Abstract
Background
Togo's National Malaria Control Programme has initiated an active home-based malaria management model for all age groups in rural areas of Bassar Health District. This report describes the model, reports its main results, and determines the factors associated with positive rapid diagnostic test results.
Methods
From 2014 to 2017, in three peripheral care units of Bassar Health District (Binaparba, Nangbani, and Baghan), community health workers visited residents' homes weekly to identify patients with malaria symptoms, perform rapid diagnostic tests in symptomatic patients, and give medication to positive cases. Univariate and multivariate logistic regression models were used to determine the factors associated with positive tests.
Results
The study covered 11,337 people (817 in 2014, 1804 in 2015, 2638 in 2016, and 6078 in 2017). The overall mean age was 18 years (95% CI 5–29; min–max: 0–112 years). The median age was 10 years (SD: 16.9). The proportions of people tested positive were 75.3% in Binaparba, 77.4% in Nangbani, and 56.6% in Baghan. The 5–10 age group was the most affected category (24.2% positive tests). Positive tests were more frequent during the rainy than during the dry season (62 vs. 38%) and the probability of positive test was 1.76 times higher during the rainy than during the dry season (adjusted OR = 1.74; 95% CI 1.60–1.90). A fever (37.5 °C or higher) increased significantly the probability of positive test (adjusted OR = 2.19; 95% CI 1.89–2.54). The risk of positive test was 1.89 times higher in passive than in active malaria detection (adjusted OR = 1.89; 95% CI 1.73–2.0).
Conclusions
This novel experimental community and home-based malaria management in Togo suggested that active detection of malaria cases is feasible within 24 h, which allows rapid treatments before progression to often-fatal complications. This PECADOM + program will help Togo's National Malaria Control Programme reduce malaria morbidity and mortality in remote and hard-to-reach communities.
Similar content being viewed by others
Background
Malaria is one of the greatest threats to human life in developing countries [1] and, in most African countries, malaria is the leading cause of death, especially in children. According to the United Nations Children's Fund (UNICEF), a child dies from malaria every 30 s [2]. In the latest World Malaria Report issued by the World Health Organization (WHO), the estimated number of malaria deaths was 619,000, of which 96% occurred in Africa [3]. At the peak of the COVID-19 pandemic (2020–2021), COVID-19-related disruption to malaria control resulted in nearly 13 million additional malaria cases and 63,000 additional malaria deaths [3]. In terms of expenditure, the annual cost of malaria in Africa was 12 billion US dollars [1].
Early and correct management of malaria cases within 24 h of symptom onset has always been a concern for "Roll Back Malaria" partners and the WHO [4,5,6,7,8,9]. The WHO reiterated this strategic principle in its latest Global Malaria Action Plan, urging countries to take initiatives to guarantee malaria control services to populations in areas without health facilities by promoting home-based management of malaria cases: programme “Prise en Charge à Domicile” (PECADOM) or home care [4,5,6]; i.e., “an array of health and social support services provided to clients in their own residence”. Since the Declaration of Alma-Ata (1978), several countries have involved community health workers (CHWs) in supporting access to primary health care in remote or deprived communities [10].
CHWs are non-medical volunteers or paid members of a community, who are able to offer early and local access to certain healthcare services. Regarding malaria, CHWs help reduce the disease burden‒transmission, morbidity, and mortality by means of: (i) case management; i.e., diagnosis with rapid diagnostic tests (RDTs), treatment of fever and uncomplicated malaria cases, and referral of complicated cases to health facilities; (ii) prevention; i.e., education about malaria and its complications, intermittent preventive treatment for pregnant women or children, and supply of insecticide-treated bed nets; and, (iii) collection of key epidemiological data needed for surveillance and control. The actions of CHWs proved successful, provided sufficient funding and remuneration, adequate training, clear role definitions and guidelines, regular supervision, constant support, motivating incentives, and better recognition by the healthcare system (and thus the community itself) [11].
