Abstract
Background
Early-life environmental exposures are suspected to be involved in the development of chronic diseases later in life. Most studies conducted so far considered single or few exposures and single-health parameter. Our study aimed to identify a childhood general health score and assess its association with a wide range of pre- and post-natal environmental exposures.
Methods
The analysis is based on 870 children (6–12 years) from six European birth cohorts participating in the Human Early-Life Exposome project. A total of 53 prenatal and 105 childhood environmental factors were considered, including lifestyle, social, urban and chemical exposures. We built a general health score by averaging three sub-scores (cardiometabolic, respiratory/allergy and mental) built from 15 health parameters. By construct, a child with a low score has a low general health status. Penalized multivariable regression through Least Absolute Shrinkage and Selection Operator (LASSO) was fitted in order to identify exposures associated with the general health score.
Findings
The results of LASSO show that a lower general health score was associated with maternal passive and active smoking during pregnancy and postnatal exposure to methylparaben, copper, indoor air pollutants, high intake of caffeinated drinks and few contacts with friends and family. Higher child’s general health score was associated with prenatal exposure to a bluespace near residency and postnatal exposures to pets, cobalt, high intakes of vegetables and more physical activity. Against our hypotheses, postnatal exposure to organochlorine compounds and perfluorooctanoate were associated with a higher child’s general health score.
Conclusion
By using a general health score summarizing the child cardiometabolic, respiratory/allergy and mental health, this study reinforced previously suspected environmental factors associated with various child health parameters (e.g. tobacco, air pollutants) and identified new factors (e.g. pets, bluespace) warranting further investigations.
Similar content being viewed by others
Introduction
It is recognized that the early-life period is particularly vulnerable to the influences of environmental factors, in particular the pregnancy period and the first years of life [1]. The concept of “exposome” is defined by all the exposures that a human being undergoes since conception [2], ranging from air pollution to chemical pollutants, the social environment etc. In recent years, an increased number of studies based on the exposome approach identified the main environmental threats for specific health parameters [3,4,5] or for a specific health domain [6,7,8]. However, this traditional approach of investigating exposures associated with single health parameter is limited. One main limit is that it fails to recognize the whole system nature of multiple interactive exposures that shape multiple health outcomes.
In addition to the outcome-wide approach previously proposed [9], an approach based on a general health indicator is relevant. While the outcome-wide approach assesses the impact of exposures on several health outcomes considered independently, a general health score aims to cover multiple health domains (e.g. cardiometabolic, respiratory/allergy and mental health) in a single indicator. This approach is based on the assumption that mental, cardiometabolic and respiratory outcomes partly share some biological pathways that are affected by environmental factors. This assumption is supported by the identification of pleiotropic genes and evidences for shared influence of major regulating systems such as inflammation and oxidative stress between these various health outcomes [10,11,12,13]. Pointing out early-life exposures associated with multiple health domains in children is needed to prioritise public health messages but also to prevent multimorbidity, i.e. the coexistence of several conditions in the same individual. This approach may lead to the identification of new environmental health risk factors as some exposure affects in a low-grade manner multiple health outcomes. As far as we know, few general health indicators exist out of the spectrum of questionnaire on quality of life related to self-perceived general health (e.g. the Child Health Questionnaire [14]) and no study has sought for environmental factors affecting a general health indicator in children.
This project aimed to compute a general health score and assess its association with multiple prenatal and postnatal environmental factors, in the large European Human Early-Life Exposome (HELIX) cohort [15, 16]. Our main hypothesis is that this approach can reinforce the significance of some suspected environmental factors and identify new risk factors simultaneously affecting various health parameters.
Materials and methods
Study population
This study is based from the HELIX project, which includes six existing population-based birth cohorts: Born in Bradford (BiB, UK) [17], Étude des Déterminants pré et postnatals du développement et de la santé de l’Enfant (EDEN, France) [18], Infancia y Medio Ambiente (INMA, Spain) [19], Kaunas Cohort (KANC, Lithuania) [20], The Norwegian Mother, Father and Child Cohort Study (MoBa, Norway) [21], and Mother–Child Cohort (RHEA, Greece) [22]. Around 32,000 mothers were recruited during pregnancy (2003–2009), from which 1,301 mother–child pairs were followed-up when the child was 6–11 years old (2014–2015). Standardized protocols were used to collect biological samples and questionnaire data, conduct health examinations and characterise a large range of exposures. The present study included 870 mother–child pairs for which data was available to build the general health score (see more details in the following part).
Health data: cardiometabolic, respiratory/allergy and mental health
Fifteen health parameters were considered for this study, covering the cardiometabolic, respiratory and mental health, as listed in Table 1 (see more details in eMethods 1). The cardiometabolic parameters considered were the child blood pressure (diastolic and systolic), the waist circumference, lipids (high-density lipoprotein (HDL) cholesterol and triglycerides) and insulin levels. The first two parameters were measured by medical staff, and the last two were obtained through blood and serum, respectively. The respiratory and allergy-related health was assessed by spirometry (Forced Expiratory Volume in one second (FEV1)) and by a questionnaire adapted from the International Study on Asthma and Allergy in Childhood (ISAAC) [23] including doctor-diagnosed asthma, food allergies, eczema, as well as rhinitis symptoms [3, 7]. The cognitive and behavioural parameters considered were the measured fluid intelligence (Raven Colour Progressive Matrix™), an index regarding symptoms of Attention Disabilities and Hyperactivity Disorders (ADHD) (Conner’s rating scales of 27 items) and internalizing and externalizing scores (99-item Child Behaviour Checklist (CBCL) [6, 8]. All these health parameters were measured at the Helix follow-up when the child was between 6–11 years old (see eTable 1).
