Abstract
Background
Corynebacterium striatum is a microorganism with an excellent capacity for biofilm production and thus has been correlated with nosocomial transmission and invasive infections. However, little is known about the mechanism of biofilm formation of this commensal pathogen. In this study, we aimed to investigate the biofilm formation abilities of multidrug-resistant Corynebacterium striatum clinical isolates and the roles of extracellular proteins, exopolysaccharides and extracellular DNA in mediating more robust biofilm formation by the isolates of C. striatum.
Methods
C. striatum isolates were identified using VITEK-2 ANC card, matrix-assisted laser desorption/ionization-time of flight mass spectrometry and 16S rRNA sequencing. The antibiotic susceptibility test was performed using the broth microdilution method. The distribution of spaDEF genes among C. striatum isolates was detected by polymerase chain reaction, and pulsed-field gel electrophoresis typing was employed to analyze the genotypes of the isolates. Crystal violet staining and scanning electron microscopy techniques were used to detect biofilm production by C. striatum isolates. Biofilm degradation assay was performed to observe the effects of extracellular matrix degradative agents (proteinase K, dispersin B, and DNase I) on C. striatum biofilms.
Results
Twenty-seven C. striatum isolates were enrolled in the study, and the resistance rates were the highest (100%, 27/27) against penicillin and ceftriaxone. Approximately 96.3% (26/27) C. striatum isolates were resistant to at least three different types of antimicrobial agents tested. All isolates were confirmed to be biofilm producers, and 74.07% (20/27) isolates presented moderate to strong biofilm production abilities. P7 genotype (44.4%, 12/27) was identified to as the predominant genotype, and all of isolates belonging to this genotype were multidrug-resistant and had stronger biofilm-forming abilities. Most C. striatum isolates (74.07%, 20/27) carry spaD, spaE, and spaF genes, which encode spa-type pili. However, the correlation between the expression of spa-type genes and the biofilm production abilities of the C. striatum isolates was not found. The biofilms of 80% (8/10), 90% (9/10), and 100% (10/10) C. striatum isolates with moderate to strong biofilm production abilities were significantly eliminated upon the treatment of dispersin B (20 μg/mL), DNase I (20 μg/mL), and proteinase K (20 μg/mL) (p < 0.05), respectively. For the combination groups with two kinds of biofilm-degradative agents, the combination of 20 μg/mL proteinase K/dispersin B showed the strongest biofilm-eliminating effects, when the biofilms of 90% (9/10) C. striatum isolates degraded more than 50%.
Conclusions
The C. striatum isolates that belonged to the predominant genotype showed a multidrug resistance (MDR) phenotype and strong biofilm formation abilities. Extracellular matrix seems to be an essential determinant in mediating biofilm formation of MDR C. striatum, since extracellular matrix degradative agents (proteinase K, dispersin B and DNase I) showed strong biofilm-eliminating effects toward multidrug-resistant C. striatum isolates. The findings of this study highlight new ideas/directions to explore the whole nature of biofilm formation of C. striatum and the function of extracellular matrix in this process.
Similar content being viewed by others
Background
Corynebacterium striatum is a gram-positive, rod-shaped bacterium, opportunistic bacterial pathogen that has caused several types of invasive infections in the last two decades [1]. C. striatum clinical isolates often show a high rate of multidrug resistance [2], and its predominant have stronger biofilm formation ability, which correlates with its widespread transmission within the hospital environment [3, 4]. Unfortunately, little is known about the actual mechanism of biofilm formation of this pathogen.
Some recent reports revealed that production of biofilm was correlated with pathogenicity potential of C. striatum, especially for some kinds of chronic infections, including lower respiratory tract infections, catheter-related infections, and meningitis [5]. C. striatum can produce biofilms on different types of abiotic surfaces, which may contribute to the enhancement of its virulence and invasion ability. For some well-known bacterial pathogens, the expression of bacterial surface structure and production of extracellular matrix were recognized to be two different kinds of crucial mechanisms contributing to biofilm formation. A recent report revealed that spa genes, encoding essential cellular surface structure pili, were highly expressed among C. striatum clinical isolates and were thought to correlate with cellular adhesion and biofilm production of C. striatum [6]. Complex extracellular matrix correlated with biofilm formation in bacterial pathogens and is mainly composed of exopolysaccharides (EPS), proteins and extracellular DNA(eDNA) [7,8,9], and corresponding degradative agents (protein degradative enzymes, polysaccharide poly-N-acetyl glucosamine degradative enzymes and eDNA degradative enzymes) usually showed significant biofilm eradicating effects [8, 9]. To date, no study reported the role of extracellular matrix degradative agents in the biofilm formation process in C. striatum.