In Africa, Senegal has led the way with various community-based initiatives [1]. In 2009, Senegal extended its community-based programmes to villages more than five km from a health facility [12]. Based initially on a passive case detection model, this initiative significantly reduced malaria-related deaths. In this passive model, community members sought treatment from CHWs only when they suspected malaria [12]. In 2012, to remedy the main weakness of that passive model (i.e., waiting for the patients to seek care), an active case detection model (named PECADOM +) was initiated in Kédougou Region where CHWs circulated and searched for malaria cases in homes [12]. The success of this new model led to its adoption by the US Presidents of Malaria Initiative (in partnership with the Senegalese Ministry of Health) and its extension to the whole of Senegal [12].
In Togo, activity decentralization and community involvement in health actions are paradigms already adopted by the Ministry of Health and Public Hygiene to optimize the results of anti-malarial interventions. After discussions with its financial and technical partners, the Togolese authorities approved PECADOM + for implementation as part of its National Malaria Strategic Plan (NMSP). However, a pilot study–limited to a part of the country–had to be carried out to evaluate the feasibility and the outcomes of the project as well as the involvement of the local actors, authorities, and beneficiaries. During this study, a full adhesion to the project was observed and a great satisfaction was expressed by the actors, the partners, and the beneficiaries.
Within this framework, inspired by the Senegalese experience, and in collaboration US Peace Corps Togo, the National Malaria Control Programme (NMCP) implemented, between 2014 and 2017, a community-based model of active malaria screening and treatment in villages located more than five km from a Peripheral Care Unit (PCU) in the Health District of Bassar (Kara Region of Northern Togo). The choice of the region was based on data showing that Bassar had the highest rate of malaria transmission [13]. Currently, the Togolese PECADOM + project is active in 17 villages, employs 21 CHWs, and reaches nearly 6,000 people.
This study aimed to describe this malaria active screening model, show its main results, and determine the factors associated with positive rapid diagnostic tests in Bassar communities.
Methods
PECADOM + in Togo
In Togo, this pilot model was set up between 2014 and 2017 after an introductory feasibility study in Bassar communities. For this feasibility study and set up, the following methodological steps were used:
-
Documentary research work: this work involved the analysis of several documents: the project protocol, workshop reports, training and coordination reports, information documents on the Bassar health district (in particular, the malaria situation), and the characteristics of the participating villages (including the number of inhabitants, distance from the reference health facility).
-
Interviews: these were carried out with stakeholders such as NMCP technical managers, project partners, department managers, local administrative authorities, community leaders, and benefiting communities.
-
Site visits: several candidate villages in Bassar Health District were visited to meet local stakeholders and agree with them on the project outline.
-
Selection of the participating villages: after an orientation workshop held with all the stakeholders, these villages were selected on of the following criteria:
-
o
Being located in the Bassar Health District area;
-
o
Being deprived of an appropriate health infrastructure;
-
o
Being temporarily or permanently located within five km from the nearest health facility (difficult-to-reach areas, islands, disaster zones).
-
o
-
Selection of the CHWs: these workers were selected according to the following criteria:
-
o
Being residents in their respective villages;
-
o
Being volunteers;
-
o
Being nominated by their respective communities;
-
o
Being available and committed to each community's interests;
-
o
Being able to read and write in French and speak local languages.
-
o
-
Training of the CHWs: this included a theoretical and a practical step. The training focused on malaria transmission, recognition of the signs of uncomplicated and severe malaria, use of RDTs with strict adherence to hygiene rules, treatment with an artemisinin-based combination therapy (ACT), reasons for case referral, and maintenance of management tools (registers, stock sheets, monthly reports).
-
Supervision, monitoring, and evaluation of the strategy: these actions were carried out at various levels by NMCP managers, regional managers, district managers, and managers of the PCU.
