Abstract
Background
Aspergillosis of Central Nervous System (CNS) is a highly lethal infection in patients with leukemia and Stem Cell Transplantation (SCT).
Methods
Case reports of CNS aspergillosis in patients with leukemia and SCT published between 1990 and August 2020 were gathered using a structured search through PubMed/Medline.
Results
Sixty-seven cases were identified over the searches of the PubMed bibliographic database and then, 59 cases were included in the final analysis. Europe had the largest share of cases at 57.6% (34 reports), followed by Americas and Asia. Affected patients were predominantly males (58.6%) and the mean age of the patients was 36.1 years, while 62.7% of the patients were under the age of 50 years. The most common leukemia types include Acute Lymphoblastic Leukemia (ALL), Chronic Lymphocytic Leukemia (CLL), and Acute Myeloid Leukemia (AML) at 43.4%, 27.4%, and 23.5%, respectively. Furthermore, stem cell transplantation was reported in 11 cases. The overall mortality was 33%; however, the attributable mortality rate of CNS aspergillosis was 24.5%. Altered mental status, hemiparesis, cranial nerve palsies, and seizures were the clearest manifestations of infection and lung involvement reported in 57% of the patients. Histopathologic examination led to the diagnosis of infection in 57% of the patients followed by culture (23.7%), galactomannan assay (8.5%), and molecular method (3.3%). Amphotericin B and voriconazole were the most frequently used drugs for infection treatment. Good results were not obtained in one-third of the patients treated by voriconazole. Finally, neurosurgical intervention was used for 23 patients (39%).
Conclusion
CNS aspergillosis is a rapidly progressive infection in leukemic patients. Thus, these patients should be followed up more carefully. Furthermore, management of induction chemotherapy, use of different diagnostic methods, and use of appropriate antifungal can lead to infection control.
Similar content being viewed by others
Introduction
Aspergillus is a branching septate filamentous fungus that can induce invasive, lethal infections in immune-deficient patients. Among the many species that are identified and recognized, Aspergillus fumigatus is by far the most common species Shariati A, Moradabadi A, Chegini Z, Khoshbayan A and Didehdar M [1]. Specifically, Aspergillus species can infect respiratory and gastrointestinal tracts and skin, and in patients with immunodeficiency, other forms of the disease can occur. It is quite rare for the Invasive Central Nervous System (CNS) to become subjected to aspergillosis and this phenomenon constitutes about 10–20% of all invasive aspergillosis cases with poor prognosis and significant mortality [2]. Aspergillus spp. are common in the environment (soil, dust, plants, and decaying vegetable matter) that are inhaled by breathing normal air, and the lungs have the highest chance of exposure to infection [3]. Thus, the portal of entry for Aspergillus usually lies in the respiratory tract and CNS involvement arises as a result of hematogenous spreading from the lung or through direct invasion of the adjacent cranial structure, surgery, contamination of indwelling catheters, and iatrogenic or penetrating trauma [4]. If aspergillosis already invades the paranasal sinuses or palate, it might penetrate the ethmoid sinuses and cribriform plate all the way into the intracranial compartment in which meninges, nerves, lymphatic channels, and blood vessels can become involved [5, 6]. Its extension to the surrounding neural tissues and the vessel wall erosion by hyphal would promote meningitis, hemorrhage, necrosis, vasculitis, and infarction. When Aspergillus wears away the arterial wall and attacks the infarcted brain, the sterile infarct will convert to septic infarct and abscess. Thus, meningitis, cerebral blood vessel invasion with secondary infection or hemorrhage, and single or multiple brain abscesses are the highly prevalent forms of CNS aspergillosis reported in patients [7, 8].
Persistent and profound neutropenia is the most significant risk factor in invasive aspergillosis; thus, this infection predominantly occurs in immune-compromised hosts. In this regard, patients with leukemia, recipients of bone marrow transplant, and patients exposed to allogeneic hematopoietic Stem Cell Transplantation (SCT) have a very high chance of developing invasive CNS aspergillosis [1, 9]. For patients with leukemia, especially Acute Lymphoblastic and Myeloid Leukemia (ALL and AML), by receiving intensive cytotoxic chemotherapy and several previous chemotherapy regimens, immunodepression with hypogammaglobinemia inherent to the primary disease and neutropenia caused by infiltration of bone marrow are at increased risk of CNS aspergillosis [10, 11]. Patients with SCT run the high risk of aspergillosis because they are being treated with immunosuppression including high-dose steroids due to the possible spread of the Graft versus Host Disease (GvHD) [12]. Besides, antifungal prophylaxis is recommended for these patients; however, prophylaxis could turn out to be unsuccessful, even with the first-line choices, in about 3–14% of all the patients exposed to invasive fungal infection [4, 13]. Therefore, patients with hematologic malignancies or SCT due to underlying disorders and immunosuppressive therapies have a very high chance of developing CNS aspergillosis and antifungal prophylaxis may not prevent this infection. In addition, given the poor penetration of antifungal agents across the brain-blood barrier, their low concentration in brain tissue and Cerebro-Spinal Fluid (CSF), and their high toxicity, its mortality rate for these patients is quite high [14]. Since little is known about CNS aspergillosis in patients with leukemia or SCT, this systematic review aims to investigate the reported CNS aspergillosis cases in these patients.
Methods
Literature search and inclusion criteria
This study carried out a Medline search (via PubMed) from January 1, 1990 to August 30, 2020 with the search keywords obtained from the National Library of Medicine’s Medical Subject Heading (MeSH) terms, abstracts, or titles by using Boolean Operators (and, or): “Aspergillus” or “Aspergillosis” and “Leukemia” or “Blood” or “Hematologic” or “Hematological” or “Haematologic” or “Haematological” or “Stem cell transplantation (SCT)” or “Bone marrow transplantation” or “Cytopenia” or “Leukopenia” or “Neutropenia” and “Cerebral” or “Cranial” or “Central Nervous System (CNS)” or “Brain” or “Meningitis”. Article references were reviewed and cross checked at length for any possible additional cases that might have been missed out or overlooked throughout the initial search. It is noteworthy to mention that non-English language studies were excluded. The review protocol used in this study centers on the paper of Hickey et al. and our recent article [15, 16].
Inclusion criteria
All reports of CNS aspergillosis in patients with leukemia or SCT, full-text or abstract-only studies in English, and research works online in Medline (via PubMed) (from 1990 until August, 2020) were eligible for study inclusion and they were carefully screened by both authors (AS and AM).
Exclusion criteria
The exclusion criteria comprised CNS infections with other fungi, review articles (either systematic or meta-analysis), non-human study, guidelines, CNS aspergillosis in patients without leukemia or SCT, non-propagation of infection into the CNS, and inadequate reported data (Fig. 1).