Hence, this study aimed to explore the biofilm formation abilities of C. striatum clinical isolates and the roles of pili or anti-biofilm activities of extracellular matrix degradative agents in the regulating biofilm formation.
Methods
Identification of C. striatum strains
C. striatum strains were isolated from the patients admitted to the Affiliated Hospital of Inner Mongolia Medical University from January 2012 to July 2021. All isolates were identified by VITEK-2 ANC card (BioMérieux, France) and were frozen at − 80℃. The isolates were sub-cultured on blood agar plates, incubated under 35 ℃ for 24 h, and validated by MALDI-TOF MS equipment (micro type MS, Tianrui, China) and 16S rRNA sequencing technology. Epidemiological data for the patients infected with C. striatum isolates were collected from the hospital information system.
Antimicrobial susceptibility test
The antibiotic susceptibility test was performed using the broth microdilution method, and the antibiotics tested include penicillin (0.06–4 μg/mL),cefatriaxone (1–64 μg/mL),
cefepime (1–4 μg/mL), meropenem (0.25–16 μg/mL), gentamicin (4–16 μg/mL), tetracycline (4–16 μg/mL), erythromycin (0.25–8 μg/mL), vancomycin (0.5–4 μg/mL), trimethoprim-sulfamethoxazole (0.5/9.5–4/76 μg/mL), ciprofloxacin (1–4 μg/mL), levofloxacin (2–8 μg/mL), clindamycin (0.25–4 μg/mL), daptomycin (0.5–1 μg/mL), and linezolid (1–2 μg/mL). The antimicrobial susceptibility test and data analysis were performed according to the Clinical and Laboratory Standards Institute guidelines (M45 A2 edition) [10] and the recommendation of the European Committee on Antimicrobial Susceptibility Testing [11].
Detection of spa genes
C. striatum isolates were inoculated on the blood agar medium at 35 ℃ for 24 h, and the colonies were picked out and transferred into a 1.5 mL Eppendorf tube with 0.5 mL sterile distilled water. Lysozyme (1 mg/ml) was added into each tube and mixed thoroughly by vortexing, and the tubes were placed into a water bath and incubated at 35 ℃ for 30 min. The total bacterial DNA was extracted according to the instructions of the bacterial genome DNA extraction kit (Beijing Tiangen Company, China). The information on the primers for spaD, spaE, and spaF used in this study is shown in Table 1. The PCR conditions were set as follows: 98 ℃ at 30 s for initial denaturation; 35 cycles including 98 ℃ for 5 s, 56 ℃ for 10 s, and 72 ℃ for 10 s and a final extension at 72 ℃ for 5 min. The primers and PCR conditions were designed in this study, and related C. striatum sequences were used as references (Genbank no., CAACYF010000001.1, UFXV01000002.1, and CTEG01000009.1).
Pulse-field gel electrophoresis (PFGE)
The optical density of the bacterial cultures of the C. striatum isolates tested in this study was adjusted to 3.5–4.0 Mcf and digested with lysostaphin (1 mg/mL) (Merck, USA) at 37 °C for 30 min. The bacterial chromosomal DNA of the isolates was extracted and cleaved using 40 U SwaI (Takara, China). The DNA of the S. braenderup H9812 standard strain was extracted and cleaved using 40 U XbaI (Takara, China) and used as the molecular mass standard. Electrophoresis was performed on the CHEF-Mapper XA PFGE system (Bio-Rad, Hercules, CA, USA), and PFGE profiles were analyzed using the Bionumerics v.7.6 software. The isolates with 100% similarity were considered indistinguishable [1, 12], and each genotype was named using a single capital letter.
