Abstract
Non-tuberculous Mycobacterial Pulmonary Disease (NTM-PD) is an increasingly recognised global health issue. Studies have suggested that neutrophils may play an important role in controlling NTM infection and contribute to protective immune responses within the early phase of infection. However, these cells are also adversely associated with disease progression and exacerbation and can contribute to pathology, for example in the development of bronchiectasis. In this review, we discuss the key findings and latest evidence regarding the diverse functions of neutrophils in NTM infection. First, we focus on studies that implicate neutrophils in the early response to NTM infection and the evidence reporting neutrophils’ capability to kill NTM. Next, we present an overview of the positive and negative effects that characterise the bidirectional relationship between neutrophils and adaptive immunity. We consider the pathological role of neutrophils in driving the clinical phenotype of NTM-PD including bronchiectasis. Finally, we highlight the current promising treatments in development targeting neutrophils in airways diseases. Clearly, more insights on the roles of neutrophils in NTM-PD are needed in order to inform both preventative strategies and host-directed therapy for these important infections.
Similar content being viewed by others
Introduction
Non-tuberculous mycobacterial pulmonary disease (NTM-PD) is an increasingly prevalent and challenging infection syndrome that causes significant morbidity, healthcare utilization and mortality [1].
Although there are over 170 NTM species, most NTM-PD results from a small number of these environmental bacteria, which act as human opportunistic pathogens [2] but differ in their pathogenicity and response to treatment [3, 4]. Common species causing NTM-PD are Mycobacterium avium complex (MAC), (most often the slower-growing M. avium, M. intracellulare and M. chimaera species), M. kansasii, and M. xenopi, and also the rapid-grower M. abscessus complex (MABC) [5, 6].Unlike tuberculosis, which typically affects younger people without other co-morbid illness, NTM lung disease commonly occurs in people aged fifty years or above, who may have other underlying conditions, e.g., bronchiectasis, chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF) [7,8,9].
The decision to treat is not straightforward: some patients appear to spontaneously clear infection and others are clinically stable without treatment over long periods of time [9]. Further, antimicrobial therapy is often prolonged, can be poorly tolerated [10] and not necessarily effective.
Studies on the host immune response to NTM have generally focused on T cells, ‘T helper-1’ cytokines and mononuclear phagocytes [11]. While these are important in protection against NTM [12], their role in either causing or preventing lung damage is less well-defined.
Generally, professional phagocytes (neutrophils, macrophages, and dendritic cells) are considered as a first-line defence against bacterial pathogens. Neutrophil polymorphonuclear granulocytes are the most prominent cell type in the bronchial lumen and are rapidly recruited to sites of infection when they sense signals from chemoattractants such as Interleukin-8 (IL-8) generated by host cells. Following migration (chemotaxis) they trap and kill the invading pathogens. Neutrophils are known to be essential for defence against extracellular bacteria [13]. However, the role of the neutrophil response in NTM-PD is still not completely understood. Recent studies have suggested that neutrophils may help control NTM infection [14, 15]; though they can also contribute to NTM-associated disease pathology, for example in the development of bronchiectasis [14, 16]. In this review we discuss the role of neutrophils in relation to non-tuberculous mycobacterial pulmonary disease (NTM-PD) and explore the apparently conflicting contributions of neutrophils in this disease.
Neutrophils and immunity
Neutrophils’ antibacterial functions include phagocytosis (ingestion), degranulation (release of soluble antimicrobials either into the phagosome or extracellularly), and the release of nuclear material in the form of neutrophil extracellular traps (NETs) [17, 18]. Initiation of neutrophil phagocytosis is significantly enhanced by opsonisation of the bacteria whereby opsonins, for example complement components and immunoglobulins (Igs), coat the bacteria and are recognized by specific surface receptors on neutrophils causing avid binding and triggering ingestion.
Generally, once phagocytosis has been initiated by engagement of opsonic receptors, internalisation of the pathogen within phagosomes inside the neutrophil occurs in seconds [19].
Subsequently, phagosomal maturation with intracellular granule fusion takes place and gives neutrophils unique advantages over other phagocytes as the granules contain powerful bactericidal proteins. Four groups of granules are found in neutrophils: azurophil (primary), containing enzymes such as neutrophil elastase (NE) and antibacterial molecules including azurocidin and human neutrophil peptides (HNP) 1–3, specific granules (secondary), gelatinase granules, and secretory vesicles, each playing specific roles during the response to infection [20]. Some of these are discussed in more detail below.
NADPH oxidase in the wall of secondary granules initiates the oxidative burst, leading to the production of antimicrobial reactive oxygen intermediates (superoxide, hydrogen peroxide, hypochlorous acid). However, neutrophil influx may also be associated with pathology through the release of these cytotoxic contents; and if neutrophils are disrupted these processes may lead to damage of neighbouring cells and tissue injury [21, 22].
Neutrophils are also professional bacteria-responsive immune cells. Toll-like receptors (TLRs) are a type of pattern recognition receptor (PRR) that trigger the innate immune response by sensing conserved molecular patterns and allowing early immunological pathogen detection [23].
These rapid antimicrobial neutrophil functions give the acquired immune system enough time to develop pathogen-specific immunity, although, as discussed later, neutrophil behaviour can itself influence the acquired immune response.
The neutrophil response to NTM
Innate phagocytic immune cells, including mononuclear phagocytes such as macrophages, rapidly eliminate mycobacteria through phagocytosis and intracellular killing, and an impairment in this process can predispose to the development of mycobacterial infection [24, 25]. Although the neutrophil response to NTM is poorly studied, previous work has proposed that granulocytes are important participants in the host defence against mycobacteria [26, 27] and these cells can kill several species of mycobacteria [28]. However they are also implicated in the pathology of mycobacterial diseases such as tuberculosis [16, 29] where they are the dominant host cell for infecting organisms in sputum, bronchoalveolar lavage (BAL) fluid, and cavity contents in patients with pulmonary TB disease [14]. Although it is clearly simplistic to translate results in Mycobacterium tuberculosis (Mtb)-based experiments to NTM, precise data on NTM are often lacking and in this review where necessary we have discussed the available data for Mtb. This highlights the urgent need for more research on NTM (and especially those species which cause most human disease).
