Abstract
Background
Intermittent fasting (IF), consisting of either a one-day (IF1) or two consecutive days (IF2) per week, is commonly used for optimal body weight loss. Our laboratory has previously shown an IF1 diet combined with 6d/week of protein pacing (P; 4–5 meals/day evenly spaced, ~ 30% protein/day) significantly enhances weight loss, body composition, and cardiometabolic health in obese men and women. Whether an IF1-P or IF2-P, matched for weekly energy intake (EI) and expenditure (EE), is superior for weight loss, body composition, and cardiometabolic health is unknown.
Methods
This randomized control study directly compared an IF1-P (n = 10) versus an IF2-P (n = 10) diet on weight loss and body composition, cardiovascular (blood pressure and lipids), hormone, and hunger responses in 20 overweight men and women during a 4-week weight loss period. Participants received weekly dietary counseling and monitoring of compliance from a registered dietitian. All outcome variables were assessed pre (week 0) and post (week 5).
Results
Both groups significantly reduced body weight, waist circumference, percent body fat, fat mass, hunger, blood pressure, lipids, glucose, and increased percent fat-free mass (p < 0.05). However, IF2-P resulted in significantly greater reductions in body weight (-29%) and waist circumference (-38%) compared to IF1-P (p < 0.05), and showed a strong tendency for greater reductions in fat mass, glucose, and hunger levels (p < 0.10) despite similar weekly total EI (IF1-P, 9058 ± 692 vs. IF2-P, 8389 ± 438 kcals/week; p = 0.90), EE (~ 300 kcals/day; p = 0.79), and hormone responses (p > 0.10).
Conclusions
These findings support short-term IF1-P and IF2-P to optimize weight loss and improve body composition, cardiometabolic health, and hunger management, with IF2-P providing enhanced benefits in overweight women and men.
Trial registration
This trial was registered March 03, 2020 at www.clinicaltrials.gov as NCT04327141.
Similar content being viewed by others
Background
Intermittent fasting (IF) is an increasingly popular and effective dietary strategy for weight loss (WL) and improved health. Numerous recent meta-analyses and reviews confirm this contention [1,2,3,4,5], although the benefits are not universal [6], and perhaps due to insufficient lengths of sustained fasting (> 16 h). Of the myriad types of IF, several are particularly common and have shown modest efficacy on WL and health improvement: a) “periodic” IF includes fasting one (IF1; < 36 h) to two (IF2; > 48 h) days/week and feeding ad libitum (eating freely) the remaining five or six days [7]; b) alternate-day fasting (ADF), consisting of no (or very low) energy (calorie) intake every second day while eating ad libitum the other days [8]; and c) time-restricted eating (TRE) consisting of fasting for 12–20 h/day and the remaining time-consuming calories freely [9]. The IF-mediated benefits are associated with increased oxidation of fatty acids (lipolysis) and ketone body formation (ketogenesis), activated cell-signaling pathways (insulin sensitivity, reduced inflammation, autophagy), and preservation of lean body mass, known as “metabolic switching” [1,2,3,4,5,6,7,8,9,10,11]. Interestingly, these mechanisms are typically not fully activated until at least 24 h of fasting [5]. Although a common feature of IF diets emphasizes the timing and quantity of calories eaten (or not eaten) during the feasting/fasting days, there is also an under-emphasis on the “quality” of calories consumed during the feeding and/or fasting period. This lack of emphasis on the quality of nutrient-dense (vitamins, minerals, antioxidants, botanicals, unprocessed, high-quality protein, etc.) energy consumed during feasting and fasting periods is a significant oversight. Indeed, this concept warrants further study, especially given the proven benefits of high-quality nutrient-dense food intake on improving weight management and overall health.
Our lab has conducted a series of experiments focused on low-sugar, nutrient-dense calorie intake during short- and long-term weight loss interventions, with and without IF protocols, in overweight/obese adults [12,13,14]. A consistent finding of these studies is the profound impact that low-sugar, nutrient-dense calorie intake, including meal replacement bars and shakes, has on WL and body composition management and health outcomes. Specifically, we have shown “protein pacing” (P, 4–6 meals/day evenly-spaced, 25–40 g protein/meal, > 30% protein/day) combined with a one-day (36 h) nutrient-dense IF (IF1-P) meal pattern significantly improves body composition and cardiometabolic health [12,13,14,15]. A low-sugar (< 60 g/day) IF1-P diet incorporates timed-daily ingestion of protein-rich meals (P), from meal replacements, supplementation, and whole food sources, providing approximately 0.25–0.4 g/kg body weight protein per meal (or > 30% protein per day; 1500–1800 kcals/day) combined with nutrient-dense (antioxidant/botanical) beverages with high protein/fiber low-sugar snacks on IF days (350–500 kcals/day) [13,14,15].
Despite the demonstrated benefits of both traditional IF and nutrient-dense IF-P regimens, there is little research comparing the effects of extended (> 24 h) IF1-P versus an IF2-P meal pattern, matched for total weekly kcals, macronutrient distribution, and physical activity energy expenditure, on body weight and composition, hormones, and hunger ratings in overweight and obese men and women during short-term (4 weeks) WL. Thus, the primary purpose of this study was to examine body composition, and cardiometabolic responses to short-term (weeks 0–4) nutrient-dense IF1-P (36 h IF) or IF2-P (60 h IF) dietary interventions. Based on previous research, we hypothesized that a short-term (4 weeks) IF2-P diet intervention would result in greater WL, and improved body composition and cardiometabolic health compared to an IF1-P regimen in overweight/obese men and women.