From the whole HELIX population (n = 1,301), at least one health parameter was missing for 11.5% (n = 150) of children regarding cardiometabolic parameters, for 32.4% (n = 294) of children regarding respiratory and allergic parameters (mostly due to FEV1), and for 0.8% (n = 23) of children regarding mental parameters. Children with all fifteen health parameters were included, leading to the inclusion of 870 mother–child pairs.
Characterisation of the exposome
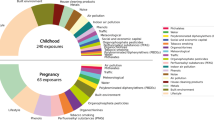
A wide range of environmental exposures was assessed in each mother–child pair, covering 21 families of exposures, with 53 prenatal and 105 postnatal exposures, as detailed in Table 2 (see also previous Helix papers [16, 24, 25]). Briefly, outdoor exposures were assessed based on remote and spatial sensing data from a geographical information system (see eMethods 2). Factors regarding the lifestyle were collected by questionnaire and included smoking habits of the mother, food intakes, the social environment (pregnancy and childhood), physical activity, sleep and the presence of pets (childhood) (see eMethods 3). Biomarkers of chemical compounds were measured through biological samples (mostly serum and urine, as detailed in eTable 2) during pregnancy and childhood (see eMethods 4). Collection time points for prenatal exposures are given in eTables 3 and 4.
Covariates
Covariates used for the prenatal analyses included cohort, child age and sex, maternal age, highest parental education (primary, secondary or higher education), parental country of birth (none, one or both parents born in the cohort country), pre-pregnancy body mass index (BMI) and season of birth (winter, spring, summer or autumn). Regarding postnatal analyses, breastfeeding duration (< 11 weeks, 11–35 weeks, > 35 weeks) was added to the set of covariates.
Creation of the general health score
The general health score averaged three sub-scores, each representing a specific health domain (cardiometabolic, respiratory/allergy and mental health). Beforehand, continuous health parameters were transformed in z-scores, using Generalize Additive Model for Location, Scale and Shape (GAMLSS) [26] to standardize on covariates (mostly age and sex, see eTables 5 and 6) and approach normality. The health parameters were not adjusted on each cohort in order to keep the between-cohort variability of the general health status for descriptive purposes. As used previously in the Helix population, the cardiometabolic sub-score was defined as (-z waist circumference) + (- z insulin) + (z HDL cholesterol – z triglycerides)/2 + (-z systolic BP – z diastolic BP)/2 [27, 28]. Following the approach of Eisenmann [29] the respiratory/allergy sub-score and the mental sub-score were defined as the first principal component of a multiple factorial analysis (see eFigures 1–2 and eTable 7–8). All of the three sub-scores were built such that a higher score means the child is in better health (see eMethods 5). The three sub-scores were scaled and aggregated into a single general health score by taking their mean. By construct, the general health score is low for children with conjointly low-to-moderate cardiometabolic, respiratory/allergy and mental health in children, as well as for children highly affected in one health domain while no or moderately affected for the other two.
Strategy of analysis for the exposome-health association
For all exposures and covariates, the optimal transformation to approach normality was applied (see eTable 9), which is necessary for following steps including imputation and penalized regression models. Imputation of the missing values on exposures and covariates was done using the method of chained equations [30]. (see more details in eMethods 6). It generated 20 imputed datasets, used in the statistical analyses with the Rubin’s rule. After imputation, continuous exposures were centred and standardized by the interquartile range (IQR).
The exposure-general health score association study was performed separately for the prenatal and postnatal exposures using the Least Absolute Shrinkage and Selection Operator (LASSO) as the main analysis [31]. This penalized regression model considers all exposures and covariates simultaneously and selects the best predictors of the outcome (note that covariates were forced in the model). Optimization of the penalizing parameter \(\lambda\) was performed by minimizing the mean cross-validated error on each of the 20 imputed dataset. The exposures selected for at least 50% of LASSO models (10 imputed datasets out of the 20) were used as the final set of exposures [32]. The main models consisted in two multivariable linear regressions (one prenatal and one postnatal) considering all the selected exposures, after removing all exposures with p-value higher than 10%. More details on the strategy of analysis can be found in eMethods 7.
As secondary analyses, an exposome-wide association study (ExWAS) was conducted. It considered each exposure in separate linear regression models [33], adjusted on the same covariates, and corrected for multiple hypothesis testing (adapted from Li [34]). Moreover, some specific hypotheses were tested: 1) For organochlorine compounds (OCs), the associations found were stratified on the terciles of the BMI because OCs are known to accumulate in fat; 2) for PFASs, the associations were adjusted on fish consumption as a correlation between PFASs and fish consumption has been noticed in the Helix population [35] 3) the final multivariable models were stratified on sex to address a potential gender-specific association; 4) the final multivariable models were stratified on cohort to address the robustness of the findings to the multicentre study design; 5) the linearity of the associations was tested using a Generalized Additive Model (GAM) with smooth functions for all selected exposures. In addition, a sensitivity analysis to assess the robustness to extreme values was conducted by fitting the multivariable model after excluding the 2% lowest and 2% highest values for the general health score (n = 836).