Using Mtb, Jones et al. and Majeed et al. found high efficacy of mycobacterial phagocytosis by neutrophils through complement-mediated opsonization [30, 31]. However, the results of in vitro studies by Irina et al. and Lenhart-Pendergrass et al. pointed out the low capacity of neutrophils to phagocytose non-opsonized M. smegmatis and M. avium respectively [32, 33]. Collectively, these data suggest that neutrophils are capable of phagocytosing mycobacteria but this is may require opsonization by complement or immunoglobulins and could vary between species [33].
TLR-2 deficient mice with M. avium infection exhibited defective neutrophil function and a subsequent impairment in controlling the infection in its early stages [34, 35], implying a potentially crucial role for neutrophils in the host immune response to NTM. Conversely, it has been shown that neutrophils might contribute to the pathological dissemination of the infection rather than early clearance among genetically-susceptible mice, though this did depend on the mycobacterial species (occurring with M. avium but not Mtb [36]). Specifically, it has been suggested that neutrophils may carry mycobacteria to the pulmonary surface [14].
Faldt et al. reported that NTM (M. avium and M. smegmatis) induced a significantly higher secretion of TNFα, IL‐6, and IL‐8 from activated neutrophils than Mtb which might suggest these species evoke innate immune reactions that can lead to effective clearance of mycobacteria [37].
Table 1 summarises mouse studies suggesting a significant role for neutrophils in the early response to NTM infection, some of which are further explored in the next section.
Animal models and human genetic studies implicate neutrophils in the host response to mycobacterial infection
Over 35 years ago, Brown and colleagues documented an interaction between neutrophils and mycobacteria [42]. Appelberg et al. subsequently demonstrated the major contribution of neutrophils to protect against intravenously inoculated mycobacterial infection when, using granulocyte-depleting monoclonal antibody (MAb) RB6-8C5 treatment, they noted a higher bacterial growth [38]. Petrofsky and Bermudez used a similar procedure for neutrophil depletion and also concluded that neutrophils provide some protection against M. avium during the early phase of infection [35]. In contrast, Saunders and Cheers did not identify a clear protective role for mouse lung neutrophils following inhalational challenge with M. avium, despite using similar experimental methods [43].
A study by Goncalves and Appelberg suggested that the CXC receptor 2 (CXCR2) may play a key role in neutrophil recruitment following mycobacterial infection. In comparison to control mice, the CXCR2 knockout mice had considerably fewer neutrophils in the peritoneal cavity over the course of a 15-day intraperitoneal infection with M. avium. However, the CXCR2 mutation had no effect on neutrophil recruitment to the lungs during an aerogenic M. avium infection over the course of the 60-day trial—suggesting that this may be a tissue site-related phenomenon [39].
Whole-Blood Gene Expression has been performed to investigate the host immune response to NTM-PD. A recent study included 25 patients with NTM-PD and 27 controls who were uninfected but had respiratory disease. Microarray analysis suggested that the NTM-PD population had decreased expression of 213 genes associated with T-cell signalling, including IFN-g. Chest CT lesion severity, lung dysfunction, and other markers of disease severity including high neutrophil count were associated with decreased IFN-g expression [44].
Collectively, this experimental evidence suggests that neutrophils play an important role in the host response to NTM infection though does not define what this might be, or whether it is protective or driving pathology.
Can neutrophils kill NTM?
Several in vitro studies (summarised in Table 2) have addressed the capacity of human neutrophils to kill NTM species with the general consensus that neutrophils can eliminate—or at least restrict the growth of—clinically important NTM.
There is limited clinical evidence reporting the NTM susceptibility in neutropenic patients or those with neutrophil disorders [44]. However, neutropenia has been associated with disseminated NTM (although not pulmonary NTM) in patients with haematological malignancy [45].
A potential pathway through which neutrophils may kill mycobacteria is via human neutrophil peptides (HNP) 1, 2 and 3. These belong to a family of endogenous cationic antimicrobial and cytotoxic peptides (defensins) localised in the azurophilic granules. HNP also function as immunomodulatory molecules influencing cytokine production as well as inflammatory and immunological responses [46]. The ability of HNP-1 to kill M. tuberculosis has been studied in vitro by Miyakawa et al., [47], Sharma et al., [48], Kalita et al., [49], and Martineau et al., [50]. These studies have suggested that neutrophils may play a substantial role in innate resistance against TB infection through the activity of HNP and that these molecules could potentially be the basis of new therapeutic approaches.
However, another study showed that high concentrations of HNP are detected in both cystic fibrosis (CF) and non-CF bronchiectatic airways and that these inhibit PMN function via interference with phagocytosis [51, 52].
Neutrophils directly influence the development of an acquired immune response to NTM
Neutrophils have the ability to shape adaptive immunity and bridge the innate and adaptive immune systems [58, 59].
Cytokine networks play significant roles in the cell mediated immune response to NTM infection. The T cell response against NTM is regulated by the production of IL-12 following endocytosis of mycobacteria by innate mononuclear phagocytes (eg dendritic cells (DC) and macrophages). In turn, activated CD4+ T cells (T-helper 1) and CD8+ T cells release IFNγ which enhances killing by mononuclear phagocytes and is essential for host defence against mycobacteria [60, 61]. Therefore, NTM infection which overcomes initial innate mechanisms may be controlled by efficient Th1 responses mediated by IL-12 and IFNγ [62].
Genetic mutations in the IL-12-IFNγ pathway increase susceptibility to NTM infection, for example; IFN-γR1 and IFN-γR2 deficiencies (both autosomal recessive and dominant forms), IL12β and IL12Rβ1 deficiencies, transcription factor STAT1 deficiency, RAR-related Orphan Receptor C (RORC) deficiency, interferon-stimulated gene 15 (ISG15) deficiency, interferon regulatory factor 8 (IRF8) deficiency and tyrosine kinase 2 (TYK2) deficiency [63,64,65,66].
In particular, It has been reported that defects in IL-12 and IFNγ pathways can predispose to pulmonary NTM infections [67]. Notably, interleukin-12-induced IFNγ production from T cells also activates neutrophils to phagocytose and/or kill NTM [68]. Moreover, IL-17, IL-21, and IL-22 produced by T-helper 17 CD4+ T cells induce neutrophil influx into inflamed disease sites which might help arrest the progression of NTM infection via direct killing [69,70,71].
