Abstract
In January 2020, SARS-CoV-2 virus was identified as a cause of an outbreak in China. The disease quickly spread worldwide, and the World Health Organization (WHO) declared the pandemic in March 2020.
From the first notifications of spread of the disease, the WHO’s Emergency Programme implemented a global COVID-19 surveillance system in coordination with all WHO regional offices. The system aimed to monitor the spread of the epidemic over countries and across population groups, severity of the disease and risk factors, and the impact of control measures. COVID-19 surveillance data reported to WHO is a combination of case-based data and weekly aggregated data, focusing on a minimum global dataset for cases and deaths including disaggregation by age, sex, occupation as a Health Care Worker, as well as number of cases tested, and number of cases newly admitted for hospitalization. These disaggregations aim to monitor inequities in COVID-19 distribution and risk factors among population groups.
SARS-CoV-2 epidemic waves continue to sweep the world; as of March 2022, over 445 million cases and 6 million deaths have been reported worldwide. Of these, over 327 million cases (74%) have been reported in the WHO surveillance database, of which 255 million cases (57%) are disaggregated by age and sex. A public dashboard has been made available to visualize trends, age distributions, sex ratios, along with testing and hospitalization rates. It includes a feature to download the underlying dataset.
This paper will describe the data flows, database, and frontend public dashboard, as well as the challenges experienced in data acquisition, curation and compilation and the lessons learnt in overcoming these. Two years after the pandemic was declared, COVID-19 continues to spread and is still considered a Public Health Emergency of International Concern (PHEIC). While WHO regional and country offices have demonstrated tremendous adaptability and commitment to process COVID-19 surveillance data, lessons learnt from this major event will serve to enhance capacity and preparedness at every level, as well as institutional empowerment that may lead to greater sharing of public health evidence during a PHEIC, with a focus on equity.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
History of COVID-19 and surveillance data collection
Global infectious disease surveillance is part of WHO’s mandate since the creation of the institution in 1948. The International Health Regulations (IHR) aim to standardize the list of a few epidemic prone diseases under immediate notification, as well as unexpected events of international concern to prevent international spread. The latest update of IHR in 2005 [1] included a list of diseases for immediate notification, definition of public health event of international concern requiring immediate notification, as well as a frame for an efficient communication between Member States and WHO. The list of epidemic prone diseases requiring immediate notification is adapted to national context. International surveillance existed for tuberculosis, smallpox, cholera, typhoid, flu, and the HIV pandemic during accelerated the development of national surveillance systems and data sharing. For acute respiratory disease with pandemic potential, the Global Influenza Surveillance and Response System [2], (GISRS) is operational since 1952, with a network of institutions in 124 countries, aiming to provide surveillance data on influenza and respiratory syndromes (Influenza Like Illness, Severe Acute Respiratory Infection).
In January 2020, SARS-CoV-2 was identified as the virus involved in an outbreak in the province of Hubei, China, as mentioned in the Disease Outbreak News [3]. The disease quickly spread worldwide, and the World Health Organization’s (WHO) Emergency Committee declared a Public Health Event of International Concern (PHEIC) on 30 January 2020 [4]. On 11 March 2020 [5], WHO declared COVID-19 as a pandemic. From the first notification of spread of the disease outside China, WHO’s Emergency Programme implemented a global COVID-19 surveillance system based on the IHR [6].
WHO’s response to previous PHEICs has provided valuable lessons and capacity building in preparedness for future emergencies. For example, in 2004, WHO guidelines for the Global Surveillance of SARS [7] recommended a minimum global dataset for surveillance, including case-based data and age and sex disaggregated data. Following this, the response to the 2009 influenza A (H1N1) pandemic emphasized the need for timely surveillance data to enable evidence-based decisions in public health [8]. From end of April 2009 to August 2010, WHO IHR contact points in WHO regional offices and IHR National Focal Points collected paper and/or electronic case-based clinical and epidemiological data from laboratory-confirmed cases. WHO headquarters (WHO-HQ) received these data operationalized as different variables and in different formats. Over 18,000 individual case reports of laboratory-confirmed influenza cases were reported to WHO from 84 of its 193 Member States of over 6 million cases allegedly reported worldwide. In comparison, considering the enormous number of cases over a much smaller period, surveillance of the COVID-19 pandemic proved to be an unprecedented challenge.
COVID-19 global surveillance aims to monitor the extension of the pandemic across countries, the severity of the disease and risk factors, and the impact of control measures. Data collection tools and standardized surveillance methods were documented in the interim guidance Public Health Surveillance for SARS-CoV-2 [9].
Standardized surveillance methods are designed to provided standard definitions, variable formats, and data collection tools to be able to merge surveillance datasets from different sources and maintain comparability and data compatibility. This is particularly of importance during a global pandemic such as COVID-19, with large case numbers, and cases and deaths reported from all over the world.
Updates of the interim guidance Public Health Surveillance for SARS-CoV-2 were released as evidence unfolded (nine such updates have been published up between January 2020 and February 2022). These updates involved notably case definition and laboratory confirmation methods, definition of reinfection, list of signs and symptoms included in case definition, inclusion of variables on vaccine status of cases.