For better comparability across exposures, estimates were expressed as an increase in interquartile range of the transformed exposure (continuous exposures). Significance level was defined as 5% for all statistical tests. Analyses were done with R version 4.2.1, using the packages mice, gamlss, FactoMineR, psych and glmnet. The main steps in the analysis are summarized in Fig. 1.
Results
Description of the population
The study population, aged between 5.4 and 12.0 years old (median = 8.1 years old) at the HELIX follow-up was 47% girls (Table 3). At birth, mothers were on average 31 years old and about half of them (51%) had a high degree of education. Tables describing the exposures and health parameters (including percent of missing data) during pregnancy and childhood are available in the supplementary materials (eTables 10, 11, 12 and 13).
Description of the general health score
The cardiometabolic, respiratory/allergy and mental sub-scores ranged between -3.20 and 3.10, -4.53 and 3.18, and -2.89 and 2.76, respectively. The three sub-scores were poorly correlated (eTable 14), with more details and descriptions in the supplementary (eFigure 3, eTable 7– 8).
The general health score, calculated as the mean of the three sub-scores, had a normal distribution (Shapiro test p-value = 0.21) with a mean (sd) of 0.03 (0.60). The median general health score varied among cohorts, with the lowest in BiB (median = -0.21) and the highest in MoBA (median = 0.42), as shown in Fig. 2. The general health score increased with parental education, breast-feeding duration and maternal age, and decreased with pre-pregnancy BMI (eTable 15). The joint distributions of the sub-scores, key health parameters and the general health score are presented in the supplementary (eTable 16).
Distribution of the general health score by cohort
Boxplot showing the distributions of the built general health score in the whole population and in each cohort. Population: study population from the HELIX subcohort (n = 870). Accronyms: BiB: Born in Bradford, EDEN: Étude des Déterminants pré et postnatals du développement et de la santé de l’Enfant, INMA: Infancia y Medio Ambiente, KANC: Kaunus Cohort, MoBa: The Norwegian Mother, Father and Child Cohort Study, RHEA: Mother–Child Cohort in Crete
Which exposures were associated with the general health score?
Three exposures during pregnancy were selected by LASSO: maternal passive smoking (assessed by questionnaire), maternal active smoking (assessed by cotinine levels) and the presence of a bluespace near residency (Fig. 3 and Table 4). In the multivariable model, maternal passive smoking remained significantly associated with a poorer general health score. Although not significant, higher levels of cotinine (> 50 µg/L vs < 18.5 µg/L) were association with a poorer score (p-value = 0.09) and the presence of a bluespace was associated with a better score (p-value = 0.07).
Results of the final multivariable models
Population: n = 870 children from the HELIX subcohort. Method: multivariable models between the general health score and the exposures selected by LASSO for 50% of models, plus the covariates, separately for prenatal and postnatal exposures. All exposures with a p-value > 10% were removed one by one from the final model. Covariates: cohort, child age, maternal education and age, parental country of birth, season of birth, pre-pregnancy BMI, plus the breastfeeding duration for postnatal exposures only. Acronyms: Co: Cobalt, Cu: Copper, DDE: Dichlorodiphenyldichloroethylene, HCB: Hexachlorobenzene, MEPA: Methyl-paraben, PFOA: Perfluorooctanoate, PM: Particulate matter
Regarding the exposures during childhood, a total of 23 variables was selected by LASSO and 16 of them were kept in the final multivariable model (p-value ≤ 10%) (Fig. 3 and Table 4). High intakes of caffeinated drinks (compared to low intakes), indoor levels of benzene and PM2.5, exposure to methylparaben and copper were significantly associated with a poorer general health score. A non-significant association (p-value = 0.07) was observed between less frequent contact with family and friends (once a week vs daily) and a poorer health score. On the other hand, intakes of vegetables (high vs low intake), owning a pet, physical activity, cobalt, exposure to perfluorooctanoate (PFOA), dichlorodiphenyldichloroethylene (DDE) and hexachlorobenzene (HCB) were significantly associated with a better score. Suggestive associations (0.05 < p < 0.10) were observed between medium intakes of sodas and bakery products and a better score.
The ExWAS approach led to similar results than LASSO, highlighting significant associations of the general health score with postnatal exposures to pets, diet, metals, indoor air pollutants, OCs and PFOA. No association with prenatal exposure remained significant after correcting on multiple testing. All estimations are available in the supplementary materials (eTables 17 and 18).
