Abstract
Background
Higher prenatal ambient air pollution exposure has been associated with impaired neurodevelopment in preschoolers and school-aged children. The purpose of this study was to explore the relationships between prenatal ambient air pollution exposure and neurodevelopment during infancy.
Methods
This study examined 161 Latino mother-infant pairs from the Southern California Mother’s Milk Study. Exposure assessments included prenatal nitrogen dioxide (NO2) and particulate matter smaller than 2.5 and 10 microns in diameter (PM2.5 and PM10, respectively). The pregnancy period was also examined as three windows, early, mid, and late, which describe the first, middle, and last three months of pregnancy. Infant neurodevelopmental outcomes at 2 years of age were measured using the Bayley-III Scales of Infant and Toddler Development. Multivariable linear models and distributed lag linear models (DLM) were used to examine relationships between prenatal exposures and neurodevelopmental scores, adjusting for socioeconomic status, breastfeeding frequency, time of delivery, pre-pregnancy body mass index, and infant birthweight and sex.
Results
Higher prenatal exposure to PM10 and PM2.5 was negatively associated with composite cognitive score (β = -2.01 [-3.89, -0.13] and β = -1.97 [-3.83, -0.10], respectively). In addition, higher average prenatal exposure to PM10 was negatively associated with composite motor (β = -2.35 [-3.95, -0.74]), scaled motor (β = -0.77 [-1.30, -0.24]), gross motor (β = -0.37 [-0.70, -0.04]), fine motor (β = -0.40 [-0.71, -0.09]), composite language (β = -1.87 [-3.52, -0.22]), scaled language (β = -0.61 [-1.18, -0.05]) and expressive communication scaled scores (β = -0.36 [-0.66, -0.05]). DLMs showed that higher prenatal air pollution exposure during mid and late pregnancy was inversely associated with motor, cognitive, and communication language scores.
Conclusions
Higher exposure to air pollutants during pregnancy, particularly in the mid and late prenatal periods, was inversely associated with scaled and composite motor, cognitive, and language scores at 2 years. These results indicate that prenatal ambient air pollution may negatively impact neurodevelopment in early life.
Similar content being viewed by others
Background
The World Health Organization estimates that more than 90% of the world’s population is exposed to particulate matter (PM) exceeding its recommended levels for healthy air and that air pollution is responsible for 7 million premature deaths annually [1]. The burden of exposure to air pollutants is unequally distributed in the US, where racial/ethnic minorities and low-income populations experience higher average levels of pollution than non-minority and high-income populations [2, 3]. For example, Latinos experience 63% more exposure to air pollution than they are responsible for creating [4]. The health consequences of these disparities are important, as epidemiological evidence suggests that polluted air is one of the leading factors associated with development of respiratory illness, cardiovascular disease, and lung cancer [5]. In addition, a growing body of evidence suggests that exposure to air pollutants has adverse influences on cognitive development in children [6, 7]. Children in general are exposed to higher levels of air pollutants due to an increased respiratory rate relative to adults [8]. Further, the emergence of cognitive capacities during childhood depends on earlier brain maturation during critical windows of development, particularly during fetal development [9]. Exposure to air pollutants during these critical periods may be more likely to adversely influence brain and cognitive development.
Studies assessing the association of prenatal air pollution exposure and cognitive development in children have predominantly focused on school aged (6–12 years) children [10,11,12,13,14,15,16] with most exceptions assessing these associations from early childhood (1–4 years) to school age [17,18,19]. Among school-aged children, prenatal exposure to polycyclic aromatic hydrocarbons (PAH) has been linked with reductions in left hemisphere white matter surface at age 8, and prenatal PM2.5 exposure has been shown to be inversely associated with cortex thickness, altered white matter organization, and reduced blood flow at 6–14 years. [12, 13, 20] Prenatal PM10 exposure has also been inversely associated with full-scale IQ at ages 4–6 years, and prenatal NO2 exposure and traffic density around the home has been inversely associated with verbal IQ scores at 7 years of age [10, 11]. Further, studies have shown that prenatal PM2.5 exposure is inversely associated with conflict network performance at ages 8–9 years, and prenatal NO2 exposure is associated with poorer attentional performance at 4–5 years of age [14, 15]. Additionally, studies have found that PM2.5 exposure between weeks 12–40 is associated with lower IQ, slower reaction times, and poorer memory at 6–7 years, [16] while NO2 exposure between weeks 6–14 and 32–35 is associated with increased hit reaction time at age 7 [21]. Studies assessing children younger than school age report that prenatal exposure to NO2 is associated with poorer cognitive development at ~14 months [19] and poorer global psychomotor development [17] between 1–6 years while prenatal PAH exposure is associated with poorer cognitive development at age 3 [18]. In addition, PM10, PM2.5, and NO2 exposure in the late-prenatal and early postnatal periods have been associated with an increased risk of ADHD-like behaviors in children around age 3 [22].
