meta-analysis due to the low number of samples. Country-specific findings indicated that Nigeria had a PPE of 33.4% and 11.9% for E. coli and K. pneumoniae, respectively. Other countries, including Sudan [29], India [9], Algeria [30], Ghana [31], Turkey [32], Japan [33], Canada [34], Finland [35], Taiwan [36], South Africa [37], Tanzania [38], Tunisia [39], India [40], and the Netherland [41] were not included in the meta-analysis due to the low number of studies.
ESBL of E. coli and K. pneumoniae isolated from the environment
This systematic review and meta-analysis showed that ESBL-producing E. coli and K. pneumoniae were isolated from various types of environmental samples, such as water, manure, hospital sewage, and raw vegetables. The PPE of ESBL-producing E. coli was 56.9% (31.6–79.0) while the PPE of ESBL-producing K. pneumoniae was 24.2%. This could be due to the possibility of traces of previously consumed antibiotics and antimicrobial-resistant bacteria in faecal and urine waste. This waste and wastewater can contribute to the spread of antimicrobial-resistant bacteria in the environment possibly having a negative impact on human health. Many of these species have genes for antibiotic resistance, which are eventually incorporated into genetic mobile platforms that can spread among bacterial populations in soil and water.
The PPE of ESBL-producing E. coli from environments was 39%, which is comparable to a previous study conducted in Bangladesh that reported a pooled prevalence of 39% for ESBL-producing E. coli from environments [16]. According to the literature, there is no meta-analysis on ESBL-producing K. pneumoniae in the environment. Most of the studies were conducted from 2011 to 2020 with a PPE of 75.6% for ESBL-producing E. coli and 11% for ESBL-producing K. pneumoniae. Data recorded from the included articles showed that the studies were from only five countries, namely Nigeria, India, Tunisia, Nepal, and Thailand.
ESBL genes in E. coli and K. pneumoniae isolated from animals, humans, and the environment
In recent years, there has been an increase in ESBL-producing E. coli and K. pneumoniae, mostly identified using phenotypic methods [42, 43]. We report the first global PPE of co-existing ESBL-producing E. coli and K. pneumoniae. A number of studies have investigated blaTEM, blaSHV, blaOXA, and blaCTX−M genes, with blaCTX−M being the type most commonly associated with ESBL [43,44,45]. These genes reside on plasmids, which can be transferred horizontally to other bacteria [46].
Among the bla-genes screened in this study, blaCTX−M and blaSHV were the most detected from ESBL-producing E. coli and K. pneumoniae from animals, humans, and the environment. The blaTEM have been reported from vegetables in Finland [47] and in southern Thailand [48]. In this review, blaCTX−M was the gene most detected in animals, humans, and the environment. It has also been reported that K. pneumoniae and E. coli from dogs and cats possess blaCTX−M type genes [49, 50]. Lately, blaCTX−M enzymes are the most common ESBL type because they have environmental origins [51]. The blaCTX−M enzyme is divided into five subgroups based on its amino acid composition: blaCTX−M−1, blaCTX−M−2, blaCTX−M−8, blaCTX−M−9, and blaCTX−M−25 [46]. Globally, carbapenem-resistant Enterobacteriaceae are increasing due to the growing usage of carbapenems to treat ESBL-producing infections [52].
In this systematic review and meta-analysis, we also report the presence of blaKPC in humans. These findings warrant further investigation of the presence of carbapenem-resistant genes since such resistant E. coli and K. pneumoniae are among top priority pathogens on the list of World Health Organization [WHO] for the development of antimicrobials [53].
“One Health” perspective
“One-Health” concept refers to the collaborative effort between local, national, and global disciplines for the purpose of attaining optimal health for humans, animals, and the environment [54,55,56]. Antimicrobial use practices in companion animals are similar to those in humans; that is, drugs are primarily administered for treating clinical infections, such as post-surgery infections, with some being used as prophylaxis- [57]. A major obstacle to addressing antimicrobial resistance has been the blame game between human medicine and the agriculture sectors regarding who is responsible for the increase in antimicrobial resistance due qof bacteria causing zoonotic diseases [58].
