Abstract
Background
Over one million yearly deaths are attributable to Streptococcus pneumoniae and people living with HIV are particularly vulnerable. Emerging penicillin non-susceptible Streptococcus pneumoniae (PNSP) challenges therapy of pneumococcal disease. The aim of this study was to determine the mechanisms of antibiotic resistance among PNSP isolates by next generation sequencing.
Methods
We assessed 26 PNSP isolates obtained from the nasopharynx from 537 healthy human immunodeficiency virus (HIV) infected adults in Dar es Salaam, Tanzania, participating in the randomized clinical trial CoTrimResist (ClinicalTrials.gov identifier: NCT03087890, registered on 23rd March, 2017). Next generation whole genome sequencing on the Illumina platform was used to identify mechanisms of resistance to antibiotics among PNSP.
Results
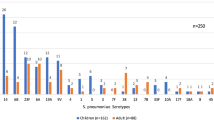
Fifty percent (13/26) of PNSP were resistant to erythromycin, of these 54% (7/13) and 46% (6/13) had MLSB phenotype and M phenotype respectively. All erythromycin resistant PNSP carried macrolide resistance genes; six isolates had mef(A)-msr(D), five isolates had both erm(B) and mef(A)-msr(D) while two isolates carried erm(B) alone. Isolates harboring the erm(B) gene had increased MIC (> 256 µg/mL) towards macrolides, compared to isolates without erm(B) gene (MIC 4-12 µg/mL) p < 0.001. Using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines, the prevalence of azithromycin resistance was overestimated compared to genetic correlates. Tetracycline resistance was detected in 13/26 (50%) of PNSP and all the 13 isolates harbored the tet(M) gene. All isolates carrying the tet(M) gene and 11/13 isolates with macrolide resistance genes were associated with the mobile genetic element Tn6009 transposon family. Of 26 PNSP isolates, serotype 3 was the most common (6/26), and sequence type ST271 accounted for 15% (4/26). Serotypes 3 and 19 displayed high-level macrolide resistance and frequently carried both macrolide and tetracycline resistance genes.
Conclusion
The erm(B) and mef(A)-msr(D) were common genes conferring resistance to MLSB in PNSP. Resistance to tetracycline was conferred by the tet(M) gene. Resistance genes were associated with the Tn6009 transposon.
Similar content being viewed by others
Background
Streptococcus pneumoniae is a transient colonizer of the nasopharynx, with colonization peaking in the early years of life and declining into adulthood. It is associated with both invasive and non-invasive pneumococcal diseases. The incidence of invasive pneumococcal diseases is most prominent at the extremes of age, as well as in immunocompromised hosts and people with chronic respiratory tract diseases. People living with HIV are particularly vulnerable to severe pneumococcal disease [1] Globally, Streptococcus pneumoniae is estimated to have caused as many as 515,000 deaths (95% uncertainty interval, UI 302,000–609,000) in children aged < 5 years in 2015, and approximately 50% of those deaths occurred in four countries in Africa (Nigeria, Democratic republic of Congo) and Asia (India, Pakistan) [2]. Globally, Streptococcus pneumoniae is estimated to cause 1,189,937 deaths (UI 690,445-1,770,660) [3].
Before 1970, pneumococci were readily susceptible to nearly all relevant antibiotics, and penicillin was the antibiotic of choice. In the late 1970s, pneumococci with non-susceptibility to penicillin emerged, resulting in treatment failures [4, 5]. The discovery of pneumococci resistant to penicillin shifted empirical treatment for suspected bacterial respiratory tract infection to macrolides and tetracycline. In Tanzania, standard treatment guidelines recommend macrolides and tetracyclines as first and second line treatments, respectively, for mild to moderate community acquired pneumonia caused by Streptococcus pneumoniae [6]. However, the recommendation is not based on current evidence of susceptibility patterns, as surveillance of the trend of antibiotic resistance in Streptococcus pneumoniae is limited in Tanzania. Data from the Network for Surveillance of Pneumococcal Disease in the East African Region in the pre-pneumococcal vaccination era reported a low rate of Streptococcus pneumoniae resistant to erythromycin and other antibiotics in Tanzania [7]. Furthermore, a meta-analysis of childhood pneumococcal diseases in Africa prior to the widespread use of the pneumococcal capsular vaccine (PCV) reported a low rate of resistance to erythromycin, but a substantially higher rate of resistance to tetracycline [8].