PECADOM + experimental study environment
Togo is a West African country with a surface area of 56,600 km2 and a population of 8,095,498 people [14]. Overall, Togo's health system has a three-tier pyramid structure. The NMCP (that supervised this project) is located at the Central level, where policies, strategies and guidelines for malaria management are set up. The Regional (or Intermediate) level comprises six health regions and is the level that provides technical support to the Health Districts. The third (or Peripheral) level is the most decentralized; it is the operational level [15].
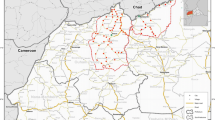
The Bassar Health District is inhabited by 33,156 people. It comprises four communes and ten cantons that cover 3,620 km2. The three PCUs of Bassar where PECADOM + was implemented were: Binaparba (1303 inhabitants), Nangbani (473 inhabitants), and Baghan (4,114 inhabitants) (Fig. 1).
Data collection
Data were collected weekly over four successive years (2014–2017) by 21 CHWs. The data collection used the following tools:
-
Information-gathering media (care booklets, stock sheets);
-
Report templates;
-
CHW supervision grids;
-
Monthly site reports;
-
Supervision reports;
-
Interviews using an interview guide adapted to each target group.
The information collected included data on the PCU, year, age, sex, presence of fever and malaria symptoms, RDT result, presence or absence of mosquito nets in the household, and type of detection: active (the CHWs track malaria cases in the community) or passive (the community seeks care from the CHWs or at a PCU).
In-house data checking and validation sessions were carried out to avoid missing data and identify/correct inconsistent data.
Community health workers’ protocol
This protocol included at-home diagnosis, test, and treatment. A diagnosis of malaria required the presence of fever (hot body) or a recent history of fever during the current episode of illness without signs of severity and diagnosis confirmation by a RDT. Temperature was measured with a thermometer (axillary temperature ≥ 37.5 °C).
The RDT used in this study was SD BIOLINETM Malaria Ag P.f (HRP2/pLDH) (Standard Diagnostics Inc., Korea). This test is usually routinely used in hard-to-reach and lab-deprived areas. Its performance characteristics are: 99.3% specificity and P.f (HRP2)/P.f (pLDH) 99.7%/97.4% sensitivity.
To ensure a high testing quality, the CHWs were, first, rigorously and intensively trained in the use (preparation and interpretation) of the RDT before being sent to the villages and, second, given a quality assurance guide on how to maintain the test performance and reduce the risks of misuse.
A CHW could administer ACT (using artemether-lumefantrine or artesunate-amodiaquine) to a patient with a positive RDT result. Otherwise, the patient was referred to the nearest health facility. During a follow-up visit, a CHW had to:
-
Reassess the patient’s clinical condition;
-
Advise on the correct use of medication;
-
Emphasize preventive measures against malaria; in particular, the use of mosquito nets (Fig. 2).
Statistical analysis
Categorical variables were described by numbers and proportions and quantitative variables by means, standard deviations, maxima, minima, and medians with interquartile ranges (IQRs). Univariate and multivariate logistic regression analyses were performed to determine the factors associated with positive RDT results. Only variables associated with a p-value ≤ 0.2 in the univariate analysis were included in the multivariate models using the bottom-up stepwise method. Interactions between the variables retained in the multivariate models were tested. The various models were then compared using the likelihood ratio test to select the more parsimonious risk model and a value of p < 0.05 was considered for statistical significance. Unadjusted and adjusted odds ratios (ORs and aORs) were reported with their 95% confidence intervals (CIs). All statistical analyses were performed using R Studio software version 14.2.2 (2022-10-31).
Results
Descriptive analysis
Data distribution by year
In all, 11,337 people participated in the study: 817 in 2014, 1,804 in 2015, 2,638 in 2016, and 6,078 in 2017. The number of participants was relatively low at the start of the project because only one PCU was included (Binaparba). This number increased after inclusion of the second and third PCUs (Nangbani then Baghan). The mean age of the participants was 18 years (SD: 16.9 years), and its median was 10 years (min–max: 0–112 years). Women accounted for 49.7% of the participants. The other participants’ characteristics are shown in Table 1.
