Abstract
Background
Mass distributions of long-lasting insecticidal nets (LLINs) have contributed to large reductions in the malaria burden. However, this success is in jeopardy due in part to the increasing pyrethroid-resistant mosquito population as well as low LLINs coverage in various areas because the lifespan of LLINs is often shorter than the interval between replenishment campaigns. New insecticide-treated nets (ITNs) containing pyrethroid and piperonyl-butoxide (PBO) have shown a greater reduction in the incidence of malaria than pyrethroid LLINs in areas with pyrethroid-resistant mosquitoes. However, the durability (attrition, bio-efficacy, physical integrity and chemical retainment) of pyrethroid-PBO ITNs under operational settings has not been fully characterized. This study will measure the durability of pyrethroid-PBO ITNs to assess whether they meet the World Health Organization (WHO) three years of operational performance criteria required to be categorized as “long-lasting”.
Methods
A prospective household randomized controlled trial will be conducted simultaneously in Tanzania, India and Côte d’Ivoire to estimate the field durability of three pyrethroid-PBO ITNs (Veeralin®, Tsara® Boost, and Olyset® Plus) compared to a pyrethroid LLIN: MAGNet®. Durability monitoring will be conducted up to 36 months post-distribution and median survival in months will be calculated. The proportion of ITNs: (1) lost (attrition), (2) physical integrity, (3) resistance to damage score, (4) meeting WHO bio-efficacy (≥ 95% knockdown after 1 h or ≥ 80% mortality after 24 h for WHO cone bioassay, or ≥ 90% blood-feeding inhibition or ≥ 80% mortality after 24 h for WHO Tunnel tests) criteria against laboratory-reared resistant and susceptible mosquitoes, and insecticidal persistence over time will be estimated. The non-inferiority of Veeralin® and Tsara® Boost to the first-in-class, Olyset® Plus will additionally be assessed for mortality, and the equivalence of 20 times washed ITNs compared to field aged ITNs will be assessed for mortality and blood-feeding inhibition endpoints in the Ifakara Ambient Chamber Test, Tanzania.
Conclusion
This will be the first large-scale prospective household randomized controlled trial of pyrethroid-PBO ITNs in three different countries in East Africa, West Africa and South Asia, simultaneously. The study will generate information on the replenishment intervals for PBO nets.
Similar content being viewed by others
Background
Long-lasting insecticidal nets (LLINs) have contributed substantially to the reduction of the malaria burden over the past 15 years [1]. Between 2004 and 2020, more than two billion LLINs have been distributed worldwide [2]. Since the 1980s [3, 4], bed nets have been treated with pyrethroid insecticide because it has low toxicity to humans and non-targeted arthropods, and has a rapid insecticidal effect against susceptible malaria mosquitoes [5, 6]. Pyrethroid LLINs provide a physical barrier between humans and host-seeking mosquitoes, and the pyrethroid kill, incapacitate (knockdown), or inhibit feeding among mosquitoes that come into contact with the net [7, 8], enhancing bite protection even when the net is physically damaged [9]. When used with high coverage, LLINs provide community protection against malaria by reducing mosquito survival [7, 10,11,12].
Pyrethroid-resistant malaria vectors are now a major threat to the effectiveness of LLINs [13]. To tackle this threat, a new generation of insecticide-treated nets (ITNs) have been developed using pyrethroids combined with a synergist, such as piperonyl-butoxide (PBO) [12, 14]. PBO has little insecticidal activity by itself but acts as a synergist by blocking the oxidases (cytochrome P450) that commonly detoxify pyrethroid insecticides inside the mosquito’s body to restore pyrethroid efficacy [15,16,17,18]. Pyrethroid-PBO ITNs were found to yield a greater reduction in the incidence of malaria cases compared to standard pyrethroid LLINs for up to 21 months of use in areas with a high level of pyrethroid-resistance in mosquitoes [19] and have shown increased mosquito mortality compared to pyrethroid only LLINs in experimental hut studies when unwashed or after 20 washes [20]. However, in the Cochrane review of pyrethroid-PBO ITNs, the authors concluded that there is no evidence that PBO content persists under operational conditions for three years [20].
