Abstract
Background
Several studies have reported the association between dietary inflammatory index (DII) and the SARS-CoV-2 infection risk, severity or mortality of COVID-19, however, the outcomes remain controversial.
Objective
We sought to examine whether a dose-response association of DII and SARS-CoV-2 infection exists.
Design
A dose-response meta-analysis was performed to investigate the association of DII and SARS-CoV-2 infection. We conducted a systematic search of PubMed, Embase and Web of Science up to March 15th, 2023. The odds ratios (OR) of DII and COVID-19 risk and severity were computed.
Results
Totally, 5 studies were included (1 from UK and 4 from Iran), consisting of 197,929 participants with 12,081 COVID-19 cases. Although there was heterogeneity among studies, the results indicated that higher DII was independently related to higher SARS-CoV-2 infection incidence (OR = 1.57, 95% CI: 1.14, 2.17) and COVID-19 severity (OR = 1.11, 95% CI: 1.07, 1.15) but not COVID-19 mortality (risk ratio = 1.13, 95% CI: 1.00, 1.27). The incidence of SARS-CoV-2 infection increased by 31% for each 1-point increase in the E-DII (OR = 1.31, 95% CI: 1.20, 1.43).
Conclusions
This meta-analysis suggests that an elevated DII score is associated with increased SARS-CoV-2 infectious risk and severity of COVID-19. There were not enough studies on COVID-19 mortality. Further large prospective studies in different countries are warranted to validate our results.
Similar content being viewed by others
Introduction
Since the initial cases of COVID-19 were reported in 2019, the world has been grappling with a swift global pandemic [1]. While vaccines can shield against severe disease and ideally contribute to herd immunity, the emergence of new SARS-CoV-2 variants (such as Omicron, XBB lineage, etc.) is likely to result in fresh outbreaks due to heightened infectivity, virulence, or enhanced potential for immunological evasion [2, 3]. COVID-19 manifests through distinct inflammatory pathways, immune responses, and potentially devastating cytokine storms [4, 5]. Being a crucial aspect of life, a high-quality daily diet and patterns have been reported to correlate with a reduced risk of SARS-CoV-2 infection and hospitalization [6]. In contrast, malnutrition serves as an ominous prognostic sign in patients hospitalized with COVID-19 [7]. Studies have shown an inverse relationship between the Mediterranean diet and the risk of COVID-19, suggesting its potential utility in mitigating COVID-19-induced inflammation [8, 9]. Food antioxidant supplements, such as vitamin C and vitamin D3, exhibit a strong correlation with decreased severity and mortality in COVID-19, as indicated by randomized controlled trials [10, 11]. Diet may participate in the inflammatory response in patients with COVID-19 [12]. So what is the specific effect of anti-inflammatory diet or pro-inflammatory diet on COVID-19?
The Dietary inflammatory index (DII) is a quantitative tool for assessing diet inflammatory potential based on the inflammatory factors from daily intake from a multiple items food frequency questionnaire (FFQ) [13]. The Energy-adjusted DII (E-DII) primarily concentrates on energy intake, and elevated DII/E-DII levels are supported by inflammatory markers like C-reactive protein [14]. Numerous studies have highlighted the crucial role of DII in assessing the inflammatory potential of diets and predicting the risk of chronic diseases, such as cardiovascular diseases or depression [15, 16]. We have also previously reported a linear dose-response relationship between DII and the risk of human cancer [17]. Therefore, to prevent and reduce the severity or mortality of COVID-19, DII may serve as a reminder for individuals to maintain healthy eating habits.