However, post-PCV surveillance studies conducted in well-organized settings have shown increased pneumococcal resistance to erythromycin and other antibiotics, partly attributed to increased consumption of macrolides [9,10,11].
Pneumococcal resistance to macrolides is mediated by erythromycin ribosomal methylase B (erm(B)) encoding enzymes that methylate the 23S rRNA, thereby inhibiting macrolide binding [12]. The erm(B) confers resistance to macrolides, lincosamides, and Streptogramin B, producing MLSB phenotypes [13, 14]. Macrolide efflux protein A and E, efflux pumps encoded by the mef(A) and mef(E) genes, and ribosomal mutations (23S rRNA), are other common causes of macrolide resistance in Streptococcus pneumoniae [13]. The mef(A/E) genes confer the M phenotype, exhibiting low level resistance to macrolides, but not resistance to lincosamides and streptogramin B. The macrolide resistance genes are commonly carried on mobile genetic elements, facilitating their easy intra- and interspecies dissemination [15, 16]. The Tn916 transposon family that contains the tetracycline resistance determinant tet(M), has frequently been reported to harbor macrolide resistance determinant genes [17].
Macrolide resistance determinants vary with geographical locations [13]. In Tanzania, where macrolides and tetracyclines are commonly used and easily accessible over the counter without prescriptions, the mechanisms of resistance to these antibiotics in Streptococcus pneumoniae has not been studied. Therefore, we performed this study using whole genome sequencing to determine mechanisms of antibiotic resistance among penicillin non-susceptible Streptococcus pneumoniae isolated from Tanzania.
Materials and methods
Bacterial isolates
Twenty-six penicillin non-susceptible Streptococcus pneumoniae were isolated by culturing nasopharyngeal swabs obtained from healthy HIV infected adults in Tanzania as part of the randomized clinical trial CoTrimResist (ClinicalTrials.gov identifier: NCT03087890, registered on 23rd March, 2017). The study population and bacterial isolates have been described previously [18]. Streptococcus pneumoniae was identified by conventional methods including optochin disk and bile susceptibility and further confirmation was done by Matrix-Assisted Laser Desorption/Ionization-Time of Flight (MALDI-TOF) mass spectrometry (MS), using the Microflex LT instrument and MALDI Biotyper 3.1 software (Bruker Daltonics, Bremen, Germany). Isolates with discordant results between MALDI-TOF and conventional identification with optochin disk and bile susceptibility were omitted.
Serotyping of Streptococcus pneumoniae was performed by latex agglutination (Immulex™ Pneumotest Kit; SSI Diagnostica A/S, Hillerød, Denmark).
Antimicrobial susceptibility testing
The E-test strip (bioMérieux, Marcy-I-Etoile, France) was used to determine the minimum inhibitory concentrations (MIC) for azithromycin, erythromycin and penicillin. The disk diffusion method was used to determine tetracycline and clindamycin susceptibility [19]. Muller Hinton supplemented with 5% sheep blood agar was used for antimicrobial susceptibility testing, and it was incubated at 35 °C in 5% CO2 for 20–24 h. The guidelines of the Clinical and Laboratory Standards Institute [19], and The European Committee on Antimicrobial Susceptibility Testing [20], were used to interpret antimicrobial susceptibility testing results. PNSP was defined according to CLSI breakpoint interpretation [19].
Whole genome sequencing and analysis
Whole genome sequencing was performed using the Next generation sequencing platform HiSeq X10 (Illumina, San Diego, CA, USA) at MicrobesNG (Microbes NG, Birmingham, UK). Quality filtering and sequencing short read trimming were performed by MicrobesNG using SPAdes and annotated in GenBank. Short read sequences were assembled using Unicycler at MicrobesNG.
For allocation of multi-locus sequence typing (MLST) and clonal complex, we used the online MLST database website https://pubmlst.org/.
Identification of acquired resistance was performed using the web-based platform ResFinder v3.2 of the Center for Genomic Epidemiology (http://www.genomicepidemiology.org/).
For identification of mobile genetic elements and their related acquired antimicrobial resistance we used the Center for Genomic Epidemiology MobileElement Finder v1.0.3 (http://www.genomicepidemiology.org/).
