Abstract
Background
Plasmodium falciparum is the dominant malaria species in the sub-Saharan Africa and the main cause of severe disease and death. Notwithstanding, severe malaria and death due to non-falciparum infections have been reported, but at much lower rates than P. falciparum infections. Following increasing use of molecular detection techniques in epidemiological studies, a higher prevalence of non-falciparum species has been reported in the region than previously thought. This article reviews the literature on the prevalence of non-falciparum malaria species in Uganda and the clinical figures of their severe diseases. It aims to elucidate the extent to which mono non-falciparum malaria infections in a highly malaria-endemic country contribute to malaria mortality and outline its policy implications on malaria case management.
Methods
The available English-language published peer-reviewed literature up to March 2024 was sought via PubMed and Google Scholar. The keywords used were severe malaria, AND P. falciparum, P. malariae, P. vivax, P. ovale spp., mixed infections AND Uganda. The review encompassed 53 articles. Articles using molecular diagnosis methods were accounted for analysis.
Results
The literature reported a substantial prevalence of non-falciparum infections in Uganda. Plasmodium malariae and Plasmodium ovale spp. were the second and third most prevalent reported malaria species respectively after P. falciparum as dominant species. Non-falciparum malaria infections often occur as mixed infections rather than mono-infections. Besides, molecular diagnostics revealed that 21% of initially reported mono-infections of P. falciparum were, in fact, mixed infections. No article was found on the prevalence of severe malaria or case fatality rate due to mixed or non-falciparum infections.
Conclusion
A critical knowledge gap exists regarding the impact of mixed and non-falciparum species on severe malaria and death in Uganda. Robust evidence on prevalence, recurrent parasitaemia, and severe clinical manifestations of mixed and non-falciparum malaria infections is crucial for evidence-based and effective policymaking regarding malaria case management.
Similar content being viewed by others
Background
Reduction of malaria mortality remains among top priorities of the endemic countries. As outlined in Target 3.3 Sustainable Development Goals, the World Health Organization (WHO) member states have targeted a minimum 90% reduction in the global malaria mortality rate by 2030 compared to 2015 [1,2,3]. In 2022, Uganda accounted for approximately 5.1% of global malaria cases, ranked third in terms of malaria burden and eighth in malaria-attributed death worldwide [4]. The country has embraced many interventions aimed at malaria mortality reduction to less than 1 death per 100,000 population by 2030 [5].
Over the last decade, the Uganda Ministry of Health reported a 27% reduction in malaria deaths [5]. While this achievement is promising, malaria remains the largest contributor to morbidity and mortality in the country. In Uganda, severe malaria is responsible for 15–20% of hospital admissions and is the leading cause of mortality among children under 5 yearswith an estimated 18,000 malaria-attributed deaths in 2022 [4]. Inadequate access to qualified and affordable case management services, poor care-seeking behaviour [6,7,8,9,10], and the emergence and spread of artemisinin and partner drug resistance are important challenges that may hinder progress [11,12,13].
In addition, an overlooked factor may also contribute to malaria deaths. Increasing alarming signals emerged from the literature are highlighting the possibility of contribution of neglected non-falciparum malaria infections to malaria mortality. Plasmodium falciparum is the main malaria species that causes severe disease and malaria-attributed mortality [14]. Notwithstanding often being considered benign, growing evidence confirms severe disease and morbidity associated with Plasmodium malariae, Plasmodium ovale spp. and Plasmodium vivax infections albeit at much lower rates than P. falciparum infection [14,15,16,17,18,19,20,21].
Non-falciparum malaria species also can present a chronic pattern of infections with frequent recrudescences or relapses that may cause serious health complications [22,23,24]. Chronic infection with P. malariae can cause severe complications in approximately 3% of cases, including refractory nephrotic syndrome, splenomegaly, and anaemia [14, 16, 25, 26]. Literature findings indicate that patients with mixed P. falciparum/P. malariae infections may have a higher proportion of multiple organ failure, severe anaemia, and pulmonary complications than those with mono-infection of P. falciparum [20]. Besides, several studies have suggested that mixed P. falciparum/P. malariae infections were associated with increased P. falciparum gametocytaemia, which may accelerate malaria transmission[16, 27, 28].