Detection of biofilm production
Crystal violet staining
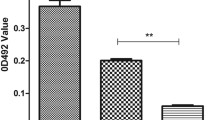
For C. striatum isolates tested in this study, the bacterial suspensions with turbidity equivalent to 0.5 Mcf were prepared and diluted 100 folds with TSB, and 200 μL of the diluted bacterial suspension was added into the wells in a 96-well polystyrene plate, and incubated at 35℃ for 24 h. The TSB with non-adherent bacteria was removed, and the wells were washed by adding 200 μL PBS. PBS was removed, and the plate was placed at room temperature for 10–20 min to dry. Methanol (200 μl) was added to each well and placed at room temperature for 20 min to dry, and 200 μl of crystal violet solution was added to each well and placed at room temperature for 5–10 min. Then, the plate was gently washed for three times with 200 μL PBS and was placed at room temperature until it was completely dry. Biofilm production was quantified by adding 160 μL glacial acetic acid to destain each well, and the solution was gently mixed for 10 min. The absorbance was measured at 620 nm to quantify the crystal violet present in the destaining solution. Each assay was performed in triplicate. Referring to Brahma U et al. [13], a cut-off value of ODc was set as the mean value of the blank control. The judgment standard was set as follows, OD ≤ ODc, indicating without biofilm formation ability; ODc < OD ≤ 2ODc, indicating weak biofilm formation ability; 2ODc < OD ≤ 4ODc, indicating moderate biofilm formation ability; OD > 4ODc, indicates strong biofilm formation ability.
Scanning electron microscopy (SEM)
The C. striatum strains with strong and weak biofilm production in the logarithmic growth period were transferred to a 6-well plate containing TSB liquid medium (the initial amount of bacteria in the well was 107 CFU). A sterile plastic coverslip (Thermanox™) was put into each well to collect the biofilm and incubate it aerobically at 35 ℃ for 24 h. After removing the culture medium, the coverslip was fixed in 0.1 M cacodylate sodium buffer (pH 7.2) with 2.5% glutaraldehyde at room temperature for 30 min, and in the same buffer with 1%OsO4 solution containing 2.5 mM CaCl2 at room temperature for 30 min. The biofilm was dehydrated in ascending acetone series and dried. Subsequently, the samples were observed with scanning electron microscope after drying [14].
Biofilm degradation assay
The degrading activities of three kinds of well-known extracellular matrix degradative agents on the biofilm of C. striatum were investigated in this study, including protein degrading enzyme (proteinase K), polysaccharide poly-N-acetylglucosamine (PNAG): degrading enzyme (dispersin B), and eDNA degrading enzyme (DNase I). The assays were performed according to the protocols recommended previously [9, 15]. Proteinase K (RT403, 20 mg/mL) was purchased from TIANGEN Biotech (Beijing) Co., Ltd. β-N-Acetylglucosaminidase (S10237, 69.3 U/mL) was purchased from Shanghai yuanye Bio-Technology Co., Ltd, and DNase I (D8071, 5 mg/ml) from Beijing Solarbio Science & Technology Co., Ltd. Biofilm formation assay was performed as above mentioned. After 24 h of incubation, the biofilm was washed with 200 μL PBS three times. Then, 200 μL of the degrading agent was carefully added to the wells with biofilm and incubated at 37 ℃ for 2 h. The wells were washed with PBS for three times and the biofilm was stained with crystal violet. The following groups were designed to observe the biofilm degrading effects of different kinds of the agents, including control group (without degrading agents), single degrading agent groups (20 μg/mL proteinase K, 20 μg/mL dispersin B, and 20 μg/mL DNase I), combination groups of degrading agents (20 μg/mL proteinase K, and 20 μg/mL dispersin B; 20 μg/mL proteinase K, and DNase I; 20 μg/mL dispersin B, and DNase I). All the tests were performed in triplicate. The data were analyzed with an independent-samples t-test using IBM SPSS Statistics 23.0 software, and p < 0.05 was deemed statistically significant.
Results
Epidemiological data showed that the average age of twenty-seven patients infected with C. striatum was 54.63-years-old, and the male/female ratio was 22:5. The average time from admission to the isolation of C. striatum isolates was 24.04 days. Around 96.30% (26/27) of the patients received antimicrobial treatment two weeks before C. striatum isolation. The most frequently prescribed antimicrobials are β-lactam antibiotic/β-lactamase inhibitor combinations (66.67%, 18/27), followed by fluoroquinolones (44.44%, 12/27), carbapenems, (44.44%, 12/27) and cephalosporins (14.81%, 4/27). Two patients died during hospitalization, and the remaining patients were discharged after recovery. The detailed epidemiological data are summarized in Additional file 1: Table S1.