After stratifying on the terciles of BMI, higher DDE exposure was associated with a better general health score in the low BMI group, but tended to be associated with a poorer score in the high BMI group (see eFigure 4). Adding fish consumption as a confounder variable did not change the results estimated for PFOA (see eTable 19). Results of the multivariable models stratified by sex showed overall similar results in boys and girls (eFigures 5 and 6), although boys-specific associations were observed for postnatal exposures to indoor benzene and HCB. When stratifying on cohorts, results were overall consistent (see eFigures 7 and 8) although some differences were observed for postnatal exposure to copper, DDE, contact with family and friends and intake of bakery products. The results of GAM did not invalidate the assumption of linearity for most exposures at the exception of child HCB (see eFigure 9). The general health score first increased with child HCB for “low” HCB levels, but was constant for “moderate-to-high” HCB level. In the sensitivity analysis where extreme values of the general health score were removed, the magnitude of the associations remained similar (see eFigure 10).
Discussion
This novel study intended to approach the complexity of multiple exposures impacting multiple health parameters by assessing the association between a wide range of pre- and post-natal exposures and a general health score in children. Three prenatal and fourteen postnatal exposures associated with the child’s general health score were identified. Environmental factors already suspected of being associated with some child’s health parameters were reinforced, such as maternal smoking exposures during pregnancy, a healthy lifestyle, indoor air pollutants and parabens. In addition, our findings pinpoint new environmental factors associated with child’s health, particularly the presence of a nearby bluespace during pregnancy and pets during childhood were associated with a better child’s general health score.
Interpretation of the results and comparison with the literature
Previously suspected environmental factors were identified in this study, in particular tobacco, diet, the social environment, metals and parabens. While tobacco, caffeinated drinks, indoor air pollutants, parabens and few contacts with family were associated with a poorer general health score, a healthy diet was associated with a better general health score. Interestingly, these six families of exposures have been highlighted as being associated with at least two health domains (among cardiometabolic, respiratory/allergy and mental health) in previous ExWAS studies conducted on the HELIX population [3,4,5,6,7,8]. It validates the assumption that using a general health score allows to identify the exposures associated with multiple health parameters.
Noteworthy, our study identified three exposures, namely pets, the presence of a bluespace and physical activity, that were not identified in previous HELIX studies on single health outcomes. It confirms our hypothesis on the added value of this approach which is able to detect exposures associated in a low-grade manner with multiple health parameters. In particular, this study indicates that the presence of pets during childhood could improve the overall child's health. The literature on pet’s exposure reports conflicting findings on its impact on allergies and asthma [36, 37]. Pets is a well-established source of allergens [38, 39] but being exposed to them early in life could actually prevent allergic diseases [40,41,42] through microbial and immune mechanisms [43]. Additionally, the literature supports that the presence of pets is associated with lower blood pressure and heart rate [44] as well as lower anxiety [45]. Moreover, our findings add to the limited but growing literature on the beneficial health impact of the presence of a bluespace nearby [46]. To the best of our knowledge, very few studies focused on the pregnancy period, but past studies in adults showed an association between better perceived health with the density of “coastal” land [47] and the proximity of coast [48]. Finally, our study confirmed the benefits of physical activity on child’s BMI [49], respiratory [50] and mental health [51, 52].
Unexpectedly, some positive associations have been found between postnatal blood concentration to three persistent organic pollutants (PFOA, HCB and DDE) and the child’s general health score. These cross-sectional associations could be due to an inverse causality phenomenon, with lower blood levels of DDE and HCB in overweight children due to accumulation in fat. When stratifying on BMI, opposite trends of associations were found for HCB in the low vs. high BMI groups, which supports that the body composition might impact these associations. Plus, a non-linear association was suggested for HCB, calling for further investigations. A confounding bias due to fish consumption could be induced for PFASs [35] but further adjusting on total fish consumption did not change the results. Our results are in agreement with similar unexpected results previously found in the HELIX population [4, 5, 8].
Strength and limitations of this study
This study has several strengths including first the longitudinal design of the HELIX project that allowed for an extended study of the exposome, with a wide range of exposures measured both during pregnancy and childhood using standardised protocols for each cohort site. A novelty of this study lies in the use of a general health score built by aggregating fifteen health parameters, covering three health domains with frequent childhood disorders: the cardiometabolic health (overweight), the respiratory health and allergies (asthma) and mental health (anxiety and behavioural disorders). A further strength relates in the ability of this approach to highlight exposures particularly harmful because affecting several health domains simultaneously, which can help prioritising public health messages.
However, we acknowledge that our study has some limitations. Some errors in exposure assessment could impact the statistical power, in particular regarding the least persistent pollutants like phenols and phthalates [53]. More generally, variability in measurement error between the exposures limits the ability to hierarchize the risk factors. Also, results regarding cross-sectional associations may suffer from reverse causality bias, for example the concentration of some persistent pollutants could be influenced by the child's health (through fat mass) instead of vice versa. In addition, the general health score, designed for etiological research but not for clinical purposes, has not been validated clinically. Finally, the same dataset has been used for the optimization of lambda and the model estimation which can be considered as a limit, even though cross-validation has been used for the first step.
Public health impact
The identified early-life environmental exposures associated with the general health of children, are suspected to have an impact on several health parameters simultaneously, calling for prioritized public health messages. In terms of public health recommendations, it is helpful to disentangle environmental risk factors affecting multiple health outcomes to those affecting a single health outcome or affecting in different direction several health outcomes.