One of key difference between P. ovale spp. or P. vivax with P. falciparum is the possibility of relapses [29, 30]. Without radical treatment using primaquine, the risk for relapse in P. vivax and P. ovale spp. is estimated to be around 33.3% and 10.0%, respectively [31]. Each recurrent episode of symptomatic malaria causes haemolysis.
Increasing evidence in many sub-Saharan African countries following introduction of molecular techniques highlights underreporting and underestimation of non-falciparum malaria species [16, 30]. This is due to the widespread use of rapid diagnostic tests (RDTs) that only detects P. falciparum. In addition, microscopy is not a sensitive tool for diagnosis of mixed infections due to the lower parasite density of P. malariae, P. vivax and P. ovale spp. compared with P. falciparum [32]. In Uganda, the proportion of suspected malaria cases who were tested using RDTs that detect only P. falciparum increased from 2% in 2010 to 78% in 2014, remaining above 75% from 2014 to 2022 [4].
Therefore, it is important to investigate the prevalence of non-falciparum malaria infections in Uganda, both mono and mixed infections as well as their disease severity and treatment outcome. The findings of the study will identify potential solutions to enhance malaria case management policy.
Methods
The available English-language published peer-reviewed literature from 2005 up to March 2024 was sought via PubMed and Google Scholar. The used keywords for the search were severe malaria AND P. falciparum, P. malariae, P. vivax, P. ovale spp., mixed infections AND Uganda, as well as Uganda AND P. malariae, P. vivax, P. ovale spp., and mixed infections. After reviewing titles of the 7005 records, the authors excluded a large number of articles that were irrelevant and removed duplicate articles. In the next steps, the authors reviewed 254 abstracts of remaining articles and excluded irrelevant items. Some articles were excluded after reviewing the full content which finally left 53 articles. The selected articles were reviewed on three main themes including (1) prevalence of non-falciparum malaria infections in Uganda and (2) Clinical manifestations of severe malaria in non-falciparum infections in Uganda, and (3) Non-falciparum malaria case management in Uganda. Given the limitation of RDTs and microscopy methods in diagnosis of non-falciparum species, studies using molecular methods have been used to estimate the prevalence of non-falciparum malaria infections and clinical manifestation of severe malaria in non-falciparum infections in Uganda. The article is structured around these three key themes. To enrich the discussion, the authors searched WHO website for relevant WHO reports and technical documents.
Results and discussion
Prevalence of Plasmodium species in Uganda
Five studies that used molecular tests for diagnosis of malaria species have been selected for further analysis of the prevalence of Plasmodium species in Uganda. The characteristics of the studies included in the review are presented in Table 1 and the included studies results are presented in Table 2.
Overall pooled analysis of Plasmodium species in 2227 positive cases in 4019 samples showed:
Non-falciparum malaria:
-
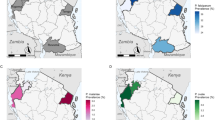
Molecular diagnostics revealed that 21% of initially reported mono-infections of P. falciparum were, in fact, mixed infections, P. falciparum/P. malariae 16%, and P. falciparum/P. ovale spp. 5% (Fig. 1).
-
Non-falciparum infections were more common as coinfections with P. falciparum rather than mono-infection.
-
Mixed infections of three species (P. falciparum, P. malariae, and P. ovale spp.) were rarely reported.
-
P. malariae was the second most prevalent species (9.7% of positive cases infected by P. malariae species, mono or mixed infections with P. falciparum)
-
P. ovale spp. was the third most prevalent species (4% of positive cases infected by P. ovale spp. species, mono or mixed infections with P. falciparum)
-
P. vivax was rarely reported. Other studies reported P. vivax in Uganda as well [33, 34].
-
A significant heterogeneity in the prevalence of non-falciparum malaria infections was found in different geographical areas and among various age groups. The range of non-falciparum infections varied from 4 to 58% of positive cases. On average non-falciparum infections accounted for 18.6% of positive cases.