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the BioProject number PRJNA918594.
Statistical analysis
Categorical variables were presented in frequencies, percentages, and proportions. Categorical variables were compared using chi square test. A p-value < 0.05 was considered as threshold for statistical significance. Statistical analysis was performed using STATA version 16 (College Station, TX).
Results
A total of 26 penicillin non-susceptible Streptococcus pneumoniae were analyzed by whole genome sequencing. Resistance to both macrolides and tetracyclines was observed in 12/26 (46%) of PNSP isolates.
Phenotypic results using both EUCAST and CLSI breakpoint interpretation showed that 13/26 (50%) of PNSP isolates were resistant to macrolides (erythromycin). Phenotypic resistance to erythromycin was in concordance with genotypic resistance determinants as shown in Table 1.
For azithromycin resistance, using EUCAST breakpoints (MIC > 0.5 µg/mL), all PNSP (26/26) were interpreted as resistant, but genetic markers conferring macrolide resistance could only be found in isolates with MIC ≥ 6 µg/mL (50%, 13/26). Using CLSI interpretation breakpoints (MIC ≥ 2 µg/mL), 58% (15/26) of PNSP were interpreted as resistant to azithromycin, while genotypic markers for macrolide resistance was found in 87% (13/15) of these isolates (Table 1).
Genes conferring resistance to the group of macrolides, lincosamides, and streptogramin B were observed in all the 13-erythromycin resistant PNSP isolates. The MLSB phenotype (resistance to macrolides, lincosamides, and streptogramin B) accounted for 54% (7/13) while the M phenotype (resistance to macrolides, but not to lincosamides or streptogramin B) accounted for the remaining 46% (6/13).
The macrolide efflux gene mef(A)-msr(D) was observed in 6 isolates. Five isolates carried both the erythromycin ribosomal methylase gene erm(B) and mef(A)-msr(D), while erm(B) alone was detected in only two isolates. Isolates carrying the erm(B) gene had increased MIC for both erythromycin and azithromycin, > 256 µg/mL, compared to isolates lacking the erm(B) gene (MIC 4-12 µg/mL), p < 0.001.
The mef(A)-msr(D) gene predicted phenotypic resistance to macrolides, but at low MIC values (4–12 µg/mL for erythromycin) and (6–96 µg/mL for azithromycin). Carrying the mef(A)-msr(D) gene alone did not predict phenotypic resistance to lincosamides (clindamycin) and the six isolates carrying mef(A)-msr(D) were all phenotypically susceptible to clindamycin.
Disc diffusion results showed that 13/26 (50%) of PNSP isolates were resistant to tetracyclines. All the 13-tetracycline resistant PNSP isolates carried the tet(M) gene which confers resistance to tetracyclines. Three PNSP isolates harbored cat (pC194) which confers resistance to chloramphenicol.
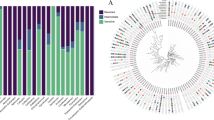
The Tn6009-like element was detected in 13 PNSP. All tetracycline resistant PNSP were associated with a Tn6009 like element, while 12/13 of the erythromycin resistant PNSP had a Tn6009 like element. Twelve out of 13 tetracycline-resistant PNSP isolates were associated with plasmid replicon type repUS43.
Serotype 3 was the most common, followed by serotype19 and 35B. The majority of serotype 3 and 19 PNSP displayed high level macrolide resistance and carried erm(B) and tet(M) genes. MLST analysis identified seventeen different sequence types (ST), among which ST271 accounted for 15% (4/26), followed by ST172 (12%, 3/26) and ST14821 (8%, 2/26). The ST271 isolate belonged to serotype 3 and carried multiple resistance-determinant genes.
Discussion
We observed a discrepancy in azithromycin susceptibility depending on whether using breakpoints from EUCAST or CLSI guidelines for interpretation. All 13 PNSP isolates harboring genetic determinants for macrolide resistance were correctly identified as resistant to erythromycin and azithromycin (all with MIC ≥ 4 µg/mL) regardless of which breakpoints were used (sensitivity 100%, 13/13). Using the EUCAST breakpoints appeared to overestimate azithromycin resistance, as all 13 erythromycin-susceptible PNSP without genetic determinants of macrolide resistance were interpreted as resistant to azithromycin (MIC-values from 0.5 to 2, specificity 0%, 0/13). Using CLSI breakpoints only misclassified two such isolates (MIC 2 µg/mL, specificity 85%, 11/13). Therefore, relying on current EUCAST guidelines appears to overestimate azithromycin resistance and could in the clinical perspective lead to unnecessary use of more broad-spectrum antibiotics. Our findings suggest that the EUCAST guidelines currently use a too low cutoff for MIC-values for azithromycin resistance in pneumococci.