Two years after the beginning of the COVID-19 pandemic, many aspects of epidemiological surveillance remain challenging despite many achievements and lessons learnt. This paper documents the construction of the WHO-HQ COVID-19 surveillance database by describing its data collection, storage, and dissemination mechanisms, followed by a discussion of the use of these data in facilitating analysis of inequalities. Finally, we discuss challenges and lessons learnt as well as future directions and proposed developments for the database.
Construction
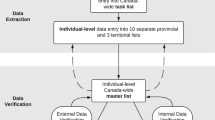
Data flow of COVID-19 surveillance data from Ministries of Health to WHO-HQ is complex, and combines multiple sources, tools, and stakeholders (Fig. 1). Despite widespread advocacy on standardized surveillance tools, Ministries of Health often rely on existing systems, that do not always include recommended standards. This leads to multiple datasets, using different data formats, rendering major challenges in interoperability and dataset consolidation. This article focuses on the database maintained and coordinated at WHO-HQ, hereafter referred to as “WHO-HQ COVID-19 surveillance database”. The data acquisition, curation and analysis will be detailed in the paragraphs below.
Data acquisition
In January 2020, WHO developed a COVID-19 case definition for surveillance and asked all countries to report cases through the IHR national focal points. WHO also started to collect and compile daily cases and death counts globally from web based public sources (public dashboards and social media) [10]. In the Interim guidance public health surveillance for SARS-CoV-2 published in August 2020, it was recommended that ministries of health share the case definition for COVID-19 case confirmation and deaths. However, there was little compliance from the Ministries of Health, and active follow-up on public websites to review case definitions.
The initial surveillance strategy aimed at collecting daily case-based data through a case report form [11], with standardized variables. The case-based data collection system was set up using existing regional surveillance networks. In several countries the case-based data was collected through the existing influenza surveillance system and in others via the routine disease surveillance system.
The influenza surveillance system was operational in 149 countries in 2019, where a network of a subset of sentinel health facilities reports Influenza like Illnesses and Severe Acute Respiratory Infection. In the beginning of the COVID-19 pandemic, emergency surveillance standards from IHR recommend reporting comprehensively all detected cases, from all health facilities, including those not included in the sentinel network. The influenza surveillance data management systems for case-based reporting, Flu ID, and Tessy, managed by ECDC in Europe, were adapted to report comprehensively all COVID-19 cases. In addition to this the virological reporting system (FluNet) was adapted to include SARS-Cov-2 detections by week in specimens received at the laboratory. In many countries, routine disease surveillance systems were adapted include COVID-19 in the list of mandatory diseases report, and the frequency of reporting was increased from weekly to daily reporting.
The objectives of the surveillance were to monitor areas and population affected by COVID-19 and to understand the epidemiological characteristics of the illness (incubation period, secondary attack rate, serial interval, case fatality rate) including age, sex, co-morbidities, settings of transmission, and nature of contacts to identify vulnerable groups at highest risk of exposure or severe disease. Variables recommended by WHO for COVID-19 surveillance evolved in accordance with latest scientific evidence on the pathogen (variants), response (vaccination), and impact on mortality, morbidity, and healthcare capacity. Geographical distribution of cases was reported at national and regional level. Localization of cases reported in case-based data was described at locality level, but challenges with data formatting, poor completeness and data curation rendered poor added value to the geographical information reported to WHO-HQ.
As the disease spread and numbers of cases increased, the burden on surveillance systems impacted the capacity to provide detailed case-based data, and in March 2020, recommendations for surveillance data reporting shifted to weekly aggregate reporting of a minimum global dataset comprising age and sex disaggregation of cases and deaths, newly hospitalized cases, and occupation (to capture cases among Health Care Workers (HCW). The set of variables for weekly aggregate reporting was further completed and amended in August 2020 [12] (see Table 1).
Recommended variables and disaggregation of COVID-19 surveillance data allow for detection and reporting on changes in the disease in the affection between men and women, and in different subgroups such as infants, children, elderly, and other high-risk groups defined by occupational such as HCWs [13], social or behavioural factors, migrants, young adults and others. Inequality monitoring in the most vulnerable groups is a priority focus. Milestones of the adaptation of surveillance recommendations are displayed in Fig. 2.
While some countries and regions shifted to aggregate reporting as recommended by WHO, others continued to report case report forms throughout the pandemic. Some regions reported case-based data to the global database while others shared aggregated case-based data according to the recommended minimum dataset. Ad hoc data acquisition, aggregation and combination using various formats is also performed as some countries have not yet stabilised the data collection and aggregation process. Publicly reported data processed through global aggregators like “FIND” [14] and “Our World in Data” [15] were also loaded and used to complement the datasets reported by countries.
Internal analyses were performed to attempt to predict increases in transmission and anticipate operations and preventive measures in countries where the health systems would risk reaching capacity. These were kept internal given political sensitivity. Modelling techniques were tested by researchers to account for under reporting, but given wide variety of situations, factors, and highly dynamic situations, validation delays were not adapted to real time surveillance data received by WHO.