-
Results of study of Murphy in the blood donors revealed that 9.3%, 4.5%, 1.6% asymptomatic cases were infected with P. falciparum, P. malariae and, P. ovale spp., respectively [35] (Fig. 2).
The findings of this review are similar to the results of studies in other countries. The prevalence of P. malariae and the total prevalence of P. malariae and P. ovale spp. in sub-Saharan Africa has been estimated at around 10% [23] and 20% [30], respectively. A higher prevalence of mixed infections of non-falciparum malaria compared with their mono-infections has been confirmed in other countries as well [16, 30]. The presence of P. malariae and P. ovale spp. both mono and mixed infections in Uganda using microscopy has been confirmed [16, 27, 36,37,38]. Besides, the transmission patterns of the non-falciparum species do not necessarily follow those of P. falciparum, stressing the need for attention towards non-falciparum malaria in Africa [39].
Clinical manifestations of severe malaria in non-falciparum infections in Uganda
The authors did not find any articles using molecular methods investigating the impact of non-falciparum malaria infections on disease severity and malaria mortality in Uganda. The reviewed literature on severe malaria and the majority of articles on uncomplicated malaria in Uganda relied on RDTs or light microscopy [5, 8,9,10, 14, 20, 29, 40,41,42,43,44,45,46,47,48,49]. Non-falciparum malaria prevalence may have been underestimated in studies relying on rapid diagnostic tests (RDTs) detecting only P. falciparum [14, 27, 32, 42, 50].
Underestimation of non-falciparum malaria infections may happen using microscopy-based methods as well. Diagnosis of P. malariae and P. ovale spp. both mixed and mono-infections by light microscopy can be difficult because non-falciparum parasitaemias often occur below detection thresholds or are masked by more visible, concurrent P. falciparum species [14, 16, 32, 42, 51, 52]. The similarity of P. malariae and P. falciparum parasites, microscopists competency, and laboratory infrastructure are other factors affecting the accuracy of diagnosis of malaria species and mixed infections by microscopy [14, 42].
Non-falciparum malaria case management in Uganda
Malaria case management policy in many sub-Saharan countries including Uganda has focused on P. falciparum [14]. The policy has been set based on this argument that non-falciparum malaria infections are mild and easily curable with common anti-malarial medicines recommended for P. falciparum.
The review found one article in Uganda that its results indicated persistent chronic multi-species malaria infections (9.2%) in children after artemether/lumefantrine treatment [16] indicating this hypothesis that artemether/lumefantrine may not be an effective medicine to treat P. malariae.
In addition, alarming literature evidence in other countries has been found on the possibility of treatment failure of non-falciparum malaria infections following treatment with mefloquine, halofantrine, quinine, and artemisinin-based combination [14, 24, 52]. A significant reduction in ex vivo susceptibility of P. malariae to lumefantrine and artemether has been reported in Mali [42].
It should be considered that recurrent episodes can occur due to recrudescence, relapse (in P. vivax and P. ovale spp. infections), or a new infection [17, 53]. Therefore, without robust evidence, recurrent episodes cannot be considered equal to reinfection.
The WHO emphasizes that the programme should ensure access to early diagnosis and prompt, effective treatment [54,55,56]. Strong surveillance, case detection, diagnosis, and treatment have direct benefits in reducing mortality and severe malaria disease but additionally can reduce transmission by diminishing the pool of infected individuals, which in return indirectly reduces malaria mortality [8, 17, 55] (Fig. 3). This recommendation covers all malaria species.
To address concerns regarding P. malariae treatment response, in some studies, using artemisinin combination therapy with a long half-life partner drug was recommended [16, 24]. The published evidence in the literature is insufficient to conclude common antimalarial medicines recommended for P. falciparum are not effective for non-falciparum malaria treatment in Uganda. Given any changes in case management policy will have policy implications, should be justified by robust evidence, and its feasibility and its pros and cons should be carefully considered.