Data distribution by PCU
The mean age of the participants was 20 years in Binaparba (median: 12; min–max: 0–112), 17 years in Nangbani (median: 10; min–max: 0–90) and 16 years in Baghan (median: 9; min–max: 0–112). According to the RDT results, people who tested positive represented 75.3% of the participants in Binaparba, 77.4% in Nangbani, and 56.6% in Baghan. Household mosquito net ownership was 82% in Binaparba, 99.1% in Nangbani, and 73.7% in Baghan. Regarding the type of detection, the active approach allowed CHWs to detect 38.1% of malaria cases in Binaparba, 68.8% in Nangbani, and 80.6% in Baghan. Other interesting data are given in Table 2.
Distribution of RDT results
Overall, the proportion of positive RDTs was 62% during the rainy season and 38% during the dry season. A decrease in positive RDTs was observed by the end of each rainy season.
The mean age at malaria case detection by RDT was 16 years (median: 9; min–max: 0–11). However, the 5–10 age group was the most affected; it represented 24.2% of all positive RDTs. Although 85% of the households in this study had mosquito nets, 77.2% of the RDTs were positive. Data relative to other variables linked with RDT results are given in Table 3.
Results of the univariate logistic regression analysis
The univariate logistic regression model showed that the probability of testing positive was 1.55 times higher during the rainy than during the dry season (OR = 1.55; 95% CI 1.43–1.68).
Men appeared to be 1.15 times more likely to have positive RDTs than women (OR = 1.15; 95% CI 1.06–1.24) and people with fever (≥ 37.5 °C) were significantly more likely to have positive RDTs than asymptomatic people (OR = 3.41; 95% CI 2.98–3.90). When fever was associated with other malaria symptoms, the probability of positive RDT was 1.26 times higher (OR = 1.26; 95% CI 1.10–1.45) than in the absence of association. Without fever but in the presence of other symptoms, the probability of testing positive was multiplied by 2.28 (OR = 2.28; 95% CI 2.00–2.60).
In passive case detection, the risk of a positive RDT was 1.89 times higher than in active detection (OR = 1.89; 95% CI 1.73–2.0). The results of the univariate analysis are shown in Table 4.
Results of the multivariate regression analysis
These results showed that, vs. Binaparba, the probability of positive RDT was significantly lower in Baghan (aOR = 0.53; 95% CI 0.47–0.59) and higher in Nangbani (aOR = 1.30; 95% CI 1.14–1.49). During the rainy season, the probability of testing positive appeared to be 1.76 times higher than during the dry season (aOR = 1.74; 95% CI 1.60–1.90).
Regarding age, there was no significant difference in the probability of positivity between children aged 0–1 years and those aged 1–2 years. However, from age 2 up to 15, the likelihood of a positive test was, on average, 1.52 times higher than in the reference age group (0 to one year). This probability decreased significantly after age 25 (aOR = 0.65; 95% CI 0.52–0.81). In terms of sex, the multivariate analysis revealed an association between being a male and the probability of testing positive (aOR = 1.11; 95% CI 1.02–1.21).
Having a fever (≥ 37.5 °C) significantly increased the probability of a positive test (aOR = 2.19; 95% CI 1.89–2.54). This increase was also observed for symptoms other than fever (aOR = 1.83; 95% CI 1.59–2.12). Also, the presence of fever along with other symptoms appeared to be associated with positive malaria detection (aOR = 1.16; 95% CI 1.00–1.35); however, this association was not significant (the CI of the aOR included value 1).
Passive case detection was found to be associated with a higher probability of positive RDT result than active case detection (aOR = 1.55; 95% CI 1.40–1.71). The results of the multivariate analysis are shown in Fig. 3.