Conclusion
This first exposome study on child’s general health attempted to approach the system nature of multiple exposures from our environment that shape multiple health outcomes. Our results reinforced the impact of several environmental risk factors (prenatal exposure to smoking, postnatal exposure to methylparaben, indoor air pollutants, caffeine and few social contacts) and protective factors (high intake of vegetables) on child’s health and identified new environmental protective factors (bluespace, pets) which calls for further investigation.
Fundings
The study received funding from the European Community’s Seventh Framework Programme (FP7/2007–206) (grant agreement no 308333 - HELIX project) and the H2020-EU.3.1.2. - Preventing Disease Programme (grant agreement no 874583 - ATHLETE project). JJ holds a Miguel Servet-II contract (grant CPII19/00015) awarded by the Instituto de Salud Carlos III (cofunded by the European Social Fund “Investing in your future”). RM and JW are supported by the NIHR Applied Research Collaboration for Yorkshire and Humber (NIHR200166). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. LM is funded by a Juan de la Cierva-Incorporación fellowship (IJC2018-035394-I) awarded by the Spanish Ministerio de Economía, Industria y Competitividad. NS has received funding from the Ministry of Science and Innovation and State Research Agency through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S) and from IJC2020-043630-I financed by MCIN/AEI/10.13039/501100011033 and the European Union “NextGenerationEU/PRTR”. The Born in Bradford (BiB) cohort study was supported by infrastructure grant 101597 from the Wellcome Trust and joint grant MR/N024391/1 from the UK Medical Research Council and UK Economic and Social Science Research Council. Data collection at Infancia y Medio Ambiente (INMA) was supported by grants from the Instituto de Salud Carlos III, Centro de Investigacion Biomedica en Red Epidemiologia y Salud Publica, and the Generalitat de Catalunya-CIRIT. The Kaunas cohort (KANC) was supported by grant 6-04-2014_31V-66 and on September 13, 2015, by No. 31V-77, from the Lithuanian Agency for Science Innovation and Technology. A full list of support for the Etude des Determinants Pre et Postnatals du Developpement et de la Sante de l’Enfant (EDEN) cohort is found in Heude B et al. Cohort profile: the EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol. 2016;45(2):353-363. The Norwegian Mother and Child Cohort Study (MoBa) is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), and NIH/NINDS (grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1). The RHEA Mother Child Cohort was supported by European projects and the Greek Ministry of Health (Program of Prevention of Obesity and Neurodevelopmental Disorders in Preschool Children, Heraklion, Crete, Greece: 2011–2014; “Rhea Plus”: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–2015).
Availability of data and materials
The datasets analysed during the current study are not available because they contain multiple sensitive identifiers.
Abbreviations
- ADHD:
-
Attention Deficit Hyperactivity Disorders
- BMI:
-
Body Mass Index
- BiB:
-
Born in Bradford
- BPA:
-
Bisphenol-A
- BUPA:
-
Butyl-paraben
- Cd:
-
Cadmium
- Co:
-
Cobalt
- Cu:
-
Copper
- DDE:
-
Dichlorodiphenyldichloroethylene
- DEHP:
-
Di EthylhexylPhthalate
- DEP:
-
Diethyl phosphate
- DMP:
-
Dimethyl phosphate
- DMTP:
-
Dimethyl thiophosphate
- EDEN:
-
Étude des Déterminants pré et postnatals du développement et de la santé de l’Enfant
- ETPA:
-
Ethylparaben
- FWER:
-
Family Wise Error Rate
- HCB:
-
Hexachlorobenzene
- Hg:
-
Mercury
- INMA:
-
Infancia y Medio Ambiente
- IOTF:
-
International Obesity Task Force
- KANC:
-
Kaunus Cohort
- KIDMED:
-
Mediterranean diet in children
- MBzP:
-
Mono benzyl phthalate
- DiNP:
-
Diisononyl phthalate
- MEP:
-
Monoethyl phthalate
- MEPA:
-
Methylparaben
- Mo:
-
Molybdenum
- MiBP:
-
Mono-iso butyl phthalate
- MoBa:
-
The Norwegian Mother Father and Child Cohort Study
- NDVI:
-
Normalized difference vegetation index
- NO2:
-
Nitrogen dioxide
- OC:
-
Organochlorine compound
- OP:
-
Organophosphate pesticide
- OXBE:
-
Oxybenzone
- Pb:
-
Plomb
- PBDE:
-
Polybrominated diphenyl ether
- PCB:
-
Polychlorobiphenyls
- PFASs:
-
Per- and polyfluoroalkyl substance
- PFHxS:
-
Perfluorohexane sulfonate
- PFNA:
-
Perfluorononanoate
- PFOA:
-
Perfluorooctanoate
- PFOS:
-
Perfluorooctane sulfonate
- PFUNDA:
-
Perfluoroundecanoate
- PM:
-
Particulate matter
- PRPA:
-
Propyl-paraben
- RHEA:
-
Mother–Child Cohort in Crete
- Se:
-
Selenium
- Tl:
-
Thalium
- TRCS:
-
Triclosan
- UV-Vit. D:
-
Vitamin-D dose from ultraviolet
References
Silveira PP, Portella AK, Goldani MZ, Barbieri MA. Developmental origins of health and disease (DOHaD). J Pediatr (Rio J). 2007;83(6):494–504. https://doi.org/10.2223/JPED.1728.
Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–50. https://doi.org/10.1158/1055-9965.EPI-05-0456.
Agier L, Basagaña X, Maitre L, et al. Early-life exposome and lung function in children in Europe: an analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet Health. 2019;3(2):e81–92. https://doi.org/10.1016/S2542-5196(19)30010-5.
Warembourg C, Maitre L, Tamayo-Uria I, et al. Early-Life Environmental Exposures and Blood Pressure in Children. J Am Coll Cardiol. 2019;74(10):1317–28. https://doi.org/10.1016/j.jacc.2019.06.069.
Vrijheid M, Fossati S, Maitre L, et al. Early-Life Environmental Exposures and Childhood Obesity: An Exposome-Wide Approach. Environ Health Perspect. 2020;128(6):67009. https://doi.org/10.1289/EHP5975.
Maitre L, Julvez J, López-Vicente M, et al. Early-life environmental exposure determinants of child behavior in Europe: A longitudinal, population-based study. Environ Int. 2021;153:106523. https://doi.org/10.1016/j.envint.2021.106523.
Granum B, Oftedal B, Agier L, et al. Multiple environmental exposures in early-life and allergy-related outcomes in childhood. Environ Int. 2020;144:106038. https://doi.org/10.1016/j.envint.2020.106038.
Julvez J, López-Vicente M, Warembourg C, et al. Early life multiple exposures and child cognitive function: A multi-centric birth cohort study in six European countries. Environ Pollut Barking Essex. 1987;2021(284):117404. https://doi.org/10.1016/j.envpol.2021.117404.
VanderWeele TJ. Outcome-wide Epidemiology. Epidemiology. 2017;28(3):399–402. https://doi.org/10.1097/EDE.0000000000000641.
Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res. 2010;690(1–2):24–39. https://doi.org/10.1016/j.mrfmmm.2009.09.005.
Halaris A. Inflammation-Associated Co-morbidity Between Depression and Cardiovascular Disease. Curr Top Behav Neurosci. 2017;31:45–70. https://doi.org/10.1007/7854_2016_28.
Friedman EM, Mroczek DK, Christ SL. Multimorbidity, inflammation, and disability: a longitudinal mediational analysis. Ther Adv Chronic Dis. 2019;10:2040622318806848. https://doi.org/10.1177/2040622318806848.
Thurston GD, Kipen H, Annesi-Maesano I, et al. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J. 2017;49(1):1600419. https://doi.org/10.1183/13993003.00419-2016.
Raat H, Botterweck A, Landgraf J, Hoogeveen W, Essink-Bot M. Reliability and validity of the short form of the child health questionnaire for parents (CHQ-PF28) in large random school based and general population samples. J Epidemiol Community Health. 2005;59(1):75–82. https://doi.org/10.1136/jech.2003.012914.
Vrijheid M, Slama R, Robinson O, et al. The human early-life exposome (HELIX): project rationale and design. Environ Health Perspect. 2014;122(6):535–44. https://doi.org/10.1289/ehp.1307204.
Maitre L, de Bont J, Casas M, et al. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open. 2018;8(9):e021311. https://doi.org/10.1136/bmjopen-2017-021311.
Wright J, Small N, Raynor P, et al. Cohort Profile: The Born in Bradford multi-ethnic family cohort study. Int J Epidemiol. 2013;42(4):978–91. https://doi.org/10.1093/ije/dys112.
Heude B, Forhan A, Slama R, et al. Cohort Profile: The EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol. 2016;45(2):353–63. https://doi.org/10.1093/ije/dyv151.
Guxens M, Ballester F, Espada M, et al. Cohort Profile: the INMA–INfancia y Medio Ambiente–(Environment and Childhood) Project. Int J Epidemiol. 2012;41(4):930–40. https://doi.org/10.1093/ije/dyr054.
Grazuleviciene R, Danileviciute A, Nadisauskiene R, Vencloviene J. Maternal Smoking, GSTM1 and GSTT1 Polymorphism and Susceptibility to Adverse Pregnancy Outcomes. Int J Environ Res Public Health. 2009;6(3):1282–97. https://doi.org/10.3390/ijerph6031282.
Magnus P, Birke C, Vejrup K, et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45(2):382–8. https://doi.org/10.1093/ije/dyw029.
Chatzi L, Leventakou V, Vafeiadi M, et al. Cohort Profile: The Mother-Child Cohort in Crete, Greece (Rhea Study). Int J Epidemiol. 2017;46(5):1392–1393k. https://doi.org/10.1093/ije/dyx084.
Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–91. https://doi.org/10.1183/09031936.95.08030483.
Haug LS, Sakhi AK, Cequier E, et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ Int. 2018;121(Pt 1):751–63. https://doi.org/10.1016/j.envint.2018.09.056.
Tamayo-Uria I, Maitre L, Thomsen C, et al. The early-life exposome: Description and patterns in six European countries. Environ Int. 2019;123:189–200. https://doi.org/10.1016/j.envint.2018.11.067.
Rigby RA, Stasinopoulos MD, Heller GZ, De Bastiani F. Distributions for Modeling Location, Scale, and Shape: Using GAMLSS in R. Chapman and Hall/CRC; 2019. https://doi.org/10.1201/9780429298547.