However, neutrophil recruitment can also contribute to negative effects on the acquired immune response. A study using a mouse model of MAC infection demonstrated that when Th1 immunity is impaired, Th17 + cells provoked neutrophil recruitment that appeared to increase susceptibility to MAC infection [69]. There may be a particular negative effect of dead neutrophils. In a whole blood model with Mtb, necrotic neutrophils impaired host control of mycobacterial growth and increased immunosuppressive IL-10 as well as growth factors and chemokines [72]. This may result in further neutrophil accumulation at the site of disease: a pathological cycle that can contribute to the undesirable impact of neutrophils on host outcome [16, 73].
‘Frustrated’ neutrophils that release granule contents extracellularly could drive tissue damage and cause profound effects on T cell differentiation and proliferation [74,75,76]. Indeed, granule constituents or production of chemokines by neutrophils can mediate a suppressive effect directly or indirectly on T-cell responses, inactivating T-cell stimulating cytokines, eg IL-2 and IL-6, and speeding up the shedding of IL-2 and IL-6 cytokine receptors on T-cells [58, 77,78,79].
As an example, neutrophil elastase selectively cleaves IL-2 receptor and IL-6 receptor, and leads to the reduction of co-stimulatory molecule expression by dendritic cells, thus limiting T cell maturation and affecting the development of the Th1 response [80]. Down-regulation of T cell receptor (TCR) expression can also occur upon release of arginase and the production of reactive oxygen species (ROS) from neutrophils [58, 78].
Conversely, the production of NETs may reduce T cells’ activation threshold [81], while an abolition of Th1-specific responses has been reported when neutrophils were depleted during BCG vaccination of mice [82].
In summary, there is a bi-directional relationship between neutrophils and the acquired immune response with neutrophil behaviour potentially influencing T cell-mediated immunity either positively or negatively depending on the immune environment.
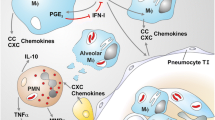
Figure 1 summarises the various potential roles for neutrophils in NTM pulmonary disease.
Summary of the potential roles of neutrophils in non-tuberculous mycobacterial lung disease. The figure is made with BioRender (https://app.biorender.com/). Abbreviations: ROS: Reactive oxygen species; NET neutrophil extracellular trap
NTM, neutrophils and the humoral immune response
Mycobacteria are intracellular organisms and thus, cell-mediated immunity is considered to be the major component of host immunological defence against these bacteria. However, understanding the interaction between innate immunity, antibody-mediated immunity and cellular immunity is useful to determine strategies (both treatments and vaccines) that might combat NTM infection and disease.
Interactions of T cells, B cells and antigen presenting cells (APCs) with neutrophils allow neutrophils to modulate humoral adaptive immunity [76]. For example, activated neutrophils have a role in B-cell development through the production of B-cell activating factor (BAFF), which is an essential cytokine for B cell development, and granulocyte colony-stimulating factor (G-CSF) [76, 83]. Reciprocally, B cells can influence neutrophil activity via the production of antibodies which opsonise mycobacteria and thereby enhance neutrophil phagocytosis (discussed above).
The protective effect of the humoral immune response against mycobacterial antigens has been demonstrated in several models using Mtb. Kunnath et al. described the contribution of the humoral immune response to the control of Mtb [84] and Hamasur et al. showed the protective effects of mouse monoclonal IgG1 antibody to lipoarabinomannan (SMITB14) against tuberculosis when mice were infected intravenously [85]. Zimmermann et al. demonstrated that IgA (but not IgG) antibodies specific for different Mtb surface antigens blocked Mtb activity [86]. It is unclear whether the same applies to NTM – and this is an area which requires further investigation.
Glycopeptidolipids (GPLs) are a class of glycolipids expressed in the outer layer of several NTM species, including MAC and M. abscessus. The GPLs of MAC are highly antigenic and serovar-specific and are associated with MAC virulence [87, 88]. A serological diagnostic test measuring the serum IgA antibody against MAC GPLs has been developed and used clinically to diagnose MAC disease. An increase of antibody levels was recorded in patients with NTM-PD caused by MAC and not in patients with Mtb [89].
A recent study has shown the utility of serological testing in the detection of culture-positive cases of M. abscessus infection in CF patients [90]. The test was based on the detection of IgA against M. abscessus protein, recombinant PLC (rPLC), and the TLR2eF extract. This IgA ELISA was able to differentiate M. abscessus from M. avium and M. chimaera infections (but not from M. intracellulare infection) based on the recognition of MABC proteins or extracts, in contrast to the older test which is based on the detection of antibodies recognizing the GPL core antigen of M. avium [90]. The prevalence of NTM infection in CF patients is currently being tested in a prospective study (clinical study number ID RCB:2017-A00025-48) using both ELISAs.
Overall, studies have identified a potential role for anti-mycobacterial antibodies during the course of infection and argue for further work to help elucidate their mechanisms of action [91,92,93,94]. Specifically, antibody-mediated opsonisation of mycobacteria with subsequent enhancement of phagocytosis by neutrophils requires investigation.
The role of neutrophils in the pathology of NTM-PD
The typical pulmonary radiological patterns seen in NTM infection include bronchiectasis and cavitation, both of which are understood to be driven in large part by neutrophils, with a particular role for neutrophil elastase [95, 96].
MAC and MABC are the most prevalent species causing NTM-PD, accounting for 95% of cases [97, 98]. MABC infection, typically seen in patients with a history of pulmonary disease such as cystic fibrosis and bronchiectasis, has the highest recorded fatality rate among rapidly growing mycobacteria [3, 99,100,101]. MAC is less clearly associated with severe disease, but around 35–42% of positive sputum cultures for MAC represent NTM-PD [102, 103].
Why NTM are so variably pathogenic in humans is unclear. This is the case even with M. avium, whose host response is probably best understood [104].
Upon entry into the body, NTM usually settle in the lower airways and, if clinical illness develops, this presents as localised inflammation (airways disease, pneumonia, cavitation) [105].
NTM-PD is frequently seen in association with bronchiectasis, which may precede or be a consequence of the infection. In general terms, bronchiectasis can be caused by an underlying condition such as CF or other disorders of ciliary function, associated with immune deficiency (especially antibody deficiency) or occurs secondary to infection [106, 107]. It usually presents with persistent productive cough and is characterised by impairment of mucus clearance from the airways. The accumulation of mucus in the damaged airways of the lungs generates a favorable site for bacteria (including NTM) to grow, leading to further inflammation with consequent damage and dilatation of the airways, often in the right middle lobe or the lingula segment. This destruction is usually accompanied by clinical manifestations and establishes a ‘vicious circle’ due to the interaction between persistent or recurrent infection and excess inflammation [108].