Discussion
In Togo, as in other malaria-affected countries, early detection of cases is of crucial importance for adequate care and optimal management of the disease as part of its eradication [16,17,18]. PECADOM + , as other CHW-led projects, aimed to improve access to malaria screening, treatment, and management services to reduce disease complications (especially in children and pregnant women), reduce transmission in remote communities, and accelerate eradication [19]. This intervention aligns with the WHO's vision of eradicating malaria worldwide by 2030 [19]. As per the study design and the WHO recommendations for such an initiative, the objective of the CHWs was not to test all household members but to identify only individuals suspected of having malaria (current or recent fever and positive RDT) so they could be rapidly evaluated and treated.
Experimenting PECADOM + in Togo highlighted differences in the risk of positive RDT between the participating PCUs. This suggests that the way of implementing community management of malaria may differ according to local characteristics such as demography, behavior, and type of intervention.
Also, the results highlighted differences in transmission and risk between seasons (rainy vs. dry), indicating the importance of a more sustained screening during the rainy season in response to the favorable conditions for mosquito vector reproduction [20]. This result is in line with recent studies conducted in Togo. Indeed, a survey of the trends in malaria morbidity and mortality in Togo from 2008 to 2017 showed that morbidity tended to increase throughout the country during the rainy seasons despite intensification of various strategies and interventions [15]. Similarly, a study conducted on chemoprevention of seasonal malaria in Togo from 2013 to 2020 reported that, before implementing this intervention, frequent increases of malaria cases were seen during the rainy seasons, especially among children under five [21]. Finally, a study conducted from 2008 to 2017 in Togo showed that maximum seasonal indices were seen during rainy seasons and minimum seasonal indices during dry seasons [22].
The results of the present experiment showed that children aged five to ten years were the most at risk of malaria. According to internal NMCP reports and a significant number of other sources, children under five are generally the most vulnerable to malaria. Here, the trend was a shift in morbidity towards slightly older children. This might be linked to the effect of implementing the Seasonal Malaria Chemoprevention (SMC) strategy in children aged 3–59 months during seasons of high malaria transmission in Bassar Health District. In some countries of the West African sub-region (Senegal, Mali), this observation has led to reconsidering SMC targets and extending them to children under ten [23,24,25].
One expected result was that fever was often present in RDT-positive subjects. This confirms that fever is a crucial symptom of malaria. However, other symptoms, such as headache, body aches, chills, dizziness, and tiredness, were also associated with positive RDT results. In an early detection approach, it is necessary to pay attention to the latter symptoms. This would help early detections and rapid treatments that save lives.
Mosquito nets are known to be efficient in reducing the risk of being bitten by malaria mosquito vectors. Therefore, mosquito nets in households are necessary to prevent malaria transmission. Within this experiment, nets were present in nearly all visited households; however, it was impossible to evaluate their exact impact because no information was collected regarding their proper use.
Finally, this experiment indicated that passive detection would increase the probability of finding positive tests vs. active home visits by CHWs. This contrasts with results obtained by Linn et al. [12] showing that the prevalence of RDT-confirmed malaria was 16 times higher in passively-screened than in actively-screened villages. One explanation is that passively-detected malaria cases in this experiment were genuine cases; sick people would come more readily to the CHWs in the villages when they feel ill. The increase in the probability of finding positive tests through active home visits underlines the importance of active case detection in the communities through regular screening and targeting specific or at-risk groups. Active home visits also help case identification before developing clinical signs; thus, early management. However, more information is needed on how the CHWs carried out the two types of detection; this will optimize the detection strategy and improve the quality of care.
The literature still needs to report more on the role of CHWs in the fight against malaria [26,27,28,29,30]. In Africa, most studies were carried out in West Africa and East sub-Saharan Africa [31,32,33]. The present study is most probably the first in Togo to describe community management of malaria and identify factors associated with positive RDT results. It presents a major asset of an exhaustive participation of the PCUs of Bassar Health District between 2014 and 2017.