Regarding diagnosis methods of suspected severe malaria cases, some studies emphasized the importance of diagnosis of suspected severe malaria cases due to infections by all malaria species including mono and mixed non-falciparum infections as well as awareness raising of physicians regarding the possibility of severe disease of neglected species in areas where more than one species is prevalent [19, 20, 26]. The WHO recommendations highlight that RDT can be used to confirm malaria rapidly however, microscopy is preferred for diagnosis of severe malaria as in addition to diagnosis species, it can provide other important parameters of prognostic relevance [54]. If quality-assured microscopy services are provided in hospitals of endemic countries, mixed infections as well as mono infections of non-falciparum malaria infections, particularly in severe malaria cases can be detected.
In the reviewed articles the authors didn’t find any published paper covering radical treatment of P. ovale spp. or P. vivax in Uganda. To prevent relapse in malaria cases infected by P. ovale spp. or P. vivax, the WHO recommends radical treatment in all transmission settings (except for those that have contraindications of primaquine) [57].
Conclusion
Non-falciparum malaria infections are neglected malaria species in sub-Saharan countries including Uganda where P. falciparum is the dominant species and the main cause of severe disease and mortality. This caused a knowledge gap in epidemiology, biology, health impact, and the role of mixed or mono-infections of non-falciparum species particularly regarding severe forms of malaria. Given mixed infection is common in Uganda, further research using reliable malaria species diagnosis methods to address this gap is recommended.
Besides, the focus of case management in Uganda is on P. falciparum. The policy has been set based on this argument that non-falciparum malaria infections are mild and easily curable with common antimalarial medicine recommended for P. falciparum. There is insufficient evidence in the literature on treatment outcomes of non-falciparum malaria of mixed infections to challenge this policy.
Finally, the health workforce in high-endemic countries where non-falciparum infections are common should be informed that mixed and mono-infections of non-falciparum malaria species can be seen. This may save the life of severe malaria cases with negative RDTs or severe malaria cases with frequent recurrent parasitaemia after discharge when its reason may not be reinfection of P. falciparum.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated during the current study.
Abbreviations
- RDT:
-
Rapid diagnostic tests
- Pf :
-
Plasmodium falciparum
- Pm :
-
Plasmodium malaria
- Pv :
-
Plasmodium vivax
- Po :
-
Plasmodium ovale Spp.
- WHO:
-
World Health Organization
References
WHO. Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015.
WHO. SDG Target 3.3 Communicable diseases. Geneva, World Health Organization; 2022. https://www.who.int/data/gho/data/themes/topics/sdg-target-3_3-communicable-diseases
WHO, RBM. High burden to high impact: a targeted malaria response. Geneva: World Health Organization; 2018.
WHO. World malaria report 2023. Geneva: World Health Organization; 2023.
Zalwango MG, Bulage L, Zalwango JF, Migisha R, Agaba BB, Kadobera D, et al. Trends and distribution of severe malaria cases, Uganda, 2017–2021: analysis of health management information system data. Uganda National Institute of Public Health, Kampala, 2023. https://uniph.go.ug/trends-and-distribution-of-severe-malaria-cases-uganda-2017-2021-analysis-of-health-management-information-system-data/
Zalwango MG, Simbwa BN, Kabami Z, Kawungezi PC, Wanyana MW, Akunzirwe R, et al. Risk factors for death among children with severe malaria, Namutumba District, Eastern Uganda, September 2021–February 2022. Research Square. 2023 (preprint).
Nabyonga Orem J, Mugisha F, Okui AP, Musango L, Kirigia JM. Health care seeking patterns and determinants of out-of-pocket expenditure for malaria for the children under-five in Uganda. Malar J. 2013;12:175.
Zalwango JF, Nankabirwa JI, Kitutu FE, Akunzirwe R, Buhuguru R, Rokani JB, et al. Malaria diagnostic and treatment practices for febrile children under 5 years at two general hospitals in Karamoja, a high transmission setting in Uganda. Malar J. 2022;21:312.