As vaccinations became available, WHO started to roll out data collection based on public websites in January 2021. A weekly system was implemented to aggregate the number of total administered doses, number of persons with at least one dose, and number of persons fully vaccinated. Additionally, data on types of vaccines used and date of vaccination rollout were collected. A monthly system of collecting more detailed data and including disaggregation by age and sex was launched based on the existing Joint Reporting Form used by WHO and UNICEF to collect immunization data from countries [16].
Given this diversity, data processing at WHO-HQ adapted accordingly, allowing for data collection through different means including form-based reporting, uploads of spreadsheets to the data platform, application programming interface (API) or direct server connections.
Data curation and storage
At the beginning of the epidemic, the case-based data was stored anonymously on public data storage tools. With rising case counts and to use a more sustainable solution it was decided to use the WHO xMart system already used in the WHO Global Influenza Programme for the storage of case-based data. As more datasets were added when new reporting recommendations were issued, WHO xMART became the main repository for all COVID-19 surveillance data. Datasets were loaded regardless of the original format and transformed to fit the proposed data model. Regional office focal points were trained on how to upload data to WHO xMart and cut-off time was communicated to ensure timeliness of reported data. The WHO xMART system allowed to continuously adapt the global surveillance system to rapidly changing environment during the pandemic: to add and withdraw variables, to merge data from different formats, to aggregate individual and aggregated surveillance data, to change age-group distribution several times. Adaptability of data acquisition and storage capacity is essential while setting up an ad hoc surveillance system during a pandemic of a new emerging disease and xMART was a major asset.
Data from Ministries of Health were in general curated at regional offices before they were uploaded to the global database, except for some instances when the data were reported directly from the country to the global database. Automated data corrections were implemented in the global database including mostly curation of dates in case report forms and checks across fields to correct or complete missing information based on information from other fields, e.g. the calculation of age based on date of birth; and checking for dates to be in chronological order, e,g, date of death not being before date of onset. Furthermore, data quality control dashboards were made available to WHO data managers at WHO-HQ and regional offices. The latter provide overviews on data completeness and highlight data inconsistencies.
The surveillance data from the different formats and datasets were merged into one single dataset using an algorithm to enable maximal data availability for each recommended variable, without double counting. Data were combined by country, week, age and sex as available in the following priority order:
-
1.
Data collected through weekly aggregated reporting (V2) [12]
-
2.
Data collected through reporting systems recommended at earlier stages of the epidemic (weekly aggregated reporting (V1), case report forms [11])
-
3.
Aggregated data from WHO regions
-
4.
Aggregated testing data from FIND [14] (testing data only for countries, areas or territories in the WHO African Region)
-
5.
Aggregated data from Our World in Data [15]
Age bands have changed in recommendations for aggregate reporting over time (age groups 15–24 and 24–64 in weekly aggregated V1 and age group 20–29 in Weekly Aggregate V2). To combine the data reported in both different age bands for analysis, national age-stratified population data was used to distribute the cases and deaths accordingly for each country following the approach used by the Max Planck Institute for their COVerAGE-DB [17].
The number of cases as reported through case report forms was aggregated to weeks using the date of report. Data from global daily case counts were used as reference to check for completeness of surveillance data received [10]. Timely sharing of data was prioritized over extensive data quality control. This has resulted in historical data changing when corrections have been applied post hoc, i.e., after the data has first been shared.
Data analysis and dissemination
At the beginning of the epidemic, surveillance data was not disseminated publicly, but rather analyzed and internally disseminated at WHO. It was also used for risk assessments and detailed analyses, some of which were made publicly available in the WHO Weekly Epi Update [3].
In March 2021, a public dashboard displaying the main trends and features in variables in the WHO COVID-19 surveillance database was released [18]. The dashboard comprised eight views reporting cases and deaths disaggregated by age and sex (as well as for HCWs specifically), hospitalization, and testing. A download feature for the database, allowing the public to access the whole dataset, was added in April 2021. Data in the public dashboard are updated once every day for aggregated data sources and once every week for case-based data. Vaccination data, also stored on the WHO xMart, is displayed on a separate dashboard on the WHO website [19].
Within the public dashboard, epidemiological curves displaying COVID-19 cases and deaths across time allow longitudinal analysis of trends, enhancing identification of departures from trends and decoupling of trends that can indicate shifts in transmission and severity dynamics, and impact of Non Pharmaceutical Interventions (NPI) and Public Health and Social Measures (PHSM) (Fig. 3).
Age disaggregation of cases and deaths is displayed both in absolute numbers and in relative proportion (Fig. 4). The trend analysis of age disaggregation allows the identification of shifts in infection, hospitalisation, deaths and vaccination rollout and coverage across age groups, and both Non Pharmaceutical Interventions (NPI) and Public Health and Social Measures (PSHM).
Age disaggregation of case fatality rate allows the detection of unusual mortality trends in specific age groups and the identification of potential high risk groups (Fig. 5).
Sex disaggregation of cases and deaths (Fig. 6) allows the highlighting of differential risk of exposure and the identification of target groups with higher risk of exposure and severe outcomes, and the subsequential tailoring of Risk Communication and Community Engagement (RCCE), communications, NPI and response. Potential gender-based factors of exposure include social and behavioural factors, and severe disease and death can be due to different physiological and clinical manifestations.
Trends in HCW infections regarding trends in general population (Fig. 7) allow to detect differential risks in infection and fatal outcome in HCWs, due to potential increased exposure.