Previous work highlights that prenatal exposure to air pollutants is critically important since cognitive and motor capacities during childhood and beyond depend upon the proper sequencing of complex maturational events during gestation, which air pollution may disrupt, including neurogenesis, neuronal migration, axonal and dendritic arborization, synaptogenesis, myelination, apoptosis, and neural circuit formation [23,24,25,26]. Many of these processes occur throughout gestation where neurogenesis can begin as early as week six and end by mid-gestation while others, such as circuit formation and myelination, are not observed until mid-gestation and continue beyond birth at rapid pace [23,24,25,26]. Additionally, a substantial body of work highlights how disruptive events (environmental, immunological, stress) during pregnancy negatively impact neurodevelopment [27,28,29], where air pollution research has primarily implicated the mid-late gestational periods, when migration, synaptogenesis, myelination, and circuit formation begin to occur [24, 26, 29, 30], as critical windows of exposure [16, 21, 22].
The primary aim of the current study was to assess the association of residential prenatal exposure to ambient air pollutants—PM2.5, PM10, NO2—and neurodevelopmental outcomes at 2 years of age in infants from the Southern California Mother’s Milk Study. We also sought to determine whether these pollutants exert their adverse influences during critical windows of prenatal development. We hypothesized that residential exposure to ambient air pollutants during pregnancy would be associated with poorer cognitive and motor development at 2 years of age. We also hypothesized that the adverse outcomes associated with exposure would be most evident with higher exposure levels in late pregnancy, the time of rapid neural circuit formation.
Methods
Participants were recruited from the Mother’s Milk Study, a longitudinal cohort of Latino mother infant pairs from Southern California [31,32,33,34,35,36]. Recruitment began in 2016 from maternity clinics associated with the University of Southern California and Children’s Hospital Los Angeles. The Mother’s Milk study was designed to examine the effects of human breast milk components on the growth and development of infants. Inclusion criteria included: 1) mothers and fathers who self-identify as Hispanic/Latino, and their infants; 2) singleton birth; 3) mother’s declared intent to breastfeed for at least 3-months; 4) mother’s enrollment within one month of the infant’s birth; 5) mother’s ability to read and comprehend English or Spanish at a 5th grade level to understand study procedures and provide informed consent. Exclusion criteria included: 1) diagnosis of significant illness (including type 1 or type 2 diabetes) or eating disorder; 2) cognitive or physical constraints that prevent participation; 3) medication use that could affect physical health, metabolism, or weight; 4) current smoking (defined as more than 1 cigarette in the past week) or use of other recreational drugs; 5) pre-term birth or diagnosis of fetal abnormalities; and 6) mothers younger than 18 years of age. Written informed consent was obtained from mothers prior to enrollment. The institutional review boards of the University of Southern California, Children’s Hospital Los Angeles, and University of Colorado Boulder each gave their approval for the study.
Clinical assessments
At the time of the current analysis, 219 participants were enrolled in the study, of whom 196 had complete residential address histories for air pollution exposure estimates during pregnancy. Of these, 166 completed the 2-year follow up visit, when neurodevelopmental outcomes were assessed in the infants. Of those with outcome data, 5 were removed due to lacking data for time of delivery, leaving us with 161 mother-infant pairs for analysis. Maternal and family medical histories, including relevant covariate data, were collected at the 1-month postpartum visit. Infant outcomes were assessed at the 2-year study visit. Maternal pre-pregnancy body mass index (BMI) (kg/m2) was measured by self-reported recall of pre-pregnancy height (m) and weight (kg). Infant birthweight (kg) was obtained from hospital records. Categorical time of delivery was self-reported by mothers to estimate gestational age. Values included on-time, early (≥ 2 weeks before due date), and late (≥ 2 weeks after due date). Infant breast feedings per day were based on questionnaire data with answer choices of 0–1, 1, 2, 3, 4, 5, 6, 7, and ≥ 8 breast feedings per day. We then assigned 0–1 as 0 feedings per day, 1–7 as their reported values, and ≥ 8 as 8 before treating breast feeding as a continuous variable. Socioeconomic status (SES) was calculated using a modified version of the Hollingshead Index, with missing values replaced by the median [37]. The Hollingshead index combines data on marital status, sex, educational attainment, and occupational prestige to create a numerical index of an individual’s SES [38].
Neurodevelopmental outcomes
Neurodevelopmental outcomes were assessed at the 2-year follow up visit using the Bayley Scales of Infant and Toddler Development – Third Edition (BSID-III). Trained research personnel administered the BSID-III under the close supervision of an expert in child developmental assessment. The BSID-III provides information on 5 domains of infant development: cognitive, motor, language, social emotional, and adaptive behavior. Cognitive, motor, and language domains were assessed in an interactive examination lasting approximately 2 h. Social emotional and adaptive behavior were assessed via parent-reported questionnaires. We elected to restrict our analysis to cognitive, motor, and language domains which each produce a scaled and composite score. In addition, the motor domain includes scaled sub-scores for fine and gross motor and the language domain includes scaled sub-scores for expressive and receptive communication. Composite scores are used to describe overall development in the relative domain of the BSID-III. Raw scores for each domain are age-scaled and then transformed to scaled and composite scores that have means of 10 and 100 and standard deviations of 3 and 15, respectively. Scores are all age-adjusted.