Methods
Participants
This study enrolled 200 individuals from the Saratoga Springs, NY area. Potential participants responded to flyers, local newspapers, or emails advertising the study. The number of subjects initially screened was 55, of which 42 were eligible for participation. Participants were healthy, nonsmoking, overweight/obese men and women. A comprehensive medical examination/history assessment was performed by their physicians to rule out any current cardiovascular or metabolic disease. For at least six months before the start of the study, all subjects were either sedentary or lightly active (< 30 min, two days/week of organized physical activity), overweight or obese (BMI > 27.5 kg/m2; % body fat > 30%), weight stable (± 2 kg), and middle-aged (30–65 years). Every participant provided informed written consent in accordance with the Skidmore College Human Subjects review board before participation. The study was approved by the Human Subjects Institutional Review Board of Skidmore College (IRB#: 1911–859). All experimental procedures were performed in adherence with related New York State regulations and the Federal Wide Assurance, consistent with the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, and in agreement with the Helsinki Declaration (revised in 1983). This trial was registered at clinicaltrials.gov as NCT04327141.
Experimental design
Study timeline
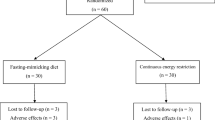
Subjects were enrolled in two separate cohorts due to COVID-19 restrictions regarding personnel laboratory access, such that half enrolled in fall 2020 and the other half in spring 2021. The current study includes a subgroup of a more extensive intervention study comparing intermittent fasting and protein pacing diet (IF-P, n = 20) versus a heart-healthy (HH, n = 19) diet on body composition, cardiometabolic, microbiome, and metabolomic outcomes over eight weeks in 39 overweight/obese women and men. Therefore, only necessary comparisons between the IF-P groups will be presented in this manuscript. The 20 IF-P study participants were matched for weight and BMI and randomly assigned to either: a) an intermittent fasting diet for one day/week (IF 36 h total) and protein pacing (P) diet for the remaining six days/week (IF1-P), or b) an IF diet for two consecutive days (IF 60 h total) and P for the remaining five days/week (IF2-P) for four weeks. (Fig. 1 shows the CONSORT study flow chart). Complete blinding was not possible given the study design, as both groups consumed identical weekly total calorie intakes and macronutrient distributions (including food and nutritional supplements as described in detail below (see lines 174–214 and Supplemental Table S1) over the entire 4 weeks. In addition, both groups received identical nutritional support from the registered dietitian during weekly meetings. Lastly, participants in both groups were not explicitly made aware of study participants in the other group. The primary justification for using a 4-week intervention was to allow for comparisons to previously published interventions. More importantly, this period is sufficient for physiological and biochemical induced pathways to be expressed and subsequent quantification of changes in all outcome measures following a calorie restricted/IF dietary protocol. Extending beyond 4-weeks reduces compliance and may be overly excessive for a caloric restriction and 2 day IF and create undue metabolic, physiologic, hormonal, and psychological stress in the study participants.
All laboratory testing procedures (see below) were performed at baseline control (CON, week 0) and week 5 (Fig. 2).
The weight loss (WL) phase began with all participants following a four-week controlled IF-P intervention, as detailed below. During the 1-week baseline control (CON), subjects maintained a stable body weight by consuming a similar caloric intake as their pre-enrollment caloric intake while maintaining their sedentary lifestyle. Following CON baseline testing, participants were provided with detailed instructions on their WL dietary guidelines and scheduled weekly 1-h meetings with a registered dietitian.
Dietary interventions
Weight loss (WL) (weeks 1–4): intermittent fasting (IF) – protein pacing (P) diets
Participants were randomly assigned to one of two different IF-P protocols beginning at week one and instructed to follow the meal plan for four weeks.
IF day diets
IF1-P consisted of ~ 400 kcals per day in which participants were provided a variety of supplements and snacks. Specifically, participants consumed each of the following supplements mixed with either cold or hot water: an adaptogen/antioxidant-rich beverage four servings, evenly spaced throughout the day (morning, noon, mid-afternoon, evening; Cleanse for Life®, 160 kcals/day); two servings of an antioxidant beverage (morning/evening; Ionix® Supreme, 40 kcals/day); one serving of a collagen bone broth beverage (dinner meal; Collagen Bone Broth, 45 kcals/day); one serving of an electrolyte beverage (AMPED™ Hydrate, 20 kcals/day); and for the dinnertime meal, one serving of snack crackers (dinner meal; Harvest/Whey Thins™, 100 kcals/day) with the bone broth. Once these basic requirements were met, participants were able to choose from a list of “options” to achieve a total of ~ 400 kcals/day, these included: half of a dark chocolate square (IsaDelight® Chocolates, 30 kcals/day); one to two servings of an anti-oxidant/caffeinated beverage (BEA™, 20 kcals/day); ½ of a nut bar (Snack Bites, 50 kcals/day); or a combination of fresh vegetables/fruits and nuts/seeds (not to exceed 50 kcals/day) as recommended by the research team to support their energy needs. IF2-P followed an identical meal pattern for both IF days, except for consuming an additional 100 kcals from the “options” list to achieve ~ 500 kcals/day for each of the two consecutive fasting days. Sample menus and meal timing for IF1-P and IF2-P intermittent fasting (IF) days are shown in Supplemental Table S1. All participants were provided a detailed cookbook including recipes and menus for the dinner meal and afternoon snacks that met the requirements for IF and P macronutrient and total kcal intakes.