As shown in Table 2, twenty-seven clinical C. striatum isolates were all sensitive to vancomycin, linezolid, daptomycin, and the resistance rate to gentamicin was lower (7.41%). However, the resistance rates to penicillin, cefepime, meropenem, tetracycline and erythromycin were high (100%, 92.59%, 85.19%, 81.48%, and 88.89%), while the resistance rates to penicillin and ceftriaxone were the highest, reaching up to 100% (27/27). Around 96.3% (26/27) of C. striatum isolates were deemed multidrug-resistant (MDR) in at least three different kinds of the antimicrobials tested in this study. CS-14 isolate was the only non-MDR isolate resistant to penicillin and ceftriaxone.
The PCR results revealed that the positive rates of spaD, spaE and spaF genes were 81.48% (22/27), 85.19% (23/27), and 85.19% (23/27), respectively. Around 74.07% (20/27) of the C. striatum isolates carried spaD, spaE and spaF genes concurrently, while 7.41% (2/27) of C. striatum isolates did not carry any of spaD, spaE or spaF genes. Around 11.11% (3/27) of the isolates were positive with spaD and spaF genes, but the spaE gene was not detected. In addition, 7.41% (2/27) of the C. striatum isolates only carried the spaD gene.
Based on the discrimination standard for PFGE genotyping proposed by Tenover et al.[12], twenty-seven C. striatum isolates were divided into eleven genotypes (P1 ~ P11), the P7 is the predominant genotype and accounted for 44.44% (12/27), as shown in Fig. 1. The P7 genotype contained twelve strains of C. striatum isolated from 31st, 2020 to 11th, 2021. Half of the samples were isolated from the intensive care unit (ICU), and 50% (6/12) of the specimens were isolated from qualified sputum samples. Six isolates were isolated from the cerebrospinal fluid (16.67%, 2/12), pus (16.67%, 2/12), and whole blood (16.67%, 2/12), respectively. In addition, most of the strains belonging to the P7 genotype presented stronger biofilm formation abilities and were all multidrug-resistant (MDR). Even worse, four isolates belonging to the P7 genotype were sequentially isolated from the patients admitted to ICU between March 31st and April 21st, 2020, indicating a potential nosocomial outbreak. Also, the isolates belonging to another genotype (P4) with weak biofilm formation ability accounted for 14.8% (4/27), three of which were collectively isolated from the patients admitted to ICU from February 8th to May 5th, 2020, which indicated a possible nosocomial transmission.
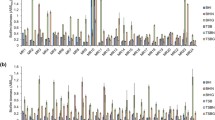
As shown in Fig. 2, twenty-seven C. striatum strains were all biofilm producers but the biofilm production abilities differed significantly. Eight isolates were potent biofilm producers (29.63%, 8/27), twelve were medium biofilm producers (44.44%, 12/27), while the remaining seven isolates showed weak biofilm production abilities (25.93%, 7/27). Most isolates (51.85%, 14/27) were isolated from the lower respiratory tract samples and all the strong biofilm producers belonged to the P7 genotype. The SEM results confirmed the presence of mature and high-density of biofilm in the strong biofilm producers (Fig. 3), compared with those with weak biofilm production abilities.
Morphological features of C. striatum strains strong and weak biofilm formation abilities. A, D. C. striatum isolate with strong biofilm formation ability; B, E. C. striatum isolate with medium biofilm forming ability. C, F. C. striatum isolate with weak biofilm forming ability. Black arrow (→) indicates the filaments produced by the C. striatum isolate with strong biofilm formation ability
As shown in Fig. 4, the biofilm of 80% (8/10), 90% (9/10), and 100% (10/10) C. striatum isolates were significantly eliminated upon the treatment with 20 μg/mL dispersin B, DNase I, and proteinase K (p < 0.05), respectively. The degradation efficiency of the above three agents differed significantly, when proteinase K showed the most potent degrading effects, followed by dispersin B and DNase I (Fig. 4). The degrading percentages of the biofilm of 50% (5/10) isolates (CS-20, CS-259, CS-2, CS-11, and CS-5) exceeded 50% when treated with proteinase K, while only 20% (2/10) and 10% (1/10) for dispersin B and DNase I groups. For combination groups, the combination of proteinase K/dispersin B showed the most potent biofilm-eliminating effects, followed by the combination of proteinase K/eDNA and dispersin B/DNase I (Fig. 5 and Additional file 2: Table S2). The degrading percentages of the biofilm of 90% (9/10) isolates exceeded 50% when treated with combination of proteinase K/dispersin B, followed by combination of proteinase K/DNase I (80%,8/10) and dispersin B/DNase I (40%, 4/10). However, the degrading percentage of the biofilm of CS-51 isolate did not exceed 50% when treated with any combinations of the degrading agents.
