Abstract
Background
Abnormal prolongation in the QT interval or long QT syndrome (LQTS) is associated with several cardiac complications such as sudden infant death syndrome (SIDS). LQTS is believed to be linked to genetic mutations which can be understood by using animal models, such as mice models. Nevertheless, the research related to fetal QT interval in mice is still limited because of challenges associated with T wave measurements in fetal electrocardiogram (fECG). Reliable measurement of T waves is essential for estimating their end timings for QT interval assessment.
Results
A mathematical model was used to estimate QT intervals. Estimated QT intervals were validated with Q-aortic closure (Q-Ac) intervals of Doppler ultrasound (DUS) and comparison between both showed good agreement with a correlation coefficient higher than 0.88 (r > 0.88, P < 0.05).
Conclusion
Model-based estimation of QT intervals can help in better understanding of QT intervals in fetal mice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The ventricular repolarization period of action potential (AP) is reflected as a T wave in the electrocardiogram (ECG). T wave is important for QT interval assessment which is considered a critical biomarker for cardiac abnormalities. QT interval is the interval starting from the QRS complex to the end of a T wave and it represents ventricular electrical systole [1]. In humans, abnormal prolongation in the QT interval or long QT interval syndrome (LQTS) is thought to be linked to Torsades de Pointes (TdP) [2] and sudden infant death syndrome (SIDS) [3]. LQTS can occur due to mutations in specific genes that make up the ionic channels responsible for the cardiac AP, and disruptions in the same channels may lead to an abnormal prolongation in the AP duration (APD) [4].
The relationships in LQTS and SIDS is not well established [5], therefore, more research is needed to understand how LQTS leads to SIDS. Since genetic manipulation of humans is not possible, animal models, such as mouse models, can be used for better understanding of cardiac abnormalities and LQTS. Up until now, fetal QT intervals in mice have received minimal attention because of the challenges associated with T wave measurements. T waves are known for their low amplitudes which make their detection from fetal ECG (fECG) challenging [6]. Furthermore, a mouse fetal heart is much smaller than a human fetal heart which imposes additional challenges on measuring T waves. Due to the difficulties in measuring T waves in fetal mice, model-based estimations of the end of T waves can help in better understanding of QT intervals in mice.
In this study, a mathematical model for the estimation of end of T waves of fECG collected from normal fetal mice is proposed. The model discussed in this study was developed from our earlier mathematical model that addressed fECG of humans [7]. The model was developed based on the similarity between the human’s repolarization phase of an AP and the discharging phase of capacitors in resistor–capacitor (RC) circuits. Similarly, the model in this study was developed due to the high similarities between mice AP and the charging and discharging phase of the capacitor [8]. Since there are differences in APD patterns between humans and mice, our earlier mathematical model was adjusted to accommodate for the difference.
In our previous study [7] model-based QT intervals had high agreement with QT intervals calculated from scalp fetal ECG (sfECG) and Q-aortic closing (Q-Ac) intervals calculated from pulsed Doppler records. In this study, we validated our model with Q-Ac.
Results
RR interval values, measurers’ evaluation of Q-Ac intervals and their difference percentages, and model-based QT interval values and their difference percentages to Q-Ac values are shown in Table 1.
In Table 1, the difference percentage for the measurers’ evaluation of Q-Ac is less than 5% which indicates high agreement. The difference percentage values between the two measurers’ Q-Ac values and the model-based QT intervals are also less than 5%. The low percentage difference between the measurers Q-Ac values and model-based QT intervals indicates that the model could estimate fetal QT intervals. For further validation of the measurers’ evaluation and model-based QT intervals, correlation and Bland–Altman (BA) analyses were conducted, and the results are shown in Fig. 1. In Fig. 1A, the correlation coefficient is high, r = 0.93 and the BA plot shows that at least 90% of the values are within the limits of agreement. High correlation coefficients and agreements are also shown in Fig. 1B, C which show the comparison between the measurer’s Q-Ac values and QT intervals.