P day diets
IF1-P consumed a protein pacing (P) diet consisting of four and five meals/day for women and men, respectively; two of which (breakfast and evening) were liquid meal replacement shakes with added whole foods (Whole Blend IsaLean® Shakes, 350/400 kcals, 30/36 g of protein/meal); a whole food evening dinner meal (450/500 kcals men), an afternoon snack (200 kcals, men only), and an evening protein snack (Whole Blend IsaLean® Shakes or Bars; 200 kcals). This dietary regimen provided 1350 and 1700 kcals/day for women and men, respectively, and a macronutrient distribution targeting 35% protein, 35% carbohydrate, and 30% fat. This macronutrient distribution has previously been used successfully in our lab to induce an energy deficit without compromising lean body mass [12, 13]. IF2-P followed a similar P meal protocol with the following modifications: breakfast/evening shakes increased to 400/450 kcals, women and men, respectively; and evening snack added 50 kcals to the evening snack from whole food “options” (250 kcals total). This dietary regimen provided 1500 and 1850 kcals/day for women and men, respectively, and similar macronutrient distribution and total weekly calorie intakes (~ 8500 kcals/week) as IF1-P. Isagenix International, LLC (Gilbert, AZ, USA) provided all meal replacement shakes, bars, beverages, and supplements. Sample menus and meal timing for IF1-P and IF2-P protein pacing (P) days are shown in Supplemental Table S1.
Compliance
All subjects met with a registered dietitian weekly during WL to facilitate healthy eating habits and adherence to the respective dietary protocols. In addition, subjects were provided detailed written instructions for each IF diet plan. They were closely monitored through daily participant-researcher communication (e.g., email, text, and mobile phone), two-day food diary analysis, weekly dietary intake journal inspections, distribution of weekly meal/supplement containers, and return of empty packets and containers. Weekly meetings were held via ZOOM (Zoom Video Communications, Qumu Corporation, San Jose, CA, USA) for each IF group separately, with the dietitian and the research team to verify compliance with the dietary meal plans, clarify dietary guidelines, and answer questions. The overall compliance rate in each group was high (> 90%), which was defined as consuming more than 90% of their respective meals/supplemented feedings. Noncompliance was defined as being absent from more than two consecutive dietitian meetings and under- or over-consuming ≥ three inappropriate meal/supplement servings a week for ≥ two consecutive weeks at a time. Two-day food records were completed by every participant at two different time points (Week 0 and 4) to further verify compliance to each IF diet (see Energy Balance Assessment, below).
Laboratory testing procedures
Body composition assessments
At weeks 0 and 5, all participants were tested between the hours of 6:00 a.m. and 9:00 a.m., after an overnight fast, and underwent body composition assessments (height, body weight, and total body composition). Body weight was obtained using a standard digital scale and, height was obtained without shoes using a stadiometer. Waist circumferences, in centimeters (cm) were obtained with a standard tape measure placed around the waist two centimeters above the iliac crest by the same investigator (K.M.A.), at each time point. Body composition was assessed by BODPod (Cosmed, Chicago, IL, USA) for the measurement of total fat mass (FM), % body fat (%BF), fat-free mass (FFM), % FFM (body weight/FFM). Standard body mass index (BMI) measurements were obtained by dividing the subject’s weight (kg) by the square of their height (m2).
Energy balance assessment
Energy balance was calculated for each individual by closely monitoring both physical activity energy expenditure (EE) (Actigraph LLC, Pensacola, FL, USA) as well as their energy intake (EI) for two days during baseline control (CON, week 0) and week 4 (1 day each of IF and P). The registered dietitian and a member of the research team instructed participants on completing detailed dietary food records of portion sizes and food items. All food logs were recorded using the Food Processor SQL Edition (version 11.6.522 ESHA Research, Salem, OR, USA, 2012). A single trained research team member (M.P.) and the PI (P.J.A.) analyzed all the food logs to reduce inter-investigator variation. Each participant was also given a checklist to help them adhere to the IF regimens. Participants were asked to maintain their current level of physical activity (sedentary/low activity) and to abstain from starting any new exercise programs throughout the entire WL intervention. To verify sedentary/low activity levels, all participants wore an Actigraph Data Analysis Software accelerometer (v. 6.13.3; Actigraph LLC, Pensacola, FL, USA) around their waist for two days during weeks 0 and 4. All EE accelerometer data were analyzed by the same research team member (M.P.) for all participants.
Cardiovasculare and plasma biomarkers
Blood pressure and heart rate was obtained with an automated blood pressure monitor (Omron Healthcare Inc., Milton Keynes, UK) following > 15 min of quiet sitting. For plasma hormone measurements, 12-h fasted venous blood samples (~ 20 mL) were collected into EDTA-coated vacutainer tubes and centrifuged (Hettich Rotina 46R5) for 15 min at 2500 rpm at -4 °C. After separation, plasma was stored at − 80 °C until analyzed. Plasma concentrations of insulin (INS), ghrelin (GRL), and glucagon-like polypeptide -1 (GLP-1) were analyzed using ELISA’s (RayBiotech, Peachtree Corners, GA, USA). Glucagon and insulin-like growth factor -1 (IGF-1) were analyzed by custom-plex immunoassays (Eve Technologies Corporation, Calgary, AB Canada) and, blood glucose and lipids were determined using a colorimetric assay (Cholestech LDX Analyzer, Abbott Laboratories, Abbott Park IL, USA). Test–retest intraclass correlation (r) and coefficient of variation (CV) in our laboratory with n = 15 was: insulin, and glucose (mg/dL) r = 0.95, CV = 3.2%, and r = 0.97, CV = 5.3%, respectively.