Neutrophils are responsible for airway damage via the release of granule contents (human NE in particular) during degranulation [109] and are strongly implicated in the development of bronchiectasis. Granule-derived molecules have antimicrobial properties that assist in combating the infection (see above), but they can also damage host tissues (leading to bronchial dilation) [58].
Neutrophil-dominant inflammation is a central feature of bronchiectasis pathogenesis. High levels of NE in the airways are associated with exacerbations and worse lung function in both CF and non-CF bronchiectasis [110].
Some studies have reported a higher neutrophil count in the sputum of bronchiectasis patients versus healthy controls which correlates with disease progression [111,112,113]. Patients with bronchiectasis, who are at a considerably increased risk of NTM-PD [114, 115], exhibit ‘reprogramming’ of peripheral blood neutrophils during the stable state and prolonged neutrophil survival with impaired ability to kill and phagocytose bacteria, thereby perpetuating the vicious circle [116, 117]. However, this appears to improve following antibiotic treatment [116]. In addition, impairment of neutrophils’ phagocytic ability and ROS production in CF airways has been reported [118].
Any impairment of neutrophils’ ability to phagocytose and kill bacteria, including NTM, could contribute to perpetuation of the vicious circle in bronchiectasis [116].
Neutrophils can extrude the contents of their nuclei extruded to the extracellular space as neutrophil extracellular traps (NETs). NETs are made up of chromatin, histones, and various neutrophil granule proteins, including NE, cathelicidin, cathepsin G, and myeloperoxidase (MPO) [119]. NETs are used to combat pathogens in a process called NETosis, a type of cell death [120]. Cytokines such as IL8, TNF, and IFN-γ can induce NETosis in addition to bacterial components, mainly lipopolysaccharide (LPS) and lipophosphoglycan (LPG) [121].
NETs were initially identified as means of preventing bacterial dissemination by trapping and killing the bacteria [120]. However, Nakamura et al. found that MAC-induced NET formation was not involved in killing but in the production of MMPs and IL-8 that promote the progression of lung infections [122]. Furthermore, NET components such as PR3, MPO, and NE, activated and released during NETosis, are cytotoxic and have been shown to cause direct damage to the endothelium.
Moreover, research has also shown that Type I IFN-induced pulmonary NETosis can have a direct impact on TB pathogenesis in TB susceptible mice. The presence of NETs in necrotic lung lesions in patients with tuberculosis also supports a causal role for NETosis in TB pathogenesis [123, 124].
Another study has demonstrated the role of NETs in disease severity and treatment response in bronchiectasis [125], with the abundance of NET-associated proteins in patients’ sputum differing between mild and severe cases.
Targeting neutrophils for treatment
Although they may help control NTM during early infection, neutrophils appear to have a pathogenic role in the bronchiectasis associated with established NTM-PD. Some treatments have therefore attempted to directly target neutrophils to limit further tissue damage. These have focused on neutrophil influx, neutrophil weaponry and neutrophil function (Table 3). Although no neutrophil-targeting strategies are currently licensed [126, 127], several chronic inflammatory conditions are managed—at least in part—by modifying neutrophil activity and numbers locally and systemically. These include asthma, ulcerative colitis, and rheumatoid arthritis [128].
In the clinical setting, therapies which reduce neutrophil number are less preferable as they have been associated with compromising the patient’s immunity and increasing the risk of recurrent infections [129]. However, reduction of neutrophil migration in COPD patients seems to reduce their risk for exacerbations [126, 130].
In bronchiectasis, neutrophilic inflammation and dysfunctional killing of pathogens are considered key factors (see above). Whilst it has been proven that sputum NE is a useful marker for bronchiectasis during both stable state and exacerbations, the treatment of bronchiectasis through the inhibition of NE is still at an early stage [131]—though, this has been proposed for patients with COPD and CF using the selective NE inhibitor AZD9668 [131,132,133].
A recent clinical trial of brensocatib, an inhibitor of dipeptidyl peptidase 1 (DPP-1), demonstrated a relationship between the activity and quantity of neutrophil serine proteases and prognosis for patients with non-cystic bronchiectasis. A strong association was found between undetectable levels of sputum neutrophil elastase and the reduction of lung exacerbations [134].
Some therapies exist which may indirectly affect neutrophils’ function; Prezzo et al. found that intravenous immunoglobulin (IVIg) replacement therapy for antibody defects affected neutrophil activation by reducing serum IL-8 concentration, the expression of its receptor CXCR1 and the release of neutrophil elastase. This study suggested that the reduction in IL-8/CXCR1 post IVIg infusion may play a protective role in neutrophil-mediated inflammation [135]. Recently, Hitoshi et al. identified that the anti-lipoarabinomannan (anti-LAM) monoclonal IgMs, TMDU3 and LA066, significantly inhibited the phagocytosis of Mycobacterium avium by human neutrophils, and that mycobacterial load was reduced in the presence of neutrophils and anti-LAM IgM (albeit in the absence of other opsonins). These mannan core-directed monoclonal antibodies (mAbs) were therefore proposed as potential therapies to target aberrant or excessive neutrophil-associated immune responses [136].
Given the often poor response and considerable toxicity seen with antimicrobial therapies directed against NTM, there is an urgent need for new treatment options.
Conclusions
In summary, available evidence suggests that neutrophils can contribute to early clearance of infection via phagocytosis and killing but may also disseminate bacilli to distant sites. Neutrophils can influence (positively or negatively) the development of acquired immune responses. In established disease, neutrophil products contribute to airway damage and are therefore appropriate targets for host-directed therapy. Currently, much of the evidence is extrapolated from research on Mtb which may not be an appropriate model for NTM, and NTM species differ between each other. Further research is required to fully characterise the diverse functions of neutrophils in NTM pulmonary disease.
Availability of data and materials
Not applicable.
References
Strollo SE, Adjemian J, Adjemian MK, Prevots DR. The burden of pulmonary nontuberculous mycobacterial disease in the United States. Ann Am Thorac Soc. 2015;12(10):1458–64.
Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax. 2017;72(Suppl 2):ii1–64.
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416.
Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16(10):1576.
Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6(3):210.
Musaddaq B, Cleverley JR. Diagnosis of non-tuberculous mycobacterial pulmonary disease (NTM-PD): modern challenges. Br J Radiol. 2020;92(1106):20190768.
Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36(1):91–9.