Study selection and data extraction
As mentioned earlier, the two researchers (AS and AM) screened the articles and in case of any discrepancy, both researchers were obligated to scan the paper or conference abstract to ensure its eligibility for the review. Individual case reports were considered so as to collect data about the epidemiology, clinical manifestations, treatment, and diagnosis of CNS aspergillosis in patients with leukemia and SCT. Finally, the following features of each pertinent article were extracted and recorded by using Excel software (Microsoft, Redmond, WA, USA): country, year of publication, age, sex, causative fungal pathogen, leukemia, clinical presentation, treatment, surgery, diagnostic methods, and outcome.
Quality assessment
A critical appraisal checklist was employed for the case reports provided by the Joanna Briggs Institute (JBI) to carry out a quality assessment of the studies [17].
Results
Epidemiology
Sixty-seven cases were detected using searches through the PubMed bibliographic database as case reports. Five additional cases were identified and the above-cited references were screened further. Then, thirteen cases were excluded because the leukemia cases of CNS aspergillosis were not analyzed. Finally, 59 patients with leukemia or SCT and CNS aspergillosis were included in the final analysis based on the study criteria (Fig. 1). These cases of individuals were published from USA (14 reports), France (eight reports), Turkey (seven reports), Japan (six cases), Germany and Italy (four each), United Kingdom (three reports), Australia (two reports), Austria, Belgium, China, Czech, Greece, India, Iran, Netherlands, Portugal, Spain, and Sweden (one each). Thus, Europe had the largest share of cases at 57.6% (34 reports), followed by the continents including Americas, Asia, and Australia at 23.7% (14 reports), 15.3% (9 reports), and 3.4% (2 reports), respectively. No case from Africa was found. Our analysis also showed that 58.6% of the patients were male and the rest were female. The mean age of the patients was 36.1 years (ranged from 1.5–90 years), 62.7% of whom were under the age of 50 years (Table 1). Notably, 38.9% of these cases were 18 or younger.
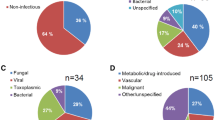
The most common type of leukemia associated with CNS aspergillosis was ALL at 43.4% followed by Chronic Lymphocytic Leukemia (CLL) and AML (two case with Acute promyelocytic leukemia (APML, APL)) at 27.4% and 23.5%, respectively (Fig. 2). Different transplantations were reported in 11 patients (18.6%) that include allogeneic bone marrow and SCT for aplastic anemia in four patients, allogeneic bone marrow transplantation for patient with CML, allogeneic SCT for two patients with multiple myeloma and AML, autologous SCT for breast cancer (two patients) and osteosarcoma and finally, cord blood transplantation for patients with AML (Table 1).
Different leukemia in patient with CNS aspergillosis ((PubMed reported cases until August 2020). CLL Chronic lymphocytic leukemia. ALL Acute lymphoblastic leukemia. AML acute myeloid leukemia. CML Chronic myelogenous leukemia. APL Acute promyelocytic leukemia. T-ALL: acute T-lymphoblastic leukemia. T-LGL: T-cell large granular lymphocytic leukemia.
Our analysis showed that the total mortality rate in this cohort of published leukemia and SCT cases of CNS aspergillosis was 33% (19 cases) (two studies were omitted from final analysis due to the patients’ failure to adhere to proper follow-up). Among the dead patients, 14 cases (24.5%) died of infection, while CNS aspergillosis was controlled in the other five patients and they, instead, died of hematologic disease progression (ALL and AML (each two cases) and CLL). The highest mortality rate was observed for patients with ALL (47.3%), followed by AML (26.4%) and SCT for Aplastic anemia and multiple myeloma (15.7%). Notably, two patients with CLL and CML also died. Among the patients who died, 52.7% were men and the rest were women. The mean age of the dead was 26.2 years (ranging from 1.5–59 years).
Only 29 studies (49%) performed species-level identification and found that A. fumigatus was the most common pathogen isolated from patients with 23 reports. A. felis, A. flavus, A. niger, A. nidulans, and A. terreus were the other pathogens isolated from patients. Noteworthy, A. fumigatus and A. niger were also isolated from the patients with pulmonary and cerebral aspergillosis and the mixed breakthrough invasive fungal infections caused the death of patients (Table 1).
Clinical manifestations
The most common presented manifestations were fever (59.3%), altered mental status (confusion, lethargy or loss of consciousness) (45.7%), headache (23.7%), hemiparesis (17%), facial palsy (8.4%), generalized seizure (10%), ataxia (5%), and focal seizure (6.7%). Imaging modalities showed lung involvement in 57% of the patients, but chest pain was reported only in 8.4% of the cases. In this section, we have divided the duration of onset of clinical symptoms in patients after treatment into three categories: patients undergoing chemotherapy, ibrutinib, and transplant patients. The duration of onset of symptoms in patients who had undergone chemotherapy was determined in 22 cases and the average duration was 27.8 days (mean ± SD = 27.8 ± 16.8 days, ranging from 4 to 80 days). The time of onset of symptoms in patients under ibrutinib therapy was reported in 10 cases with a mean duration of 5.9 months (mean ± SD = 5.8 ± 6 month, ranging from 15 days to 18 month). Finally, in transplant patients, symptoms developed over 4.9 months on average (mean ± SD = 4.8 ± 6.5 month, ranging from 1 week to 22 months) after transplantation. As mentioned, in more than half of the patients, pulmonary infection was also reported and the duration of the onset of CNS aspergillosis symptoms following the detection of lung involvement was reported in 21 cases, which was 18.5 days on average (mean ± SD = 18.5 ± 14.6 days, ranging from 2 days to 2 months). Noteworthy, based on various clinical and laboratory findings, brain lesions (abscess or multiple abscesses), cerebral blood vessel invasion with secondary infection or hemorrhage, and meningitis were the most common CNS disorders caused by Aspergillus species in patients at 86.4%, 18.6%, and 3.3%, respectively.
Diagnoses
The diagnosis was performed by histopathologic examination of different specimens in 23 of 59 (39%) instances. Furthermore, results obtained from culture and histopathologic examination led to the diagnosis of the infection in 11 (18.7%) patients. In three other cases, CNS aspergillosis was diagnosed post-mortem (5%) and culture results confirmed infection in three (5%) other patients. The samples used for diagnosis were obtained by craniotomy, stereotactic and burr hole biopsy, and laminectomy. In light of the formation of abscess capsule, transcranial puncture was not performed on one of the patients [18].