Kiguba R, Karamagi C, Bird SM. Quality of care for adult in-patients with malaria in a tertiary hospital in Uganda. Malar J. 2021;20:178.
Ampadu HH, Asante KP, Bosomprah S, Akakpo S, Hugo P, Gardarsdottir H, et al. Prescribing patterns and compliance with World Health Organization recommendations for the management of severe malaria: a modified cohort event monitoring study in public health facilities in Ghana and Uganda. Malar J. 2019;18:36.
Conrad MD, Asua V, Garg S, Giesbrecht D, Niaré K, Smith S, et al. Evolution of partial resistance to artemisinins in malaria parasites in Uganda. N Engl J Med. 2023;389:722–32.
Ebong C, Sserwanga A, Namuganga JF, Kapisi J, Mpimbaza A, Gonahasa S, et al. Correction to: Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated P. falciparum malaria and prevalence of molecular markers associated with artemisinin and partner drug resistance in Uganda. Malar J. 2022;21:37.
Tumwebaze PK, Katairo T, Okitwi M, Byaruhanga O, Orena S, Asua V, et al. Drug susceptibility of Plasmodium falciparum in eastern Uganda: a longitudinal phenotypic and genotypic study. Lancet Microbe. 2021;2:e441–9.
Ayo D, Odongo B, Omara J, Andolina C, Mulder O, Staedke SG, Bousema T. Plasmodium malariae infections as a cause of febrile disease in an area of high Plasmodium falciparum transmission intensity in Eastern Uganda. Malar J. 2021;20:425.
Oriero EC, Demba MA, Diop MF, Ishengoma DS, Amenga-Etego LN, Ghansah A, et al. Plasmodium malariae structure and genetic diversity in sub-Saharan Africa determined from microsatellite variants and linked SNPs in orthologues of antimalarial resistance genes. Sci Rep. 2022;12:21881.
Betson M, Clifford S, Stanton M, Kabatereine NB, Stothard JR. Emergence of nonfalciparum Plasmodium infection despite regular artemisinin combination therapy in an 18-month longitudinal study of Ugandan children and their mothers. J Infect Dis. 2018;217:1099–109.
Geleta G, Ketema T. Severe malaria associated with Plasmodium falciparum and P. vivax among children in Pawe Hospital, Northwest Ethiopia. Malar Res Treat. 2016;2016:1240962.
Siagian FE. Complications of kidney in severe malaria. Asian J Res Infect Dis. 2022;11:6–17.
Kotepui M, Kotepui KU, Milanez GD, Masangkay FR. Severity and mortality of severe Plasmodium ovale infection: a systematic review and meta-analysis. PLoS ONE. 2020;15: e0235014.
Kotepui M, Kotepui KU, De Jesus Milanez G, Masangkay FR. Plasmodium spp. mixed infection leading to severe malaria: a systematic review and meta-analysis. Sci Rep. 2020;10:11068.
Hwang J, Cullen KA, Kachur SP, Arguin PM, Baird JK. Severe morbidity and mortality risk from malaria in the United States, 1985–2011. Open Forum Infect Dis. 2014;1:ofu034.
Langford S, Douglas NM, Lampah DA, Simpson JA, Kenangalem E, Sugiarto P, et al. Plasmodium malariae infection associated with a high burden of anemia: a hospital-based surveillance study. PLoS Negl Trop Dis. 2015;9: e0004195.
Culleton R, Pain A, Snounou G. Plasmodium malariae: the persisting mysteries of a persistent parasite. Trends Parasitol. 2023;39:113–25.
Grande R, Antinori S, Meroni L, Menegon M, Severini C. A case of Plasmodium malariae recurrence: recrudescence or reinfection? Malar J. 2019;18:169.
Proietti C, Pettinato DD, Kanoi BN, Ntege E, Crisanti A, Riley EM, et al. Continuing intense malaria transmission in northern Uganda. Am J Trop Med Hyg. 2011;84:830.
Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale–the ‘bashful’malaria parasites. Trends Parasitol. 2007;23:278–83.
Roh ME, Oyet C, Orikiriza P, Wade M, Kiwanuka GN, Mwanga-Amumpaire J, et al. Asymptomatic Plasmodium infections in children in low malaria transmission setting, southwestern Uganda. Emerg Infect Dis. 2016;22:1494.
Bousema JT, Drakeley C, Mens PF, Arens T, Houben R, Omar SA, et al. Increased Plasmodium falciparum gametocyte production in mixed infections with P. malariae. Am J Trop Med Hyg. 2008;78:442–8.
Groger M, Fischer HS, Veletzky L, Lalremruata A, Ramharter M. A systematic review of the clinical presentation, treatment and relapse characteristics of human Plasmodium ovale malaria. Malar J. 2017;16:112.
Hawadak J, Dongang Nana RR, Singh V. Global trend of Plasmodium malariae and Plasmodium ovale spp. malaria infections in the last two decades (2000–2020): a systematic review and meta-analysis. Parasit Vectors. 2021;14:297.
Wångdahl A, Sondén K, Wyss K, Stenström C, Björklund D, Zhang J, et al. Relapse of Plasmodium vivax and Plasmodium ovale malaria with and without primaquine treatment in a nonendemic area. Clin Infect Dis. 2022;74:1199–207.
Subissi L, Kanoi BN, Balikagala B, Egwang TG, Oguike M, Verra F, et al. Plasmodium malariae and Plasmodium ovale infections and their association with common red blood cell polymorphisms in a highly endemic area of Uganda. Trans R Soc Trop Med Hyg. 2019;113:370–8.
Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Greenhouse B, Staedke SG, et al. Incidence of malaria and efficacy of combination antimalarial therapies over 4 years in an urban cohort of Ugandan children. PLoS ONE. 2010;5: e11759.
Dhorda M, Nyehangane D, Rénia L, Piola P, Guerin PJ, Snounou G. Transmission of Plasmodium vivax in south-western Uganda: report of three cases in pregnant women. PLoS ONE. 2011;6: e19801.
Murphy KJ, Conroy AL, Ddungu H, Shrestha R, Kyeyune-Byabazaire D, Petersen MR, et al. Malaria parasitemia among blood donors in Uganda. Transfusion. 2020;60:955–64.
Agaba BB, Rugera SP, Mpirirwe R, Atekat M, Okubal S, Masereka K, et al. Asymptomatic malaria infection, associated factors and accuracy of diagnostic tests in a historically high transmission setting in Northern Uganda. Malar J. 2022;21:392.
Pullan RL, Bukirwa H, Staedke SG, Snow RW, Brooker S. Plasmodium infection and its risk factors in eastern Uganda. Malar J. 2010;9:2.
Hergott DE, Owalla TJ, Staubus WJ, Seilie AM, Chavtur C, Balkus JE, et al. Assessing the daily natural history of asymptomatic Plasmodium infections in adults and older children in Katakwi, Uganda: a longitudinal cohort study. Lancet Microbe. 2024;5:e72-80.
Yman V, Wandell G, Mutemi DD, Miglar A, Asghar M, Hammar U, et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLoS Negl Trop Dis. 2019;13: e0007414.
Mpimbaza A, Ndeezi G, Katahoire A, Rosenthal PJ, Karamagi C. Demographic, socioeconomic, and geographic factors leading to severe malaria and delayed care seeking in Ugandan children: a case–control study. Am J Trop Med Hyg. 2017;97:1513–23.
Kigozi SP, Kigozi RN, Epstein A, Mpimbaza A, Sserwanga A, Yeka A, et al. Rapid shifts in the age-specific burden of malaria following successful control interventions in four regions of Uganda. Malar J. 2020;19:128.
Moffitt CA, Olupot-Olupot P, Onen JW, O’Brien N. Adherence to severe malaria treatment guidelines in children at a Ugandan regional hospital: a baseline assessment for a malaria treatment quality improvement project. Malar J. 2023;22:67.