Faverio P, Stainer A, Bonaiti G, Zucchetti SC, Simonetta E, Lapadula G, et al. Characterizing non-tuberculous mycobacteria infection in bronchiectasis. Int J Mol Sci. 2016;17(11):1913.
Rawson TM, Abbara A, Kranzer K, Ritchie A, Milburn J, Brown T, et al. Factors which influence treatment initiation for pulmonary non-tuberculous mycobacterium infection in HIV negative patients; a multicentre observational study. Respir Med. 2016;120:101–8.
Egelund EF, Fennelly KP, Peloquin CA. Medications and monitoring in nontuberculous mycobacteria infections. Clin Chest Med. 2015;36(1):55–66.
Lake MA, Ambrose LR, Lipman MC, Lowe DM. ‘” Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med. 2016;14(1):54.
Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15(8):968–80.
Aleyd E, van Hout MW, Ganzevles SH, Hoeben KA, Everts V, Bakema JE, et al. IgA enhances NETosis and release of neutrophil extracellular traps by polymorphonuclear cells via Fcα receptor I. J Immunol. 2014;192(5):2374–83.
Eum S-Y, Kong J-H, Hong M-S, Lee Y-J, Kim J-H, Hwang S-H, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137(1):122–8.
Malcolm KC, Caceres SM, Pohl K, Poch KR, Bernut A, Kremer L, et al. Neutrophil killing of Mycobacterium abscessus by intra-and extracellular mechanisms. PLoS ONE. 2018;13(4):e0196120.
Lowe DM, Bandara AK, Packe GE, Barker RD, Wilkinson RJ, Griffiths CJ, et al. Neutrophilia independently predicts death in tuberculosis. Eur Respir J. 2013;42(6):1752–7.
Hickey MJ, Kubes P. Intravascular immunity: the host–pathogen encounter in blood vessels. Nat Rev Immunol. 2009;9(5):364–75.
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89.
Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002;366(3):689–704.
Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5(14):1317–27.
Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78(5):1025–42.
Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–70.
Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–73.
Galkina E, Kondratenko I, Bologov A. Mycobacterial Infections in Primary Immunodeficiency Patients. New York: Springer New York; 2007.
Andrews T, Sullivan KE. Infections in patients with inherited defects in phagocytic function. Clin Microbiol Rev. 2003;16(4):597–621.
Loukides S, Bouros D, Papatheodorou G, Lachanis S, Panagou P, Siafakas NM. Exhaled H2O2 in steady-state bronchiectasis: relationship with cellular composition in induced sputum, spirometry, and extent and severity of disease. Chest. 2002;121(1):81–7.
Silva MT, Silva MN, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989;6(5):369–80.
Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012;33(1):14–25.
Ong CW, Elkington PT, Brilha S, Ugarte-Gil C, Tome-Esteban MT, Tezera LB, et al. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog. 2015. https://doi.org/10.1371/journal.ppat.1004917.
Jones GS, Amirault HJ, Andersen BR. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990;162(3):700–4.
Majeed M, Perskvist N, Ernst JD, Orselius K, Stendahl O. Roles of calcium and annexins in phagocytosis and elimination of an attenuated strain ofMycobacterium tuberculosisin human neutrophils. Microb Pathog. 1998;24(5):309–20.
Miralda I, Klaes CK, Graham JE, Uriarte SM. Human neutrophil granule exocytosis in response to Mycobacterium smegmatis. Pathogens. 2020;9(2):123.
Lenhart-Pendergrass P, Malcolm K, Wheeler E, Rysavy N, Poch K, Caceres S, et al. Opsonization promotes efficient Mycobacterium avium killing by human neutrophils. D6 D006 CLINICAL AND TRANSLATIONAL ADVANCES IN TB AND NTM: American Thoracic Society; 2021. p. A1193-A.
Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P, et al. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)-and TLR4-deficient animals. J Immunol. 2003;171(9):4758–64.
Petrofsky M, Bermudez LE. Neutrophils fromMycobacterium avium-infected mice produce TNF-α, IL-12, and IL-1β and have a putative role in early host response. Clin Immunol. 1999;91(3):354–8.
Kondratieva E, Logunova N, Majorov K, Averbakh M Jr, Apt A. Host genetics in granuloma formation: human-like lung pathology in mice with reciprocal genetic susceptibility to M. tuberculosis and M. avium. PloS one. 2010. https://doi.org/10.1371/journal.pone.0010515.
Fäldt J, Dahlgren C, Ridell M. Difference in neutrophil cytokine production induced by pathogenic and non-pathogenic mycobacteria. APMIS. 2002;110(9):593–600.
Appelberg R, Castro AG, Gomes S, Pedrosa J, Silva MT. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995;63(9):3381–7.
Goncalves AS, Appelberg R. The involvement of the chemokine receptor CXCR2 in neutrophil recruitment in LPS-induced inflammation and in Mycobacterium avium infection. Scand J Immunol. 2002;55(6):585–91.
Bermudez L, Petrofsky M, Stevens P. Treatment with recombinant granulocyte colony-stimulating factor (FilgrastinTM) stimulates neutrophils and tissue macrophages and induces an effective non-specific response against Mycobacterium avium in mice. Immunology. 1998;94(3):297–303.
Caverly LJ, Caceres SM, Fratelli C, Happoldt C, Kidwell KM, Malcolm KC, et al. Mycobacterium abscessus morphotype comparison in a murine model. PLoS One. 2015. https://doi.org/10.1371/journal.pone.0117657.
Brown AE, Holzer TJ, Andersen BR. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156(6):985–9.
Saunders BM, Cheers C. Intranasal infection of beige mice with Mycobacterium avium complex: role of neutrophils and natural killer cells. Infect Immun. 1996;64(10):4236–41.
Cowman SA, Jacob J, Hansell DM, Kelleher P, Wilson R, Cookson WO, et al. Whole-blood gene expression in pulmonary nontuberculous mycobacterial infection. Am J Respir Cell Mol Biol. 2018;58(4):510–8.
Chen C-Y, Sheng W-H, Lai C-C, Liao C-H, Huang Y-T, Tsay W, et al. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31(6):1059–66.
Fu L. The potential of human neutrophil peptides in tuberculosis therapy. Int J Tuberc Lung Dis. 2003;7(11):1027–32.
Miyakawa Y, Ratnakar P, Rao AG, Costello ML, Mathieu-Costello O, Lehrer RI, et al. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect Immun. 1996;64(3):926–32.
Sharma S, Verma I, Khuller G. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur Respir J. 2000;16(1):112–7.