Regarding the relationship between an inflammatory diet and COVID-19, researchers have discovered that a high-quality diet can lower the levels of inflammatory factors [18]. Research has demonstrated the relevance of the Mediterranean diet to a reduced risk of SARS-CoV-2 infection and improved outcomes in patients with COVID-19 [19]. Additionally, diets rich in antioxidants, such as vitamin D, with immunomodulatory potential, may contribute to prophylactically mitigating the severity of COVID-19 [20]. Meanwhile, an unhealthy diet should not be neglected as a potential source of inflammation, given that chronic inflammation has adverse effects on health. The Western diet, characterized by high consumption of processed foods, may induce hyperglycemia and hyperlipidemia. Diets with different DIIs may exhibit different effects on regulating the level of inflammation in the body, which in turn affects the rate and severity of COVID-19 infections. Li et al. found that hyperglycemia is associated with a high risk of mortality in patients with severe COVID-19 [21, 22]. Furthermore, the Western diet may be associated with adaptive immunity impairment in patients with COVID-19, potentially contributing to aggravation of the disease [23]. Over the past decades, certain strategies, such as the Healthy Eating Index and Alternative Healthy Eating Index, have been investigated for their association with the risk of all-cause mortality [24, 25]. The DII, a comprehensive, reproducible and quantitative method of FFQ calculation, can recognize the exact inflammatory potential of diet. The low DII items contain deleted n-3 fatty acids, β-carotenes, phytochemicals, and plant fiber, whereas the Western diet (high DII) means a high consumption of red and processed meat, trans-fatty acid or saturated fat acid [26].
To date, the results from studies on DII and COVID-19 remain inconclusive and controversial. For instance, individuals with higher DII had a higher SARS-CoV-2 infectious risk and mortality of COVID-19 in a UK-biobank cohort [27]. However, the results from Tavassoli and Cols indicated no association between DII score and SARS-CoV-2 infection incidence (OR = 1.08, 95% CI: 0.92–1.27) [28]. Here we systematically summarize the currently available evidence on DII and the incidence of SARS-CoV-2 infection, severity and mortality of COVID-19. Meta-analyses were performed on the incidence and severity, and a dose-response analysis was conducted on the incidence. Additionally, meta-regression and sensitivity analysis were carried out to assess stability. To date, this is the first meta-analysis concerning dietary-related inflammation and COVID-19, as far as we know.
Methods
The study was preregistered with PROSPERO under project number CRD42023407410.
Search strategy
According to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), we carried out this study [29]. We comprehensively searched for relevant literature published in PubMed, Embase and Web of Science from January 1st, 2020 to March 15th, 2023. The main terms were as follows: (‘pro-inflammatory diet’ OR ‘dietary inflammatory index’ OR ‘anti-inflammatory diet’ OR ‘inflammatory potential intake’) AND (‘COVID-19’ OR ’SARS-CoV-2 ’) AND (‘risk’ OR ‘incidence’ OR ‘odds’ OR ‘hazards’ OR ‘mortality’ ). There were no language restrictions, and we manually searched reference lists for additional relevant publications.
Inclusion and exclusion criteria
The eligible criteria were under the guide by the Population, Intervention, Comparison, Outcome (PICO) question: In general adult population (P), DII or EDII score (I), higher DII/E-DII group compared to lower DII/E-DII group (C), and COVID-19 incidence (primary outcome), severity or mortality (secondary outcomes) (O). The exclusion criteria were: (1) Reporting DII during or after the pandemic; (2) Repeated publications; 3.Less than 30 participants; 4. Laboratory articles, non-human animal studies, or review articles.
Assessment of study quality
Two authors (X.H. and S.L.) independently screened titles and abstracts, meanwhile, they evaluated the methodological quality of potential studies using the Newcastle–Ottawa Quality Assessment Scale (NOS) tool [30]. When the NOS score was none less than 7, the study was regarded as high quality. If the two authors had a disagreement, a third author made the final decision (D.L.).
Data extraction
After a full-text review, we extracted the following variables from each included study: the first author’s name, publication year, study design, country of the participants, sample size (number of COVID-19 patients), age, sex, multivariate adjustments, outcomes and ORs with 95% CI. Only the multivariate ORs were adopted for meta-analysis because of the higher objectivity than univariate ORs. The corresponding author was contacted for further information under the data-deficiency circumstance.
Statistical analysis
We synthesized odds ratios (ORs) with 95% confidence intervals (CIs) using the meta-analysis method to investigate the association between the Dietary Inflammatory Index (DII) and the incidence and severity of COVID-19. The DII scores from eligible studies were continuous variables or categorical (the highest quartile versus the lowest). Heterogeneity among studies was assessed using the Cochrane Q test (P value < 0.10 represented heterogeneity) and Inconsistency(I2 value > 50% indicated heterogeneity) [31]. Due to potential heterogeneity, the DerSimonian and Laird inverse variance random-effects model was applied to calculate pooled results [32]. Subgroup analyses were conducted to explore the causes of heterogeneity, considering study design, DII or E-DII, study quality and ethnic differences. In addition, we used sample size and sex proportion as co-variates to do a meta-regression.