The results of culturing the CNS tissue samples were reported in 19 patients, of whom only three patients (15.7%) were tested negative. On the other hand, the results of CSF culturing were stated in 15 cases and all of them were negative and only one positive case was reported in one patient with thoracic spinal cord intramedullary Aspergillus invasion. Our analysis also showed positive cultures from initial vitrectomy, subcutaneous nodules, and surgical joint biopsy. Finally, it should be noted that all blood cultures were negative.
With respect to laboratory tests, Aspergillus galactomannan (GM) antigen assay was another diagnostic method that detected CNS aspergillosis in five (8.5%) other patients (lower limit for a positive result 0.5 ng/mL). In one of these patients, negative CSF culture and polymerase chain reaction (PCR) disrupted the diagnosis process; however, serum and CSF samples tested positive for Aspergillus GM (more than 5.0), which led to correct identification of the cause of the infection and choice of an appropriate treatment [19]. Aspergillus GM results were stated in 28 cases. In this regard, the most positive results of this test were reported for serum (18 (72%) positive, 7 negative), bronchoalveolar lavage (BAL) (2 (50%) positive, 2 negative), and CSF (7 (43%) positive, 9 negative) samples, respectively. Aspergillus GM index was reported in 12 cases with an average of 3 ng/mL (Additional file 1: Table S3).
Positive results of CSF Aspergillus GM assay and PCR led to the diagnosis of infection in a patient, while Aspergillus was not isolated in the CSF [20]. On the other hand, in another case of a 15-year-old girl undergoing autologous SCT, parents were reluctant to agree to proceeding with brain biopsy and serologic markers of fungal infection were negative. In this situation, fungal DNA was detected in the CSF by panfungal PCR assay using the primers derived from fungal 18S ribosomal RNA (rRNA) genes [21]. Of note, only 11 (18.6%) patients were diagnosed with PCR, and CSF (four (57%) positive, three negative), tissue (two positive), BAL (one positive), and serum (two negative) samples were used for diagnosis (Fig. 3). It is noteworthy to mention that CSF examination (cell count, glucose level, and protein content) was carried out in 19 cases, the result of which was normal in ten patients (52.6%).
Medical imaging modalities including Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) did not yield a definitive diagnosis for any of the patients. However, among the patients with imaging modalities, abscesses appearing as ring-enhancing lesions (post-infarct abscess formation) (57.6%), perifocal edema on MRI scan (marked T2-hyperintense peripheral edema and marked T1-hypointense peripheral edema) (56%), brain hemorrhages (3.3%), and nonspecific hyper intense foci (1.7%) were the most common signs reported. In the case of a Japanese patient with AML and Aspergillus meningitis, CT of the brain was unremarkable; however, MRI scans showed abnormal meningeal enhancement [20].
In 12 other patients (20.4%), pulmonary aspergillosis was diagnosed using BAL, bronchial washing and sputum culture, and lung biopsy. In this regard, CNS aspergillosis was diagnosed only after pulmonary aspergillosis and observations of lesions in the CNS, and no specific diagnostic method was used to confirm CNS aspergillosis. Finally, thirty-six cases (61%) of CNS aspergillosis were presented with additional non-CNS sites of invasive aspergillosis. The affected sites among these cases were the lungs (34 cases), bone, kidneys, heart and thyroid gland (two each), eyes, sinus, liver, and intramuscular and subcutaneous abscesses (one each) (Additional file 1: Table S1).
Treatment
Various antifungal drugs have been used to treat CNS aspergillosis. The prevalent use of drug for treating this infection is amphotericin B (AMB) (76%), either deoxycholate (DAMB) or liposomal AMB. In five patients, the use of DAMB showed a good therapeutic function. DAMB was combined with itraconazole and voriconazole (two cases) and was administered locally in the surgical cavity for two other patients. The combined use of liposomal AMB with voriconazole and isavuconazole (every two cases) controlled infection in patients (Table 1). Notably, the AMB-resistant infection was reported in two patients. One of these patients had a DAMB-resistant pulmonary and cerebral aspergillosis (during treatment with humanized monoclonal antibody anti CD52), which was treated with voriconazole. Another patient in a case suffered a fatal CNS aspergillosis caused by A. terreus, an amphotericin-resistant mold, and he was mistakenly treated by empirical antifungal therapy including intrathecal AMB and then, died as a result [11, 22].
Voriconazole was another drug administered to a large number of patients (64%). According to our analysis results, voriconazole has been used to treat CNS aspergillosis since 2003, whereas before this year, it was used only for a patient in a phase-II clinical trial. In this patient, DAMB was changed to LAMB due to renal toxicity; then, antifungal therapy was continued with itraconazole; however, due to the development of the paraventricular lesion and an additional lesion in the cerebellum, the patients were subsequently enrolled in a phase-II trial and then, received voriconazole [23]. On the other hand, voriconazole has not been administered to 11 patients after 2003. In one of these patients, voriconazole could not be administered because of the hyperbilirubinemia; instead, DAMB was used, which did not affect the treatment process and the patient died as a result [14] (the antifungal drugs used for these patients are listed in Table 1). Side effects of voriconazole were reported in nine patients (23%) such as severe cytolytic hepatitis, transient visual disturbances, reversible elevation of the alkaline phosphatase, respiratory insufficiency, gastrointestinal disturbances, significant photosensitivity, and nail changes. It should be noted that, voriconazole monitoring was performed in 14 (36.8%) patients and the duration of using this drug by patients was reported in 16 cases with an average of 10.7 months.
Among the patients that were treated by voriconazole, the antifungal agent did not exhibit proper therapeutic function effects in 12 patients (31.5%) [3, 4, 10, 22, 24,25,26,27,28,29,30,31]. In four of these patients, the antifungal agent was replaced with other drugs due to its possible side effects [10, 24,25,26]. Besides, in another patient, voriconazole was replaced by isavuconazole due to coinfection with Mucorales [32]. Voriconazole in 58.3% of these patients was not monitored. Finally, the combined use of voriconazole and liposomal AMB (three cases), caspofungin (there cases), DAMB (two cases), and micafungin led to controlling CNS aspergillosis [2, 33,34,35,36,37,38,39,40]. In addition, in a patient with CLL, signs of intracranial hyper-tension with generalized seizure were developed despite taking voriconazole. Prednisone (40 mg/day) was added to this antifungal agent which led to the gradual improvement of the patient’s condition [37]. In addition to AMB and voriconazole, other antifungals such as caspofungin (11 cases), itraconazole (nine cases), isavuconazole (five cases), flucytosine and micafungin (four each), fluconazole, and posaconazole (three each) were used (Table 2). Notably, prophylactic and empirical antifungal agents were used for treatment to control fungal infections in patients with leukemia or SCT. However, in almost all of the cases, no improvement was achieved (Additional file 1: Table S2).