Ambient air pollution exposure
Participants provided residential address histories that included the entire pregnancy period. Residential addresses were geocoded using the Texas A&M Geocoder (http://geoservices.tamu.edu/Services/Geocode/). Individual residential estimates for ambient air pollutants were modeled from geocoded address data and data from the US Environmental Protection Agency’s Air Quality System (AQS) via an inverse distance-squared weighting algorithm. These estimates use data from up to four AQS stations within 50 km of participants homes and have been previously shown to exhibit reasonable error and low bias in California [39]. These pollutants include nitrogen dioxide (NO2) and particulate matter less than 10 and 2.5 microns in aerodynamic diameter (PM10 and PM2.5, respectively). NO2 was measured in parts per billion (ppb) while PM10 and PM2.5 were measured in micrograms per meter cubed (μg/m3). Pollution estimates are recorded hourly and daily and were then averaged across the prenatal period. We first examined the entire prenatal (pregnancy) period, estimated as the average exposure during the 9 months prior to birth since information regarding timing of conception was unavailable. We then looked at each individual monthly lag of pregnancy exposure to search for critical windows. We refer to the pregnancy period in terms of three windows, early, mid, and late, which describe the first, middle, and last three months of pregnancy, respectively.
Statistical analysis
Two approaches were used to examine the associations of prenatal ambient pollution with developmental outcomes at 2 years. First, multivariable linear regression was used to examine the associations of prenatal air pollution exposure (9-month pregnancy period) with neurodevelopmental outcomes. Effect estimates for these analyses are reported for a one standard deviation (SD) difference in exposure. Second, distributed lag linear models (DLMs) were used to assess the associations of monthly ambient air pollution exposures during the 9 months prior to birth with developmental outcomes at 2 years. Briefly, DLMs allow for the analysis of correlated time-varying exposure variables that can have differential effects over time rather than all at once. DLMs fit a regression that simultaneously includes all monthly exposures, such that the association for a specific month is adjusted for all other exposure windows. Effect estimates for the DLMs were scaled to an interquartile range (IQR) increase across all monthly exposures for each pollutant. IQR was used in place of SD for DLMs to aid in the comparison of effect estimates between each monthly exposure window since standard deviations varied by month. Effect estimates for each modeling approach were reported with 95% confidence intervals (CIs). Primary scores from the cognitive, motor, and language domains of the BSID-III were assessed in addition to motor and language subscale scores (fine motor, gross motor; receptive communication, expressive communication). The associations between average prenatal air pollution exposures with developmental outcomes were also examined for non-linearity by fitting generalized additive models (GAMs). Models with statistically significant smooth terms (p < 0.05) and effective degrees of freedom greater than one (edf > 1) were flagged as non-linear and investigated based on tertiles of exposure using multivariable linear regression. This included the associations of prenatal PM10 exposure with composite, scaled, and gross motor (pGAM < 0.05). For these associations, we also fitted GAMs for each individual monthly exposure during the prenatal period to assess the validity of applying linear DLMs. Among the three outcomes assessed (composite, scaled, and gross motor), each had only one month of PM10 exposure that showed some evidence of non-linearity. Therefore, we proceeded to use linear DLMs in all analyses.
All statistical models adjusted for SES, number of breast feedings per day, infant gestational age (early/late/on time), pre-pregnancy BMI (kg/m2), infant birthweight (kg) and infant sex. These covariates were chosen by examining choice covariates in relevant literature. However, univariate associations between prenatal exposures and neurodevelopment outcomes are also reported in Supplemental Table 2. Based on previous studies, [14, 16] infant sex was also examined as a potential effect modifier of the relationships between prenatal ambient air pollution and neurodevelopmental outcomes via an interaction term in multivariable linear regression models. Reported values for descriptive statistics are shown as means ± standard deviations for continuous variables and as percentages (%) for categorical variables. Correlations among pollutants and exposure windows were examined using Spearman’s rank tests. Statistical significance for this study was defined as a two-sided p-value less than 0.05 for our models. However, we also report p-values that were adjusted for multiple hypothesis testing when examining the associations between the three average prenatal exposures and the 10 neurodevelopmental outcomes using a false discovery rate (FDR) of 10% with the Benjamini-Hochberg (BH) procedure (PFDR < 0.10). All statistical analyses were performed in R (Version 4.0.2) and figures were made in R or PRISM (Version 9.4).
Results
This analysis examined 161 Latino mother-infant pairs (Table 1). Briefly, 57% of infants were female. Average maternal age was 29.02 years (range: 18–45) at enrollment. Mothers in our sample were on average overweight, with an average pre-pregnancy BMI of 28.55 kg/m2. Most families were of a low SES according to the Hollingshead index with scores ranging from 3–68 (average: 26.81). Average ambient air pollution exposure for each individual prenatal monthly lag is shown in Table 2. The correlations between average prenatal exposures of PM10, PM2.5, and NO2 ranged from 0.33 to 0.76 (Table 3) while correlations among individual monthly lags of exposure ranged from -0.30 to 0.68 for PM10, -0.29 to 0.46 for PM2.5, and -0.79 to 0.82 for NO2 (Fig. 1). Correlations among individual monthly lags of exposure between pollutants are shown in Supplemental Table 1.