PNSP susceptibility to erythromycin, on the other hand, was similar using both EUCAST and CLSI breakpoints, and the phenotypic findings correlated well with the identified genotypic resistance markers. To avoid variations in interpretation, our findings call for AST guidelines to be harmonized. Both CLSI and EUCAST state that erythromycin susceptibility can predict susceptibility to clarithromycin, azithromycin, dirithromycin, and roxithromycin [19, 20]. Because almost all PNSP resistant to erythromycin carried genetic determinants for macrolide resistance, our study supports that erythromycin determines susceptibility to other macrolides.
In Tanzania, macrolides are commonly used to treat respiratory tract infections. In the treatment of community-acquired pneumonia, erythromycin and azithromycin are used as first and second line treatment, respectively [6]. In this study, however, we observed that 50% and 58% of PNSP were resistant to erythromycin and azithromycin, respectively. Consequently, macrolides appear potentially ineffective for treating PNSP infections in this setting. This calls for prudent use of antibiotics including the use of narrow-spectrum penicillin. But for treatment failure or infections likely caused by resistant pneumococci/PNSP, options are difficult, with azithromycin, the currently preferred treatment covering just half of the PNSP.
The most common phenotype was MLSB and isolates with this phenotype harbored the erm(B) gene conferring a high level of resistance to macrolides (> 256 µg/mL) and clindamycin. All but two isolates with the MLSB phenotype carried the mef(A) and msr(D) genes as well. The erm(B) gene has been reported as the most common macrolide resistance determinant in Streptococcus pneumoniae in studies from Africa [14, 21, 22] and some part of Asia [11]. Macrolide resistance genes in Streptococcus pneumoniae have marked geographical variability [13]. The mef(A) has been reported to be the predominant mechanism of pneumococcal macrolide resistance in North America and some parts of Europe [13]. Our study found the macrolide efflux genes mef(A)/msr(D) to be more prevalent (11/13) than the erm(B) genes (7/13). Still, considering the high number of isolates harboring both types of resistance genes (5/13), the dominant MLSB phenotype was more frequent (7/13, erm(B) with or without mef(A)/msr(D)) than the M-phenotype (6/13, mef(A)/msr(D) alone).
Previous studies have shown that tetracycline and macrolide resistance genes are carried on mobile genetic elements, composite conjugative transposons, Tn916-like elements, which facilitate their dissemination between different bacteria [23, 24]. Tn916 and Tn917-like composite elements have been documented to facilitate dissemination of erm(B) and mef(A/E) in Streptococcus pneumoniae [17]. However, our study found a Tn6009-like element in all 13 PNSP isolates carrying the tet(M) gene which confers resistance to tetracycline, and in 12/13 (92%) of PNSP isolates carrying macrolide resistance determinants. Tn6009 is a member of the Tn916–Tn1545 transposon family previously detected in Gram-positive and Gram-negative bacteria [25]. Tn6009 has been reported to carry genes conferring resistance against tetracycline tet(M), and inorganic and organic mercury [25]. Through horizontal gene transfer, the conjugative mobile elements enable bacteria to acquire and disseminate DNA between related and unrelated bacteria. The presence of transposons containing macrolide and tetracycline resistance genes in PNSP in our study could indicate an increased risk of dissemination of these resistance determinants.
Conclusion
Macrolides and tetracyclines have only about 50% chance of being effective against PNSPs recovered from nasopharynx from people living with HIV in Dar es Salaam. The erm(B) and mef(A)-msr(D) were common genes conferring resistance to macrolides and clindamycin, while resistance to tetracycline was conferred by the tet(M) gene. Detection of the composite conjugate transposon Tn6009 associated with macrolides and tetracycline genes could indicate the possibility of horizontal transfer of resistant genes. Using EUCAST guidelines for interpretation overestimates azithromycin resistance in PNSP compared to genetic correlates of resistance.