This study confirmed the co-existence of ESBL-producing E. coli and K. pneumoniae prevalence in animals, humans, and the environment (water, manure, hospitals sewage, and raw vegetables). Our results highlight the significance of “One Health,” as ESBL-producing E. coli and K. pneumoniae were detected in humans, animals, and the environment. Moreover, the blaCTX−M, and blaSHV were detected in ESBL-producing E. coli and K. pneumoniae isolated from animals, humans, and the environment, hence the “One Health” concern. Future researchers should be encouraged to use the “One Health” approach to develop methodologies that explicitly examine interlinks between human-animal-environment frameworks, with a particular emphasis on antibiotic resistance in zoonotic diseases.
Limitations
To assess ESBL-producing E. coli and K. pneumoniae levels globally, this study relied on peer-reviewed publications. Reviews, theses, and preprint articles that potentially include essential information on ESBL-producing E. coli and K. pneumoniae were excluded from our review as per PRISMA guidelines for writing systematic reviews and meta-analysis. There may have been articles published in other languages that were missed since the search strategy was limited to articles published in English. The pooled prevalence of some countries and continents was not calculated because there are few published studies. Some antimicrobial resistance genes were also not included in this meta-analysis due to the small number of studies. In a meta-analysis, publication bias may be evaluated using funnel plots, regressions, and Begg’s adjusted rank correlation test [59]. However, meta-analyses that include fewer than 10 studies or high amounts of heterogeneity between studies can lead to misleading results from these evaluation tools. In the presence of a high level of heterogeneity, it is very difficult to evaluate the true results of statistically significant publication bias tests. Since there is high heterogeneity across all analyses, readers should be cautious when interpreting pooled analyses and subgroups.
Conclusion
This study is the first meta-analysis that estimates the prevalence of co-existing ESBL-producing E. coli and K. pneumoniae in animals, humans, and the environment worldwide. We further observed that blaCTX−M, and blaSHV were the most frequently detected genes from ESBL-producing E. coli and K. pneumoniae infecting animals, humans, and the environment. This study presents robust and valuable data that can serve as a useful reference for doctors, veterinarians, and environmental scientists, as it informs them about the prevalence of co-existing ESBL-producing E. coli and K. pneumoniae recovered from humans, animals, and the environment. “One-health” surveillance is vital for source tracking and mitigating the spread of antimicrobial-resistant bacteria. ESBL-containing bacteria should be included in emerging state and national surveillance systems. Globally, alternative treatments for antimicrobial drug resistance should be well researched, planned, and implemented.
Data Availability
Data will be made available on request.
Abbreviations
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- WHO:
-
World Health Organization
- ESBL:
-
Extended Spectrum Beta Lactamase
- PPE:
-
Pooled prevalence estimates
- CI:
-
Confidence interval
- PP:
-
Pooled prevalence
- AMR:
-
Antimicrobial Resistance
- MDR:
-
Multi-drug resistant
- JBI:
-
Joanna Briggs Institute
References
Salgado-Caxito M, Benavides JA, Adell AD, Paes AC, Moreno-Switt AI. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing-Escherichia coli in dogs and cats–a scoping review and meta-analysis. One Health. 2021;12:100236. https://doi.org/10.1016/j.onehlt.2021.100236.
Akenten CW, Ofori LA, Khan NA, Mbwana J, Sarpong N, May J, Thye T, Obiri-Danso K, Paintsil EK, Fosu D, Philipps RO. Prevalence, characterization, and Antimicrobial Resistance of extended-spectrum beta-lactamase-producing Escherichia coli from Domestic Free-Range Poultry in Agogo, Ghana. Foodborne Pathog Dis. 2023;20(2):59–66. https://doi.org/10.1089/fpd.2022.0060.
Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–66. https://doi.org/10.1007/s40265-019-01180-3.
Castanheira M, Simner PJ, Bradford PA. Extended-spectrum β-lactamases: an update on their characteristics, epidemiology and detection. JAC Antimicrob Resist. 2021;3(3):dlab092. https://doi.org/10.1093/jacamr/dlab092.
Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–76. https://doi.org/10.1128/AAC.01009-09.
Meijs AP, Gijsbers EF, Hengeveld PD, Dierikx CM, de Greeff SC, van Duijkeren E. ESBL/pAmpC-producing Escherichia coli and Klebsiella pneumoniae carriage among veterinary healthcare workers in the Netherlands. Antimicrob Resist Infect Control. 2021;10(1):1–12. https://doi.org/10.1186/s13756-021-01012-8.
Pitout JD. Infections with extended-spectrum β-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70:313–33. https://doi.org/10.2165/11533040-000000000-00000.