Correlations Among Exposure Windows During the 9-Month Pregnancy Period. Figures show the Spearman correlation structure among individual monthly lags of prenatal ambient air pollution exposure for NO2 (left), PM10 (middle), and PM2.5 (right). Blue colors indicate positive correlations while red colors indicate negative correlations. Statistical significance is denoted as *p < 0.05, **p < 0.01, and ***p < 0.001, respectively
Prenatal exposure to ambient air pollution was associated with neurodevelopmental outcomes at 2 years
Higher prenatal PM10 and PM2.5 exposure were associated with lower cognitive outcome scores at 2 years of age (Table 4). Specifically, prenatal exposure to PM10 and PM2.5 were negatively associated with composite cognitive score (β = -2.01, p = 0.04; β = -1.97, p = 0.04, respectively). In addition, prenatal PM10 exposure was negatively associated with all measures related to motor function, including fine motor scaled score (β = -0.40, p = 0.01), gross motor scaled score (β = -0.37, p = 0.03), total motor scaled score (β = -0.77, p < 0.01), and composite motor score (β = -2.35, p < 0.01). Prenatal exposure to PM10 was also inversely associated with composite language score (β = -1.87, p = 0.03), scaled language score (β = -0.61, p = 0.03), and expressive communication score (β = -0.36, p = 0.02). Of note, and as shown in Fig. 2, PM10 was negatively associated with the composite scores for each of the neurodevelopmental domains examined in this study. Average prenatal exposure to NO2 was not associated with neurodevelopmental outcomes at 2 years of age. After correction for multiple hypothesis testing, only prenatal PM10 exposure remained significantly associated with motor scaled score (PFDR = 0.069) and composite motor score (PFDR = 0.069) at a 10% false discovery rate. Results from univariate models were largely consistent except for the association between prenatal PM2.5 and composite cognitive score, which was no longer statistically significant when not adjusting for our a priori covariates (Supplemental Table 2). Additionally, only the associations between prenatal NO2 with gross motor scaled score and language composite score differed by infant sex (Pint = 0.02 and Pint = 0.05, respectively). Specifically, prenatal NO2 was positively associated with gross motor scaled score among females (β = 0.20, p = 0.03) but not males (β = -0.16, p = 0.14). Also, prenatal NO2 tended to be negatively associated with gross motor scaled score among males (β = -0.96, p = 0.06) but not females (β = 0.62, p = 0.20). No other associations between prenatal ambient air pollution exposure and neurodevelopmental outcomes differed by infant sex (Pinteractions > 0.05). Lastly, GAMs for the associations between prenatal PM10 exposure and motor scores demonstrated some evidence of non-linearity (pGAM = 0.007, edf = 3.03; pGAM = 0.04, edf = 2.95 and pGAM = 0.01, edf = 3.0, respectively) (Supplement Fig. 1). To identify the best modeling approach, we ran GAMs containing separate terms for the linear and non-linear effects of PM10 and found that the non-linear effects of PM10 with composite, scaled, and fine motor scores were statistically significant (p = 0.04, p = 0.04, p = 0.05, respectively), while the linear effects were not. Plots of the fitted associations appeared cubic, therefore we examined prenatal PM10 exposure in tertiles (n = 54 and 18.7–27.1 µg/m3, n = 53 and 27.1–31.2 µg/m3, n = 54 and 31.2–38.2 µg/m3) to explore these non-linear associations. Overall, positive, yet non-statistically significant, associations were observed between prenatal PM10 exposure with composite, scaled, and fine motor scores in the bottom (β = 1.60, β = 0.57, β = 0.03, respectively) and top tertiles (β = 3.35, β = 1.10, β = 0.58, respectively). Whereas negative associations between prenatal PM10 exposure with composite, scaled, and gross motor scores were observed in the middle tertile (β = -6.54, β = -2.12, β = -0.86, respectively).
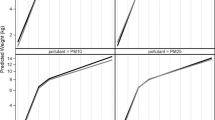
Associations Between Prenatal PM10 Exposure and Composite Cognitive, Motor, and Language Scores at 2 Years. Average prenatal exposure to PM10 (µg/m3) was inversely associated with composite cognitive score (A), composite motor score (B), and composite language score (C). Unadjusted plots and regression lines for neurodevelopmental scores and prenatal PM10 are shown. Figures show betas (β) and 95% confidence intervals (CIs) that were scaled to a 1SD difference in exposure (SD NO2 = 2.43 ppb, SD PM10 = 3.94 µg/m3, SD PM2.5 = 1.24 µg/m3) from multivariable linear regression models that adjusted for socioeconomic status (SES), breast feedings per day, gestational age, pre-pregnancy BMI, infant birthweight, and infant sex
Mid/Late prenatal ambient air pollution was inversely associated with neurodevelopmental outcomes at 2 years
DLMs were used to examine monthly prenatal ambient air pollution exposures to identify critical windows of exposure that may be related to neurodevelopmental outcomes at 2 years of age. Results from this analysis indicated that prenatal exposure during the 1 to 5 months prior to birth was associated with neurodevelopmental outcomes at 2 years of age. For instance, we observed significant negative associations between prenatal exposure to PM10 and PM2.5 and both composite and scaled cognitive scores during mid/late pregnancy (Fig. 3). As shown in Fig. 4, significant negative associations were also found between prenatal PM10 and composite, scaled, gross, and fine motor scores during mid/late pregnancy. In addition, higher prenatal PM2.5 during late pregnancy was significantly associated with lower composite, scaled, and fine motor scores during late pregnancy (Fig. 5). Lastly, as shown in Table 5 we observed several other significant negative associations between NO2, PM10, and PM2.5 during specific prenatal months and other neurodevelopmental scores. Results from all the DLM models can be found in Supplemental Table 3.