Bed nets that retain their bio-efficacy thresholds (the proportion of mosquitoes (≥ 95%) knocked down after one hour or (≥ 80%) mortality after 24 hours for cone bioassay, or if cone bio-efficacy thresholds are unmet, the proportion of mosquitoes (≥ 90%) blood-feeding inhibition or (≥ 80%) mortality after 24 h for tunnel tests) for at least 20 World Health Organization (WHO) standard washes under laboratory conditions and three years of recommended use under operational conditions are qualified as LLINs [8, 14]. However, it is not known if the 20 times WHO laboratory washed ITNs correspond to the field-used ITNs in inducing mosquito mortality. Also, although the WHO has prequalified several pyrethroid-PBO ITNs, there is currently limited data available on their physical and insecticidal durability under operational conditions in different geographical regions [8]. Understanding the durability of pyrethroid-PBO ITNs under operational conditions is important to guide procurement decisions and for devising replacement policies [21].
The physical integrity of a LLIN under operational conditions is one of the key determinants of net retention in the household, and more than 75% of discarded LLINs have damage and are perceived as failing to protect the user [22]. Factors associated with the loss of the physical integrity of LLINs include the environment in which the net is used [23,24,25], the users’ net care behaviour and attitude towards the LLIN [26], the type of net material [27,28,29,30], snag strength, bursting strength, abrasion resistance and resistance to hole enlargement [31], the number and age of people sleeping under the net [32, 33], and the geographical location in which the net is used [34]. Therefore, it is recommended to conduct ITN durability studies in multiple geographical locations [8]. The current study aims to measure the insecticidal and physical durability of three PBO ITNs alongside a pyrethroid only net over three years of household use in three different regions.
Study objectives
Overall aim
To evaluate the physical and insecticidal durability of PBO ITNs (Veeralin®, Tsara® Boost, Olyset® Plus) and a standard pyrethroid LLIN (MAGNet®) over 3 years of household use in Tanzania, East Africa, Côte d’Ivoire, West Africa and India, South Asia using standard WHO methods [8], and additionally to assess the non-inferiority [35, 36] of the PBO ITNs against Olyset® Plus.
Secondary objectives
-
i.
Estimate the attrition rate of each ITN product at 6, 12, 24 and 36 months of household use in each location.
-
ii.
Estimate fabric integrity of each ITN product at 6, 12, 24 and 36 months of household use in each location.
-
iii.
Estimate the bio-efficacy of each ITN product after 6, 12, 18, 24, 30 and 36 months of household use in each location.
-
iv.
Measure the insecticide content of each ITN product after 12, 24 and 36 months of household use in each location.
-
v.
Assess non-inferiority of field used Veeralin® and Tsara® Boost compared to Olyset® Plus and superiority of PBO ITNs compared to pyrethroid only MAGNet® from the Tanzania site after 12 and 24 months, upon the 24-h mortality primary endpoint.
-
vi.
Assess the equivalence of unwashed and 20 times laboratory washed nets and field-used ITNs after 12 and 24 months of each net type from the Tanzania site upon the 24-h mortality and blood-feeding inhibition endpoints.
Methods
Study area
This study will be conducted in selected villages in Tanzania, Côte d’Ivoire and India (Table 1). Study villages will be selected based on the availability of a large number of households required for the study, their accessibility throughout the year, the high abundance of malaria vectors that promotes mosquito net usage [37] and proximity to the bio-efficacy testing laboratory in each study country.
Study design
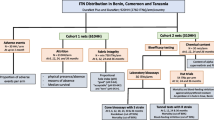
This will be a prospective household-randomized controlled trial following WHO guidelines [8] for monitoring the durability of ITNs with slight modifications to the sample size and study procedures. A minimum of 6500 households from the selected villages in the study area will be enrolled in each country. In this study, a household is defined as a group of people who share living accommodation and who are eating from one pot. The households will be the unit of ITNs randomization and the individual nets as a unit of observation. At baseline, a household survey will be conducted to collect households’ information including demographic and socioeconomic characteristics and the location of the household. At the same time, mosquito nets will be distributed by a study team in each household to cover all sleeping places, to ensure maximum coverage of ITNs for all members of the household. Follow-up surveys will be conducted to assess the rate of net loss in the receiving households (attrition rates due to all causes, as well as for wear and tear), physical integrity, bio-efficacy and chemical residual content of ITNs collected from households at the time intervals up to 36 months (Table 2). The study flow diagram is shown below (Fig. 1). All participating households will be blinded to which net product they receive, and study investigators will also be blinded to intervention allocation.