Feelings of hunger and satiety
Visual analog scales (VAS) were administered at weeks 0 and 5 to evaluate the effects of the IF-P protocols on hunger, satiation, quantity-of-food-to-eat, and desire-to-eat. Briefly, participants were instructed, using a pen and paper, to mark their levels of hunger, satiety, quantity-of-food-to-eat, and desire-to-eat on a 100 mm line that was anchored at either end with “0” (none) to “100” (extreme). For each of these measures, the degree of sensation was quantified by the distance from the “0” mm point. All VAS scoring was measured by the same investigator (M.P.).
Statistical analysis
Statistical analysis was performed using SPSS software (Ver. 27; IBM-SPSS, Armonk NY, USA). Before starting the study, the sample size was determined through power analysis based on the primary outcome variables body weight and body composition to achieve an effect size of 0.25 with 80% power at alpha 0.05 based on previous data [12, 16, 17]. This analysis determined that n = 10 was required to detect a significant mean difference of 1.4 kg weight loss between the two diet intervention groups (IF1-P vs. IF2-P) during WL. Absolute changes in body weight (kg) and composition, biomarkers, and hunger ratings were calculated. Two way (2 × 2) factorial mixed model Analysis of Variance (ANOVA) were performed for WL parameters using IF-P (IF1-P vs. IF2-P) and time (week 0 vs. 5) to determine the main effects. Data analysis was not performed blinded but each intervention group was assigned a number code. A per-protocol approach was used on data for all compliant study participants and an intent-to-treat analyses was performed on data (pre and post) from all randomized study participants. The per-protocol analysis is presented in the results and the ITT analysis is presented in the Supplemental Table S2. One-tailed tests were utilized for this study, and the significance was set at p < 0.05. All values are reported as means ± standard error (SE) unless stated otherwise.
Results
Weight loss (WL; weeks 0–4)
Subject characteristics
One individual in IF1 did not adhere to the dietary guidelines and was dropped due to non-compliance. ITT analysis was conducted including this individual and is presented in Supplemental Table S2). Thus, descriptive baseline characteristics of the twenty subjects (14 women and six men) who completed WL are reported in Table 1. Both groups were similar for all variables at baseline.
Dietary intake and physical activity during WL
The IF-P WL diet interventions significantly altered both groups’ dietary energy and macronutrient intake (Table 2).
Specifically, total energy intake following IF-P diets decreased significantly (p < 0.05) in both groups by ~ 40% (1000 kcals/day) during WL, with no differences between groups. This reduction was due to significant (p < 0.05) decreases in percentage and grams of dietary fat (5%-10%; 50–60 g) and carbohydrate (7%–10%; 138–152 g) intake, as well as increased percentage and amount of protein intake (17%; 21–25 g, respectively; p < 0.05) in both groups. In addition, IF-P protocols significantly (p < 0.05) increased dietary fiber (8–12 g/day) and decreased sugar (57–77 g/day) and sodium (1500–2000 mg/day) intake. Both groups maintained similar amounts of physical activity energy expenditure throughout the WL period.
Body weight and composition during WL
Effects of the IF-P protocol on body weight and body composition are shown in Table 3.
Relative to baseline, both IF-P groups had significant reductions (p < 0.05) in all outcome measures (body weight, waist circumference, BMI, total body fat %, fat mass, and fat-free mass), however, IF2-P resulted in greater (p < 0.05) body weight and waist circumference loss compared to IF1-P (Fig. 3). IF1-P and IF2-P lost 4.7 kg and 7.1 kg or 5.2% and 7% of body weight from baseline, respectively (p < 0.05). Similarly, IF1-P and IF2-P reduced waist circumference by 5 cm and 7.6 cm or 4.8% and 8% from baseline, respectively (p < 0.05). Of special note, when expressed as a percentage of body weight, FFM increased by 2%, with no differences between groups.
Plasma hormones during WL
Plasma biomarkers are reported in Table 4 and show no significant change from baseline or between groups. This observation suggests that hormone changes were not responsible for the body composition and cardiometabolic responses between IF1-P and IF2-P during the 4-week intervention.
Cardiovascular responses during WL
Both groups experienced significant reductions in blood pressure (SBP, 8/5 mmHg; DBP, 6/3 mmHg; IF1-P / IF2-P, respectively, p < 0.05), fasting total cholesterol, LDL-C, and triglycerides (17%/16%; 10%/14%, and 21%/11%; IF1-P and IF2-P, respectively, p < 0.01), with no differences between groups during the 4-week WL intervention (Table 5). HDL-C decreased significantly in both groups; however, the TC:HDL-C remained unchanged by the dietary interventions.
Hunger ratings during WL
Self-reported feelings of hunger, desire to eat, the quantity of food to eat, and fullness are reported in Table 6. Both groups experienced significant reductions in desire to eat (44%) and quantity of food to eat (30%), with a tendency of hunger ratings to be lower in IF2-P (40%) compared to IF1-P (p = 0.10).