Ahrens W, Moreno LA, Mårild S, et al. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int J Obes. 2005;2014(38 Suppl 2):S4–14. https://doi.org/10.1038/ijo.2014.130.
Stratakis N, V Conti D, Jin R, et al. Prenatal Exposure to Perfluoroalkyl Substances Associated With Increased Susceptibility to Liver Injury in Children. Hepatol Baltim Md. 2020;72(5):1758–70. https://doi.org/10.1002/hep.31483.
Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7(1):17. https://doi.org/10.1186/1475-2840-7-17.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–99. https://doi.org/10.1002/sim.4067.
Tibshirani R. Regression Shrinkage and Selection via the Lasso. J R Stat Soc Ser B Methodol. 1996;58(1):267–88.
Wood AM, White IR, Royston P. How should variable selection be performed with multiply imputed data? Stat Med. 2008;27(17):3227–46. https://doi.org/10.1002/sim.3177.
Patel CJ, Bhattacharya J, Butte AJ. An Environment-Wide Association Study (EWAS) on Type 2 Diabetes Mellitus. PLoS ONE. 2010;5(5):e10746. https://doi.org/10.1371/journal.pone.0010746.
Li MX, Yeung JMY, Cherny SS, Sham PC. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet. 2012;131(5):747–56. https://doi.org/10.1007/s00439-011-1118-2.
Papadopoulou E, Haug LS, Sakhi AK, et al. Diet as a Source of Exposure to Environmental Contaminants for Pregnant Women and Children from Six European Countries. Environ Health Perspect. 2019;127(10):107005. https://doi.org/10.1289/EHP5324.
Fretzayas A, Kotzia D, Moustaki M. Controversial role of pets in the development of atopy in children. World J Pediatr WJP. 2013;9(2):112–9. https://doi.org/10.1007/s12519-013-0412-6.
Chen CM, Tischer C, Schnappinger M, Heinrich J. The role of cats and dogs in asthma and allergy–a systematic review. Int J Hyg Environ Health. 2010;213(1):1–31. https://doi.org/10.1016/j.ijheh.2009.12.003.
AlShatti KA, Ziyab AH. Pet-Keeping in Relation to Asthma, Rhinitis, and Eczema Symptoms Among Adolescents in Kuwait: A Cross-Sectional Study. Front Pediatr. 2020;8:331. https://doi.org/10.3389/fped.2020.00331.
Apelberg BJ, Aoki Y, Jaakkola JJ. Systematic review: Exposure to pets and risk of asthma and asthma-like symptoms. J Allergy Clin Immunol. 2001;107(3):455–60. https://doi.org/10.1067/mai.2001.113240.
Dharmage SC, Lodge CL, Matheson MC, Campbell B, Lowe AJ. Exposure to cats: update on risks for sensitization and allergic diseases. Curr Allergy Asthma Rep. 2012;12(5):413–23. https://doi.org/10.1007/s11882-012-0288-x.
Korppi M, Hyvärinen M, Kotaniemi-Syrjänen A, Piippo-Savolainen E, Reijonen T. Early exposure and sensitization to cat and dog: different effects on asthma risk after wheezing in infancy. Pediatr Allergy Immunol. 2008;19(8):696–701. https://doi.org/10.1111/j.1399-3038.2008.00758.x.
Carlsten C, Dimich-Ward H, Becker AB, et al. Indoor allergen exposure, sensitization, and development of asthma in a high-risk birth cohort. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e740–746. https://doi.org/10.1111/j.1399-3038.2010.01021.x.
Kim H, Sitarik AR, Woodcroft K, Johnson CC, Zoratti E. Birth Mode, Breastfeeding, Pet Exposure, and Antibiotic Use: Associations With the Gut Microbiome and Sensitization in Children. Curr Allergy Asthma Rep. 2019;19(4):22. https://doi.org/10.1007/s11882-019-0851-9.
Ein N, Li L, Vickers K. The effect of pet therapy on the physiological and subjective stress response: A meta-analysis. Stress Health J Int Soc Investig Stress. 2018;34(4):477–89. https://doi.org/10.1002/smi.2812.
Gadomski AM, Scribani MB, Krupa N, Jenkins P, Nagykaldi Z, Olson AL. Pet Dogs and Children’s Health: Opportunities for Chronic Disease Prevention? Prev Chronic Dis. 2015;12:E205. https://doi.org/10.5888/pcd12.150204.
Gascon M, Zijlema W, Vert C, White MP, Nieuwenhuijsen MJ. Outdoor blue spaces, human health and well-being: A systematic review of quantitative studies. Int J Hyg Environ Health. 2017;220(8):1207–21. https://doi.org/10.1016/j.ijheh.2017.08.004.
Wheeler BW, Lovell R, Higgins SL, et al. Beyond greenspace: an ecological study of population general health and indicators of natural environment type and quality. Int J Health Geogr. 2015;14:17. https://doi.org/10.1186/s12942-015-0009-5.
Wheeler BW, White M, Stahl-Timmins W, Depledge MH. Does living by the coast improve health and wellbeing? Health Place. 2012;18(5):1198–201. https://doi.org/10.1016/j.healthplace.2012.06.015.