Besides antifungal therapy, surgical intervention appears to have a key role in the treatment of CNS aspergillosis. Surgery was used in 23 (39%) patients. These surgeries included Craniotomy with aspiration and resection of brain abscesses, stereotactic resection, aspiration, and drainage. Besides, one female patient with ALL underwent T11–L1 laminectomy and ultrasound-guided aspiration for her intramedullary and extra medullary abscesses [28]. The main surgical finding was a vascularized thick capsule (soft capsule) containing a necrotic purulent component, pinkish white pus or viscous fluid [25, 41, 42]. In one patient, after five weeks of DAMB use, the patient’s symptoms worsened; therefore, resection of the inferior temporal lobe abscesses and debridement of the external canal, petrous apex, and mastoid air cells were performed, which led to control of the infection and recovery of the patient [5]. On the other hand, a patient with SCT underwent total surgical removal of the cerebellar abscess with suboccipital craniectomy; however, after 5 weeks, the scan again revealed an intra-cerebellar abscess. In this condition, the patient underwent reoperation and was treated with locally DAMB and itraconazole [43]. Stereotactic or open surgery were recommended in five patients, which was not possible due to severe thrombocytopenia and underlying hematological conditions, critical localization of the lesion, in light of the formation of abscess capsule and the patient’s general condition [2, 3, 18, 31, 33]. In another patient, the neurosurgical procedure was not considered due to the risk that the organism would penetrate the brain; however, the patient successfully recovered with catheter coil embolization and long-term antifungal agents [44].
Discussion
Aspergillus species are common contaminants of the upper respiratory tract with initial colonization occurring in the nasopharynx or lower respiratory tree. However, in patients with leukemia or prolonged neutropenia, hematogenous dissemination from the lung and secondary cerebral aspergillosis cause a significant mortality rate [6, 7]. Recent study reported acute leukemia as the most common underlying disease in patients with fungal infections of the CNS and paranasal sinuses [62] and our results also showed ALL as the most common leukemia in patients. In the present study, the overall mortality was 34%; however, the mortality attributed to CNS aspergillosis was 24.5%. On the other hand, in a systematic review of reported cases (ninety cases recorded up to June 2005) on CNS aspergillosis in children, as published by Dotis et al., the overall mortality rate was 65.4% [63]. Such a high mortality rate can be related to the screening of the disease in infants and children, the unavailability of antifungal such as voriconazole, and the screening of patients with all the underlying disorders. In this regard, two recent studies have reported 33% and 48% mortality rates for invasive fungal infections of the CNS. They suggested that the mortality remained high; however, compared to previous historical data, it seemed to have been reduced, probably due to the availability of newer antifungal drugs, immune response in histopathology, absence of co-infections, corticosteroid tapering, and possibly surgical drainage [62, 64].
From the data available in the literature, altered mental status, hemiparesis, cranial nerve palsies, and seizures were the clearest manifestations of CNS aspergillosis. Besides, our results showed lung involvement in more than half of the patients and 61% of the cases presented with additional non-CNS sites of invasive aspergillosis. These results should motivate clinicians to rule out CNS aspergillosis quickly and efficiently in patients suffering pulmonary aspergillosis [41]. In this regard, in a patient with AML, before the initiation of induction chemotherapy, MRI of the brain was conducted and it did not detect any intraparenchymal brain abnormalities. However, 16 days after induction chemotherapy, the patient developed pulmonary symptoms and three days later, brain involvement occurred [22]. Therefore, the onset of CSN aspergillosis in patients with leukemia and immunodeficiency is very rapid, which requires greater control and following up of patients. Ibrutinib has been used in recent years to treat CLL patients. Our results showed that, CNS aspergillosis in patients occurs 6 months, on average, after ibrutinib use, suggesting that CNS is a safe haven for invasive aspergillosis in all CLL-induced patients treated with ibrutinib. In this respect, meticulous and repeated neurological examinations and fast diagnosis are needed for patients with invasive aspergillosis after ibrutinib treatment, with a very low threshold for prescribing MRI of the brain [37].
Given that the clinical signs of CNS aspergillosis are usually nonspecific and similar to other diseases, differential diagnosis such as lung cancer, cerebral infection or abscesses such as listeriosis, cryptococcal and tuberculous meningitis, metastatic disease and cerebral malignancy should be considered when imaging modalities are used for diagnosis in patients [39]. Noteworthy, the MRI appearance of CNS aspergillosis depends on different factors such as the timing of neuroradiologic assessment, immunologic status of the patient, and the characteristics of the fungus [42]. However, our results showed that on conventional MRI sequences in patients with leukemia, CNS aspergillosis appears as ring-enhancing lesions, with perifocal edema on MRI. Brain CT has not proven useful in the case of Aspergillus meningitis, which has no parenchymal lesions, while Gadolinium-enhanced MRI of the brain ensures a more efficient diagnosis of the infection [20, 65]. As mentioned earlier, CSF analysis was normal in most of the patients because neutropenic patients with fungal meningitis do not always show elevation of the CSF cell count [20]. Therefore, imaging modalities are subject to many limitations for accurate diagnosis of CNS aspergillosis; but, if CT and MRI are indicative of cerebral lesions and infarction and vascular inflammation in an immune-compromised host, a fungal etiology must be considered, even if CSF examination does not reveal any abnormalities [7].
Histopathological examination and the use of brain biopsy have been the most commonly used diagnostic methods for patients; however, given that a large number of patients are children, parents are often reluctant to proceed with brain biopsy [21]. In addition, due to coagulation issues and underlying hematological conditions, applying an invasive diagnostic procedure is not always feasible for patients with leukemia [3]. In this regard, a study reported that performing MR-guided biopsy of the suspected brain lesion can yield a more precise tissue diagnosis and its feasibility is proven for sick leukemia patients during remission induction and it allows for intra-lesional local instillation of drugs required [10]. On the other hand, even with a proper biopsy, histopathological examinations may not show the diagnostic features of fungal infections [45]. Besides, it is quite challenging to make a diagnosis of CNS aspergillosis on a histomorphological basis and the most prevalent cause for incorrect morphological diagnosis is the misidentification of Mucorales as Aspergillus spp [66, 67]. Due to mixed mold infection and antifungal resistance, identification of Aspergillus at the species level should be considered in multiple site involvement [4]. Therefore, histopathological examination of different samples has limitations for diagnosis and for some patients, other ways such as culture and molecular methods should be used for the species-level identification and definitive diagnosis of infection.