Subsequently, we conducted a dose-response analysis to examine the relationship between E-DII and the incidence of COVID-19. The OR, 95% CI and corresponding dose values for each category of E-DII (lowest to the highest) were extracted. We assigned the midpoint of each category when the dose values were unavailable [33]. If the highest (lowest) category was open-ended, we adopted the lower end value of the category and added (reduced) the closest neighboring category dose interval value [34]. We applied the ‘glst’ command and a random-effect four knots cubic spline (non-linear) model in STATA software to do a conservative dose-response analysis [35, 36]. Four dose knots at 5% (0), 35% (dose1), 65% (dose2) and 95%(dose3), in the dose range were generated before evaluating the dose-response via the restricted cubic spline method [17].
For evaluating the reliability of the main pooled outcome, a sensitivity analysis was conducted by removing a single study in turn. Egger’s and Begg’s tests, along with funnel plots, were adopted to assess publication bias [37]. A P value > 0.1 indicated no potential publication bias. All statistical analyses mentioned above were performed using STATA 12.0 software (Stata Corporation, TX, USA). A two-sided P value < 0.05 was considered statistically significant.
Results
Study search and characteristics
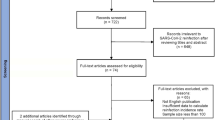
A comprehensive literature search was performed as shown in Fig. 1. Initially, 139 papers were identified through database searches. Subsequently, 19 potential studies were retained through reading the titles and abstracts. Afterward, 14 studies were excluded due to insufficient data (1 study) or not being research-oriented articles (13 studies). Finally, 5 articles [27, 28, 38,39,40] met the inclusion criteria for this meta-analysis.
Table 1 presents the fundamental research information of the included studies. The publication years ranged from 2021 to 2023. In total, these studies contained 197,929 participants with 12,081 COVID-19 cases. Participant sexes included male and female, and ages ranged from 18 to 73 years. All 5 included studies employed the same way of scoring the DII based on a self-reported or face-to-face recorded food frequency questionnaire (FFQ). The rationale and description of the DII methodology were previously published [13]. In brief, the DII was calculated from every inflammatory item in the FFQ. Among them, 4 studies were launched in Iran, while only 1 study was performed in a Western country (the UK). In general, 1 prospective cohort and 4 case-control studies reported the association between DII and the incidence of COVID-19, whereas 2 studies investigated the relation between DII and the severity of COVID-19. There was only 1 study about DII and COVID-19 mortality.
Quality assessment
Despite potential methodological deficiencies in the quality of the included studies, all were assessed as medium to high quality using the NOS assessment tool, with scores ranging from 5 to 7 (Table 1).
DII and incidence, severity, and mortality of COVID-19
As demonstrated in Fig. 2, the forest plot indicates that a higher DII was independently associated with the increasing odds of COVID-19 (pooled OR = 1.57, 95% CI: 1.14, 2.17, Fig. 2A). Only 2 studies investigated the association of DII and COVID-19 severity (defined as the length of hospital-stay in both studies). The result of meta-analysis suggested that elevated DII was also related to more severe COVID-19 (pooled OR = 1.11, 95% CI: 1.07, 1.15, Fig. 2B). Given that only 1 study reported the potential association of DII and the mortality of COVID-19 after our systematic screening, we were unable to do further meta-analysis. Therefore, we adopted the result that the peak value group DII (the highest quintile DII vs. the lowest quintile) was associated with a slightly higher risk ratio (RR) of COVID-19 mortality (RR = 1.43, 95% CI: 1.01, 2.01, P = 0.04). However, when considered as a continuous variable, DII was not significantly associated with COVID-19 mortality (RR = 1.13, 95% CI: 1.00, 1.27, P = 0.37).
Afterwards, we did additional subgroup analyses to explore the association between DII and the infectious risk of SARS-CoV-2 infection incidence. First, we performed subgroup analysis based on different countries. Among the 5 included studies, 4 studies were from an Asian country, Iran. The pooled OR in this group was 1.80 (95% CI: 1.23, 2.63, Fig. 3A). Second, a subgroup analysis was conducted based on study design. There were 4 case-control studies, the pooled OR was 1.80 (95% CI: 1.23, 2.63, Fig. 3B). Third, we respectively performed meta-analysis on DII and energy-adjusted DII. Neither DII (OR = 1.23, 95% CI: 0.87, 1.73, Fig. 3C) nor energy-adjusted DII (OR = 1.98, 95% CI: 0.85, 4.64, Fig. 3D) were significantly associated with the odds of SARS-CoV-2 infection.