Bland–Altman and correlation analyses were performed to compare the Q-Ac interval values that were estimated by the two measurers a, and to compare model-based QT interval values with Q-Ac interval values of the two measurers b–c. QT and Q-Ac intervals were estimated beat by beat and the total number of heart beats was 309 from five fetal mice. a 95% of the values are within the limits of agreement (r = 0.93, P < 0.05). b 93% of the values are within the limits of agreement (r = 0.89, P < 0.05). c 92% of the values are within the limits of agreement (r = 0.91, P < 0.05)
Discussion
The AP consists of depolarization, plateau phase and repolarization. APD patterns in murine ventricular myocardium differ from that of humans [9]. In mice, the APD decreases with fetal age, whereas the opposite occurs in humans [8]. In addition, mice APD is shorter than humans’ which explains the higher heart rates in mice [9]. Differences in the APD between the two species are mainly attributed to the differences in the ion channels responsible for the repolarization phase. The repolarization phase is controlled by the flow of potassium ion channels (K+) out of the cells [9, 10]. K+ currents are usually divided into transient outward currents (Ito) and delayed rectifier currents (IK). Ito plays a major role in the repolarization phase in mice [11]; on the other hand, the same current is prominent in the plateau phase in humans. In adult mice, the IK current, though still controversial, seems to be negligible. In contrast, the same current is important for the completion of the repolarization phase in humans [9]. According to L. Wang et al. [12], the Ik current is mostly prominent in fetal mice, and it reduces with fetal age and almost disappears during adulthood [12].
Due to the shorter APD in mice compared to humans, the model described in this study was adjusted to accommodate for the shorter repolarization period which signifies earlier occurrence of a T wave end. In our earlier model [7], the end of a T wave was calculated by subtracting a factor of \(\frac{6\pi }{{x^{2} }}\) from the term \(\frac{{{\text{mean}} \,\left( {R\left( t \right)} \right)}}{x}\) (Eq. 3). On the other hand, in mice, a factor of \(\frac{2\pi }{{x^{2} }}\) was added to the same term to accommodate for the faster termination of a T wave in mice AP. To evaluate the accuracy of the model, the QT interval values were compared with the Q-Ac interval values estimated by two measurers from Doppler Ultrasound (DUS) records. The both measurers provided similar evaluations of Q-Ac interval with difference percentage less than 5% (Table 1). In addition, the similarity between the two measurers was further confirmed with correlation and BA plots in Fig. 1a which shows that around 95% of the values are within the limits of agreement with r = 0.91 (P < 0.05). After confirming the high agreement between the two measurers’ Q-Ac interval values, they were used for validating model-based QT-intervals. Table 1 reveals that the difference percentages between model-based QT intervals and Q-Ac intervals are less than 5%, and the correlation and BA analyses in Fig. 1b, c show high agreement between the same intervals with high correlation, r > 0.90 (P < 0.05). The mean values in the BA plots in Fig. 1b, c indicate that the model tends to provide QT interval values that are less than the Q-Ac interval values. The latter could be due the time difference between the mechanical activity and electrical activity of the heart [13]. Up until now, little information is available about the time lag between mechanical and electrical activities in fetal mice, therefore, it is difficult to further comment on the expected difference between the QT and Q-Ac intervals.
The model proposed in this study can be useful in thorough QT/corrected QT interval (QTc) studies which involve assessments of drugs that may cause LQTS [14]. For example, one can compare the predicted QT interval with the measured QT interval value after administration of a drug to determine if the drug is causing abnormalities to the QT interval or not. However, although the model showed high agreement with Q-Ac interval, it needs to be tested on more subjects. The model was tested on a low number of heart beats because of the difficulties that were involved in the experiment. Although the fECG recordings were carried out for 15 min, only heart beats that had clear pulsed Doppler images were selected for analysis in this study. Pulsed Doppler images of fetal heart were particularly challenging to obtain because of the small size of the fetal mouse. During the experiment, several attempts were made to obtain clear Doppler data and eventually only a few DUS images were clear enough for Q-Ac assessments.
Conclusion
So far, it is unknown how repolarization patterns change in human fetuses. Therefore, it is important to utilize animal models, such as mice models, for better understanding of cardiac repolarization patterns. Repolarization patterns can be assessed by calculating QT intervals from ECG records. Nevertheless, due to the difficulties in measuring QT intervals in fetal mice, we proposed a model to estimate QT intervals by using RR intervals only. The model showed good accuracy with Q-Ac interval values of DUS. However, due to the low number of analyzed heart beats, more research is needed for further validation of the model.