Culture was used to diagnose CNS aspergillosis in 23% of the patients. It should be mentioned that in some patients, prolonging the culture time of the microorganism reduces the diagnostic value of this method. For example, in a patient with APL, the histologic evaluation proved diagnostic for aspergillosis, while the cultures became positive only 3 weeks later [5]. In addition, even after identifying a mold on the culture media, it still requires several more days to detect the fungus at the species level [22]. Our results showed that when samples obtained from biopsy or surgery were used for culture, there was a higher chance of isolating Aspergillus, while the use of CSF was not very desirable for culture. In this context, as mentioned, obtaining tissue samples in patients with leukemia is highly restricted. Therefore, because early identification of opportunistic invasive fungal pathogens has been shown to guide interventions and affect prognosis, culture may be limited in patients with challenging conditions.
In this regard, the use of molecular methods for diagnosing the cause of infection and drug resistance can be helpful. The use of PCR should be considered in two situations: (A) when the levels of fungi in both blood and CSF are below the lower limit of detection by conventional diagnostic assays; (B) when an uncommon fungal pathogen, which remains undetected by conventional diagnostic assays, infects the CNS [21]. In a patient with AML, A. terreus was detected using PCR and Electrospray Ionization with Mass Spectrometry. This pathogen is inherently resistant to AMB and rapid diagnosis can prevent therapeutic fractures in patients [22]. Therefore, timely identification of CNS aspergillosis by molecular methods can lead to the institution of pathogen-specific and directed therapy and should be used more in patients.
Aspergillus GM assay was another diagnostic method for diagnosing infection in 8.4% of the patients. The GM test is an enzyme-based immunological method used to determine the GM exo-antigen of Aspergillus species in the cell wall [35]. Recent studies have reported low sensitivity to PCR, considering that only a small number of fungal cells are observed in the CSF. Alternatively, GM assay in the CSF was considered to be the most useful [19, 65, 68]. However, our results showed the superiority of PCR in detecting CNS aspergillosis from CSF samples. Moreover, present study demonstrated that serum and BAL samples were more suitable for performing GM assay than CSF. Notably, GM assay showed cross-reactivity with other hyalohyphomycetes such as Fusarium [69, 70]. However, when culture and PCR of CSF were negative in one patient, GM assay alone led to a correct diagnosis of the infection. Furthermore, decline of the GM antigen titer during treatment corresponded to the clinical response to treatment [19, 65]. More importantly, accurate diagnosis using GM assay demands multiple sampling and serial Aspergillus GM monitoring is useful in the early detection of relapse and reinitiation of antifungal therapy [27]. Thus, as mentioned before, each of the diagnostic methods of CNS aspergillosis in patients with blood malignancies has advantages and limitations. Therefore, if possible, using the most appropriate sample for each test can increase the chances of detecting a fungal infection. Clinicians should use diagnostic methods according to the patients’ condition to ensure correct diagnosis of the infection.
After proper and timely diagnosis, the use of appropriate antifungal drugs is also very important. AMB and voriconazole are the most commonly used antifungals in patients with CNS aspergillosis. AMB, the echinocandins, itraconazole, and posaconazole are large molecules and the penetration of these drugs across the blood–brain barrier is mainly limited. Fluconazole and 5-fluorocytosine penetrate well into the CNS; however, Aspergillus frequently exhibits resistance to these antifungal agents [71]. Voriconazole displays a broad range of antifungal activities and facilitates CNS penetration. The 2017 ESCMID guidelines recommend voriconazole as the first-line agent for “proven” or “probable” aspergillosis treatment in all children [72]. However, our analysis showed that good results were not obtained in one-third of patients treated with voriconazole.
Therapeutic Drug Monitoring (TDM) is highly recommended when voriconazole is used, because achieving therapeutic concentrations in a timely manner can be challenging due to nonlinear pharmacokinetics and observed inter-patient variability. In this regard, it is still difficult to find the most effective, yet tolerated, dose, primarily due to the poor correlation between dose and serum concentration. Our results showed that TDM of voriconazole has not been performed for a range of patients which could be due to limited access to the serum voriconazole level testing and slow turnaround time. Most significantly, individuals with sub-therapeutic concentrations are at increased risk of mortality. On the other hand, high voriconazole concentrations may cause adverse effects like neurotoxicity and hepatotoxicity [3, 61, 73]. Furthermore, clinicians should be cognizant of the drug-drug interaction between voriconazole and corticosteroids for cytochrome P450 isoenzymes, CY3A4, CYP2C9, and CYP2C19, which can lead to decreased plasma voriconazole concentrations and, thus, limited efficacy against the Aspergillus [10, 31]. Therefore, to prevent voriconazole therapy failure, it is imperative to attain therapeutic voriconazole plasma concentrations promptly in order to achieve a favorable response and also, is necessary to perform a CYP2C19 genotype test to determine the genetically predicted metabolizer status can prevent therapeutic failures when voriconazole is used.
In some patients, the use of combination therapy showed good performance. However, characterization of patients benefiting from a combination antifungal therapy is required and confirmatory results of further prospective studies are needed before the combination therapy of antifungal agents can be fully accepted as standard strategies for CNS aspergillosis. Lastly, there are no clear recommendations as to the exact duration of antifungal treatment of mold infections of the CNS. However, antifungal chemotherapy is usually recommended until the resolution of all clinical, laboratory, and radiographic findings of active infection [52]. Besides, following the treatment of CNS aspergillosis in leukemia patients, prolonged and, in some cases, lifelong secondary prophylaxis may be necessary after the initial treatment [27].
Neurosurgical intervention was used in 39% of the patients. One study reported that the use of image-guided stereotactic neurosurgery provided a safe and vital component in the successful treatment of patients’ devastating conditions [41]. In another patient with bone marrow transplantation, despite the administration of AMB, flucytosine, and micafungin, the patient died 2 months after transplantation. The authors suggested that if the infected lesion remains after antifungal agent’s therapy, surgical drainage or resection of infected tissue along with systemic therapy may be important. In this regard, Infectious Diseases Society of America (IDSA) guidelines recommend surgical drainage and infected tissue removal along with systemic antifungal therapy for patients suffering from CNS aspergillosis [52]. Therefore, using a combination of antifungals along with surgery can help control the infection. In some cases, due to the critical localizations of the lesion and underlying hematological conditions, surgery is not possible. In this situation, the use of antifungal agents continues for a very long time [2].