Meta-regression
An obvious heterogeneity was found in the included studies (Fig. 2A, I2 = 75.2%), so we performed a meta-regression to seek potential influencing factors. Owing to the insufficient data on other parameters, such as baseline cardiovascular diseases, we chose sample size and sex proportion as covariates to estimate between-study variance. The results of this regression model indicated that the 2 factors above (sample size: P = 0.292, sex proportion: P = 0.310) were not the direct source of heterogeneity.
Dose-response analysis
As shown in the dose-response plot, the pooled dose-response OR of the non-linear model was 1.31 (95% CI: 1.20, 1.43, Z value = 5.91, P < 0.001, Fig. 4), demonstrating that human COVID-19 risk increased by 31% after E-DII increasing by 1-point increase in the score. Unfortunately, dose-response analysis was not conducted on the severity or mortality of COVID-19 due to the lack of original data.
Sensitivity analysis
A sensitivity analysis was performed by removing 1 study in sequence to test the stability of our pool results. The removal of a single study did not significantly impact the main results, as shown in Fig. 5.
Publication bias
In order to gauge publication bias, we performed both Egger’s and Begg’s tests. Neither the Egger’s test (P = 0.301, > 0.1) nor the Begg’s test (P = 0.933, > 0.1) indicated potential publication bias in the association between DII and COVID-19 risk. The corresponding plots of the upon tests are displayed in Fig. 6.
Discussion
In this study, we systematically summarized the existing evidence from the 5 included studies with 197,929 participants. The results indicate that a high DII score is independently associated with an increased incidence of SARS-CoV-2 infection incidence (OR = 1.57, 95% CI: 1.14, 2.17). Additionally, individuals with an elevated DII score had an 11% increased odds ratio of COVID-19 hospital-stay severity compared to those with a lower DII. Although individuals with the highest quintile DII may have a tiny increased risk of COVID-19 deaths, we should not draw the conclusion that DII is associated with the mortality of COVID-19 based on current evidence. Importantly, we objectively concluded that the incidence of SARS-CoV-2 infection increased by 31% for each 1-point increase in the E-DII based on a non-linear dose-response analysis. However, the correlation of high DII and COVID-19 should be interpreted with caution. Subgroup analyses based on study design and different countries did not significantly affect the main results. Although the energy-adjusted DII subgroup showed a similar rising trend with higher COVID-19 incidence, the results in this group were not statistically significant. We consider that the obvious heterogeneity among studies may account for this situation to some extent. Different SARS-CoV-2 variants and various local lockdown prevention policies may contribute to the heterogeneity. Based on all the aforementioned results, we believe that DII can be utilized as an objective and reliable tool in evaluating dietary inflammatory potential and preventing COVID-19.
A pro-inflammatory condition is positively correlated with COVID-19, potentially leading to increased nitrogen species and reactive oxygen levels, serving as a crucial factor in the development of COVID-19 severity [5]. Viral infection and replication can lead to inflammation through cytopathic effect directly [41]. During this period, there is an upregulation of pro-inflammatory cytokines and chemokines, leading to the recruitment of monocytes, eosinophils, and T lymphocytes. This phenomenon can explain the state of lymphocytopenia observed in some patients with COVID-19 [42]. Furthermore, in the cases of dysregulated immune response, elevated levels of inflammatory cytokine secretion such as interferon-gamma (IFN-γ), monocyte chemo-attractant protein-1 (MCP-1) and interleukin-6 (IL-6) may lead to an excessive inflammatory response, known as cytokine storm [43, 44].
Interestingly, researchers observed a reduction in the consumption of high DII foods, such as fast food, soft drinks, and alcoholic beverages during the COVID-19 pandemic, which was probably reduced by mobility restrictions and social gatherings [45]. In view of the above, this study may be timely and worthwhile. It suggests that promoting a healthy anti-inflammatory dietary pattern with a lower Dietary Inflammatory Index (DII) score could be a non-pharmacological approach to prevent COVID-19.