Discussion
The primary aim of this study was to compare short-term IF – one day ((IF1-P, 36 h) per week vs. two days (IF2-P, 60 h) per week on body weight and composition, and cardiometabolic responses in overweight/obese women and men. We observed that both IF1-P and IF2-P produced significant weight loss accompanying reductions in waist circumference and body fat. This weight loss was also associated with reductions in blood lipids, blood pressure, and desire and quantity-of-food-to-eat measures. Comparatively, IF2-P yielded greater weight loss and reduction in waist circumference than IF1-P, despite similar reductions in total energy intake (-1000 kcals/day) and unchanged levels of energy expenditure (200–300 kcals/day) and similar increases in fat-free mass.
These favorable effects appear independent of alterations in circulating hormones, which remained largely unchanged throughout the WL period.
Body weight and composition
Our finding of significant weight loss and improved body composition outcomes with IF agrees with recent meta-analyses and reviews [2, 5,6,7,8,9,10,11], although this is not a universal finding [6,7,8,9,10,11,12,13,14,15,16,17,18]. Some data also suggest a substantial loss of lean body mass may occur with IF depending on the length and degree of fasting [6, 18] which is a common observation during weight loss [18]. However, in our study, the proportion of FFM increased more than 2%, with a total FFM loss of only ~ 1 kg, or less than 20% of the total weight lost. This favorable response is likely due to the emphasis on high-quality protein pacing feedings (whey and plant-based). During an energy deficit, muscle protein synthesis is reduced [19], and higher protein intake may attenuate this reduction or even increase MPS [20,21,22]. Our laboratory has consistently demonstrated favorable fat mass and attenuated loss or retention of lean body mass in overweight individuals engaged in energy restriction or IF interventions [12, 13].
Explanations for the greater weight and waist circumference loss and body fat reductions with IF2-P may be attributed to several molecular and cellular adaptations associated with intermittent fasting. First, as characterized in detail by Anton et al. [5], intermittent fasting induces a “metabolic switch” with fuel utilization and nutrient partitioning selectively transitioning from glucose to fat oxidation. With prolonged fasting of 60 h in IF2 subjects’ metabolism likely further progressing from lipolysis to ketogenesis [23]. Mechanistically, the additional day of fasting per week in IF2 participants may have likely elevated ketone production, which may further activate AMP-activated protein kinase (AMPK), a key energy-sensing modulator within the cell [24].
The current findings are the first to compare two short-term IF regimens combined with protein pacing on anthropometric and cardiometabolic measures. The novelty of these findings is underscored by three distinct factors: 1) matching weekly total energy intake, including macronutrient distribution and energy expenditure in both groups; 2) the clearly defined length of IF between groups (36 versus 60 h); and 3) consumption of nutrient-dense energy intake during the IF and protein pacing (P) days. Most previous research examined IF according to the length of time or the quantity of food allowed during the fasting period, with less emphasis on the quality of the energy (calories) consumed during fasting and feeding days. The current study provided the unique opportunity to directly quantify the effects of two different fasting periods on body composition in a group of overweight women and men.
Plasma biomarkers
Neither IF protocol significantly affected plasma hormones, glucose, or insulin levels; however, the IF2-P group trended towards lower plasma glucose, and both groups trended towards lower plasma insulin. Our findings agree with several recent IF interventions that report plasma glucose levels are tightly regulated and relatively unresponsive to IF [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. In contrast, IF interventions typically result in reductions in plasma insulin [7]. Indeed, our previous intervention [13], which included an IF-1 dietary pattern during WL, noted reductions in both plasma glucose (approximately 10%) and insulin (approximately 40%). Potential contributing factors accounting for differences between the two studies include the significantly greater level of WL of subjects in the previous study compared to this one (11.6 kg vs. 5.8 kg) and the duration of the WL phases (12 weeks vs. four weeks). Finally, it must be noted that our intervention included a low carbohydrate/sugar intake which generally does not impact plasma insulin concentrations [26], and that reduction in insulin levels may not occur during short-term interventions [27].
Cardiovascular responses
Both IF interventions significantly reduced lipid concentrations and blood pressure. These findings agreed with our previous study [13] and others [28,29,30]. HDL-C also decreased during WL, supporting other observations [27]; however, this reduction did not affect the TC: HDL-C ratio. The hypotriglyceridemic effect of IF has also been noted in some [28,29,30,31] but not all studies [32]. Blood pressure reductions in response to IF have also been reported by many researchers [27,28,29, 33, 34], although these reductions are typically associated with longer interventions.
Hunger ratings
Both IF1 and IF2 significantly reduced self-reported desire and quantity-of-food-to-eat. Interestingly, feelings of hunger and satiety were unchanged. This finding was surprising considering that participants experienced a 40% reduction in total energy intake (primarily from carbohydrate and fat; protein intake modestly increased) compared to baseline. In contrast, acute and longer-term energy restriction in overweight and obese individuals has been reported to increase appetite and appetite-associated hormones [35,36,37,38]. Therefore, the current study’s results have important compliance implications for future recommendations as an effective weight loss strategy.
Macronutrient composition and energy intake shifted dramatically relative to dietary protein and fiber intake. In line with the protein leverage hypothesis, increasing protein density may promote satiety, which can aid in preventing energy overconsumption and obesity [39]. Dietary fiber and the resistant starches present in many of the supplements utilized in this study also have positive satiety and satiety-related hormone effects [40, 41].