Longitudinal analysis of hospitalization and deaths include trend decoupling between cases, hospitalization, and deaths (Fig. 8), which infers links between severity of clinical manifestation, and burden on healthcare systems.
Utility and discussion
Data platform
It became quickly evident in the beginning of the COVID-19 pandemic that with the volume of cases, a switch from spreadsheet data collection to a more stable system was vital. The WHO has its own warehousing solution with WHO xMART, which allows for data integration, curation, storage, and harmonization of data from different sources and formats to standard models. Access to the data through the web-based WHO xMart user interface was configured in a very granular way from overall data management capacity to only allowing data upload access for selected datasets or permission to only view selected tables. The system was continuously enhanced to resolve issues identified and to fulfill needs for effective COVID-19 surveillance data management on WHO xMart. Thus, the system facilitated collaboration across WHO offices, helped to build and maintain the WHO-HQ COVID-19 surveillance database despite changing in reporting recommendations and to automate data flows. The user interface improved significantly over time, but further efforts to complement data management functions and to ease the development of upload pipelines would be beneficial.
Current data availability, coverage, and completeness
Data availability and participation in the system was variable over time and some data only became available retrospectively, but in comparison to the influenza A (H1N1) pandemic in 2009, the collection, aggregation and dissemination of disease surveillance data improved due to higher flexibility of the systems, and collaboration between WHO-HQ, regional offices, and the Global Influenza Programme.
As of 8 March 2022, a total of 184 countries, territories and areas had shared detailed data via case report forms or weekly aggregate surveillance with WHO. Of the 440 million cases reported globally, 327 million cases (74%) were reported with detailed information to WHO-HQ COVID-19 surveillance. Data was reported by sex for over 258 million cases (59%), by age for 268 million cases (61%) and by age and sex combined for 255 million cases (58%). There were also data on COVID-19 cases among health care workers, with just over 4 million cases, and 8700 deaths, recorded in the system.
Comparing data from March 2021 (the date of public release of the WHO COVID-19 detailed surveillance dashboard) and March 2022, the proportion of cases for which detailed surveillance data has been reported remained stable, at 72 to 74% respectively, while the overall proportion of cases for which disaggregated data by age and/or sex has been reported increased (see Fig. 9). The completeness of disaggregated data varies considerably across WHO regions and countries, but availability of age and sex disaggregated data has improved overall from 36% of countries in March 2021 to 57% in March 2022.
Utility of data disaggregation by age and sex, for health care workers and for hospitalizations
Age and sex disaggregation
Age disaggregation enables risk assessment for specific age groups who are considered at risk of severe disease, and death (for instance of infants, children, and people over 65), and can also provide insight on increasing risk of transmission. For example, the data showed that young males in Middle Eastern and Asian countries had higher rates of COVID-19 infection compared to the general population in early 2020. After field investigation, it appeared that these populations were mainly comprised of young male migrant workers, living in crowded conditions [20]. In another example, a greater proportion of young adults as compared to older adults were testing positive for COVID-19 in European countries in the summer of 2021.
Sex disaggregation of cases and deaths showed two patterns: the first was that the majority of cases were in women while the majority of deaths were reported among men; the second was that both cases and deaths were higher among men than women (Fig. 6). Gender-based differences in exposure and access to healthcare and testing facilities have been hypothesised as causes for these differences and are still under investigation. Social and behavioural differences based on gender have also been examined [21,22,23,24]. It has been established that men have a higher risk of severe disease and death attributable to COVID-19 than women [25].
Comparative analysis with seroprevalence data allows the comparison of the infections reported by surveillance systems against the real range of infections as detected by seroprevalence. The comparison of the proportion of infections in both methods highlights the proportion of infections that were undetected or unreported by the surveillance system at the time of infection. This method allows the assessment of the ascertainment of COVID-19 surveillance systems, i.e. the capacity of a surveillance system to accurately and timely reflect the reality of disease burden. The proportion of asymptomatic COVID-19 cases who did not seek care was the leading cause of under-ascertainment [26]. These comparisons could potentially highlight under-ascertainment linked to equity factors, for instance in populations with lower access to testing or healthcare.
Going forward, a comparative review of age and sex disaggregated data between epidemiological surveillance and vaccine coverage would allow the investigation of unexpected increases in severe cases and deaths due to suboptimal vaccine coverage in specific groups.
Health care workers
Health care workers (HCWs) are a population group with high risk of COVID exposure and have consequently been included as a specific category in surveillance recommendations. The aim of surveillance is to follow trends in HCW infections as compared to trends in the general population and to assess relative risk of infection and death, with the caveat that the place of exposure is not documented for HCWs. According to the WHO COVID-19 surveillance data base, COVID-19 cases among HCWs were higher than the general population in the early stages of the epidemic. However, after Mid 2020, trends in cases among HCWs have been following general population, and the case fatality ratio among HCWs is also lower than among the general population. Several factors explaining these trends are under investigation, including access to testing, access and use of personal protective equipment, a high proportion of women in the health workforce, and the healthy worker effect [27,28,29].