Materials and methods
The experimental protocol of this study was approved by the Center for Laboratory Animal Research, Tohoku University (animal experiment approval numbers are 2013 ido-501 and 2016 ido-079). The study protocol followed the Regulations for Animal Experiments and Related Activities of Tohoku University.
Experimental procedures and data collection
C57BL/6N mice were purchased from CLEA Japan Inc. All mice had access to unlimited drinking water and were fed mouse pellets. All mice were kept in a room with a light–dark cycles of 12 h per cycle, in addition, mice were exposed to artificial light from 8:00 to 20:00. The room temperature was 22–26 °C, with a relative humidity of 50–60%.
2 female mice aged 7–20 weeks were housed together with 2 male mice of the same strain, separately, for one night; the next morning was considered a 0.5 embryonic day (E0.5). The experiment was performed at E18.5 and anesthetics were injected to maternal mice. The anesthetics composed of 0.2% ketamine and 0.05% xylazine for induction of anesthesia, and 0.5% isoflurane for maintenance of anesthesia. The maternal mice were kept on a supine position on a warm pad at 37 °C during the experiment. In addition, a far infrared heater was placed beside the maternal body to maintain a warm environment. Mice abdominal hair was removed using a hair removal cream (Veet, Reckitt Benckiser Group plc, Slough, England, UK). After confirming the mice were under anesthesia by disappearance of movements, the abdomen was open at the peritoneal cavity with fine scissors, and the uterus was exposed.
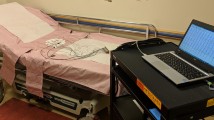
The fECG was measured at a sampling rate of 1000 Hz for 15 min by attaching two electrodes, one at the fetal chest and the other at the back and fetuses were selected randomly (the ground was the maternal body). The fECG data were recorded by a portable multi-purpose bio signal amplifier monitoring system (Polymate AP1532 and AP Monitor; Miyuki Giken, Tokyo, Japan). Simultaneously, pulsed-wave Doppler measurement was conducted by using an ultrasound imaging system (VEVO 2100 Imaging System, FUJIFILM VisualSonics Inc., Tokyo, Japan). Since the fECG and DUS data were obtained using independent systems, it is necessary to match and align the two records afterwards for analysis. Hence, a pulse generator was connected to both systems to generate the same pulsed signals at random timings during the recording time (Fig. 2A). The timing at which the pulsed wave was generated, by pressing the switch button, was recorded in a sheet. fECG and DUS data were collected from a total of 5 fetuses that belonged to 2 mothers.
A fECG and pulsed Doppler images were obtained from the fetal mouse by using different systems. In order to match Doppler images with fECG records, a signal generator was connected to both systems to input pulsed signals. B The pulse signal that was recorded with fECG is aligned with the same signal that was recorded simultaneously with DUS. Q-Ac intervals were calculated by drawing vertical lines from the Ac timings of DUS to fECG. The red asterisks in fECG indicate the estimated end of T waves. End of T waves were calculated from R(t), Eq. 3. Ac timings were determined by drawing tangent lines where blood flow crosses the base line
Model description
The fetal mouse model is an adjustment to our previous fetal human model which is discussed in detail in [7]. Our previous model was developed based on the similarities between the capacitor discharging phase in a RC circuit and the repolarization phase of a human AP. Since the decay in repolarization phase of fetal mice AP looks similar to the discharging phase of the capacitor [8]., we made use of the equations that are used for the calculations of the discharging phase of the capacitor in a RC circuit, Eqs. 1, 2, to develop our end of T wave model:
t is the time in seconds, R is the resistance in ohms and C is the capacitance in farads.
fc is the cut-off frequency in Hertz.