As noted above, there was a trend for a greater reduction in hunger during IF2. This observation was intriguing considering the longer consecutive fasting duration and a more significant decrease in body weight and waist circumference. In addition, both groups had similar weekly energy intake and expenditure and the objective measures of appetite, including ghrelin (hunger-associated hormone) and GLP-1 (fullness-associated hormone), remained unchanged from baseline levels. Other fasting research incorporating calorie restriction has reported similar findings in obese adults using IF [42, 43] and ADF regimens [44, 45]. As noted previously, we postulate IF2-P participants experienced enhanced ketogenesis compared to IF1-P participants, and previous research suggests a complex ketone-mediated suppression of some measures of hunger or appetite [46]. Finally, our findings may be mediated through gut microbiome alterations as the gut-brain axis is emerging as an important regulator of appetite and food reward signaling [47], an understudied and nascent topic of intense investigation [48].
Strengths and limitations
Strengths of the present study include: (a) direct comparison within and between interventions; (b) careful weekly monitoring and counseling with a registered dietitian; (c) direct measurement of physical activity by accelerometry; (d) high compliance (> 95%); and, perhaps most interesting, (e) the extent of the weight loss during the COVID-19 pandemic in early fall of 2020 through the spring of 2021. Indeed, recent literature has highlighted the adverse impact of COVID-19 on weight [49, 50]. We are also aware of several limitations For example, one of the IF2-P participants may have strongly influenced the group’s response. Moreover, a non-compliant participant from IF1-P was dropped in the presented results. Per our ITT analysis, including this individual obscured the significant interaction effect for BW. Importantly, this individual significantly restricted their dietary intake (< 2 SD, mean calorie intake) which contrasts with the normal non-compliance issues associated with caloric restriction clinical research. We contend the presented per-protocol results are more reflective and accurate of the intended intervention as this individual invariably produced an additional weight loss effect due to their severe caloric restriction which impacted the group mean and statistical analysis. The greater weight loss with IF-2 vs IF-1 may have been mediated by increased resting metabolism, which was not measured in the current study. Our previous investigation [13] did not observe an increased resting metabolic rate in response to 12-weeks of IF1-P in obese men and women, and therefore, this consideration requires further investigation. Also, circulating ketones were also not measured. Unfortunately, this was not feasible in the current study due to scheduling conflicts and stringent COVID laboratory restrictions preventing time-sensitive analysis following a fasting day. Finally, the short-term design of our study does not allow speculation about long-term benefits, compliance, or adverse responses to the dietary protocol.. As the study was a subgroup analysis, additional research is necessary to confirm these findings in free-living overweight/obese adults following an IF-P over more extended intervention periods.
Conclusions
Our results support the combination of intermittent fasting and protein pacing as effective short-term nutritional interventions for weight loss and improvements in body composition. These changes facilitate additional cardiometabolic benefits without adversely impacting hunger or appetite. Although our study design cannot distinguish between benefits afforded to intermittent fasting versus protein pacing, both likely contribute to significant, perhaps independent benefits. A long-term investigation of this nutritional eating pattern for addressing obesity and cardiovascular health is warranted.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
22 June 2022
ORCID has been added to the first author.
Abbreviations
- IF:
-
Intermittent fasting
- P:
-
Protein Pacing
- HH:
-
Heart healthy
- WL:
-
Weight loss
- FFM:
-
Fat-free mass
- FM:
-
Fat mass
- %BF:
-
Percent body fat
- BMI:
-
Body mass index
- %FFM:
-
Percent fat-free mass
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- IGF:
-
1-Insulin-like growth factor -1
References
de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381:2541–51.
Yang F, Liu C, Liu X, Pan X, Li X, Tian L, Sun J, Yang S, Zhao R, An N, Yang X, Gao Y, Xing Y. Effect of epidemic intermittent fasting on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2021;8: 669325.
Queiroz JDN, Macedo RCO, Tinsley GM, Reischak-Oliveira A. Time-restricted eating and circadian rhythms: the biological clock is ticking. Crit Rev Food Sci Nutr. 2021;61:2863–75.
Parr EB, Heilbronn LK, Hawley JA. A time to eat and a time to exercise. Exerc Sport Sci Rev. 2020;48:4–10.
Anton, SD; Moehl, K; Donahoo, WT; Marosi, K; Lee, SA; Mainous, AG 3rd; Leeuwenburgh, C; Mattson, MP. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity 2018, 26, 254–268.
Templeman I, Smith HA, Chowdhury E, Chen YC, Carroll H, Johnson-Bonson D, Hengist A, Smith R, Creighton J, Clayton D, Varley I, Karagounis LG, Wilhelmsen A, Tsintzas K, Reeves S, Walhin JP, Gonzalez JT, Thompson D, Betts JA. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med. 2021;13:eabd8034.
Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, Contaldo F, Pasanisi F, Ghigo E, Bo S. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018;16:371.
Harris L, Hamilton S, Azevedo LB, Olajide J, De Brún C, Waller G, Whittaker V, Sharp T, Lean M, Hankey C, Ells L. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2018;16:507–47.
Allaf M, Elghazaly H, Mohamed OG, Fareen MFK, Zaman S, Salmasi AM, Tsilidis K, Dehghan A. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2021;1:CD013496.
Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11:1234.