Atterby C, Osbjer K, Tepper V, Rajala E, Hernandez J, Seng S, Holl D, Bonnedahl J, Börjesson S, Magnusson U, Järhult JD. Carriage of carbapenemase-and extended‐spectrum cephalosporinase‐producing Escherichia coli and Klebsiella pneumoniae in humans and livestock in rural Cambodia; gender and age differences and detection of blaOXA‐48 in humans. Zoonoses Public Health. 2019;66(6):603–17. https://doi.org/10.1111/zph.12612.
Bandyopadhyay S, Bhattacharyya D, Samanta I, Banerjee J, Habib M, Dutta TK, Dutt T. Characterization of multidrug-resistant biofilm-producing Escherichia coli and Klebsiella pneumoniae in healthy cattle and cattle with diarrhea. Microb Drug Resist. 2021;27(11):1457–69. https://doi.org/10.1089/mdr.2020.0298.
Guenther S, Ewers C, Wieler LH. Extended-spectrum beta-lactamases producing E. coli in wildlife, yet another form of environmental pollution? Front Microbiol. 2011;2:246. https://doi.org/10.3389/fmicb.2011.00246.
Partridge SR, Hall RM. Evolution of transposons containing blaTEM genes. “Antimicrob Agents Chemother. 2005;49(3):1267. https://doi.org/10.1128/AAC.49.3.1267-1268.2005.
Banerjee R, Johnson JR. A new clone sweeps clean: the enigmatic emergence of Escherichia coli sequence type 131. Antimicrob Agents Chemother. 2014;58(9):4997–5004. https://doi.org/10.1128/AAC.02824-14.
Peirano G, Richardson D, Nigrin J, McGeer A, Loo V, Toye B, Alfa M, Pienaar C, Kibsey P, Pitout JD. High prevalence of ST131 isolates producing CTX-M-15 and CTX-M-14 among extended-spectrum-β-lactamase-producing Escherichia coli isolates from Canada. Antimicrob Agents Chemother. 2010;54(3):1327–30. https://doi.org/10.1128/AAC.01338-09.
Stone TJ, DeWitt M, Johnson JW, Beardsley JR, Munawar I, Palavecino E, Luther VP, Ohl CA, Williamson JC. Analysis of infections among patients with historical culture positive for extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli or Klebsiella pneumoniae: is ESBL-targeted therapy always needed? AMS Antimicrob Stewardship. 2023;3(1):e47. https://doi.org/10.1017/ash.2022.363.
Bastidas-Caldes C, Romero-Alvarez D, Valdez-Vélez V, Morales RD, Montalvo-Hernández A, Gomes-Dias C, Calvopiña M. Extended-spectrum beta-lactamases producing Escherichia coli in South America: a systematic review with a one health perspective. Infect Drug Resist. 2022;5759–79. https://doi.org/10.2147/IDR.S371845.
Islam MS, Rahman AT, Hassan J, Rahman MT. Extended-spectrum beta-lactamase in Escherichia coli isolated from humans, animals, and environments in Bangladesh: a One Health perspective systematic review and meta-analysis. One Health”. 2023;100526. https://doi.org/10.1016/j.onehlt.2023.100526.
McDanel J, Schweizer M, Crabb V, Nelson R, Samore M, Khader K, Blevins AE, Diekema D, Chiang HY, Nair R, Perencevich E. Incidence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella infections in the United States: a systematic literature review. Infect Control Hosp Epidemiol. 2017;38(10):1209–15. https://doi.org/10.1017/ice.2017.156.
Thomas KM, de Glanville WA, Barker GC, Benschop J, Buza JJ, Cleaveland S, Davis MA, French NP, Mmbaga BT, Prinsen G, Swai ES. Prevalence of Campylobacter and Salmonella in African food animals and meat: a systematic review and meta-analysis. Int J Food Microbiol. 2020;315:108382. https://doi.org/10.1016/j.ijfoodmicro.2019.108382.
Buccheri RK, Sharifi C. Critical appraisal tools and reporting guidelines for evidence-based practice. Worldviews Evid Based Nur. 2017;14(6):463–72. https://doi.org/10.1111/wvn.12258.
Afzal MA. Antibiotic resistance pattern of Escherichia coli and Klebsiella species in Pakistan: a brief overview. J Microb Biochem Technol. 2017;9:277–9. https://doi.org/10.4172/1948-5948.1000377.