Discussion
In this study, almost all of the C. striatum strains investigated in this study were multidrug -resistant. They were only sensitive to vancomycin, linezolid and daptomycin, consistent with some previous global studies [5, 16, 17]. C. striatum isolates were mainly isolated from lower respiratory tract specimens (51.85%, 14/27). A dominant genotype, P7, was identified during this study, triggering a nosocomial outbreak among ICU patients between March and April, 2020. Furthermore, all of the isolates belonging to P7 genotype were confirmed to be medium or strong biofilm producers and multidrug-resistant. Rapid and accurate discrimination of predominant C. striatum clones may help clinicians implement more potent measures to control its nosocomial transmission or treat of infections caused by C. striatum.
Some previous studies revealed that MDR C. striatum could produce mature biofilms on several kinds of surfaces, such as medical catheters [3], leading to chronic infections. Even worse, a recent report revealed significant resistance of some dominant C. striatum clones to some widely used biocides (such as glutaraldehyde), especially for biofilm-associated forms of C. striatum [18]. Exploration of new kinds of anti-biofilm agents for biofilm-producing C. striatum seems to be urgent. In this study, all C. striatum isolates were confirmed to be biofilm producers, and 74.1% (20/27) isolates were confirmed to have medium and strong biofilm producing abilities. Also, the isolates belonging to the same PFGE type or closely similar types presented identical or similar biofilm formation abilities. However, the consistency between resistance features and clonality of the isolates was not observed, which is inconsistent with another previous study [4].
Biofilm formation ability is essential for some bacterial pathogens [19]. Cellular surface components (such as pili or fimbriae [6]) and extracellular matrix [7] are two critical determinants in some well-known pathogens. Fouzia Nasim et al. [20] and Sana Alibi et al. [6] demonstrated that the adhesion ability in Corynebacterium spp. was generally mediated by fimbriae, which is usually encoded by three separate fimbriae gene clusters, spaDEF gene. Also, C. striatum fimbriae can also mediate its specific adhesion to host cells. Sana Alibi et al. revealed that Spa-type genes were detected in all C. striatum strains. However, not all the C. striatum strains (74.07%, 20/27) carried spaD, spaE, and spaF genes in this study. In addition, eight C. striatum strains with strong biofilm-forming ability carried spaDEF. At the same time, five C. striatum strains with weak biofilm-forming ability were also positive with spaDEF genes, which indicates an indefinite relationship between biofilm formation abilities and expression of pili in C. striatum. For some well-known gram-positive bacteria (including S. aureus, and Bacillus subtilis), the bacterial extracellular matrix has been recognized to be an essential determinant in mediating biofilm formation and participating in the protection process from external insults [21, 22]. The extracellular matrix composition was similar among different bacteria, including EPA, proteins, and eDNA, all of which participated in the process of biofilm formation in different manners [21, 23,24,25]. Correspondingly, degradative agents directed against the above components were developed to deter biofilm formation, including dispersin B, proteinase K, and DNase I. Biofilm matrix degradation with enzymatic degradative agents toward polysaccharides, proteins, and nucleic acids paves an efficient way of controlling or treating infections caused by biofilm-producing bacterial pathogens [26,27,28]. A previous report revealed that adding exogenous DNA would promote biofilm formation in Corynebacterium glutamicum, which suggests a nucleic acids’ potential role in regulating biofilm formation in Corynebacterium spp. [29]. Nevertheless, to the best of our knowledge, the actual composition of the extracellular matrix of C. striatum and the potential roles of biofilm matrix degradative agents was not reported yet.