Finally, in addition to surgical intervention and antifungal treatment, patient induction chemotherapy management, parallel resolution of neutropenia, and complete remission of leukemia undoubtedly play an important role in treating patients. A female patient with CLL was treated with voriconazole after being diagnosed with CNS aspergillosis. Then, she received bendamustin for CLL progression, leading to more profound neutropenia and clinical deterioration [26]. Therefore, along with the mentioned treatments, it is important to control the patient’s underlying conditions, which can facilitate the treatment process. For instance, discontinuing immunosuppressive drugs, if possible, can help control CNS aspergillosis.
Conclusion
CNS aspergillosis is a highly lethal disease in patients with blood malignancies and is subject to a very poor prognosis. Patients with leukemia are very sensitive to fungal infections due to underlying disorders and several previous chemotherapy regimens. In this regard, pulmonary involvement in these patients usually occurs shortly after the start of chemotherapy and after that, CNS infections may occur as an occult asymptomatic extra-pulmonary involvement during the diagnostic evaluation of febrile neutropenic patients or symptomatic form, which usually develop after a few weeks of pulmonary manifestation. Therefore, systematic full screening including CT scan and enhanced MRI for CNS lesions should be performed for every diagnosis of invasive fungal infection; in addition, when infection is suspected in these patients, a definitive and differential diagnosis should be made using various diagnostic methods. If possible, species identification of the fungus is suggested because the occurrence of antibiotic resistance in some species can completely change the treatment regimen. In addition, the use of combination therapies should be considered in future studies so that if the first line of the treatment fails, the most appropriate treatment strategy can be adopted for patients. Furthermore, in addition to using appropriate antifungal therapy and TDM, control of patients’ chemotherapy should also be considered because the outcome of invasive aspergillosis is poor unless immunologic status improves.
Availability of data and materials
The authors confirm that the data supporting the findings of this study is available within the article and its supplementary materials.
References
Shariati A, Moradabadi A, Chegini Z, Khoshbayan A, Didehdar M. An overview of the management of the most important invasive fungal infections in patients with blood malignancies. Infect Drug Resist. 2020;13:2329.
Patiroglu T, Unal E, Karakukcu M, Ozdemir MA, Tucer B, Yikilmaz A, Deniz K. Multiple fungal brain abscesses in a child with acute lymphoblastic leukemia. Mycopathologia. 2012;174:505–9.
De Leonardis F, Novielli C, Giannico B, Mariggiò MA, Castagnola E, Santoro N. Isavuconazole treatment of cerebral and pulmonary aspergillosis in a pediatric patient with acute lymphoblastic leukemia: case report and review of literature. J Pediatr Hematol Oncol. 2020;42:e469–71.
Amanati A, Lotfi M, Masoudi MS, Jafarian H, Ghasemi F, Bozorgi H, Badiee P. Cerebral and pulmonary aspergillosis, treatment and diagnostic challenges of mixed breakthrough invasive fungal infections: case report study. BMC Infect Dis. 2020;20:535.
Epstein NE, Hollingsworth R, Black K, Farmer P. Fungal brain abscesses (aspergillosis/mucormycosis) in two immunosuppressed patients. Surg Neurol. 1991;35:286–9.
Koh S, Ross LA, Gilles FH, Nelson MD Jr, Mitchell WG. Myelopathy resulting from invasive aspergillosis. Pediatr Neurol. 1998;19:135–8.
Christophe C, Azzi N, Bouche B, Dan B, Levivier M, Ferster A. Magnetic resonance imaging and angiography in cerebral fungal vasculitis. Neuropediatrics. 1999;30:218–20.
Miaux Y, Ribaud P, Williams M, Guermazi A, Gluckman E, Brocheriou C, Laval-Jeantet M. MR of cerebral aspergillosis in patients who have had bone marrow transplantation. AJNR Am J Neuroradiol. 1995;16:555–62.
Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis. Drugs. 2007;67:1567–601.
Sterba J, Prochazka J, Ventruba J, Kren L, Valik D, Burgetova D, Mudry P, Skotakova J, Blatny J. Successful treatment of aspergillus brain abscess in a child with acute lymphoblastic leukemia and liver failure. Pediatr Hematol Oncol. 2005;22:649–55.
Marbello L, Nosari A, Carrafiello G, Anghilieri M, Cesana C, Cafro AM, D’Avanzo G, Morra E. Successful treatment with voriconazole of cerebral aspergillosis in an hematologic patient. Haematologica. 2003;88:Ecr05.
Lacerda JF, Martins C, Carmo JA, Lourenço F, Guedes MM, Sequeira P, Lacerda JM. Invasive aspergillosis of the central nervous system after allogeneic stem cell transplantation. J Infect. 2005;51:e191-194.
Lamoth F, Chung SJ, Damonti L, Alexander BD. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin Infect Dis. 2017;64:1619–21.
Prakash G, Thulkar S, Arava SK, Bakhshi S. Cerebral aspergillus infection in pediatric acute lymphoblastic leukemia induction therapy. Indian J Med Paediatr Oncol. 2012;33:236–8.
Hickey AJ, Gounder L. Moosa M-YS, Drain PK: A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infect Dis. 2015;15:209.
Chegini Z, Didehdar M, Khoshbayan A, Rajaeih S, Salehi M, Shariati A: Epidemiology, clinical features, diagnosis and treatment of cerebral mucormycosis in diabetic patients: a systematic review of case reports and case series. Mycoses 2020.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K: Chapter 7: Systematic reviews of etiology and risk. Joanna Briggs Institute Reviewer's Manual The Joanna Briggs Institute 2017:2019–2005.
Peng HL, Yi YF, Shen XH, Yin YF, Zhang GS. Dramatic response to itraconazole in central nervous system aspergillosis complicating acute promyelocytic leukemia. Infect Dis (Lond). 2015;47:104–6.
Matsuo T, Mori N, Sakurai A, Furukawa K. Aspergillus meningitis in a patient with chronic lymphocytic leukemia. J Infect Chemother. 2020;26:622–4.
Saitoh T, Matsushima T, Shimizu H, Yokohama A, Irisawa H, Handa H, Tsukamoto N, Karasawa M, Nojima Y, Murakami H. Successful treatment with voriconazole of Aspergillus meningitis in a patient with acute myeloid leukemia. Ann Hematol. 2007;86:697–8.
Komatsu H, Fujisawa T, Inui A, Horiuchi K, Hashizume H, Sogo T, Sekine I. Molecular diagnosis of cerebral aspergillosis by sequence analysis with panfungal polymerase chain reaction. J Pediatr Hematol Oncol. 2004;26:40–4.
Modi DA, Farrell JJ, Sampath R, Bhatia NS, Massire C, Ranken R, Bonomo RA. Rapid identification of Aspergillus terreus from bronchoalveolar lavage fluid by PCR and electrospray ionization with mass spectrometry. J Clin Microbiol. 2012;50:2529–30.