Kalita A, Verma I, Khuller G. Role of human neutrophil peptide-1 as a possible adjunct to antituberculosis chemotherapy. J Infect Dis. 2004;190(8):1476–80.
Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Investig. 2007;117(7):1988–94.
Voglis S, Quinn K, Tullis E, Liu M, Henriques M, Zubrinich C, et al. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am J Respir Crit Care Med. 2009;180(2):159–66.
Schäfer J, Griese M, Chandrasekaran R, Chotirmall SH, Hartl D. Pathogenesis, imaging and clinical characteristics of CF and non-CF bronchiectasis. BMC Pulm Med. 2018;18(1):1–11.
Newman GW, Guarnaccia JR, Remold HG, Kazanjian PH Jr. Cytokines enhance neutrophils from human immunodeficiency virus-negative donors and AIDS patients to inhibit the growth of Mycobacterium avium in vitro. J Infect Dis. 1997;175(4):891–900.
Hartmann P, Becker R, Franzen C, Schell-Frederick E, Römer J, Jacobs M, et al. Phagocytosis and killing of Mycobacterium avium complex by human neutrophils. J Leukoc Biol. 2001;69(3):397–404.
Nibbering PH, Pos O, Stevenhagen A, Van Furth R. Interleukin-8 enhances nonoxidative intracellular killing of Mycobacterium fortuitum by human granulocytes. Infect Immun. 1993;61(8):3111–6.
Corleis B, Korbel D, Wilson R, Bylund J, Chee R, Schaible UE. Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell Microbiol. 2012;14(7):1109–21.
Malcolm KC, Caceres SM, Pohl K, Poch KR, Bernut A, Kremer L, et al. Neutrophil killing of Mycobacterium abscessus by intra-and extracellular mechanisms. PloS one. 2018. https://doi.org/10.1371/journal.pone.0196120.
Leliefeld PH, Koenderman L, Pillay J. How neutrophils shape adaptive immune responses. Front Immunol. 2015;6:471.
Lindestam CA, Lewinsohn D, Sette A. Antigens for CD4 and CD8 T cells in tuberculosis. Cold Spring Harbor Perspect Med. 2014;4(7):a018465-a.
Serbina NV, Flynn JL. CD8+ T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect Immun. 2001;69(7):4320–8.
Shu C-C, Wu M-F, Pan S-W, Wu T-S, Lai H-C, Lin M-C. Host immune response against environmental nontuberculous mycobacteria and the risk populations of nontuberculous mycobacterial lung disease. J Formos Med Assoc. 2020. https://doi.org/10.1016/j.jfma.2020.05.001.
Bhanothu V. An outline on the aetiopathological association of genetic polymorphisms in women with female genital tuberculosis. Fertil Sci Res. 2016;3(2):66.
Rosain J, Kong XF, Martinez-Barricarte R, Oleaga-Quintas C, Ramirez-Alejo N, Markle J, et al. Mendelian susceptibility to mycobacterial disease: 2014–2018 update. Immunol Cell Biol. 2019;97(4):360–7.
Al-Herz W, Bousfiha A, Casanova J-L, Chatila T, Conley ME, Cunningham-Rundles C, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2014;5:162.
Lake MA, Ambrose LR, Lipman MC, Lowe DM. ‘” Why me, why now?” Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med. 2016;14(1):1–13.
Ratnatunga CN, Lutzky VP, Kupz A, Doolan DL, Reid DW, Field M, et al. The rise of non-tuberculosis mycobacterial lung disease. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.00303.
Wu U-I, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15(8):968–80.
Shamaei M, Mirsaeidi M. Nontuberculous mycobacteria, macrophages, and host innate immune response. Infect Immun. 2021;89(8):e00812-e820.
Matsuyama M, Ishii Y, Yageta Y, Ohtsuka S, Ano S, Matsuno Y, et al. Role of Th1/Th17 balance regulated by T-bet in a mouse model of Mycobacterium avium complex disease. J Immunol. 2014;192(4):1707–17.
Wang Y-H, Liu Y-J. The IL-17 cytokine family and their role in allergic inflammation. Curr Opin Immunol. 2008;20(6):697–702.
Yamazaki Y, Kubo K, Takamizawa A, Yamamoto H, Honda T, Sone S. Markers indicating deterioration of pulmonary Mycobacterium avium-intracellulare infection. Am J Respir Crit Care Med. 1999;160(6):1851–5.
Lowe DM, Demaret J, Bangani N, Nakiwala JK, Goliath R, Wilkinson KA, et al. Differential effect of viable versus necrotic neutrophils on Mycobacterium tuberculosis growth and cytokine induction in whole blood. Front Immunol. 2018;9:903.
Pohl K, Grimm XA, Caceres SM, Poch KR, Rysavy N, Saavedra M, et al. Mycobacterium abscessus clearance by neutrophils is independent of autophagy. Infect Immun. 2020;88(8):e00024-e120.
Rotondo R, Bertolotto M, Barisione G, Astigiano S, Mandruzzato S, Ottonello L, et al. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011;89(5):721–7.
Hilda JN, Das S, Tripathy SP, Hanna LE. Role of neutrophils in tuberculosis: a bird’s eye view. Innate Immun. 2020;26(4):240.
Li Y, Wang W, Yang F, Xu Y, Feng C, Zhao Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signal. 2019;17(1):147.
Bank U, Ansorge S. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity control. J Leukoc Biol. 2001;69(2):197–206.
Bank U, Reinhold D, Schneemilch C, Kunz D, Synowitz H-J, Ansorge S. Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. J Interferon Cytokine Res. 1999;19(11):1277–87.
Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care. 2016;20(1):1–9.
Clayton AR, Prue RL, Harper L, Drayson MT, Savage CO. Dendritic cell uptake of human apoptotic and necrotic neutrophils inhibits CD40, CD80, and CD86 expression and reduces allogeneic T cell responses: relevance to systemic vasculitis. Arthritis Rheum. 2003;48(8):2362–74.
Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188(7):3150–9.
Trentini MM, de Oliveira FM, Kipnis A, Junqueira-Kipnis AP. The role of neutrophils in the induction of specific Th1 and Th17 during vaccination against tuberculosis. Front Microbiol. 2016;7:898.
Perobelli S, Galvani R, Gonçalves-Silva T, Xavier C, Nóbrega A, Bonomo A. Plasticity of neutrophils reveals modulatory capacity. Braz J Med Biol Res. 2015;48(8):665–75.