Namayanja C, Eregu EE, Ongodia P, Okalebo CB, Okiror W, Okello F, et al. Unusual clinical spectra of childhood severe malaria during malaria epidemic in eastern Uganda: a prospective study. Malar J. 2023;22:169.
Olupot-Olupot P, Engoru C, Nteziyaremye J, Chebet M, Ssenyondo T, Muhindo R, et al. The clinical spectrum of severe childhood malaria in Eastern Uganda. Malar J. 2020;19:322.
Kalyesubula R, Sekitoleko I, Tomlin K, Hansen CH, Ssebunya B, Makanga R, et al. Association of impaired kidney function with mortality in rural Uganda: results of a general population cohort study. BMJ Open. 2022;12: e051267.
Paasi G, Ndila C, Okiror W, Namayanja C, Okalebo BP, Abongo G, et al. Characterising childhood blackwater fever and its clinical care at two tertiary hospitals in Eastern Uganda. Research Square. 2021 (preprint).
Olupot-Olupot P, Engoru C, Uyoga S, Muhindo R, Macharia A, Kiguli S, et al. High frequency of blackwater fever among children presenting to hospital with severe febrile illnesses in eastern Uganda. Clin Infect Dis. 2017;64:939–46.
Namazzi R, Opoka R, Datta D, Bangirana P, Batte A, Berrens Z, et al. Acute kidney injury interacts with coma, acidosis, and impaired perfusion to significantly increase risk of death in children with severe malaria. Clin Infect Dis. 2022;75:1511–9.
Opoka RO, Waiswa A, Harriet N, John CC, Tumwine JK, Karamagi C. Blackwater fever in Ugandan children with severe anemia is associated with poor postdischarge outcomes: a prospective cohort study. Clin Infect Dis. 2020;70:2247–54.
Idro R, Aloyo J, Mayende L, Bitarakwate E, John CC, Kivumbi GW. Severe malaria in children in areas with low, moderate and high transmission intensity in Uganda. Trop Med Int Health. 2006;11:115–24.
Dembele L, Aniweh Y, Diallo N, Sogore F, Sangare CP, Haidara AS, et al. Plasmodium malariae and Plasmodium falciparum comparative susceptibility to antimalarial drugs in Mali. J Antimicrob Chemother. 2021;76:2079–87.
Oriero EC, Amenga-Etego L, Ishengoma DS, Amambua-Ngwa A. Plasmodium malariae, current knowledge and future research opportunities on a neglected malaria parasite species. Crit Rev Microbiol. 2021;47:44–56.
WHO. Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance (2010–2019). Geneva: World Health Organization; 2020.
WHO. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2015.
WHO. From malaria control to malaria elimination: a manual for elimination scenario planning. Geneva: World Health Organization; 2014.
WHO. Management of severe malaria; handbook. Geneva: World Health Organization; 2012.
WHO. Guidelines for malaria. Geneva: World Health Organization; 2023.
Betson M, Sousa-Figueiredo JC, Atuhaire A, Arinaitwe M, Adriko M, Mwesigwa G, et al. Detection of persistent Plasmodium spp. infections in Ugandan children after artemether-lumefantrine treatment. Parasitology. 2014;141:1880–90.
Asua V, Tukwasibwe S, Conrad M, Walakira A, Nankabirwa JI, Mugenyi L, et al. Plasmodium species infecting children presenting with malaria in Uganda. Am J Trop Med Hyg. 2017;97:753.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Mansour Ranjbar wrote the manuscript, Dr. Yonas Tegegn Woldemariam supervised and led the literature review and All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not necessary for this study because it involved information freely available in the public domain. Note: The views expressed in this article are the author's views and do not necessarily reflect the policies of the World Health Organization.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ranjbar, M., Tegegn Woldemariam, Y. Non-falciparum malaria infections in Uganda, does it matter? A review of the published literature. Malar J 23, 207 (2024). https://doi.org/10.1186/s12936-024-05023-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-05023-9