Availability of data and materials
Data are available on reasonable request.
References
Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2000;31(1):170–6. https://doi.org/10.1086/313925.
Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–57. https://doi.org/10.1016/s2214-109x(18)30247-x.
Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. https://doi.org/10.1016/s1473-3099(18)30310-4.
Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3(2):171–96. https://doi.org/10.1128/cmr.3.2.171.
Tleyjeh IM, Tlaygeh HM, Hejal R, Montori VM, Baddour LM. The impact of penicillin resistance on short-term mortality in hospitalized adults with pneumococcal pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2006;42(6):788–97. https://doi.org/10.1086/500140.
Tanzania standard treatment guidelines & national essential medicines list. STG/NEMLIT 2021. Ministry of Health, Dar es Salaam, Tanzania
Mudhune S, Wamae M. Report on invasive disease and meningitis due to Haemophilus influenzae and Streptococcus pneumonia from the Network for Surveillance of Pneumococcal Disease in the East African Region. Clin Infect Dis. 2009;48(Suppl 2):S147–52. https://doi.org/10.1086/596494.
Iroh Tam PY, Thielen BK, Obaro SK, Brearley AM, Kaizer AM, Chu H, et al. Childhood pneumococcal disease in Africa—a systematic review and meta-analysis of incidence, serotype distribution, and antimicrobial susceptibility. Vaccine. 2017;35(15):1817–27. https://doi.org/10.1016/j.vaccine.2017.02.045.
Sader HS, Mendes RE, Le J, Denys G, Flamm RK, Jones RN. Antimicrobial Susceptibility of Streptococcus pneumoniae from North America, Europe, Latin America, and the Asia-Pacific Region: results from 20 years of the SENTRY antimicrobial surveillance program (1997–2016). Open Forum Infect Dis. 2019;6(Suppl 1):S14-s23. https://doi.org/10.1093/ofid/ofy263.
Yu YY, Xie XH, Ren L, Deng Y, Gao Y, Zhang Y, et al. Epidemiological characteristics of nasopharyngeal Streptococcus pneumoniae strains among children with pneumonia in Chongqing, China. Sci Rep. 2019;9(1):3324. https://doi.org/10.1038/s41598-019-40088-6.
Beheshti M, Jabalameli F, Feizabadi MM, Hahsemi FB, Beigverdi R, Emaneini M. Molecular characterization, antibiotic resistance pattern and capsular types of invasive Streptococcus pneumoniae isolated from clinical samples in Tehran, Iran. BMC Microbiol. 2020;20(1):167. https://doi.org/10.1186/s12866-020-01855-y.
Schroeder MR, Lohsen S, Chancey ST, Stephens DS. High-Level macrolide resistance due to the mega element [mef(E)/mel] in Streptococcus pneumoniae. Front Microbiol. 2019;10:868. https://doi.org/10.3389/fmicb.2019.00868.
Schroeder MR, Stephens DS. Macrolide resistance in Streptococcus pneumoniae. Front Cell Infect Microbiol. 2016;6:98. https://doi.org/10.3389/fcimb.2016.00098.
Raddaoui A, Tanfous FB, Chebbi Y, Achour W, Baaboura R, Benhassen A. High prevalence of multidrug-resistant international clones among macrolide-resistant Streptococcus pneumoniae isolates in immunocompromised patients in Tunisia. Int J Antimicrob Agents. 2018;52(6):893–7. https://doi.org/10.1016/j.ijantimicag.2018.04.015.
Korona-Glowniak I, Siwiec R, Malm A. Resistance determinants and their association with different transposons in the antibiotic-resistant Streptococcus pneumoniae. Biomed Res Int. 2015;2015:836496. https://doi.org/10.1155/2015/836496.
Talebi M, Azadegan A, Sadeghi J, Ahmadi A, Ghanei M, Katouli M, et al. Determination of characteristics of erythromycin resistant Streptococcus pneumoniae with preferred PCV usage in Iran. PLoS ONE. 2016;11(12):e0167803. https://doi.org/10.1371/journal.pone.0167803.
Chancey ST, Agrawal S, Schroeder MR, Farley MM, Tettelin H, Stephens DS. Composite mobile genetic elements disseminating macrolide resistance in Streptococcus pneumoniae. Front Microbiol. 2015;6:26. https://doi.org/10.3389/fmicb.2015.00026.