Kunnath-Velayudhan S, Gennaro ML. Immunodiagnosis of tuberculosis: a dynamic view of biomarker discovery. Clin Microbiol Rev. 2011;24(4):792–805.
Hamasur B, Haile M, Pawlowski A, Schröder U, Källenius G, Svenson S. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F (ab′) 2 fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138(1):30–8.
Zimmermann N, Thormann V, Hu B, Köhler AB, Imai-Matsushima A, Locht C, et al. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol Med. 2016;8(11):1325–39.
Schorey JS, Sweet L. The mycobacterial glycopeptidolipids: structure, function, and their role in pathogenesis. Glycobiology. 2008;18(11):832–41.
Maekura R, Okuda Y, Hirotani A, Kitada S, Hiraga T, Yoshimura K, et al. Clinical and prognostic importance of serotyping Mycobacterium avium-Mycobacterium intracellulare complex isolates in human immunodeficiency virus-negative patients. J Clin Microbiol. 2005;43(7):3150–8.
Kitada S, Kobayashi K, Ichiyama S, Takakura S, Sakatani M, Suzuki K, et al. Serodiagnosis of Mycobacterium avium–complex pulmonary disease using an enzyme immunoassay kit. Am J Respir Crit Care Med. 2008;177(7):793–7.
Le Moigne V, Roux A-L, Mahoudo H, Christien G, Ferroni A, Dumitrescu O, et al. IgA serological response for the diagnosis of mycobacterium abscessus infections in patients with cystic fibrosis. Microbiol Spectr. 2022. https://doi.org/10.1128/spectrum.00192-22.
Glatman-Freedman A. Advances in antibody-mediated immunity against Mycobacterium tuberculosis: implications for a novel vaccine strategy. FEMS Immunol Med Microbiol. 2003;39(1):9–16.
Chambers M, Whelan A, Lloyd K, Jahans K, Glatman-Freedman A, Hewinson R, editors. A monoclonal antibody to MPB83 protects mice from lethal infection with Mycobacterium bovis. Third International Conference on Mycobacterium bovis, St John’s College, Cambridge, August; 2000.
Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci. 1998;95(26):15688–93.
Glatman-Freedman A, Mednick AJ, Lendvai N, Casadevall A. Clearance and organ distribution ofmycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect Immun. 2000;68(1):335–41.
Chan SC, Shum DK, Ip MS. Sputum sol neutrophil elastase activity in bronchiectasis: differential modulation by syndecan-1. Am J Respir Crit Care Med. 2003;168(2):192–8.
Barry S, Breen R, Lipman M, Johnson M, Janossy G. Impaired antigen-specific CD4+ T lymphocyte responses in cavitary tuberculosis. Tuberculosis. 2009;89(1):48–53.
Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee J-H, Zhang Y, et al. Nontuberculous mycobacteria: I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):828–34.
Roux A-L, Catherinot E, Ripoll F, Soismier N, Macheras E, Ravilly S, et al. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol. 2009;47(12):4124–8.
Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann J-L, Nick JA, et al. US cystic fibrosis foundation and european cystic fibrosis society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71(Suppl 1):i1–22.
Lee M-R, Sheng W-H, Hung C-C, Yu C-J, Lee L-N, Hsueh P-R. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015;21(9):1638.
Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region: report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Investig Dermatol. 1953;20(2):133–69.
Van Ingen J, Bendien SA, De Lange WC, Hoefsloot W, Dekhuijzen PR, Boeree MJ, et al. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region. The Netherlands Thorax. 2009;64(6):502–6.
Koh W-J, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006;129(2):341–8.
Orme IM, Ordway DJ. Host response to nontuberculous mycobacterial infections of current clinical importance. Infect Immun. 2014;82(9):3516–22.
Bethencourt Mirabal A, Ferrer G. Lung Nontuberculous Mycobacterial Infections. 2022 Jan 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 31869064.
Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn JS, Floto RA, et al. British thoracic society guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1–69.
King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis. 2009;4:411.
Cole P. Host-microbe relationships in chronic respiratory infection. Respiration. 1989;55(Suppl. 1):5–8.
Margaroli C, Garratt LW, Horati H, Dittrich AS, Rosenow T, Montgomery ST, et al. Elastase exocytosis by airway neutrophils is associated with early lung damage in children with cystic fibrosis. Am J Respir Crit Care Med. 2019;199(7):873–81.
Law SM, Gray RD. Neutrophil extracellular traps and the dysfunctional innate immune response of cystic fibrosis lung disease: a review. J Inflamm. 2017;14(1):1–8.
Watt A, Brown V, Courtney J, Kelly M, Garske L, Elborn J, et al. Neutrophil apoptosis, proinflammatory mediators and cell counts in bronchiectasis. Thorax. 2004;59(3):231–6.
Wilson C, Jones P, O’leary C, Hansell D, Dowling R, Cole P, et al. Systemic markers of inflammation in stable bronchiectasis. Eur Respir J. 1998;12(4):820–4.
Dente FL, Bilotta M, Bartoli ML, Bacci E, Cianchetti S, Latorre M, et al. Neutrophilic bronchial inflammation correlates with clinical and functional findings in patients with noncystic fibrosis bronchiectasis. Mediat Inflamm. 2015. https://doi.org/10.1155/2015/642503.
Kwak N, Lee JH, Kim H-J, Kim SA, Yim J-J. New-onset nontuberculous mycobacterial pulmonary disease in bronchiectasis: tracking the clinical and radiographic changes. BMC Pulm Med. 2020;20(1):1–7.
Wagner D, van Ingen J, van der Laan R, Obradovic M. Non-tuberculous mycobacterial lung disease in patients with bronchiectasis: perceived risk, severity and guideline adherence in a European physician survey. BMJ Open Respir Res. 2020;7(1):e000498.
Bedi P, Davidson DJ, McHugh BJ, Rossi AG, Hill AT. Blood neutrophils are reprogrammed in bronchiectasis. Am J Respir Crit Care Med. 2018;198(7):880–90.
Chakravarti A, Rusu D, Flamand N, Borgeat P, Poubelle PE. Reprogramming of a subpopulation of human blood neutrophils by prolonged exposure to cytokines. Lab Invest. 2009;89(10):1084–99.
Houston N, Stewart N, Smith DS, Bell SC, Champion AC, Reid DW. Sputum neutrophils in cystic fibrosis patients display a reduced respiratory burst. J Cyst Fibros. 2013;12(4):352–62.