Koutnik AP, Poff AM, Ward NP, DeBlasi JM, Soliven MA, Romero MA, Roberson PA, Fox CD, Roberts MD, D’Agostino DP. Ketone bodies attenuate wasting in models of atrophy. J Cachexia Sarcopenia Muscle. 2020;11:973–96.
Arciero PJ, Ormsbee MJ, Gentile CL, Nindl BC, Brestoff JR, Ruby M. Increased protein intake and meal frequency reduces abdominal fat during energy balance and energy deficit. Obesity. 2013;21:1357–66.
Arciero PJ, Edmonds R, He F, Ward E, Gumpricht E, Mohr A, Ormsbee MJ, Astrup A. Protein-Pacing Caloric-restriction enhances body composition similarly in obese men and women during weight loss and sustains efficacy during long-term weight maintenance. Nutrients. 2016;8:476.
Zuo L, He F, Tinsley GM, Pannell BK, Ward E, Arciero PJ. Comparison of high-protein, intermittent fasting low-calorie diet and heart healthy diet for vascular health of the obese. Front Physiol. 2016;7:350.
He F, Zuo L, Ward E, Arciero PJ. Serum Polychlorinated biphenyls increase and oxidative stress decreases with a protein-pacing caloric restriction diet in obese men and women. Int J Environ Res Public Health. 2017;14:59.
Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J. 2012;11:98.
Arciero PJ, Baur D, Connelly S, Ormsbee MJ. Timed-daily ingestion of whey protein and exercise training reduces visceral adipose tissue mass and improves insulin resistance: the PRISE study. J Appl Physiol. 2014;117:1–10.
Laurens C, Grundler F, Damiot A, Chery I, Le Maho AL, Zahariev A, Le Maho Y, Bergouignan A, Gauquelin-Koch G, Simon C, Blanc S, Wilhelmi de Toledo F. Is muscle and protein loss relevant in long-term fasting in healthy men? a prospective trial on physiological adaptations. J Cachexia Sarcopenia Muscle. 2021;12(6):1690-703. https://doi.org/10.1002/jcsm.12766.
Pasiakos SM, Vislocky LM, Carbone JW, Altieri N, Konopelski K, Freake HC, Anderson JM, Ferrando AA, Wolfe RR, Rodriguez NR. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J Nutr. 2010;140:745–51.
Hector AJ, Marcotte GR, Churchward-Venne TA, Murphy CH, Breen L, von Allmen M, Baker SK, Phillips SMW. Whey protein supplementation preserves postprandial myofibrillar protein synthesis during short-term energy restriction in overweight and obese adults. J Nutr. 2015;145:246–52 (Erratum In: J Nutr 2015 145 1373).
Murphy CH, Churchward-Venne TA, Mitchell CJ, Kolar NM, Kassis A, Karagounis LG, Burke LM, Hawley JA, Phillips SM. Hypoenergetic diet-induced reductions in myofibrillar protein synthesis are restored with resistance training and balanced daily protein ingestion in older men. Am J Physiol Endocrinol Metab. 2015;308:E734–43.
Areta JL, Burke LM, Camera DM, West DW, Crawshay S, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab. 2014;306:E989–97.
Ludwig DS, Aronne LJ, Astrup A, de Cabo R, Cantley LC, Friedman MI, Heymsfield SB, Johnson JD, King JC, Krauss RM, Lieberman DE, Taubes G, Volek JS, Westman EC, Willett WC, Yancy WS, Ebbeling CB. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. Am J Clin Nutr. 2021;114:1873–85.
González A, Hall MN, Lin SC, Hardie DG. AMPK and TOR: the yin and yang of cellular nutrient sensing and growth control. Cell Metab. 2020;31(3):472–92. https://doi.org/10.1016/j.cmet.2020.01.015 (PMID: 32130880).
Pinto AM, Bordoli C, Buckner LP, Kim C, Kaplan PC, Del Arenal IM, Jeffcock EJ, Hall WL. Intermittent energy restriction is comparable to continuous energy restriction for cardiometabolic health in adults with central obesity: a randomized controlled trial; the Met-IER study. Clin Nutr. 2020;39:1753–63.
Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS, Jr; Kelly, TN; He, J; Bazzano, LA. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176(Suppl 7):S44–54.
Kalam F, Gabel K, Cienfuegos S, Wiseman E, Ezpeleta M, Steward M, Pavlou V, Varady KA. Alternate day fasting combined with a low-carbohydrate diet for weight loss, weight maintenance, and metabolic disease risk reduction. Obes Sci Pract. 2019;5:531–9.
Kroeger CM, Klempel MC, Bhutani S, Trepanowski JF, Tangney CC, Varady KA. Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: relationship to adipokine modulations. Nutr Metab. 2012;9:98.
Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35:714–27.
Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371–93.
Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015;73:661–74.
Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S, Wang R, Carlson OD, Egan JM, Mattson MP, Howell A. The effect of intermittent energy and carbohydrate restriction v daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110:1534–47.
Sundfør TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr Metab Cardiovasc Dis. 2018;28:698–706.
Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Cardiometabolic benefits of intermittent fasting. Annu Rev Nutr. 2021;41:333–61.
Brennan IM, Seimon RV, Luscombe-Marsh ND, Otto B, Horowitz M, Feinle-Bisset C. Effects of acute dietary restriction on gut motor, hormone and energy intake responses to duodenal fat in obese men. Int J Obes. 2011;35:448–56.
Deighton K, Batterham RL, Stensel DJ. Appetite and gut peptide responses to exercise and calorie restriction the effect of modest energy deficits. Appetite. 2014;81:52–9.