Hospitalizations
The number of new hospitalized cases allows the estimation of the risk of severe disease and hospitalization and anticipation of the burden on healthcare capacity. However, this has shifted with the appearance of the Omicron variant: the large scale of Omicron transmission resulted in an unprecedented number of cases, and while the proportion of severe cases was small, they were still substantial in terms of absolute number of cases and led to hospital capacity being challenged in many parts of the world. Furthermore, it appeared that an increasing volume of hospitalized cases were tested incidentally for COVID-19, and since these were added to the newly hospitalized case tallies the difference in the cause of the hospitalization risked going undetected [30]. At present, hospitalization is a staple indicator in the estimation of burden of disease and is under extreme scrutiny regarding the future of COVID-19 surveillance, in conjunction with healthcare capacity. Notwithstanding this, currently, hospitalization data is still sparse for many countries in the WHO COVID-19 surveillance database.
Challenges
Surveillance strategy and adaptability to variable changes over time
The scale of the pandemic resulted in changes to surveillance strategies during the pandemic, shifting from case-based data to weekly aggregated data. Furthermore, as Member States and WHO regional offices upgraded and adapted their surveillance strategies, the COVID-19 surveillance data came through WHO regional offices to WHO-HQ in various data flows and data formats, which required assessment for data acquisition automation and occasionally manual data processing to populate the combined dataset. The use of existing influenza systems to collect COVID-19 surveillance data was a great asset, as it contributed to rapid implementation and automation of data flows.
However, the pace of transmission dynamics due to Variants of Concern, such as Omicron, and the scale of the burden of disease hampered the adaptability of surveillance systems to properly assess the impact of vaccine status and seroprevalence on infection and risk of death.
Volume of data
The ad hoc data collection solutions implemented at the early stages of the pandemic, such as shared spreadsheets for case-based data, could not accommodate the scale of the pandemic spread and the number of cases at country level, even less at WHO regional and HQ levels given the volume of surveillance data to process and curate. As some countries kept up case-based surveillance, the case-based dataset volume kept increasing. The volume of case report forms warranted a huge scale-up in processing and memory capacity, with the database reaching over 100 million individual case reports. Although the WHO xMart system was continuously adapted and enhanced in order to meet needs, queries to the case-based dataset and data curation of those data are still time-consuming and represent an ongoing challenge and a drawback in data analysis.
Sustaining multi-level collaboration with WHO regional offices and member states
The pandemic brought a huge reporting burden on IHR focal points in regional offices and Member States, as well as on public health surveillance systems. WHO-HQ and regional offices have collaborated closely to build the most effective dataflow. This, together with the possibility to tailor the data flows to country needs and practices and keeping a close line with countries, helped greatly to resolve bottlenecks and reporting issues and had a positive impact on the data quality. Standardization data formatting reporting across regional offices has remained a challenge, as some decided to keep the case-based reporting even as weekly aggregated data was encouraged as an attempt to alleviate reporting burden given the scale of the pandemic. Feedback from countries and regions will feed into future updates of global COVID-19 surveillance recommendations. Adaptability at country level to updates in surveillance recommendations, variables, and age groups proved challenging with an estimated six-month delay for countries to roll out new recommendations and report accordingly.
Data sensitivity and reporting
While reporting of COVID-19 cases and deaths fell under the IHR legal requirement to be reported to WHO, the reporting of detailed disaggregated data fell under a grey area, resulting in some countries withholding COVID-19 surveillance data. Furthermore, given the impact of epidemiological spread of the disease in Member States on PHSM, international travel and economic response, COVID-19 surveillance data was very sensitive, especially during the emergence phase of Variants of Concern (VOC). Sensitivity on equity issues were noted, such as COVID-19 transmission in children, sex disaggregation of cases and deaths, and hospitalization data. Reporting of surveillance data on HCWs was also particularly sensitive. All those sensitivity issues were directly reflected in data reporting, data completeness and availability of data for analysis.
Variability between countries: definitions used for surveillance, completeness of reporting
There is variability between surveillance case definitions applied in countries, as well as testing strategies and eligibility, availability and type of tests varied between countries (RDT versus PCR, etc). Hospitalization data was also reported differently - some countries reported new admissions, some reported current hospitalized cases, others counted only admissions for COVID-19 treatment, while some included incidental COVID-19 cases (tested on admission for medical reasons other than COVID-19). The differences in reporting also vary across time in accordance with transmission dynamics, burden of disease and strain on public health systems. In the WHO COVID-19 surveillance database these inconsistencies are highlighted mainly when comparing the completeness of the detailed surveillance data to the overall number of cases and deaths reported.
On the matter of recording and reporting of deaths related to COVID-19, the WHO definition of COVID-19 death for surveillance purposes [9] is very sensitive and intended to capture broadly deaths associated with COVID for surveillance perspective on the impact of the disease, rather than assess clinical causality, as physiopathology was not well understood in the early stages of the pandemic. Despite this sensitivity, COVID-19 death toll reported to WHO has been widely described as underreported.
Indeed, the underestimation of the death toll has been documented by WHO in the document Revealing the toll of COVID-19 [31], and on the webpage The true death toll of COVID: estimating excess mortality [32]. More recent work on the matter includes COVID’s true death toll: much higher than official records [33] and The pandemic’s true death toll [34].