Associations Between PM10 and PM2.5 Exposure During Mid to Late Pregnancy and Composite Cognitive Scores at 2 Years. Figures show effect sizes and 95% confidence intervals (CI) at each monthly lag of exposure during the pregnancy period. Results were obtained from distributed lag models (DLMs) that adjusted for socioeconomic status (SES), breast feedings per day, gestational age, pre-pregnancy BMI, infant birthweight, and infant sex. Panels show associations between PM2.5 and composite cognitive score (A), PM10 and composite cognitive score (B). Effect sizes are scaled by the IQR of each respective pollutant (PM10 = 8 µg/m3, PM2.5 = 3 µg/m3). Statistically significant windows are denoted by red squares (p < 0.05)
Associations Between PM10 Exposure During Mid and Late Pregnancy and Motor Scores at 2 Years. Figures show effect sizes and 95% confidence intervals (CI) at each monthly lag of exposure during the pregnancy period. Results were obtained from distributed lag models (DLMs) that adjusted for socio-economic status, breast feedings per day, gestational age, pre-pregnancy BMI, infant birthweight, and infant sex. Panels show associations between PM10 and composite motor score (A), scaled motor score (B), gross motor score (C) and fine motor score (D). Effect sizes are scaled by the IQR (PM10 = 8 µg/m3). Statistically significant windows are shown in red (p < 0.05)
Associations between PM2.5 Exposure During Mid to Late Pregnancy and Motor Scores at 2 Years. Figures show effect sizes and 95% confidence intervals (CI) at each monthly lag of exposure during the pregnancy period. Results were obtained from distributed lag models (DLMs) that adjusted for socio-economic status, breast feedings per day, gestational age, pre-pregnancy BMI, infant birthweight, and infant sex. Panels show associations between PM2.5 and composite motor score (A), scaled motor score (B), and fine motor score (C). Effect sizes are scaled by the IQR (PM2.5 = 3 µg/m3). Statistically significant windows are shown in red (p < 0.05)
Discussion
Our analysis revealed that higher average prenatal exposure to PM10 and PM2.5 was adversely associated with functional neurodevelopmental outcomes at 2 years. As an example, those exposed to PM10 levels at the 75th percentile (32.50 µg/m3) compared to the 25th percentile (26.64 µg/m3) of exposure were predicted to have a three point lower composite cognitive score. For context, in the current study, 16% of participants had a composite cognitive score that indicated some degree of impairment [40, 41]. If all participants had PM10 levels at the 75th percentile of exposure, the prevalence of cognitive impairment would be predicted to increase to 22%, which highlights the importance of even moderate increases in early-life exposure. Lastly, distributed lag modeling suggested that exposures during mid/late were inversely associated with neurodevelopment. These exposure periods overlap with developmental processes such as myelination, neuronal migration, synaptogenesis, apoptosis, and rapid neurogenesis and circuit formation that support the emergence of functional sensory systems, motor systems, and connectivity networks [23,24,25,26]. These findings support the growing body of work that indicates adverse effects of prenatal air pollution exposure on cognitive development and further suggest the presence of critical neurodevelopmental windows which exhibit increased sensitivity to environmental exposures. [10,11,12,13,14,15,16,17,18,19,20]
While the exact mechanisms by which prenatal ambient air pollution may impact neurodevelopment have yet to be fully characterized, current evidence suggests that these exposures can increase neuroinflammation and oxidative stress that may interfere with brain development. In utero, air pollutants may come into direct contact with the fetus where black carbon has been found on the fetal side of the placenta, [42] and rodent studies have found nanoparticles in fetal blood following prenatal exposure to diesel exhaust [43]. Further, exposure to ambient air pollution has been associated with increased activation of astroglia and microglia in vitro [44] that may negatively impact synaptic pruning [45, 46]. PM exposure has also been associated with increased levels of inflammatory mediators in brain tissue, cerebrospinal fluid, and serum from individuals with chronic exposure to air pollution [47,48,49,50]. Lastly, while there is mixed evidence regarding whether pro-inflammatory cytokines can successfully cross the placenta, [51,52,53] it is well established that the placenta is capable of its own cytokine production [54,55,56,57] where acute and chronic maternal inflammation during pregnancy has been associated with neurodevelopmental disorders [58,59,60,61,62]. This is important since increased levels of neuroinflammation have been linked to morphological changes in the brain in animal models [63, 64]. Together, these studies suggest several mechanisms by which prenatal ambient air pollution may impact neurodevelopment in early life.