In this study, biofilm-degradative agents (dispersin B, DNase I, and proteinase K) were employed in C. striatum biofilm degrading assays, all of which were commonly used in the assays for some other well-known bacterial pathogens, such as S. aureus [9, 15]. Interestingly, significant degradative effects of the above biofilm-degradative agents were observed. Overall, proteinase K showed the most potent biofilm degradative effects. The most effective biofilm-eliminating effect was observed in proteinase K and dispersin B or DNase I groups. Meanwhile, significant diversity of biofilm-eliminating effects of biofilm matrix degradative agents on C. striatum biofilm was observed during this study, which suggests a significant strain or clonal heterogenicity of the composition of extracellular matrix in C. striatum. Specific constituents of the extracellular matrix in determining the biofilm formation capability of C. striatum and possible molecular mechanisms need to be further investigated, especially for extracellular proteins, which may be potent anti-biofilm targets.
Conclusions
Multidrug-resistant C. striatum isolates accounted for the most of the isolates investigated in this study. The predominant clone identified in this study presented a more potent biofilm formation ability, and a nosocomial outbreak was observed. Also, this study firstly revealed the potential roles of the extracellular matrix in mediating C. striatum biofilm formation, since significant biofilm-eliminating effects of extracellular matrix degradative agents toward C. striatum were observed, especially for protein degradative agents. Further exploration of the extracellular matrix composition of C. striatum and a deep understanding of the molecular mechanism of extracellular matrix in mediating the biofilm formation of C. striatum will pave a new way for us to control and treat of colonization or infections caused by biofilm-producing C. striatum strains.
Availability of data and materials
None.
Abbreviations
- PFGE:
-
Pulsed-field gel electrophoresis
- PCR:
-
Polymerase chain reaction
- MIC:
-
Minimum inhibitory concentration
- ICU:
-
Intensive care unit
- MDR:
-
Multi-drug resistant
- SEM:
-
Scanning electron microscope
- EPS:
-
Exopolysaccharides
- eDNA:
-
Extracellular DNA
References
Alibi S, Ferjani A, Boukadida J, Cano ME, Fernández-Martínez M, Martínez-Martínez L, et al. Occurrence of Corynebacterium striatum as an emerging antibiotic-resistant nosocomial pathogen in a Tunisian hospital. Sci Rep. 2017;7(1):9704.
Asgin N, Otlu B. Antimicrobial resistance and molecular epidemiology of Corynebacterium striatum isolated in a Tertiary Hospital in Turkey. Pathogens. 2020;9(2):136.
Ramos JN, Souza C, Faria YV, da Silva EC, Veras JFC, Baio PVP, et al. Bloodstream and catheter-related infections due to different clones of multidrug-resistant and biofilm producer Corynebacterium striatum. BMC Infect Dis. 2019;19(1):672.
Souza Cd, Faria YV, Sant’Anna Lde O, Viana VG, Seabra SH, Souza MC, et al. Biofilm production by multiresistant Corynebacterium striatum associated with nosocomial outbreak. Mem Inst Oswaldo Cruz. 2015;110(2):242–8.
Milosavljevic MN, Milosavljevic JZ, Kocovic AG, Stefanovic SM, Jankovic SM, Djesevic M, Milentijevic MN. Antimicrobial treatment of Corynebacterium striatum invasive infections: a systematic review. Rev Inst Med Trop Sao Paulo. 2021;63: e49.
Alibi S, Ramos-Vivas J, Ben Selma W, Ben Mansour H, Boukadida J, Navas J. Virulence of clinically relevant multidrug resistant Corynebacterium striatum strains and their ability to adhere to human epithelial cells and inert surfaces. Microb Pathog. 2021;155: 104887.
Limoli DH, Jones CJ, Wozniak DJ. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr. 2015. https://doi.org/10.1128/microbiolspec.MB-0011-2014.
Sharma K, Pagedar SA. Antibiofilm effect of DNase I against single and mixed species biofilm. Foods. 2018;7(3):42.
Frank KL, Patel R. Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect Immun. 2007;75(10):4728–42.
Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. 2rd ed. CLSI guideline M45. Wayne, PA: CLSI; 2010.
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0. www.eucast.org. valid from 2022-01-01.
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33(9):2233–9.
Brahma U, Sharma P, Murthy S, Sharma S, Chakraborty S, Appalaraju SN, et al. Decreased expression of femXAB genes and fnbp mediated biofilm pathways in OS-MRSA clinical isolates. Sci Rep. 2019;9(1):16028.