It is worth mentioning that, among the strategies already used by the NMCP to combat malaria in Togo, PECADOM + is not yet adopted. At the time of this study, it was only experimented in a few PCUs in Bassar Health District (Kara Region) before a more extended implementation. Thus, this experimentation’s results cannot be generalized to the entire Bassar Health District, entire Kara Region, or entire Togo.
The results obtained here are interesting and place Togo among the countries that have experimented with this innovative strategy [12, 35, 36]. These results are similar to those found in the scientific literature. Indeed, in Togo, the CHWs detected more malaria cases in intervention villages [36]. Based on these results, the NMCPs of the countries that experimented with this new approach were able to provide workable guidelines to improve the implementation of community management of malaria, as well as guidance for other NMCPs that consider adopting it.
To be properly implemented and maintained, Togo's PECADOM + needs to be adequately funded to be correctly implemented and maintained. Given the financial difficulties already met in carrying out this experiment, one may expect major problems in obtaining funds for the programme extension to whole regions.
Conclusion
PECADOM + is a proactive community-based malaria prevention and control strategy that is strongly recommends by the WHO. In Togo, it is currently in an experimental phase before implementation across the whole country. The present experiment showed that important measures should be taken to improve its implementation and follow-up. These include adopting an active targeted approach to malaria case detection, optimizing data collection, checking the reproducibility of the current implementation conditions in other Health Districts, and, finally, overcoming all financial challenges by mobilizing domestic resources and lobbying all possible funders.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to their belonging to the Togolese Ministry of Health and Public Hygiene but are available from the corresponding author on reasonable request.
Abbreviations
- ACT:
-
Artemisinin-based combination therapy
- AOR:
-
Adjusted odds ratio
- CHW:
-
Community health worker
- CI:
-
Confidence interval
- COVID:
-
Coronavirus infection disease
- IQR:
-
Interquartile range
- NMCP:
-
National Malaria Control Programme
- OR:
-
Odds ratio
- PCU:
-
Peripheral care unit
- RDT:
-
Rapid diagnostic test
- SD:
-
Standard deviation
- SMC:
-
Seasonal malaria chemoprevention
- UNICEF:
-
United Nations Children's Fund
- WHO:
-
World Health Organization
References
Centers for Disease Control and Prevention. Malaria’s Impact Worldwide. 2024. https://www.cdc.gov/malaria/malaria_worldwide/impact.html. Accessed 14 Mar 2024.
UNICEF. Paludisme chez l’enfant. https://www.unicef.fr/convention-droits-enfants/sante/maladies-infantiles/paludisme. Accessed 20 Nov 2023.
WHO. World malaria report 2022. Geneva: World Health Organization; 2022.
OMS. Programme mondial de lutte antipaludique: stratégie pour améliorer l’accès au traitement par la prise en charge du paludisme à domicile. Genève: Organisation Mondiale de la Santé; 2006.
WHO. Global Malaria Programme. Geneva: World Health Organization; 2023.
WHO. Guidelines for malaria. Geneva: World Health Organization; 2023.
Grueninger H, Hamed K. Transitioning from malaria control to elimination: the vital role of ACTs. Trends Parasitol. 2013;29:60–4.
Nsungwa-Sabiiti J, Tomson G, Pariyo G, Ogwal-Okeng J, Peterson S. Community effectiveness of malaria treatment in Uganda–a long way to Abuja targets. Ann Trop Paediatr. 2005;25:91–100.
RBM Partnership to End Malaria. A Decade of Partnership and Results. Progress and Impact Series Number 7. 2011. https://reliefweb.int/report/world/progress-and-impact-series-number-7-decade-partnership-and-results-september-2011-enfr. Accessed 20 Nov 2023.
Agarwal S, Sripad P, Johnson C, Kirk K, Bellows B, Ana J, et al. A conceptual framework for measuring community health workforce performance within primary health care systems. Hum Resour Health. 2019;17:1–20.