While this study adds to a growing body of evidence that prenatal ambient air pollution exposure is associated with neurodevelopmental outcomes in early life, these results should be interpreted in the context of the study’s limitations. Upon adjustment for multiple hypothesis testing, only the associations between PM10 and composite and scaled motor score remained statistically significant as determined by a 10% false discovery rate. However, this may be due to our relatively small sample since many of the associations that we observed in the current study are consistent with other work [10, 11, 16, 65]. Further, our small sample size limits our ability to draw conclusions regarding single sex-specific associations as well as the interpretability of our non-linear models. Since this study was restricted to Latino infants, generalizability of our results may be limited; however, Latino communities are known to experience higher disease burden, disproportionate levels of air pollution exposure that may adversely impact human health, and remain understudied in biomedical research [2,3,4, 66,67,68]. For example, average 9-month prenatal exposure levels observed in our cohort were higher than the most recent World Health Organization guidelines for yearly air quality where average PM2.5, PM10, and NO2 exposures were approximately 6.88 µg/m3, 14.74 µg/m3, and 12 ppb higher than current guidelines, respectively. [1]
In the current study, neurodevelopmental assessments were made using the BSID-III, which is one of the most commonly used assessments for infant development [69, 70]. Nevertheless, the accuracy of the BSID-III in predicting future outcomes is limited; BSID-III cognitive scores at 2 years, for example, have been found to overestimate performance when compared with full-scale IQ at 4 years. [71] Also, while we did not have data regarding home environment or maternal IQ, all models adjusted for SES in an attempt to capture some of these potentially important factors in child neurodevelopment [72, 73]. Additionally, since prenatal pollution estimates were based on geocoded residential addresses, they may not fully reflect maternal time-activity patterns and do not capture indoor sources of air pollution. While these factors may contribute to exposure misclassification, this would be random amongst the sample and would likely bias results to the null [74]. Further, since the current study focused on prenatal exposures, we cannot rule out the potential importance of postnatal exposures and their impact on neurodevelopment. Future work in this cohort will aim to expand these analyses by examining the relative importance of pre- and postnatal exposures on neurodevelopment. Lastly, while this study looked at several air pollutants, we were unable to assess multipollutant models during pregnancy due to the correlation among NO2, PM10, and PM2.5 exposure. However, by using DLMs for each pollutant, we were able to identify specific time windows of exposure that appear to be important for neurodevelopment in infants.
Conclusion
Prenatal exposure to ambient air pollutants was inversely associated with functional neurodevelopmental outcomes at 2 years, raising concern for child health and future functional impairment. In addition, our findings indicate that exposures during mid to late pregnancy may be especially detrimental to neurodevelopment, which suggests the need for limiting air pollution exposure, especially during the latter half of pregnancy. In summary, this study adds to the growing body of literature cataloging the negative health consequences of both pre- and postnatal ambient air pollution exposure that should be used to inform policy efforts to limit human exposure to air pollutants.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. World Health Organization; 2021. https://apps.who.int/iris/handle/10665/345329. License: CC BY-NC-SA 3.0 IGO
Liu J, et al. Disparities in Air Pollution Exposure in the United States by Race/Ethnicity and Income, 1990–2010. Environ Health Perspect. 2021;129:127005.
Hajat A, Hsia C, O’Neill MS. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr Environ Health Rep. 2015;2:440–50.
Tessum C, et al. Inequity in consumption of goods and services adds to racial–ethnic disparities in air pollution exposure. Proc Natl Acad Sci. 2019;116:201818859.
Almetwally AA, Bin-Jumah M, Allam AA. Ambient air pollution and its influence on human health and welfare: an overview. Environ Sci Pollut Res. 2020;27:24815–30.
Suades-González E, Gascon M, Guxens M, Sunyer J. Air Pollution and Neuropsychological Development: A Review of the Latest Evidence. Endocrinology. 2015;156:3473–82.
Calderón-Garcidueñas L, Leray E, Heydarpour P, Torres-Jardón R, Reis J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Rev Neurol (Paris). 2016;172:69–80.
Fleming S, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years: a systematic review of observational studies. Lancet. 2011;377:1011–8.
Thompson R, Nelson C. Developmental Science and the Media: Early Brain Development. Am Psychol. 2001;56:5–15.
Loftus CT, et al. Prenatal air pollution and childhood IQ: preliminary evidence of effect modification by folate. Environ Res. 2019;176:108505.
Porta D, et al. Air Pollution and Cognitive Development at Age 7 in a Prospective Italian Birth Cohort. Epidemiol Camb Mass. 2016;27:228–36.
Peterson BS, et al. Effects of Prenatal Exposure to Air Pollutants (Polycyclic Aromatic Hydrocarbons) on the Development of Brain White Matter, Cognition, and Behavior in Later Childhood. JAMA Psychiat. 2015;72:531–40.
Guxens M, et al. Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biol Psychiatry. 2018;84:295–303.
Rivas I, et al. Association between Early Life Exposure to Air Pollution and Working Memory and Attention. Environ Health Perspect. 2019;127:57002.
Sentís A, et al. Prenatal and postnatal exposure to NO2 and child attentional function at 4–5years of age. Environ Int. 2017;106:170–7.
Chiu Y-HM, et al. Prenatal Particulate Air Pollution and Neurodevelopment in Urban Children: Examining Sensitive Windows and Sex-specific Associations. Environ Int. 2016;87:56–65.