Kayastha K, Dhungel B, Karki S, Adhikari B, Banjara MR, Rijal KR, Ghimire P. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species in pediatric patients visiting International Friendship Children’s hospital, Kathmandu, Nepal. J Infect Dis. 2020;13:1178633720909798. https://doi.org/10.1177/1178633720909798.
Allocati N, Masulli M, Alexeyev MF, Di Ilio C. Escherichia coli in Europe: an overview. Int J Environ Res Public Health. 2013;10(12):6235–54. https://doi.org/10.3390/ijerph10126235.
Badger-Emeka LI, Al-Sultan AA, Bohol MF, Al-Anazi MR, Al-Qahtani AA, Al-Qahtani. Genetic analysis, population structure, and characterisation of multidrug-resistant Klebsiella pneumoniae from the Al-hofuf region of Saudi Arabia. Pathogens. 2021;10(9):1097. https://doi.org/10.3390/pathogens10091097.
Shyaula M, Khadka C, Dawadi P, Banjara MR. Systematic review and Meta-analysis on extended-spectrum β-lactamases producing Klebsiella pneumoniae in Nepal. Microbiol Insights. 2023;16:11786361221145179. https://doi.org/10.1177/11786361221145179.
Chang D, Sharma L, Dela Cruz CS, Zhang D. Clinical epidemiology, risk factors, and control strategies of Klebsiella pneumoniae infection. Front Microbiol. 2021;3955. https://doi.org/10.3389/fmicb.2021.750662.
Chapelle C, Gaborit B, Dumont R, Dinh A, Vallée M. Treatment of UTIs due to Klebsiella pneumoniae carbapenemase-producers: how to use new antibiotic drugs? A narrative review. Antibiotics. 2021;10(11):1332. https://doi.org/10.3390/antibiotics10111332.
Couturier MR, Lee B, Zelyas N, Chui L. Shiga-toxigenic Escherichia coli detection in stool samples screened for viral gastroenteritis in Alberta, Canada. J Clin Microbiol. 2011;49(2):574–8. https://doi.org/10.1128/JCM.01693-10.
Kuralayanapalya SP, Patil SS, Hamsapriya S, Shinduja R, Roy P, Amachawadi RG, Amachawadi. Prevalence of extended-spectrum beta-lactamase producing bacteria from animal origin: a systematic review and meta-analysis report from India. PLoS ONE. 2019;14(9):e0221771. https://doi.org/10.1371/journal.pone.0221771.
Badri A, Williams A, Awofiranye A, Datta P, Xia K, He W, Fraser K, Dordick JS, Linhardt RJ, Koffas MA. Complete biosynthesis of a sulfated chondroitin in Escherichia coli. Nat Commun. 2021;12(1):1389. https://doi.org/10.1038/s41467-021-21692-5.
Chenouf NS, Carvalho I, Messaï CR, Ruiz-Ripa L, Mama OM, Titouche Y, Zitouni A, Hakem A, Torres C. Extended spectrum β-Lactamase-producing Escherichia coli and Klebsiella pneumoniae from broiler liver in the Center of Algeria, with detection of CTX-M-55 and B2/ST131-CTX-M-15 in Escherichia coli. Microb Drug Resist. 2021;27(2):268–76. https://doi.org/10.1089/mdr.2020.0024.
Eibach D, Dekker D, Boahen KG, Akenten CW, Sarpong N, Campos CB, Berneking L, Aepfelbacher M, Krumkamp R, Owusu-Dabo E, May J. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in local and imported poultry meat in Ghana. Vet Microbiol. 2018;217:7–12. https://doi.org/10.1016/j.vetmic.2018.02.023.
Gundogan N, Avci E. Prevalence and antibiotic resistance of extended-spectrum beta-lactamase (ESBL) producing Escherichia coli and Klebsiella species isolated from foods of animal origin in Turkey. Afr J Microbiol Res. 2013;7(31):4059–64. https://doi.org/10.1016/j.bjm.2015.11.034.
Hiroi M, Harada T, Kawamori F, Takahashi N, Kanda T, Sugiyama K, Masuda T, Yoshikawa Y, Ohashi N. A survey of β-lactamase-producing Escherichia coli in farm animals and raw retail meat in Shizuoka Prefecture, Japan. Jpn J Infect Dis. 2011;64(2):153–5. https://doi.org/10.7883/yoken.64.153.