Seimon RV, Taylor P, Little TJ, Noakes M, Standfield S, Clifton PM, Horowitz M, Feinle-Bisset C. Effects of acute and longer-term dietary restriction on upper gut motility, hormone, appetite, and energy-intake responses to duodenal lipid in lean and obese men. Am J Clin Nutr. 2014;99:24–34.
Dorling JL, Das SK, Racette SB, Apolzan JW, Zhang D, Pieper CF, Martin CK, CALERIE Study Group. Changes in body weight, adherence, and appetite during 2 years of calorie restriction the CALERIE 2 randomized clinical trial. Eur J Clin Nutr. 2020;74:1210–20.
Raubenheimer D, Simpson SJ. Protein leverage: theoretical foundations and ten points of clarification. Obesity. 2019;27:1225–38.
Amini S, Mansoori A, Maghsumi-Norouzabad L. The effect of acute consumption of resistant starch on appetite in healthy adults; a systematic review and meta-analysis of the controlled clinical trials. Clin Nutr ESPEN. 2021;41:42–8.
Hassanzadeh-Rostami Z, Faghih S. Effect of dietary fiber on serum leptin level: a systematic review and meta-analysis of randomized controlled trials. Exp Clin Endocrinol Diabetes. 2021;129:322–33.
Klempel MC, Bhutani S, Fitzgibbon M, Freels S, Varady KA. Dietary and physical activity adaptations to alternate day modified fasting: implications for optimal weight loss. Nutr J. 2010;9:35.
Bhutani S, Klempel MC, Kroeger CM, Aggour E, Calvo Y, Trepanowski JF, Hoddy KK, Varady KA. Effect of exercising while fasting on eating behaviors and food intake. J Int Soc Sports Nutr. 2013;10:50.
Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MPA. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free RadicBiol Med. 2007;42:665–74.
Hoddy KK, Gibbons C, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Gabel K, Finlayson G, Varady KA. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin Nutr. 2016;35:1380–5.
Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol. 2015;2(6):27. https://doi.org/10.3389/fpsyg.2015.00027.PMID:25698989;PMCID:PMC4313585.
Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2:747–56.
Mohr AE, Gumpricht E, Sears DD, Sweazea KL. Recent advances and health implications of dietary fasting regimens on the gut microbiome. Am J Physiol Gastrointest Liver Physiol. 2021;320:G847–63.
Bennett G, Young E, Butler I, Coe S. The impact of lockdown during the COVID-19 outbreak on dietary habits in various population groups: a scoping review. Front Nutr. 2021;8: 626432.
Westerterp KR. Lockdown induced change in energy balance. Eur J Clin Nutr. 2021;75:1416–7.
Acknowledgements
We are thankful for the time and effort put forth by all study volunteers during both phases of the study. We are grateful for the assistance of Jamie Martin RD throughout the entire intervention.
Funding
This study was supported by a grant (IRB#: 1911–859) from Isagenix International, LLC through an unrestricted research grant to Skidmore College and P.J.A.
Author information
Authors and Affiliations
Contributions
P.J.A. assisted in the design of the study, subject recruitment, data collection and analysis, manuscript preparation, obtained the grant, and served as the study PI; K.M.A., M.P., assisted in the design of the study, subject recruitment, data collection and analysis and manuscript preparation; E.G., A.E.M., assisted with manuscript preparation; S.J.I., assisted with data analysis and manuscript preparation; M.B., J.Z., M.H., E.V., D.C., K.J., A.S., O.F., and M.W., assisted with manuscript preparation. AEM and EG assisted on all manuscript drafts including the final version. All authors read and approved the final manuscript.
Authors’ information
Corresponding author P.J.A. is a fellow of the American College of Sports Medicine (FACSM), The Obesity Society (FTOS), and the International Society of Sport Nutrition (FISSN) and has published extensively on the topics of caloric restriction, intermittent fasting, and protein pacing for optimal weight loss, cardiometabolic and body composition health in normal weight, overweight, and obese participants. He is an advisory board member of the International Protein Board (iPB).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Every participant provided informed written consent in accordance with the Skidmore College Human Subjects review board before participation. The study was approved by the Human Subjects Institutional Review Board of Skidmore College (IRB#: 1911–859). All experimental procedures were performed in adherence with related New York State regulations and the Federal Wide Assurance, consistent with the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, and in agreement with the Helsinki Declaration (revised in 1983).
Consent for publication
Not applicable.
Competing interests
P.J.A. is a member of the scientific advisory board at Isagenix International LLC, the study’s sponsor. E.G. and A.E.M. are employed by Isagenix International LLC. No authors have financial interests regarding the outcomes of this investigation. All other authors declare no conflict of interest. The funder had no roles in the study design, data collection and analysis, and decision to publish.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Sample menus and meal timing for Protein Pacing (P) and Intermittent Fasting (IF) Days for (IF1-P) and (IF2-P) Study Participants during 4 Week Weight Loss (WL). Table S2. Intention-to-Treat (ITT) analysis including the non-compliant participant on primary outcomes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Arciero, P.J., Arciero, K.M., Poe, M. et al. Intermittent fasting two days versus one day per week, matched for total energy intake and expenditure, increases weight loss in overweight/obese men and women. Nutr J 21, 36 (2022). https://doi.org/10.1186/s12937-022-00790-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-022-00790-0