In accordance with WHO regional offices, the recommended weekly aggregated dataset included metadata on the surveillance strategies and definitions used in the countries, as well as detailed situation reports, but these were seldom reported by the Member States, leading WHO to rely on event-based data and investigation through public health websites to provide context to the data provided and support analysis.
Timeliness of data
This database was not intended to provide real-time data but rather provide a macro-level understanding of the trend over time. Thus data is reported with a delay ranging between a week and several months, and has been monitored as a quality attribute that has improved over time.
Lessons learnt and recommendations for the future
WHO’s role in a PHEIC, including surveillance recommendations and guidance dissemination, as well as the network of WHO offices and Member States confer a unique advantage for data collection and dissemination. The COVID-19 pandemic showed tremendous capacity surge, adaptability and commitment in displaying surveillance data.
Requested by WHO, an external review of WHO COVID-19 surveillance was conducted in 2021 by Resolve to Save Lives and provided a first set of recommendations [35]. Further recommendations on surveillance data acquisition based on the COVID-19 surveillance experience at WHO-HQ include:
-
Investment in national disease surveillance strengthening, with technical support from WHO country and regional offices.
-
Investment in interoperability of existing systems of different data entry and management tools to further ease integration of data from different settings.
Agreement on a staple minimal outbreak dataset, integrated in existing surveillance systems (such as Early Warning Alert and Response Systems EWARS [36], DHIS2 [37], the Outbreak Toolkit [38]), which can be adaptable through interconnectivity. In the same spirit and objectives as the HL7 initiative designed for patients care and clinical information [39], the outbreak toolkit project developed a list of variables that represent the essential and non-exhaustive information needed to investigate an outbreak. WHO supported by an international working group is proposing a list of essentials variables and its standards. Those variables were included into a standard questionnaire, the T0 questionnaire available on WHO website (https://www.who.int/emergencies/outbreak-toolkit/data-collection-standards/t0-initial-case-investigation-form). This T0 questionnaire was used to develop the initial case reporting form for COVID-19 case based surveillance that WHO shared with regions and countries as soon as 21 of January 2020. Description of the process to identify essential variables is provided in the article: Data collection for outbreak investigations: Process for defining a minimal data set using a Delphi approach [40]. The Outbreak toolkit project aims at providing tools and standards to increase interoperability of databases and facilitate the work of field epidemiologist investigating outbreak in remote settings: https:/www.who.int/emergencies/outbreak-toolkit
-
Investment in existing systems and data platforms and enforcement of emergency Standard Operating Procedures, to ensure agility and surge reactivity to implement data collection requirements during an emergency.
-
Investment in human resources data management and analytical skills and institutional capacity to ensure surge reactivity.
-
Identification and implementation of a strategic information plan including data standards, data sharing, and minimum reportable data by Member States, that builds on existing systems, is scalable and can inform evidence-based decision-making across Member States to develop global public health information goods during major events.
-
Age and sex disaggregation, as well as a focus on vulnerable groups, should be included in the minimum reportable dataset to provide equity insight on transmission dynamics and risk factors, especially in in lower income countries and fragile settings.
-
Centralization of emergency health information system with shared data management responsibilities designed for global events and potential Public Health Emergencies as to contribute to timely curation and availability of data and to ensure consistency and longitudinal comparability of data analysis.
Setting up a centralized database during a PHEIC may take a few weeks as it requires agreement on the minimum datasets, frequency of reporting, setting up the data pipelines, communication and training of the regions, and data validation. As part of preparedness for future outbreaks with known or unknown pathogens, it would be still important to actively collect data even before such system can be set up to characterize the epidemiological features to inform prompt decision-making.
Conclusions
Two years after the pandemic was declared, SARS-CoV-2 continues to spread, and is still considered a PHEIC. Ensuring a responsive and relevant global platform for COVID-19 surveillance, although challenging at the global level, has paramount importance for both prospective planning and for retrospective longitudinal studies. To this end, the WHO-HQ COVID-19 surveillance database is continuously maintained, and data analyzed and displayed in the public dashboard. Important aspects of pandemic risk and response have become apparent through analysis of disaggregated data even as reporting completeness, standardization and timeliness of data sharing remain a major constraint for lower income countries and fragile settings. While WHO offices have demonstrated tremendous adaptability and commitment to process COVID-19 surveillance data, lessons learnt from this major event would greatly serve to enhance capacity and preparedness at every level, as well as institutional empowerment towards improvement of shared public health evidence during a PHEIC, with a focus on equity.
Availability of data and materials
The dataset(s) supporting the conclusions of this article is(are) available in the [repository name] repository : published dashboard [18]:
Data in this report are combined from the following sources:
- Official reporting to WHO through regional offices: case report forms (CRFs) (referred to as data source CRF) [41].
- Official reporting to WHO (HQ and regional offices: daily cases/deaths count (referred to as data source DAILY or “daily counts”) [10].
- Official reporting to WHO: weekly aggregate reporting (2 versions, switch of systems in October 2020) (referred to as data source WEEKLY) [12].
- Our World In Data (referred to as data source OWID) [15].
- FIND (referred to as data source FIND) [14].
- Taken from official public websites, not officially reported to WHO (referred to as data source OTHER).