In Eq. 2, fc was considered as similar to HR, hence the term \(\frac{1}{fc}\) was replaced with RR since RR is the inverse of HR. Therefore, the term RC in Eq. 1 was replaced with the expression in Eq. 2 with replacing \(\frac{1}{fc}\) with RR and setting v0 to 100 to obtain Eq. 3:
where t is the time in milliseconds and RR is the interval between the sequential R waves. By using Eq. 3, a constant was calculated to estimate an end of a T wave, which is denoted k as:
where x is the reciprocal of RR in seconds for one beat. The expression in Eq. 4 is slightly different than the one in our previous study to accommodate for the difference in HRs between mice and humans. In our previous mode, we subtracted a factor of \(\frac{6\pi }{{x^{2} }}\) from the term \(\frac{{{\text{mean}} \,\left( {R\left( t \right)} \right)}}{x}\). Finally, by using the constant k, the median of a range in which an end of a T wave, TE, is expected to exist was calculated by using Eq. 5:
Model validation
QT interval values estimated by the model were validated by using DUS as was done in our previous study [7]. In DUS, the end of a T wave is believed to occur close to Ac [15], 16]. Hence, Q-Ac intervals were calculated and compared with QT intervals. For validation purposes, Q-Ac intervals were evaluated by two measurers. R and Q timings were determined automatically using a MATLAB code (based on “findpeaks” function) and Q timings were considered as the lowest peak preceding an R peak. Detected R peaks were checked manually to ensure that R peaks were detected properly. Ac timings were evaluated manually by drawing a tangent line at the point when the blood flow waveform crosses the baseline as indicated by the yellow line denoted as Ac in Fig. 2B. The Ac timing values were read from MATLAB by aligning the pulsed Doppler images with fECG tracings as shown in Fig. 2B. To read the timing values in MATLAB, a line (dotted yellow line in Fig. 2B) was drawn from Ac (Doppler) to the fECG signal (MATLAB) [lines were drawn by using Microsoft Power Point (Office 365)]. The exact values were ready by using the “Data tips” tool in MATLAB in which the values were displayed after clicking at a point in the fECG signal where the yellow dotted line matched with the Ac in Doppler. QT and Q-Ac intervals calculations were performed beat by beat and the total number of analyzed heart beats was 309 from five fetal mice. Before comparing Q-Ac intervals with QT intervals, Q-Ac intervals that were estimated by the two measurers were compared with each other to confirm consistency. The comparison was performed by calculating the difference percentage per fetus and performing BA analysis [17, 18] for all the 309 heart beats. Difference percentages were calculated by using Eq. 6:
Following the Q-Ac interval consistency analysis, Q-Ac intervals were compared with model-based QT intervals by using the same previously mentioned comparison analyses, difference percentage and BA plots.
Availability of data and materials
Data are available upon a proper request by contacting Dr. Yoshiyuki Kasahara: kasa@med.tohoku.ac.jp.
Abbreviations
- Ac:
-
Aortic closing
- APD:
-
Action potential duration
- BA:
-
Bland–Altman
- DUS:
-
Doppler ultrasound
- E0.5:
-
Embryonic day 0.5
- E18.5:
-
Embryonic day 18.5
- ECG:
-
Electrocardiogram
- fECG:
-
Fetal electrocardiogram
- I to :
-
Transient outward currents
- I K :
-
Delayed rectifier currents
- K+ :
-
Potassium ion channels
- LQTS:
-
Long QT syndrome
- ms:
-
Millisecond
- RC:
-
Resistor–capacitor
- sfECG:
-
Scalp fetal electrocardiogram
- SIDS:
-
Sudden infant death syndrome
- TdP:
-
Torsades de pointes
References
Ambhore A, Teo S-G, Omar AR, Poh K-K. ECG series. Importance of QT interval in clinical practice. Singapore Med J. 2014;55(12):607–12.
Topilski I, et al. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J Am Coll Cardiol. 2007;49(3):320–8.
Schwartz P, et al. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338(24):1709–14.
Crotti L, Celano G, Dagradi F, Schwartz P. Congenital long QT syndrome. Orphanet J Rare Dis. 2008. https://doi.org/10.1186/1750-1172-3-18.
Ioakeimidis N, Papamitsou T, Meditskou S, Iakovidou-Kritsi Z. Sudden infant death syndrome due to long QT syndrome: a brief review of the denetic substrate and prevalence. J Biol Res (Thessalon). 2017. https://doi.org/10.1186/s40709-017-0063-1.
Shang H, et al. An improved sliding window area method for T wave detection. Comput Math Methods Med. 2019. https://doi.org/10.1155/2019/3130527.
Widatalla N, Kasahara Y, Kimura Y, Khandoker A. Model based estimation of QT intervals in non-invasive fetal ECG signals. PLoS ONE. 2020. https://doi.org/10.1371/journal.pone.0232769.