Guxens M, et al. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiol Camb Mass. 2014;25:636–47.
Perera FP, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–92.
Guxens M, et al. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect. 2012;120:144–9.
Peterson BS, et al. Prenatal exposure to air pollution is associated with altered brain structure, function, and metabolism in childhood. J Child Psychol Psychiatry. 2022;63:1316-31.
Guxens M, et al. Prenatal air pollution exposure and child’s attentional function at 7 years old: exploring windows of susceptibility. Environ Epidemiol. 2019;3:146.
Liu B, et al. Fetal Exposure to Air Pollution in Late Pregnancy Significantly Increases ADHD-Risk Behavior in Early Childhood. Int J Environ Res Public Health. 2022;19:10482.
Kostović I, Judaš M, Petanjek Z, Šimić G. Ontogenesis of goal-directed behavior: anatomo-functional considerations. Int J Psychophysiol. 1995;19:85–102.
Borsani E, Della Vedova AM, Rezzani R, Rodella LF, Cristini C. Correlation between human nervous system development and acquisition of fetal skills: An overview. Brain Dev. 2019;41:225–33.
Konkel L. The Brain before Birth: Using fMRI to Explore the Secrets of Fetal Neurodevelopment. Environ Health Perspect. 2018;126:112001.
Stiles J, Jernigan TL. The Basics of Brain Development. Neuropsychol Rev. 2010;20:327–48.
Meyer U, Yee BK, Feldon J. The Neurodevelopmental Impact of Prenatal Infections at Different Times of Pregnancy: The Earlier the Worse? Neuroscientist. 2007;13:241–56.
Boulanger-Bertolus J, Pancaro C, Mashour GA. Increasing Role of Maternal Immune Activation in Neurodevelopmental Disorders. Front Behav Neurosci. 2018;12:230.
Roy P. Martin & Stefan C. Dombrowski. Prenatal Exposures: Psychological and Educational Consequences for Children. (Springer Science+Business Media, LLC, 2008).
Andescavage NN, et al. Complex Trajectories of Brain Development in the Healthy Human Fetus. Cereb Cortex. 2017;27:5274–83.
Alderete TL, et al. Early life gut microbiota is associated with rapid infant growth in Hispanics from Southern California. Gut Microbes. 2021;13:1961203.
Alderete TL, et al. Added sugar and sugar-sweetened beverages are associated with increased postpartum weight gain and soluble fiber intake is associated with postpartum weight loss in Hispanic women from Southern California. Am J Clin Nutr. 2020;112:519–26.
Berger PK, et al. Maternal blood pressure mediates the association between maternal obesity and infant weight gain in early postpartum. Pediatr Obes. 2019;14:e12560.
Patterson WB, et al. Prenatal exposure to ambient air pollutants and early infant growth and adiposity in the Southern California Mother’s Milk Study. Environ Health. 2021;20:67.
Wild LE, et al. Specific amino acids but not total protein attenuate postpartum weight gain among Hispanic women from Southern California. Food Sci Nutr. 2021;9:1842–50.
Wild LE, et al. Risk of Micronutrient Inadequacy among Hispanic, Lactating Mothers: Preliminary Evidence from the Southern California Mother’s Milk Study. Nutrients. 2021;13:3252.
Hollingshead A. Four-factor Index of Social Status. N. Hav: Yale Univ; 1975.
Adams J, Weakliem D, August B. Hollingshead’s ‘Four Factor Index of Social Status’: From Unpublished Paper to Citation Classic. Yale J Sociol. 2011;8:11–9.
Eckel SP, et al. Air pollution affects lung cancer survival. Thorax. 2016;71:891–8.
Çelik P, AyranciSucakli I, Yakut H I. Which Bayley-III cut-off values should be used in different developmental levels? Turk. J Med Sci. 2020;50:764–70.
Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. 2014;75:670–4.
Bové H, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10:3866.
Valentino SA, et al. Maternal exposure to diluted diesel engine exhaust alters placental function and induces intergenerational effects in rabbits. Part Fibre Toxicol. 2016;13:39.
Block, M. L. et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 18, 1618–1620 (2004).
Paolicelli RC, et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011;333:1456–8.
Mottahedin A, et al. Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front Cell Neurosci. 2017;11:190.
Block ML, Calderón-Garcidueñas L. Air Pollution: Mechanisms of Neuroinflammation & CNS Disease. Trends Neurosci. 2009;32:506–16.
Calderón-Garcidueñas L, Torres-Jardón R, Kulesza RJ, Park S-B, D’Angiulli A. Air pollution and detrimental effects on children’s brain. The need for a multidisciplinary approach to the issue complexity and challenges. Front Hum Neurosci. 2014;8:613.
Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Transl Neurosci. 2016;7:24–30.
Calderón-Garcidueñas L, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310.
Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546–50.
Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106:802–7.
Dahlgren J, Samuelsson A-M, Jansson T, Holmäng A. Interleukin-6 in the Maternal Circulation Reaches the Rat Fetus in Mid-gestation. Pediatr Res. 2006;60:147–51.
Chen HL, et al. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991;139:327–35.
Hu X-L, Yang Y, Hunt JS. Differential distribution of interleukin-1α and interleukin-1β proteins in human placentas. J Reprod Immunol. 1992;22:257–68.