WHO Member States select the reporting system they prefer to use and data from different reporting systems are reconciled. Individual countries, area and territories may decline to allow country-level disaggregation.
Change history
21 August 2023
After publication of the original article, it was noted that the Creative Commons license URL was incorrect, and has been updated.
17 May 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12939-023-01870-1
Abbreviations
- API:
-
Application Programming Interface
- COVID-19:
-
Coronavirus disease 2019
- HCW:
-
Health Care Workers
- IHR:
-
International Health Regulations
- IMST:
-
Incident Management Support Team
- NPI:
-
Non Pharmaceutical Intervention
- PHEIC:
-
Public health emergency of international concern
- PHSM:
-
Public health and social measures
- RCCE:
-
Risk Communication and Community Engagement
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- UNICEF:
-
United Nations International Children’s Emergency Fund
- WHO:
-
World Health Organization
- WHO-HQ:
-
World Health Organization Headquarters
- VOC:
-
Variant of concern
References
International Health Regulations (2005) Third Edition. [cited 2021 Jul 16]. Available from: https://www.who.int/publications/i/item/9789241580496
Global Influenza Surveillance and Response System (GISRS). [cited 2022 Aug 5]. Available from: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system
Coronavirus Disease (COVID-19) Situation Reports. [cited 2022 Feb 15]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). [cited 2022 Feb 16]. Available from: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
A year without precedent: WHO’s COVID-19 response. [cited 2022 Jan 31]. Available from: https://www.who.int/news-room/spotlight/a-year-without-precedent-who-s-covid-19-response
International Health Regulations (2005). [cited 2021 Nov 15]. Available from: https://www.who.int/publications/i/item/9789241580410
Global Surveillance during an Influenza Pandemic.
Williams S, Fitzner J, Merianos A, Mounts A. The challenges of global case reporting during pandemic a(H1N1) 2009. Bull World Health Organ. 2014.
Public health surveillance for COVID-19: interim guidance. [cited 2022 Feb 14]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-SurveillanceGuidance-2022.1
WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. [cited 2022 Mar 2]. Available from: https://covid19.who.int/
Case-based reporting form. [cited 2022 Jan 31]. Available from: https://www.who.int/publications/i/item/case-based-reporting-form
Global surveillance of COVID-19: WHO process for weekly reporting aggregated data. [cited 2022 Jan 31]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-surveillance-aggr-CRF-2020.3
Protocol for assessment of potential risk factors for 2019-novel coronavirus (COVID-19) infection among health care workers in a health care setting. [cited 2021 Nov 11]. Available from: https://www.who.int/publications/i/item/protocol-for-assessment-of-potential-risk-factors-for-2019-novel-coronavirus-(2019-ncov)-infection-among-health-care-workers-in-a-health-care-setting
Home - FIND. [cited 2022 Feb 22]. Available from: https://www.finddx.org/
COVID-19 Data Explorer - Our World in Data. [cited 2022 Feb 22]. Available from: https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&time=2020-03-01..latest&country=USA~GBR~CAN~DEU~ITA~IND®ion=World&pickerMetric=location&pickerSort=asc&Interval=7-day+rolling+average&Relative+to+Population=true&Metric=Confirmed+cases&Color+by+test+positivity=false
Monitoring COVID-19 vaccination: Considerations for the collection and use of vaccination data. [cited 2021 Nov 15]. Available from: https://www.who.int/publications/i/item/monitoring-covid-19-vaccination-interim-guidance
Riffe T, Acosta E, team the CovD, Acosta EJ, Manuel Aburto D, Alburez-Gutierrez A, et al. Data resource profile: COVerAGE-DB: a global demographic database of COVID-19 cases and deaths. Int J Epidemiol. 2021;50(2):390–390f.
WHO COVID-19 surveillance data detailed dashboard. [cited 2022 Jan 31]. Available from: https://app.powerbi.com/view?r=eyJrIjoiYWRiZWVkNWUtNmM0Ni00MDAwLTljYWMtN2EwNTM3YjQzYmRmIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9
WHO. WHO COVID-19 Vaccination dashboard. [cited 2022 Feb 22]. Available from: https://app.powerbi.com/view?r=eyJrIjoiMWNjNzZkNjctZTNiNy00YmMzLTkxZjQtNmJiZDM2MTYxNzEwIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9
Chew MH, Koh FH, Wu JT, Ngaserin S, Ng A, Ong BC, et al. Clinical assessment of COVID-19 outbreak among migrant workers residing in a large dormitory in Singapore. J Hosp Infect. 2020;106(1):202.
Griffith DM, Sharma G, Holliday CS, Enyia OK, Valliere M, Semlow AR, et al. Men and COVID-19: a biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Prev Chronic Dis. 2020;17.
Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol 2020;20(7):442–447.
Muurlink OT, Taylor-Robinson AW. COVID-19: cultural predictors of gender differences in global prevalence patterns. Front Public Health. 2020;8:174.
Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046–53.
Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020 Apr;29(8):152.
Russell TW, Golding N, Hellewell J, Abbott S, Wright L, Pearson CAB, et al. Reconstructing the early global dynamics of under-ascertained COVID-19 cases and infections. BMC Med. 2020;18(1):1–9.
Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475–83.
Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Update alert 10: epidemiology of and risk factors for coronavirus infection in health care workers. Ann Intern Med. 2022;175(1):W8–9.
Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173(2):120–36.
Harris JE, Harris JE. Estimated fraction of incidental COVID hospitalizations in a cohort of 250 high-volume hospitals located in 164 counties. medRxiv. 2022; 2022.01.22.22269700.
Revealing the toll of COVID-19. [cited 2021 Nov 15]. Available from: https://www.who.int/publications/i/item/revealing-the-toll-of-covid-19
The true death toll of COVID-19: estimating global excess mortality. [cited 2021 Nov 15]. Available from: https://www.who.int/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality
Adam D. COVID’s true death toll: much higher than official records. Nature. 2022;603(7902):562.
The pandemic’s true death toll | The Economist. [cited 2022 Apr 11]. Available from: https://www.economist.com/graphic-detail/coronavirus-excess-deaths-estimates
Rapid review of WHO COVID-19 surveillance: External review, 27 October 2021. [cited 2022 Jan 31]. Available from: https://www.who.int/publications/m/item/rapid-review-of-who-covid-19-surveillance-external-review-27-october-2021
Early Warning, Alert and Response System (EWARS). [cited 2022 Apr 4]. Available from: https://www.who.int/emergencies/surveillance/early-warning-alert-and-response-system-ewars
Covid Surveillance - DHIS2. [cited 2021 Nov 15]. Available from: https://dhis2.org/covid-surveillance/
Outbreak toolkit. [cited 2022 Apr 4]. Available from: https://www.who.int/emergencies/outbreak-toolkit
Health Level Seven International - Homepage | HL7 International. [cited 2022 Aug 10]. Available from: https://www.hl7.org/
Perrocheau A, Brindle H, Roberts C, Murthy S, Shetty S, Martin AIC, et al. Data collection for outbreak investigations: process for defining a minimal data set using a Delphi approach. BMC Public Health. 2021;21(1):1–9 Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-021-12206-5. [cited 2022 Aug 5].
Case-based reporting form. [cited 2022 Mar 2]. Available from: https://www.who.int/publications/i/item/case-based-reporting-form
Acknowledgements
Marc Riner.
Fatima Mato Cores.
Sébastien Antoni.
Ravi Shankar Santhana Gopala Krishnan.
Christopher Tantillo.
Christopher Faulkner.
Bikram Maharjan.
Boris Pavlin.
Stephane Hugonnet.
Devaki Nambiar.
Katherine Kirkby.
Ahmadreza Hosseinpoor.
About this supplement
This article has been published as part of International Journal for Equity in Health Volume 21 Supplement 3, 2022: COVID-19 and inequality. The full contents of the supplement are available online at https://equityhealthj.biomedcentral.com/articles/supplements/volume-21-supplement-3.
Funding
The WHO surveillance database and dashboards are funded by the WHO. Funding for the journal special issue has been provided by Global Affairs Canada (GAC).
Author information
Authors and Affiliations
Contributions
Maya Allan, Maja Lièvre, Henry Laurenson-Schafer, Stéphane de Barros, Yuka Jinnai, Sophie Andrews, Thomas Stricker, Jesus Perez Formigo, Craig Schultz, Anne Perrocheau, Julia Fitzner. All authors were affiliated with WHO-HQ WHE COVID-19 IMST, Geneva, Switzerland, for the work described in this article. MA wrote the manuscript. She monitored the surveillance strategy after October 2021, and was involved in the analysis and dashboard design. AP and JF led the development and implementation of the global surveillance system, architecture and design of the database, analysis, and dissemination. AP revised the manuscript and added history of the project and insights of surveillance. JF participated to the writing of the manuscript. YJ was involved in the design of global surveillance, the early construction of the database, curation and analysis. H L-S was involved in the design of the database, maintenance, curation and analysis of data. ML developed and maintained the combined analysis dataset and the dashboard. ML participated to the writing of the manuscript. SdB has been responsible for the case-based data. SA was involved with the setup of the aggregated surveillance data (V2) on xMart and with dashboard development. TS was involved with data management and dashboard development. JPF contributed to the regular reviews of the data and drafted the first version of the article. CS has been responsible for the vaccination data and since November 21 for the whole database. All authors reviewed, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the statement in the Funding section was corrected, and the author name Henry Laurenson-Schafer was incorrectly written as Henry Laurenson-Schaefer.
The original online version of this article was revised: the Creative Commons license URL was incorrect, and has been updated.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO License (https://creativecommons.org/licenses/by/3.0/igo), which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. If you remix, transform, or build upon this article or a part thereof, you must distribute your contributions under the same license as the original. In any reproduction of this article there should not be any suggestion that World Health Organisation or this article endorse any specific organization or products. The use of the World Health Organisation logo is not permitted. This notice should be preserved along with the article’s original URL.
About this article
Cite this article
Allan, M., Lièvre, M., Laurenson-Schafer, H. et al. The World Health Organization COVID-19 surveillance database. Int J Equity Health 21 (Suppl 3), 167 (2022). https://doi.org/10.1186/s12939-022-01767-5
Published:
DOI: https://doi.org/10.1186/s12939-022-01767-5