Schwartz S, Milatovic D, Thiel E. Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute leukaemia. Br J Haematol. 1997;97:663–5.
Furtwängler R, Schlotthauer U, Gärtner B, Graf N, Simon A. Nosocomial legionellosis and invasive aspergillosis in a child with T-lymphoblastic leukemia. Int J Hyg Environ Health. 2017;220:900–5.
Beresford R, Dolot V, Foo H. Cranial aspergillosis in a patient receiving ibrutinib for chronic lymphocytic leukemia. Med Mycol Case Rep. 2019;24:27–9.
Rouzaud C, Jullien V, Herbrecht A, Palmier B, Lapusan S, Morgand M, Guéry R, Dureault A, Danion F, Puget S, et al. Isavuconazole diffusion in infected human brain. Antimicrob Agents Chemother. 2019;63:1.
Davoudi S, Anderlini P, Fuller GN, Kontoyiannis DP. A long-term survivor of disseminated Aspergillus and mucorales infection: an instructive case. Mycopathologia. 2014;178:465–70.
McCaslin AF, Lall RR, Wong AP, Lall RR, Sugrue PA, Koski TR. Thoracic spinal cord intramedullary aspergillus invasion and abscess. J Clin Neurosci. 2015;22:404–6.
Sakata N, Okano M, Masako R, Tanaka A, Yamashita Y, Karasuno T, Imadome KI, Okada M, Sugimoto K: Donor-derived myelodysplastic syndrome after allogeneic stem cell transplantation in a family with germline GATA2 mutation. Int J Hematol 2020.
McCarter SJ, Vijayvargiya P, Sidana S, Nault AM, Lane CE, Lehman JS, Wilson JW, Parikh SA, Nowakowski GS, Al-Kali A. A case of ibrutinib-associated aspergillosis presenting with central nervous system, myocardial, pulmonary, intramuscular, and subcutaneous abscesses. Leuk Lymphoma. 2019;60:559–61.
Nyga R, Delette C, Mabille C, Bennis Y, Chouaki T, Boone M, Maizel J, Marolleau JP, Joseph C. Ibrutinib related cerebral aspergillosis successfully treated with isavuconazole: a case report. Leuk Lymphoma. 2020;61:1760–2.
Pouvaret A, Guery R, Montillet M, Molina TJ, Duréault A, Bougnoux ME, Galliot R, Lanternier F, Delarue R, Lortholary O. Concurrent cerebral aspergillosis and abdominal mucormycosis during ibrutinib therapy for chronic lymphocytic leukaemia. Clin Microbiol Infect. 2019;25:771–3.
Damaj G, Ivanov V, Le Brigand B, D’Incan E, Doglio MF, Bilger K, Faucher C, Vey N, Gastaut JA. Rapid improvement of disseminated aspergillosis with caspofungin/voriconazole combination in an adult leukemic patient. Ann Hematol. 2004;83:390–3.
Zwitserloot AM, Warris A. van’t Hek LG, van Die LE, Verweij PE, Mavinkurve-Groothuis AM: Disseminated aspergillosis in an adolescent with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51:423–6.
Sav H, Atalay MA, Demir G, Akif Ozdemir M, Nedret Koc A. Early diagnosis of cerebral aspergillosis with various methods: a case report. Infez Med. 2013;21:134–8.
Sadarangani M, Harvey M, McDonald A, Speert DP, Dix D. Brain Abscesses Due to Aspergillus nidulans Infection During Induction Chemotherapy for Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol. 2015;37:e384-386.
Gaye E, Le Bot A, Talarmin JP, Le Calloch R, Belaz S, Dupont M, Tattevin P. Cerebral aspergillosis: An emerging opportunistic infection in patients receiving ibrutinib for chronic lymphocytic leukemia? Med Mal Infect. 2018;48:294–7.
Peddada K, Khan NM, Rubin J, Zakaryan H, Liu Y, Popnikolov N, Sangani R, Li W. Diagnosis of Vitreoretinal Aspergillosis with Transvitreal Retinochoroidal Biopsy. Case Rep Ophthalmol Med. 2018;2018:8306163.
Faisal MS, Shaikh H, Khattab A, Albrethsen M, Fazal S. Cerebral aspergillosis in a patient on ibrutinib therapy-A predisposition not to overlook. J Oncol Pharm Pract. 2019;25:1486–90.
Peri AM, Bisi L, Cappelletti A, Colella E, Verga L, Borella C, Foresti S, Migliorino GM, Gori A, Bandera A. Invasive aspergillosis with pulmonary and central nervous system involvement during ibrutinib therapy for relapsed chronic lymphocytic leukaemia: case report. Clin Microbiol Infect. 2018;24:785–6.
Middelhof CA, Loudon WG, Muhonen MD, Xavier C, Greene CS Jr. Improved survival in central nervous system aspergillosis: a series of immunocompromised children with leukemia undergoing stereotactic resection of aspergillomas. Report of four cases. J Neurosurg. 2005;103:374–8.
Mardari R, Della Puppa A, Rotilio A, Sgarabotto D, Baracchini C, Carollo C, Manara R. Pontocerebellar angle aspergillosis: clinical and radiological findings. Neurologist. 2011;17:75–8.
Erdogan E, Beyzadeoglu M, Arpaci F, Celasun B: Cerebellar aspergillosis: case report and literature review. Neurosurgery 2002, 50:874–876; discussion 876–877.
Watanabe T, Okada T, Okada C, Onishi T, Watanabe H, Okamoto Y, Kitamura Y, Manabe S, Matsubara S, Kageji T, Iwai A. An aspergillotic aneurysm of the internal carotid artery following allogeneic bone marrow transplantation: successful management with catheter coil embolization and long-term antifungal agents. Transpl Infect Dis. 2009;11:49–53.
Coleman JM, Hogg GG, Rosenfeld JV, Waters KD. Invasive central nervous system aspergillosis: cure with liposomal amphotericin B, itraconazole, and radical surgery–case report and review of the literature. Neurosurgery. 1995;36:858–63.
Gunsilius E, Lass-Flörl C, Mur E, Gabl C, Gastl G, Petzer A. Aspergillus osteoarthritis in acute lymphoblastic leukemia. Ann Hematol. 1999;78:529–30.
Guermazi A, Benchaib N, Zagdanski AM, Hocqueloux L, Rili M, Molina JM, de Kerviler E. Cerebral and spinal cord involvement resulting from invasive aspergillosis. Eur Radiol. 2002;12:147–50.