Hamaguchi S, et al. Developmental changes in excitation-contraction mechanisms of the mouse ventricular myocardium as revealed by functional and confocal imaging analyses. J Pharmacol Sci. 2013;123(2):167–75.
Edwards A, Louch W. Species-dependent mechanisms of cardiac arrhythmia: a cellular focus. Clin Med Insights Cardiol. 2017. https://doi.org/10.1177/1179546816686061.
Boukens B, Rivaud M, Rentschler S, Coronel R. Misinterpretation of the mouse ECG: ‘musing the waves of mus musculus.’ J Physiol. 2014. https://doi.org/10.1113/jphysiol.2014.279380.
Itoh H, Naito Y, Tomita M. Simulation of developmental changes in action potentials with ventricular cell models. Syst Synth Biol. 2007. https://doi.org/10.1007/s11693-006-9002-4.
Wang L, Feng Z, Kondo C, Sheldon RDH. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ Res. 1996;79(1):79–85.
Kording F, et al. Doppler ultrasound compared with electrocardiogram and pulse oximetry cardiac triggering: a pilot study. Magn Reson Med. 2015;74(5):1257–65.
Darpo B. The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol. 2010;159(1):49–57.
Khandoker A, et al. Antepartum non-invasive evaluation of opening and closing timings of the cardiac valves in fetal cardiac cycle. Med Biol Eng Comput. 2009;47(10):1075–82.
Marzbanrad F, et al. Assessment of fetal development using cardiac valve intervals. Front Physiol. 2017. https://doi.org/10.3389/fphys.2017.00313.
Bland J, Altman D. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60.
Bland J, Altman D. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. 2003;22(1):85–93.
Acknowledgements
The authors would like to thank Prof. Toshiyuki Hayase at Institute of Fluid Science (IFS), Tohoku University, for the part of experiments carried out under the Collaborative Research Project of IFS. Kiyoe F. acknowledges JSPS KAKENHI Grant Number 25860902. The work in this paper has been supported by RIKEN Healthcare and Medical Data Platform Project, the funding for basic medical research by Shiguredo Inc and collaborative CIRA grant (2019-023) awarded to Ahsan Khandoker by Khalifa University Abu Dhabi. Also, the research is partially supported by the Project for Baby and Infant in Research of Health and Development to Adolescent and Young adult from Japan Agency for Medical Research and development, AMED.
Funding
The work in this paper has been supported by RIKEN Healthcare and Medical Data Platform Project, the funding for basic medical research by Shiguredo Inc. and collaborative CIRA Grant (2019-023) awarded to Ahsan Khandoker by Khalifa University Abu Dhabi. Also, the research is partially supported by the Project for Baby and Infant in Research of Health and Development to Adolescent and Young adult from Japan Agency for Medical Research and development, AMED. Also, part of the experiments was supported by the Institute of Fluid Science (IFS). Tohoku University and Japan society of the promotion of science (JSPS) (KAKENHI Grant Number 25860902).
Author information
Authors and Affiliations
Contributions
NW: contributed to the methodology, formal analysis, and writing and editing of the manuscript. KiF: contributed to the methodology of the study and performed the experiment and edited the final version of the manuscript. MK: worked on the experiment and reviewed the final version of the manuscript. CY: worked on the experiment and sorted the data and reviewed the final version of the manuscript. KeF: contributed to the methodology of the study and obtained resources to perform the experiment and edited and reviewed the final version of the manuscript. YKa: contributed to the methodology of the study and obtained resources and funding for the experiment and reviewed the final version of the manuscript. MS: supervised the study and analysis and reviewed and edited the final version of the manuscript. AK: contributed to the methodology and analysis of the study and obtained resources to perform the study and edited and reviewed the final version. YKi: contributed to the methodology and obtained resources to perform the experiment and reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental protocol of this study was approved by the Center for Laboratory Animal Research, Tohoku University (animal experiment approval numbers are 2013 ido-501 and 2016 ido-079). The study protocol followed the Regulations for Animal Experiments and Related Activities of Tohoku University. Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Widatalla, N., Funamoto, K., Kawataki, M. et al. Model-based estimation of QT intervals of mouse fetal electrocardiogram. BioMed Eng OnLine 21, 45 (2022). https://doi.org/10.1186/s12938-022-01015-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12938-022-01015-5