Marques VF, Motta CC, Soares BD, Melo DA, Coelho SM, Coelho ID, et al. Biofilm production and beta-lactamic resistance in Brazilian Staphylococcus aureus isolates from bovine mastitis. Braz J Microbiol. 2017;48(1):118–24.
Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74(2):470–6.
Wang J, Wang Y, Du X, Cui J, Wang K, Zhang L, et al. Rapid transmission of multidrug-resistant Corynebacterium striatum among susceptible patients in a tertiary hospital in China. J Infect Dev Ctries. 2016;10(12):1299–305.
Wang Y, Shi X, Zhang J, Wang Y, Lv Y, Du X, et al. Wide spread and diversity of mutation in the gyrA gene of quinolone-resistant Corynebacterium striatum strains isolated from three tertiary hospitals in China. Ann Clin Microbiol Antimicrob. 2021;20(1):71.
Souza C, Mota HF, Faria YV, Cabral FO, Oliveira DR, Sant’Anna LO, et al. Resistance to Antiseptics and Disinfectants of Planktonic and Biofilm-Associated Forms of Corynebacterium striatum. Microb Drug Resist. 2020;26(12):1546–58.
Venkatesan N, Perumal G, Doble M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015;10(11):1743–50.
Nasim F, Dey A, Qureshi IA. Comparative genome analysis of Corynebacterium species: The underestimated pathogens with high virulence potential. Infect Genet Evol. 2021;93: 104928.
Breslawec AP, Wang S, Li C, Poulin MB. Anionic amino acids support hydrolysis of poly-β-(1,6)-N-acetylglucosamine exopolysaccharides by the biofilm dispersing glycosidase Dispersin B. J Biol Chem. 2021;296: 100203.
Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59(4):1229–38.
Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, et al. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178(1):175–83.
Campoccia D, Montanaro L, Arciola CR. Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int J Mol Sci. 2021;22(16):9100.
Svarcova V, Zdenkova K, Sulakova M, Demnerova K, Pazlarova J. Contribution to determination of extracellular DNA origin in the biofilm matrix. J Basic Microbiol. 2021;61(7):652–61.
Fong JNC, Yildiz FH. Biofilm Matrix Proteins. Microbiol Spectr. 2015. https://doi.org/10.1128/microbiolspec.MB-0004-2014.
Erskine E, MacPhee CE, Stanley-Wall NR. Functional amyloid and other protein fibers in the biofilm matrix. J Mol Biol. 2018;430(20):3642–56.
Taglialegna A, Lasa I, Valle J. Amyloid structures as biofilm matrix scaffolds. J Bacteriol. 2016;198(19):2579–88.
Ren P, Chen T, Liu N, Sun W, Hu G, Yu Y, et al. Efficient biofilm-based fermentation strategies by eDNA formation for l-proline production with Corynebacterium glutamicum. ACS Omega. 2020;5(51):33314–22.
Acknowledgements
We thank all Lab Medicine Department staff for assisting with isolates collection, identification and storage.
Funding
This study is supported by Major project of Affiliated hospital of Inner Mongolia Medical University (Grant no.NYFY ZD 012), National Natural Science Foundation of China (Grant no.82260416), and Science and Technology Plan Project of Inner Mongolian Autonomous Region (2022YFSH0072).
Author information
Authors and Affiliations
Contributions
JW is responsible for the biofilm assay and PCR assay. ZW is responsible for antibiotic sensitivity test. XD is responsible for PFGE typing assay. RL is responsible for isolation, identification of C. striatum strains and SEM assay. JW is responsible for the design and review of this study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work is exempt from formal ethical approval and informed consent according to the local ethical guidelines, and was approved by Ethics committee of Affiliated hospital of Inner Mongolian medical university (Reference Number KY2020029).
Consent for publication
No individual person’s data was included in this study, and consent for publication is not required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Epidemiology of twenty-seven patients infected with multi-drug resistant Corynebacterium striatum.
Additional file 2: Table S2.
The results of statistical analysis of the biofilm eliminating effects of combinations of biofilm degrading agents (proteinase K, dispersin B and DNase I).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wen, J., Wang, Z., Du, X. et al. Antibioflm effects of extracellular matrix degradative agents on the biofilm of different strains of multi-drug resistant Corynebacterium striatum. Ann Clin Microbiol Antimicrob 21, 53 (2022). https://doi.org/10.1186/s12941-022-00546-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-022-00546-y