Kaplan J. Neutrophil extracelullar traps (NETs): Double-edged swords of innate immunity 1. Neutrophil. 2013;189(6):2689–95. https://doi.org/10.4049/jimmunol.
Mesa MA, Vasquez G. NETosis. Autoimmune Dis. 2013. https://doi.org/10.1155/2013/651497.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5.
Nakamura K, Nakayama H, Sasaki S, Takahashi K, Iwabuchi K. Mycobacterium avium-intracellulare complex promote release of pro-inflammatory enzymes matrix metalloproteinases by inducing neutrophil extracellular trap formation. Sci Rep. 2022;12(1):1–12.
Moreira-Teixeira L, Stimpson PJ, Stavropoulos E, Hadebe S, Chakravarty P, Ioannou M, et al. Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis. Nat Commun. 2020;11(1):1–18.
Haldule S, Chatterjee M, Goswami RP, Vadsaria I, Gaur P, Kavadichanda C, et al. A systematic review and meta-analysis of Mycobacterial infections in patients with idiopathic inflammatory myopathies. Rheumatology. 2022. https://doi.org/10.1093/rheumatology/keac041.
Keir HR, Shoemark A, Dicker AJ, Perea L, Pollock J, Giam YH, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med. 2021;9(8):873–84.
Mårdh CK, Root J, Uddin M, Stenvall K, Malmgren A, Karabelas K, et al. Targets of neutrophil influx and weaponry: therapeutic opportunities for chronic obstructive airway disease. J Immunol Res. 2017. https://doi.org/10.1155/2017/5273201.
Giam YH, Shoemark A, Chalmers JD. Neutrophil dysfunction in bronchiectasis: an emerging role for immunometabolism. Eur Respir J. 2021;58(2):2003157.
Ndlovu LN, Peetluk L, Moodley S, Nhamoyebonde S, Ngoepe AT, Mazibuko M, et al. Increased neutrophil count and decreased neutrophil CD15 expression correlate with TB disease severity and treatment response irrespective of HIV co-infection. Front Immunol. 2020;11:1872.
Jasper AE, McIver WJ, Sapey E, Walton GM. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Research. 2019. https://doi.org/10.12688/f1000research.18411.1.
Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER, et al. CXCR2 antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Res Critic Care Med. 2015;191(9):1001–11.
Gramegna A, Amati F, Terranova L, Sotgiu G, Tarsia P, Miglietta D, et al. Neutrophil elastase in bronchiectasis. Respir Res. 2017;18(1):1–13.
Virtala R, Ekman AK, Jansson L, Westin U, Cardell L-O. Airway inflammation evaluated in a human nasal lipopolysaccharide challenge model by investigating the effect of a CXCR 2 inhibitor. Clin Exp Allergy. 2012;42(4):590–6.
Stephens L, Eguinoa A, Corey S, Jackson T, Hawkins P. Receptor stimulated accumulation of phosphatidylinositol (3, 4, 5)-trisphosphate by G-protein mediated pathways in human myeloid derived cells. EMBO J. 1993;12(6):2265–73.
Chalmers JD, Haworth CS, Metersky ML, Loebinger MR, Blasi F, Sibila O, et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med. 2020;383(22):2127–37.
Prezzo A, Cavaliere FM, Milito C, Bilotta C, Iacobini M, Quinti I. Intravenous immunoglobulin replacement treatment reduces in vivo elastase secretion in patients with common variable immune disorders. Blood Transfus. 2019;17(2):103.
Nakayama H, Oshima E, Hotta T, Hanafusa K, Nakamura K, Yokoyama N, et al. Identification of anti-lipoarabinomannan antibodies against mannan core and their effects on phagocytosis of mycobacteria by human neutrophils. Tuberculosis. 2022;132:102165.
Kuna P, Jenkins M, O’Brien CD, Fahy WA. AZD9668, a neutrophil elastase inhibitor, plus ongoing budesonide/formoterol in patients with COPD. Respir Med. 2012;106(4):531–9.
von Nussbaum F, Li VMJ, Allerheiligen S, Anlauf S, Bärfacker L, Bechem M, et al. Freezing the bioactive conformation to boost potency: the identification of BAY 85–8501, a selective and potent inhibitor of human neutrophil elastase for pulmonary diseases. ChemMedChem. 2015;10(7):1163–73.
von Nussbaum F, Li VM, Meibom D, Anlauf S, Bechem M, Delbeck M, et al. Potent and selective human neutrophil elastase inhibitors with novel equatorial ring topology: in vivo efficacy of the polar pyrimidopyridazine BAY-8040 in a pulmonary arterial hypertension rat model. ChemMedChem. 2016;11(2):199–206.
Stenvall K, Mo J, Russel M, Gardiner P, Palmer R, Jauhiainen A, et al. Target engagement confirmed in man with a dipeptidyl peptidase 1 inhibitor. Switzerland: European Respiratory Society; 2017.
Dahl R, Titlestad I, Lindqvist A, Wielders P, Wray H, Wang M, et al. Effects of an oral MMP-9 and-12 inhibitor, AZD1236, on biomarkers in moderate/severe COPD: a randomised controlled trial. Pulm Pharmacol Ther. 2012;25(2):169–77.
Cahn A, Hamblin J, Begg M, Wilson R, Dunsire L, Sriskantharajah S, et al. Safety, pharmacokinetics and dose-response characteristics of GSK2269557, an inhaled PI3Kδ inhibitor under development for the treatment of COPD. Pulm Pharmacol Ther. 2017;46:69–77.
Yoo EJ, Ojiaku CA, Sunder K, Panettieri RA Jr. Phosphoinositide 3-kinase in asthma: novel roles and therapeutic approaches. Am J Respir Cell Mol Biol. 2017;56(6):700–7.
Acknowledgements
We would like to acknowledge our patients with non-tuberculous mycobacterial disease who have taught us so much over the years.
Funding
MA is funded by a King Saud University scholarship offered by the Saudi Government.
Author information
Authors and Affiliations
Contributions
MA performed the literature search and drafted the manuscript. ML and DML assisted with writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests..
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alkarni, M., Lipman, M. & Lowe, D.M. The roles of neutrophils in non-tuberculous mycobacterial pulmonary disease. Ann Clin Microbiol Antimicrob 22, 14 (2023). https://doi.org/10.1186/s12941-023-00562-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00562-6