Nyga R, Simon L, Chouaki T, Delette C, Bennis Y, Joseph C, Marolleau JP, Slama M, Zogheib E, Maizel J. The pharmacokinetic challenge of voriconazole therapy for cerebral aspergillosis in patients treated with ibrutinib. Crit Care. 2019;23:88.
Mahlknecht U, von Lintig F, Mertelsmann R, Lindemann A, Lübbert M. Successful treatment of disseminated central nervous aspergillosis in a patient with acute myeloblastic leukemia. Leuk Lymphoma. 1997;27:191–4.
Turki AT, Rashidi-Alavijeh J, Dürig J, Gerken G, Rath P-M, Witzke O. Successful treatment of cerebral aspergillosis: case report of a patient with T-cell large granular lymphocytic leukemia (T-LGL). BMC Infect Dis. 2017;17:797.
Matis GK, Voultsinou D, Chrysou O, Birbilis T, Geroukis T. Cerebral aspergillosis and acute myeloid leukemia. J Neurosci Rural Pract. 2013;4:134.
Sato T, Kaneda M, Ichikawa M, Suzuki D, Nakagawa A, Kobayashi R. Current approaches to management of cerebral fungal infection in pediatric patients with hematologic disorders. J Pediatr Hematol Oncol. 2008;30:249–53.
Sancho JM, Ribera JM, Rosell A, Muñoz C, Feliu E. Unusual invasive bronchial aspergillosis in a patient with acute lymphoblastic leukemia. Haematologica. 1997;82:701–2.
Björkholm M, Kalin M, Grane P, Celsing F. Long-term treatment of invasive sinus, tracheobroncheal, pulmonary and intracerebral aspergillosis in acute lymphoblastic leukaemia. Infection. 2012;40:81–5.
Başlar Z, Soysal T, Hanci M, Aygün G, Ferhanoğlu B, Sarioğlu AC, Ulkü B. Successful outcome of aspergillus brain abscess in a patient who underwent bone marrow transplantation for aplastic anemia. Haematologia (Budap). 1997;28:265–71.
Kural C, Ozer MI, Ezgu MC, Mehtiyev R, Yasar S, Kutlay AM, Daneyemez MK, Onguru O, Erdogan E, Izci Y. Intracavitary amphotericin B in the treatment of intracranial aspergillosis. J Clin Neurosci. 2018;51:75–9.
Ng A, Gadong N, Kelsey A, Denning DW, Leggate J, Eden O. Successful treatment of Aspergillus brain abscess in a child with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2000;17:497–504.
Wandroo F, Stableforth P, Hasan Y. Aspergillus brain abscess in a patient with acute myeloid leukaemia successfully treated with voriconazole. Clin Lab Haematol. 2006;28:130–3.
Lin C, Barrio GA, Hurwitz LM, Kranz PG. Cerebral air embolism from angioinvasive cavitary aspergillosis. Case Rep Neurol Med. 2014;2014:1.
Eichenberger EM, Saullo J, Brander D, Wang S-H, Perfect JR, Messina JA. A case of CNS aspergillosis in a patient with chronic lymphocytic leukemia on first-line ibrutinib therapy. Med Mycol Case Rep. 2020;27:17–21.
Le TH, Kumar V, Gondal K, Barnes M, Siddique H, Buttar B, Kaell A. Isolated central nervous system Aspergillosis infection in a chronic lymphocytic leukemia patient on Ibrutinib: a case report. BMC Infect Dis. 2020;20:175.
Candoni A, Klimko N, Busca A, Di Blasi R, Shadrivova O, Cesaro S, Zannier ME, Verga L, Forghieri F, Calore E, et al. Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses. 2019;62:252–60.
Dotis J, Iosifidis E, Roilides E. Central nervous system aspergillosis in children: a systematic review of reported cases. Int J Infect Dis. 2007;11:381–93.
Economides MP, Ballester LY, Kumar VA, Jiang Y, Tarrand J, Prieto V, Torres HA, Kontoyiannis DP. Invasive mold infections of the central nervous system in patients with hematologic cancer or stem cell transplantation (2000–2016): Uncommon, with improved survival but still deadly often. J Infect. 2017;75:572–80.
Verweij PE, Brinkman K, Kremer HP, Kullberg B-J, Meis JF. Aspergillus meningitis: diagnosis by non-culture-based microbiological methods and management. J Clin Microbiol. 1999;37:1186–9.
Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, Hoenigl M, Jensen HE, Lagrou K, Lewis RE. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–21.
Kung V, Chernock R, Burnham C-A. Diagnostic accuracy of fungal identification in histopathology and cytopathology specimens. Eur J Clin Microbiol Infect Dis. 2018;37:157–65.
Bretagne S, Costa J-M, Delabesse EB, Dhédin N, Rieux C, Cordonnier C. Comparison of serum galactomannan antigen detection and competitive polymerase chain reaction for diagnosing invasive aspergillosis. Clin Infect Dis. 1998;26:1407–12.
Tortorano AM, Esposto MC, Prigitano A, Grancini A, Ossi C, Cavanna C, Cascio GL. Cross-reactivity of Fusarium spp. in the Aspergillus galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol. 2012;50:1051–3.
Nucci M, Carlesse F, Cappellano P, Varon AG, Seber A, Garnica M, Nouer SA, Colombo AL. Earlier diagnosis of invasive fusariosis with Aspergillus serum galactomannan testing. PLoS ONE. 2014;9:e87784.
Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Troke P, Thiel E. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 2005;106:2641–5.
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Flörl C, Lewis RE, Munoz P, Verweij PE. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24:e1–38.
Hicks JK, Crews KR, Flynn P, Haidar CE, Daniels CC, Yang W, Panetta JC, Pei D, Scott JR, Molinelli AR. Voriconazole plasma concentrations in immunocompromised pediatric patients vary by CYP2C19 diplotypes. Pharmacogenomics. 2014;15:1065–78.
Acknowledgements
Not applicable
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AS and MD conceived and designed the study. AS and ZC contributed in comprehensive research. AS and SR analyzed the cases. AS, MD, and AM wrote the paper. SR, VF and MG participated in manuscript editing. Notably, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Various findings that led to the diagnosis of CNS aspergillosis in patients with leukemia or stem cell transplantation. Table S2. Prophylaxis and empirical antibiotic therapy for patients with inducing chemotherapy or stem cell transplantation. Table S3. Aspergillus Galactomannan assay in patients with CNS aspergillosis and leukemia or stem cell transplantation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shariati, A., Didehdar, M., Rajaeih, S. et al. Aspergillosis of central nervous system in patients with leukemia and stem cell transplantation: a systematic review of case reports. Ann Clin Microbiol Antimicrob 20, 44 (2021). https://doi.org/10.1186/s12941-021-00452-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-021-00452-9