Jamborova I, Janecko N, Halova D, Sedmik J, Mezerova K, Papousek I, Kutilova I, Dolejska M, Cizek A, Literak I. Molecular characterization of plasmid-mediated AmpC beta-lactamase-and extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae among corvids (Corvus brachyrhynchos and Corvus corax) roosting in Canada. FEMS Microbiol Ecol. 2018;94(11):1160. https://doi.org/10.1093/femsec/fiy166.
Johansson V, Nykäsenoja S, Myllyniemi AL, Rossow H, Heikinheimo A. Genomic characterization of ESBL/AmpC-producing and high-risk clonal lineages of Escherichia coli and Klebsiella pneumoniae in imported dogs with shelter and stray background. J Glob Antimicrob Resist. 2022;30:183–90. https://doi.org/10.1016/j.jgar.2022.05.021.
Kuan NL, Chang CW, Lee CA, Yeh KS. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates from the urine of dogs and cats suspected of urinary tract infection in a veterinary teaching hospital. Taiwan Vet J. 2016;42(03):143–8. https://doi.org/10.1142/S1682648515500274.
Montso KP, Dlamini SB, Kumar A, Ateba CN. Antimicrobial resistance factors of extended-spectrum beta-lactamases producing Escherichia coli and Klebsiella pneumoniae isolated from cattle farms and raw beef in North-West Province, South Africa. Biomed Res Int 2019:2019. https://doi.org/10.1155/2019/4318306.
Mwanginde LW, Majigo M, Kajeguka DC. High carriage rate of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species among poultry meat vendors in Dar es Salaam: the urgent need for intervention to prevent the spread of multidrug-resistant pathogens. Int J Microbiol. 2021;2021. https://doi.org/10.1155/2021/6653993.
Saidani M, Messadi L, Chaouechi A, Tabib I, Saras E, Soudani A, Daaloul-Jedidi M, Mamlouk A, Ben Chehida F, Chakroun C, Madec JY. High genetic diversity of Enterobacteriaceae clones and plasmids disseminating resistance to extended-spectrum cephalosporins and colistin in healthy chicken in Tunisia. Microb Drug Resist. 2019;25(10):1507–13. https://doi.org/10.1089/mdr.2019.0138.
Sivaraman GK, Sudha S, Muneeb KH, Shome B, Holmes M, Cole J. Molecular assessment of antimicrobial resistance and virulence in multi drug resistant ESBL-producing Escherichia coli and Klebsiella pneumoniae from food fishes, Assam, India. Microb Pathog. 2020;149:104581. https://doi.org/10.1016/j.micpath.2020.104581.
Vo AT, van Duijkeren E, Fluit AC, Gaastra W. Characteristics of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates from horses. Vet Microbiol. 2007;124(3–4):248–55. https://doi.org/10.1016/j.vetmic.2007.04.027.
Parveen RM, Khan MA, Menezes GA, Harish BN, Parija SC, Hays JP. Extended-spectrum β-lactamase producing Klebsiella pneumoniae from blood cultures in Puducherry, India. Indian J Med Res. 2011;134(3):392. PMCID: PMC3193723.
Ghenea AE, Zlatian OM, Cristea OM, Ungureanu A, Mititelu RR, Balasoiu AT, Vasile CM, Salan AI, Iliuta D, Popescu M, Udriștoiu AL. TEM, CTX-M, SHV genes in ESBL-Producing Escherichia coli and Klebsiella pneumoniae isolated from clinical samples in a County Clinical Emergency Hospital Romania-Predominance of CTX-M-15. Antibiotics. 2022;11(4):503. https://doi.org/10.3390/antibiotics11040503.
Bush K, Fisher JF. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annu Rev Microbiol. 2011;65:455–78. https://doi.org/10.1146/annurev-micro-090110-102911.
Seo KW. “Development of a Method for the Fast Detection of Extended-Spectrum β-Lactamase-and Plasmid-Mediated AmpC β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Dogs and Cats in the USA. Animals. 2023; 13(4): 649. https://doi.org/10.3390/ani13040649.
Riwu KH, Effendi MH, Rantam FA. A review of Extended Spectrum β-Lactamase (ESBL) producing Klebsiella pneumoniae and Multidrug Resistant (MDR) on companion animals. Syst Rev Pharm. 2020;11(7):270–7. https://doi.org/10.31838/srp.2020.7.43.