Cesa CC, Sbruzzi G, Ribeiro RA, et al. Physical activity and cardiovascular risk factors in children: meta-analysis of randomized clinical trials. Prev Med. 2014;69:54–62. https://doi.org/10.1016/j.ypmed.2014.08.014.
Lochte L, Nielsen KG, Petersen PE, Platts-Mills TAE. Childhood asthma and physical activity: a systematic review with meta-analysis and Graphic Appraisal Tool for Epidemiology assessment. BMC Pediatr. 2016;16:50. https://doi.org/10.1186/s12887-016-0571-4.
Korczak DJ, Madigan S, Colasanto M. Children’s Physical Activity and Depression: A Meta-analysis. Pediatrics. 2017;139(4):e20162266. https://doi.org/10.1542/peds.2016-2266.
Álvarez-Bueno C, Pesce C, Cavero-Redondo I, Sánchez-López M, Martínez-Hortelano JA, Martínez-Vizcaíno V. The Effect of Physical Activity Interventions on Children’s Cognition and Metacognition: A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(9):729–38. https://doi.org/10.1016/j.jaac.2017.06.012.
Casas M, Basagaña X, Sakhi AK, et al. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ Int. 2018;121(Pt 1):561–73. https://doi.org/10.1016/j.envint.2018.09.046.
Acknowledgments
We are grateful to all the participating families, practitioners, schools and researchers in France, Greece, Lithuania, Spain, Norway and UK who made this study happen. This project is only possible because of their enthusiasm and commitment. Additionnaly, ISGlobal acknowledges support from the “Centro de Excelencia Severo Ochoa 2019-2023” Program 2018-000806-S from the Spanish Ministry of Science and Innovation, and from the Generalitat de Catalunya through the Centres de Recerca de Catalunya (CERCA) Program.
Author information
Authors and Affiliations
Contributions
M.Vr., R.S., M.N., J.W., X.B. and B.G. have made substantial contributions to the conception of the Helix study. M.C., M.dC., A.D., B.G., R.G., B.H., L.H., J.J., M.L., R.M., L.M., R.S., V.S., M.N., M.Va., M.Vr., J.W., T.Y. have made substantial contributions to the acquisition of the data. I.A., A.G. and V.S. defined the strategy of analysis, conducted the statistical analysis, wrote the main manuscript text and prepared the figures and tables. I.A., A.G., C.P. and V.S. have made substantial contributions to the interpretation of the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Local ethics committee approved the consent form for each cohort and we obtained written informed consent from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
eMethods 1. Health outcomes assessment. eMethods 2. Urban exposure assessment methods (extracted from Maitre12). eMethods 3. Assessment methods for lifestyle factors and other (extracted from Maitre12). eMethods 4. Biomarker assessment methods (extracted from Maitre12). eMethods 5. Creation of the general health score. eMethods 6. Data pre-processing. eMethods 7. Exposome-health association study. eTable 1. Collection time points of health parameters in childhood (mean, SD). eTable 2. Biological matrices of maternal and child samples. eTable 3. Collection time points of prenatal exposures, eTable 4.Collection time points of maternal blood and urine samples (mean, SD). eTable 5. Standardization of the cardiometabolic parameters. eTable 6. Standardization of the mental and cognitive parameters. eTable 7. Factor analysis on respiratory and allergy-related parameters – Dimension 1. eTable 8. Factor analysis on mental and cognitive parameters – Dimension 1. eTable 9. Transformation applied to continuous postnatal exposures and IQR. eTable 10. Description of transformed urban exposures. eTable 11. Description of exposures regarding the lifestyle. eTable 12. Description of biomarkers in the Helix population. eTable 13. Description of the health parameters considered. eTable 14. Correlations between the three sub-scores. eTable 15. Description of the general health score by covariates. eTable 16. Description of the general health score divided in terciles with health parameters and- sub-scores. eTable 17. Complete results of the ExWAS on prenatal exposures. eTable 18. Complete results of the ExWAS on postnatal exposures. eTable 19. Final multivariable models with and without fish consumption as a confounder (results for postnatal PFOA). eFigure 1. Variable plot - Mixed Factorial Analysis on standardized variables of mental health (Helix subcohort, n=1278 with mental health data). eFigure 2. Variable plots - Mixed Factorial Analysis on standardized variables of respiratory health and allergies (Helix subcohort, n=1009 with respiratory and allergic data). eFigure 3. Distribution of each sub-score by cohort. eFigure 4. Multi-exposure model on postnatal exposures stratifiedon the terciles of z-BMI. eFigure 5. Multi-exposure model on prenatal exposures stratified on sex. eFigure 6. Multi-exposure model on postnatal exposures stratified on sex. eFigure 7. Multi-exposure model on postnatal exposures stratified on cohorts. eFigure 8. Multi-exposure model on postnatal exposures stratified on cohorts. eFigure 9. GAM estimated curve for postnatal selected exposures by LASSO. eFigure 10. Multi-exposure model without the 4% most extreme values for the general health score.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amine, I., Guillien, A., Philippat, C. et al. Environmental exposures in early-life and general health in childhood. Environ Health 22, 53 (2023). https://doi.org/10.1186/s12940-023-01001-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-023-01001-x