Adhikari B, Bayo M, Peto TJ, Callery JJ, Tripura R, Dysoley L, et al. Comparing the roles of community health workers for malaria control and elimination in Cambodia and Tanzania. BMJ Glob Health. 2023;8:e013593.
Linn AM, Ndiaye Y, Hennessee I, Gaye S, Linn P, Nordstrom K, et al. Reduction in symptomatic malaria prevalence through proactive community treatment in rural Senegal. Trop Med Int Health. 2015;20:1438–46.
Republic of Cameroon, Ministry of Public Health. Rapport d'activités 2013 du Programme National de Lutte contre le Paludisme. 2014. https://www.pnlp.cm/wp-content/uploads/2020/05/Rapport-annuel-PNLP-2013-du-21-12-2014.pdf. Accessed 20 Nov 2023.
Institut national de la statistique et de la démographie (INSD). Cinquième Recensement Général de la Population et de l’Habitation du Burkina Faso, Synthèse des Résultats Définitifs. 2022. http://cns.bf/IMG/pdf/rapport_resultats_definitifs_rgph_2019.pdf. Accessed 20 Nov 2023.
Bakai TA, Thomas A, Iwaz J, Atcha-Oubou T, Tchadjobo T, Khanafer N, et al. Changes in registered malaria cases and deaths in Togo from 2008 to 2017. Int J Infect Dis. 2020;101:298–305.
Republic of Togo, Ministry of Public Health. Plan Strategique National de Lutte contre le Paludisme 2017–2022. 2018. https://platform.who.int/docs/default-source/mca-documents/policy-documents/plan-strategy/TGO-CC-37-01-PLAN-STRATEGY-2018-fra-PSN-de-lutte-contre-le-paludisme-2017-2022.pdf. Accessed 20 Nov 2023.
Stanisic DI, Fowkes FJI, Koinari M, Javati S, Lin E, Kiniboro B, et al. Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children and the influence of age, force of infection, and magnitude of response. Infect Immun. 2015;83:646–60.
République du Sénégal, Ministère de la Santé et de l'Action Sociale. Manuel du DSDOM sur la prise en charge du paludisme de la diarrhée et des Infections Respiratoires Aigües. (not dated). https://pdf.usaid.gov/pdf_docs/PA00KRXM.pdf. Accessed 20 Nov 2023.
OMS. Stratégie technique mondiale de lutte contre le paludisme 2016–2030. Genève: Organisation Mondiale de la Santé; 2015.
Mouchet J, Carnevale P. Les vecteurs et la transmission. In: Danis M, Mouchet J, editors. Paludisme. Paris: Ellipses; 1991. p. 35–59.
Bakai TA, Thomas A, Iwaz J, Atcha-Oubou T, Tchadjobo T, Khanafer N, et al. Effectiveness of seasonal malaria chemoprevention in three regions of Togo: a population-based longitudinal study from 2013 to 2020. Malar J. 2022;21:400.
Thomas A, Bakai TA, Atcha-Oubou T, Tchadjobo T, Bossard N, Rabilloud M, et al. Seasonality of confirmed malaria cases from 2008 to 2017 in Togo: a time series analysis by health district and target group. BMC Infect Dis. 2021;21:1189.
Diawara SI, Konaté D, Kayentao K, Mihigo J, Shaffer JG, Sangare M, et al. Effect of seasonal malaria chemoprevention in children between 5 and 9 years old in Kita and Bafoulabe districts. Mali Parasite Epidemiol Control. 2022;18:e00258.
Cissé B, Ba EH, Sokhna C, NDiaye JL, Gomis JF, Dial Y, et al. Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: a stepped-wedge cluster-randomised trial. PLoS Med. 2016;13:e1002175.