Kameda T, et al. Production of interleukin-6 by normal human trophoblast. Placenta. 1990;11:205–13.
Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–8.
Han VX, et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry. 2021;11:1–12.
Allswede DM, Yolken RH, Buka SL, Cannon TD. Cytokine concentrations throughout pregnancy and risk for psychosis in adult offspring: a longitudinal case-control study. Lancet Psychiatry. 2020;7:254–61.
Knuesel I, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10:643–60.
Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353:772–7.
Buka SL, et al. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–7.
Solek CM, et al. Early Inflammation Dysregulates Neuronal Circuit Formation In Vivo via Upregulation of IL-1β. J Neurosci. 2021;41:6353–66.
Vallières L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci Off J Soc Neurosci. 2002;22:486–92.
Kim E, et al. Prenatal exposure to PM10 and NO2 and children’s neurodevelopment from birth to 24 months of age: mothers and Children’s Environmental Health (MOCEH) study. Sci Total Environ. 2014;481:439–45.
Grann VR. Erasing Barriers to Minority Participation in Cancer Research. J Womens Health. 2010;19:837–8.
CDC (Centers for Disease Control). CDC Health Disparities and Inequalities Report - United States 2011. MMWR 60, 1–114 (2011).
Ceballos, R. et al. Latino Beliefs about Biomedical Research Participation: A Qualitative Study on the US-Mexico Border. J. Empir. Res. Hum. Res. Ethics JERHRE 9, 10–21 (2014).
Campbell JM, Brown RT, Cavanagh SE, Vess SF, Segall MJ. Evidence-based Assessment of Cognitive Functioning in Pediatric Psychology. J Pediatr Psychol. 2008;33:999–1014.
Albers, C. A. & Grieve, A. J. Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development– Third Edition. San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 25, 180–190 (2007).
Flynn, R. S., Huber, M. D. & DeMauro, S. B. Predictive Value of the BSID-II and the Bayley-III for Early School Age Cognitive Function in Very Preterm Infants. Glob. Pediatr. Health 7, 2333794X20973146 (2020).
Ronfani L, et al. The Complex Interaction between Home Environment, Socioeconomic Status, Maternal IQ and Early Child Neurocognitive Development: A Multivariate Analysis of Data Collected in a Newborn Cohort Study. PLoS ONE. 2015;10:e0127052.
Coscia JM, et al. Effects of Home Environment, Socioeconomic Status, and Health Status on Cognitive Functioning in Children With HIV-1 Infection. J Pediatr Psychol. 2001;26:321–9.
Crouse DL, et al. Risk of Nonaccidental and Cardiovascular Mortality in Relation to Long-term Exposure to Low Concentrations of Fine Particulate Matter: A Canadian National-Level Cohort Study. Environ Health Perspect. 2012;120:708–14.
Acknowledgements
Research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. CR 83998101) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers.
Funding
This work was supported by the NIH NIDDK (R01 DK11079), The Gerber Foundation (15PN-013), NIH NIEHS (R00 ES027853), the Health Effects Institute (HEI) Rosenblith Award, and the NIH NIMHD Southern California Center for Latino Health (P50 MD17344). Design of the study; collection, analysis, and interpretation of data; and writing the manuscript was strictly the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
Zachariah Morgan helped with development of the analytical approach, conducted all analyses, and prepared the original manuscript draft. Maximillian Bailey, William Patterson, and Diana Trifonova assisted with data analysis and manuscript preparation. Noopur Naik helped prepare datasets and performed geocoding for ambient air pollution estimates. Howard Chang advised on analytical approaches. Frederick Lurmann modeled air pollutant exposure estimates. Bradley Peterson oversaw evaluation of developmental outcomes. Michael Goran supervised all primary data collection and acquired funding. Tanya Alderete conceived the hypothesis and data analysis plan, supervised the formal analysis, and acquired funding. All authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Boards of the University of Colorado Boulder, Children’s Hospital of Los Angeles, and the University of Southern California approved this study, and all participants gave informed consent.
Consent for publication
Not applicable.
Competing interests
Michael I. Goran receives book royalties. Michael I. Goran is a scientific advisor for Yumi. The other authors declare they have no actual or potential competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Figure 1.
Average Prenatal PM10 Exposure Demonstrated Non-Linear Associations with Composite, Fine, and Scaled Motor Scores at 2 Years.
Additional file 2: Supplemental Table 1.
Correlations of Monthly Exposure Lags Between Ambient Air Pollutants During the 9-Month Pregnancy Period.
Additional file 3: Supplemental Table 2.
Univariate Associations Between Average Prenatal Exposure to Ambient Air Pollutants and Neurodevelopmental Outcomes at 2 Years.
Additional file 4: Supplemental Table 3.
Associations Between All Windows of Prenatal Ambient Air Pollution Exposure and Neurodevelopmental Outcomes at 2 years.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Morgan, Z.E.M., Bailey, M.J., Trifonova, D.I. et al. Prenatal exposure to ambient air pollution is associated with neurodevelopmental outcomes at 2 years of age. Environ Health 22, 11 (2023). https://doi.org/10.1186/s12940-022-00951-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-022-00951-y