Manyahi J, Moyo S, Aboud S, Langeland N, Blomberg B. High rate of antimicrobial resistance and multiple mutations in the dihydrofolate reductase gene among Streptococcus pneumoniae isolated from HIV-infected adults in a community setting in Tanzania. J Glob Antimicrob Resist. 2020;22:749–53. https://doi.org/10.1016/j.jgar.2020.06.026.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-eighth informational supplement CLSI document M100–S20. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0, 2021. http://www.eucast.org.
Midouni Ayadi B, Mehiri E, Draoui H, Ghariani A, Essalah L, Raoult D, et al. Phenotypic and molecular characterization of macrolide resistance mechanisms among Streptococcus pneumoniae isolated in Tunisia. J Med Microbiol. 2020;69(4):505–20. https://doi.org/10.1099/jmm.0.001151.
Diawara I, Zerouali K, Katfy K, Barguigua A, Belabbes H, Timinouni M, et al. Phenotypic and genotypic characterization of Streptococcus pneumoniae resistant to macrolide in Casablanca, Morocco. Infect Genet Evol. 2016;40:200–4. https://doi.org/10.1016/j.meegid.2016.03.003.
Roberts AP, Mullany P. Tn916-like genetic elements: a diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol Rev. 2011;35(5):856–71. https://doi.org/10.1111/j.1574-6976.2011.00283.x.
Sansevere EA, Robinson DA. Staphylococci on ICE: overlooked agents of horizontal gene transfer. Mob Genet Elements. 2017;7(4):1–10. https://doi.org/10.1080/2159256x.2017.1368433.
Soge OO, Beck NK, White TM, No DB, Roberts MC. A novel transposon, Tn6009, composed of a Tn916 element linked with a Staphylococcus aureus mer operon. J Antimicrob Chemother. 2008;62(4):674–80. https://doi.org/10.1093/jac/dkn255.
Acknowledgements
Authors thank members of the bacteriology research laboratory of the Muhimbili University of Health and Allied Science in Dar es Salaam, Tanzania, for their assistance during data collection and preliminary laboratory procedures.
Funding
This research was supported by (i) Helse Bergen HF, Haukeland University Hospital, Bergen, Norway, project number 912132, , (ii) National Advisory Unit on Tropical Infectious Diseases, Haukeland University Hospital, Bergen, Norway, (iii) CAMRIA - Combatting Anti-Microbial Resistance with Interdisciplinary Approaches, Centre for Antimicrobial Resistance in Western Norway, funded by Trond Mohn Stiftelse, grant number TMS2020TMT11, and (iv) STRESST - Antimicrobial Stewardship in Hospitals, Resistance Selection and Transfer in a One Health Context, Liverpool School of Tropical Medicine, Liverpool, United Kingdom, funded by JPIAMR grant number NFR333432.
Author information
Authors and Affiliations
Contributions
BB and NL conceived the study. JM collected study data. JM and SM performed the microbiological investigations. BB and JM performed statistical analysis. JM drafted the manuscript. BB, SJM, and NL revised the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval to conduct the study were obtained from Muhimbili University of Heath and Allied Sciences Senate Research and Publications Committee (Ref. No. 2015-10-27/AEC/Vol.X/54), National Health Research Ethics Committee (Ref. No. NIMRlHQ/R. SaJVol. 1X12144), Tanzania Medicines and Medical Devices Authority (Ref. No. TZ16CT007), and Regional Committee for Medical and Health Research Ethics of Western Norway (Ref. No. REK2015/540).
Written informed consent was obtained from each study participant before the enrollment in the study.
Consent for publication
Consent to publish was obtained from National Health Research Ethics Committee (Ref. No. NIMRlHQ/R. SaJVol. 1X12144).
Competing interests
Authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Manyahi, J., Moyo, S.J., Langeland, N. et al. Genetic determinants of macrolide and tetracycline resistance in penicillin non-susceptible Streptococcus pneumoniae isolates from people living with HIV in Dar es Salaam, Tanzania. Ann Clin Microbiol Antimicrob 22, 16 (2023). https://doi.org/10.1186/s12941-023-00565-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00565-3