Despite conducting the first dose-response meta-analysis on DII and COVID-19, there are still several limitations. First, the publication language was not restricted, but all the 5 studies included were written in English. We identified no relevant articles published in Chinese or other languages which were qualified for the inclusion criteria. An extent of language bias may exist. Second, although supported the main results and there was no evidence of publication bias, the number of included studies was small in some subgroups. For instance, only 2 studies provided data on and DII and the severity of COVID-19. Moreover, the methodology had some limitations. Therefore, we emphasize the need for careful interpretation of the results. Third, within the 5 included studies, 4 were case-control studies, in which recall bias might exist. As a matter of fact, participants from only 1 prospective cohort also fulfilled the FFQ in an electronic self-reported way rather than a face-to-face solid-recorded interview, which may lead to unstable or exaggerated results. Fourth, the 5 included studies were from Iran (4 studies) or the UK (1 study). No studies on DII and COVID-19 from America, Oceania, East-Asian, or Africa were found to date. Therefore, further large prospective cohort studies in diverse populations are urgently needed.
In conclusion, this dose-response meta-analysis reveals that elevated DII is associated with a higher infectious incidence and severity of COVID-19, with the SARS-CoV-2 infectious odds increasing by 31% for each 1-point increase in the Enhanced DII (E-DII) score. However, there is insufficient evidence on COVID-19 mortality. Therefore, large prospective cohort studies in diverse regions or countries are warranted to validate the findings of this study.
Data availability
This is a meta-analysis, and all data in this article were in published articles.
References
Li J, Lai S. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature. 2021;600:408–18.
McIntyre PB, Aggarwal R, Jani I, et al. COVID-19 vaccine strategies must focus on severe disease and global equity. Lancet (London England). 2022;399:406–10.
Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet (London England). 2021;397:1023–34.
Kunnumakkara AB, Rana V, Parama D, et al. COVID-19, cytokines, inflammation, and spices: how are they related? Life Sci. 2021;284:119201.
Tay MZ, Poh CM, Rénia L. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74.
Rahmati M, Fatemi R, Yon DK. The effect of adherence to high-quality dietary pattern on COVID-19 outcomes: a systematic review and meta-analysis. J Med Virol. 2023;95:e28298.
Boaz M, Kaufman-Shriqui V. Systematic review and Meta-analysis: Malnutrition and In-Hospital death in adults hospitalized with COVID-19. Nutrients. 2023;15.
El Khoury CN, Julien SG. Inverse Association between the Mediterranean Diet and COVID-19 risk in Lebanon: a case-control study. Front Nutr. 2021;8:707359.
Milton-Laskibar I, Trepiana J. Potential usefulness of Mediterranean diet polyphenols against COVID-19-induced inflammation: a review of the current knowledge. J Physiol Biochem. 2022:1–12.
Olczak-Pruc M, Swieczkowski D. Vitamin C supplementation for the treatment of COVID-19: a systematic review and Meta-analysis. Nutrients. 2022;14.
Petrelli F, Oldani S, Borgonovo K et al. Vitamin D3 and COVID-19 outcomes: an Umbrella Review of systematic reviews and Meta-analyses. Antioxidants (Basel, Switzerland). 2023;12.
Morais AHA, Aquino JS. Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Br J Nutr. 2021;125:851–62.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96.
Shivappa N, Steck SE, Hurley TG, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. 2014;17:1825–33.
Shivappa N, Godos J, Hébert JR et al. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients. 2018;10.
Jiang C, Yin H, Liu A, Liu Q, Ma H, Geng Q. Dietary inflammatory index and depression risk in patients with chronic diseases and comorbidity. J Affect Disord. 2022;301:307–14.
Li D, Hao X, Li J, et al. Dose-response relation between dietary inflammatory index and human cancer risk: evidence from 44 epidemiologic studies involving 1,082,092 participants. Am J Clin Nutr. 2018;107:371–88.
Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):5–78.
Ponzo V, Pellegrini M, D’Eusebio C, Bioletto F, Goitre I, Buscemi S. Mediterranean Diet and SARS-COV-2 infection: is there any Association? A proof-of-Concept Study. Nutrients. 2021;13.