NDiaye JL, Cissé B, Ba EH, Gomis JF, Ndour CT, Molez JF, et al. Safety of seasonal malaria chemoprevention (SMC) with sulfadoxine-pyrimethamine plus amodiaquine when delivered to children under 10 years of age by district health services in Senegal: results from a stepped-wedge cluster randomized trial. PLoS One. 2016;11:0162563.
Uneke CJ. Impact of home management of Plasmodium falciparum malaria on childhood malaria control in sub-Saharan Africa. Trop Biomed. 2009;26:182–99.
Nzayirambaho M, Dieu JD, Freund RJ, Millet P, Merrien FX, Potel G, et al. Impact of home-based management of malaria combined with other community-based interventions: what do we learn from Rwanda? Pan Afr Med J. 2013;14:50.
Marita EO, Gichuki R, Watulo E, Thiam S, Karanja S. Determinants of quality in home-based management of malaria by community health volunteers in rural Kenya. J Infect Dev Ctries. 2021;15:897–903.
Ajayi IO, Browne EN, Bateganya F, Yar D, Happi C, Falade CO, et al. Effectiveness of artemisinin-based combination therapy used in the context of home management of malaria: a report from three study sites in sub-Saharan Africa. Malar J. 2008;7:190.
Moreno-Gutierrez D, Rosas-Aguirre A, Llanos-Cuentas A, Bilcke J, Barboza JL, Hayette MP, et al. Economic costs analysis of uncomplicated malaria case management in the Peruvian Amazon. Malar J. 2020;19:161.
Chukwuocha U. Rapid assessment of home management of malaria among caregivers in parts of South-eastern Nigeria. Pan Afr Med J. 2011;10:29.
Ajayi IO, Falade CO, Bamgboye EA, Oduola AM, Kale OO. Assessment of a treatment guideline to improve home management of malaria in children in rural South-west Nigeria. Malar J. 2008;7:24.
Hopkins H, Talisuna A, Whitty CJ, Staedke SG. Impact of home-based management of malaria on health outcomes in Africa: a systematic review of the evidence. Malar J. 2007;6:134.
Kanamori S, Kohi TW, Nyamhanga T, Mkude S. Assessing the performance of nurses in the management of malaria patients in Tanzania. J Trop Pediatr. 2011;57:378–81.
Conner RO, Dieye Y, Hainsworth M, Tall A, Cissé B, Faye F, et al. Mass testing and treatment for malaria followed by weekly fever screening, testing and treatment in Northern Senegal: feasibility, cost and impact. Malar J. 2020;19:252.
Gaye S, Kibler J, Ndiaye JL, Diouf MB, Linn A, Gueye AB, et al. Proactive community case management in Senegal 2014–2016: a case study in maximizing the impact of community case management of malaria. Malar J. 2020;19:166.
Acknowledgements
The authors would like to thank the staff of Togo's National Malaria Control Program (NMCP), the NGO "Peace Corps Togo", and the participating community health workers for their fieldwork.
Funding
This work was supported by EPIMOD Company and the American governmental organization "Peace Corps Togo".
Author information
Authors and Affiliations
Contributions
TAB, MG, NV, and NK designed the study. TAB, TT, and TA collected the data. MG, NK and NV analysed the data. MG, NK, NV, JI, and TAB interpreted the results. TAB and NK wrote the manuscript. NK, NV, AT, PV, JI, and TAB revised the manuscript to its final version. NK and NV supervised the study. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
For this study, an ethical approval was obtained from the MSHP. In accordance with the partnership standards in Togo, RDTs and ACTs were provided free of charge by "Peace Corps Togo". Verbal consents were obtained before RDTs and, in accordance with the National malaria management guidelines, free treatments were offered to those who tested positive.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bakai, T.A., Gense, M., Vanhems, P. et al. Proactive home-based malaria management in rural communities of Bassar Health District in northern Togo from 2014 to 2017: PECADOM + , a pilot experiment. Malar J 23, 203 (2024). https://doi.org/10.1186/s12936-024-04988-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-024-04988-x