Kurittu P, Khakipoor B, Aarnio M, Nykäsenoja S, Brouwer M, Myllyniemi AL, Vatunen E, Heikinheimo A. Plasmid-borne and chromosomal ESBL/AmpC genes in Escherichia coli and Klebsiella pneumoniae in global food products. Front Microbiol. 2021;12:592291. https://doi.org/10.3389/fmicb.2021.592291.
Romyasamit C, Sornsenee P, Chimplee S, Yuwalaksanakun S, Wongprot D, Saengsuwan P. “Prevalence and characterization of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolated from raw vegetables retailed in Southern Thailand. PeerJ. 2021;9: e11787. https://doi.org/10.7717/peerj.11787.
Hong JS, Song W, Park HM, Oh JY, Chae JC, Shin S, Jeong SH. Clonal spread of extended-spectrum cephalosporin-resistant Enterobacteriaceae between companion animals and humans in South Korea. Front Microbiol. 2019;10:1371. https://doi.org/10.3389/fmicb.2019.01371.
Sivaraman GK, Rajan V, Vijayan A, Elangovan R, Prendiville A, Bachmann TT. Antibiotic resistance profiles and molecular characteristics of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae isolated from shrimp aquaculture farms in Kerala, India. Front Microbiol. 2021;12:622891. https://doi.org/10.3389/fmicb.2021.622891.
Palmeira JD, Ferreira HM. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in cattle production–a threat around the world. Heliyon. 2020;6(1):e03206. https://doi.org/10.1016/j.heliyon.2020.e03206.
Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(1):28–S36. https://doi.org/10.1093/infdis/jiw282.
Gundran RS, Cardenio PA, Villanueva MA, Sison FB, Benigno CC, Kreausukon K, Pichpol D, Punyapornwithaya V. Prevalence and distribution of bla CTX-M, bla SHV, bla TEM genes in extended-spectrum β-lactamase-producing E. coli isolates from broiler farms in the Philippines. BMC Vet Res. 2019;15:1–8. https://doi.org/10.1186/s12917-019-1975-9.
Kim DW, Cha CJ. Antibiotic resistome from the one-health perspective: understanding and controlling antimicrobial resistance transmission. Exp Mol Med. 2021;53(3):301–9. https://doi.org/10.1038/s12276-021-00569-z.
Rubin C, Myers T, Stokes W, Dunham B, Harris S, Lautner B, Annelli J. “Review of institute of medicine and national research council recommendations for one health initiative. Emerg Infect Dis. 2013;19(12): 1913. https://doi.org/10.3201/eid1912.121659.
Cocker D, Chidziwisano K, Mphasa M, Mwapasa T, Lewis JM, Rowlingson B, Sammarro M, Bakali W, Salifu C, Zuza A, Charles M. Investigating one Health risks for human colonisation with extended spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in malawian households: a longitudinal cohort study. Lancet Microbe. 2023;4(7):E534–43. https://doi.org/10.1016/S2666-5247(23)00062-9.
McEwen SA, Collignon PJ, Collignon. Antimicrobial resistance: a one health perspective. Microbiol Spectr. 2017;6(2). https://doi.org/10.1128/microbiolspec.ARBA-0009-2017.
Kahn LH. Antimicrobial resistance: a one health perspective. Trans R Soc Trop Med Hyg. 2017;111(6):255–60. https://doi.org/10.1093/trstmh/trx050.
Golden CE, Mishra A. Prevalence of Salmonella and Campylobacter spp. in alternative and conventionally produced chicken in the United States: a systematic review and Meta-analysis. J Food Prot. 2020;83(7):1181–97. https://doi.org/10.4315/JFP-19-538.
Funding
This research work did not receive any specific grant from funding agencies.
Open access funding provided by North-West University.
Author information
Authors and Affiliations
Contributions
TR, KL, OT, GK and CB conceived the idea. TR contributed to literature search, screening, data extraction, data analysis, and development of manuscript drafts for the systematic review and meta-analysis protocol. TR, TM, KL and GK performed the screening, extraction of data and developed the first draft of the manuscript. TM, KL, GK, MM, GK, NS, JN, KL, OT, and CB provided critical review of the manuscript and contributed to the final version. All the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ramatla, T., Mafokwane, T., Lekota, K. et al. “One Health” perspective on prevalence of co-existing extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae: a comprehensive systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 22, 88 (2023). https://doi.org/10.1186/s12941-023-00638-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00638-3