Skrajnowska D, Brumer M, Kankowska S, Matysek M, Miazio N, Bobrowska-Korczak B. Covid 19: Diet Composition and Health. Nutrients. 2021;13.
Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–8.
Zabetakis I, Lordan R. COVID-19: the inflammation link and the role of Nutrition in potential mitigation. Nutrients. 2020;12.
Butler MJ, Barrientos RM. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun. 2020;87:53–4.
Guenther PM, Reedy J, Krebs-Smith SM. Development of the healthy eating Index-2005. J Am Diet Assoc. 2008;108:1896–901.
Schwingshackl L, Bogensberger B, Hoffmann G. Diet Quality as assessed by the healthy eating index, alternate healthy eating Index, Dietary approaches to stop hypertension score, and Health outcomes: an updated systematic review and Meta-analysis of Cohort studies. J Acad Nutr Dietetics. 2018;118:74–100e111.
Gobbi M, Brunani A, Arreghini M, et al. Nutritional status in post SARS-Cov2 rehabilitation patients. Clin Nutr. 2022;41:3055–60.
Zhao L, Wirth MD, Petermann-Rocha F. Diet-Related Inflammation Is Associated with Worse COVID-19 Outcomes in the UK Biobank Cohort. Nutrients. 2023;15.
Tavassoli M, Askar G, Hadi V, et al. The Association between Dietary Inflammatory Index with risk of coronavirus infection and severity: a case–control study. Int J Prev Med. 2023;14:14.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Res ed). 2009;339:b2535.
da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. The PICO strategy for the research question construction and evidence search. Rev Latinoam Enferm. 2007;15:508–11.
Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J evidence-based Med. 2015;8:2–10.
Kwok CS, Kontopantelis E, Kuligowski G, et al. Self-reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: a dose-response Meta-analysis. J Am Heart Association. 2018;7:e008552.
Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38:1497–511.
Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol. 2004;159:1077–86.
Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Annals Oncology: Official J Eur Soc Med Oncol. 2012;23:843–52.
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Moludi J, Qaisar SA, Alizadeh M, et al. The relationship between Dietary Inflammatory Index and disease severity and inflammatory status: a case-control study of COVID-19 patients. Br J Nutr. 2022;127:773–81.
Mohajeri M, Mohajery R, Nemati A, Pourfarzi F. The difference in the dietary inflammatory index, functional food, and antioxidants intake between COVID – 19 patients and healthy persons. Mediterranean J Nutr Metabolism. 2022;15:219–27.
Firoozi D, Masoumi SJ. The Association between Energy-Adjusted Dietary Inflammatory Index, body composition, and Anthropometric indices in COVID-19-Infected patients: a case-control study in Shiraz, Iran. Int J Clin Pract. 2022;2022:5452488.
Clemente-Suárez VJ, Bustamante-Sanchez Á. Inflammation in COVID-19 and the effects of non-pharmacological interventions during the pandemic: a review. Int J Mol Sci. 2022;23.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Med. 2020;8:475–81.
Wang Y, Perlman S. COVID-19: Inflammatory Profile. Annu Rev Med. 2022;73:65–80.
Toor SM, Saleh R. T-cell responses and therapies against SARS-CoV-2 infection. Immunology. 2021;162:30–43.
Montero López MDP, Mora Urda AI. Changes in eating behaviors during the COVID-19 Lockdown and the impact on the potential Inflammatory effects of Diet. Int J Environ Res Public Health. 2022;19.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
1) D.L. designed and supervised this research. 2) X.H. & S.L. conducted literature search. X.H. & S.L. & D.L. evaluated the quality of studies. 3) D.L. registered this research. 4) X.H & S.L. & Y.D. extracted and calculated the data. D.L.&Yy.Y. & Ym.Y. analyzed the results. 5)X.H. & S.L. wrote paper. 6) D.L. had primary responsibility for final content.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Source of support
This study didn?t receive any external funding.
PROSPERO Registry number and websites
PROSPERO :CRD42023407410 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=407410
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hao, X., Li, S., Yang, Y. et al. Association of dietary inflammatory index and the SARS-CoV-2 infection incidence, severity and mortality of COVID-19: a systematic review and dose-response meta-analysis. Nutr J 23, 21 (2024). https://doi.org/10.1186/s12937-024-00927-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-024-00927-3