Study flow diagram showing the study procedures; attrition, fabric integrity assessment, bio-efficacy and chemical residual content will be done as per WHO guidelines [38]. The cross-sectional assessment of the non-inferiority and equivalences analysis will be conducted in Tanzania. B bioefficacy, C chemical analysis, HH household, ITNs insecticidal trated nets, pHI propotionate hole index, TZ Tanzania, IACT Ifakara Ambient Chember Test
The durability monitoring components that will be evaluated are: attrition, bioefficacy, chemical residual content, and damage to fabric. In addition, the Ifakara ambient chamber testing (IACT) will be conducted as an additional bioassay using nets sampled from the field in Tanzania, 20 times laboratory washed and unwashed nets.
Study ITNs
Four different products of ITNs will be evaluated: Olyset® Plus, Veeralin®, Tsara® Boost and MAGNet® (Table 3). All test items have been prequalified by the WHO [39]. To ensure households and field workers are blinded to intervention allocation during the study, all ITNs will be rectangular in shape, white in colour, and of standard dimensions (190 cm × 180 cm × 150 cm) and will be labelled with a water-resistant six digits numeric self-laminating code attached to one of the six hanging loops. This code will uniquely identify each ITN and contain numbers representing the country, product, and individual net. Each product of ITN will be assessed at baseline in each country to ensure that they meet WHO bio-efficacy criteria before distribution (Table 4) using well-characterized laboratory-reared Anopheles mosquitoes [40] in the WHO cone bioassay or WHO tunnel tests if ITN do not meet the WHO cone bioassay criteria [8]. Insecticide content at baseline will be measured using appropriate Collaborative International Pesticides Analytical Council (CIPAC) methods, at an accredited laboratory.
Community sensitization
Community sensitization will be conducted to inform community leaders and community members about the study objectives, study rationale and to request their co-operation during study implementation. All attendees of the community sensitization meetings will be encouraged to ask questions from the study investigators to enable the community to understand the study objectives and the risks and benefits of study participation.
Baseline surveys and distribution of ITNs
Upon obtaining written informed consent from the head of household or adult resident, field workers that have been trained on the study protocol and procedures will conduct the baseline questionnaire as outlined in Lorenz et al. [41] and will distribute the nets required for each household with one net distributed per sleeping space. Non-study nets found in the households will be withdrawn from households to ensure only study ITNs will be present and used in the households. In Tanzania, the withdrawn nets will be stored in bags labelled with the name of the household head and the study village to be returned to their respective households after the study, while the withdrawn nets will be discarded in Côte d’Ivoire and India following specific country procedure. Handling of withdrawn nets is different between countries because of differences in ethical considerations and regulations between countries.
The questionnaire forms will be written in local languages; Kiswahili for Tanzania, French for Côte d’Ivoire and Telugu for India, and will be pre-loaded in Open Data Kit (ODK) Collect software installed on a hand-held Samsung Tab A tablet computer. Data collected during the baseline surveys will be used as the household roster (names, ages, sex, education, occupation and the relationship to the household head), household wealth indicators [42], house characteristics, number of sleeping areas, ownership of mosquito nets, use of mosquito nets, net attitude indicators [43] and household coordinates in the global positioning system (GPS).
Randomization to study arms
ITNs distribution
After the baseline survey questionnaire has been completed, on the same day one out of the four products of ITNs will be assigned to the household based on block randomization using a lottery method. All net types are packaged in identical cloth bags. Each field interviewer is given a large bag containing all four products that they select at random and once the net is selected, additional nets of the same product (starting with the same digit code) will be provided to cover all sleeping areas in the household.
Generating master lists for follow-up: attrition, fabric integrity and bio-efficacy
The unique identifiers (UID) consist of six numbers generated by the study statistician—the first number identifies the country, the second identifies the treatment arm and the remaining four numbers identify nets distributed per arm. At baseline, the UID of each ITN given to the household will be recorded in the baseline questionnaire and in the ITNs master list with each unique net identification code linked to the household identifying codes and GPS coordinates. Two lists will be generated using a random number generator from the household masterlist: (1) attrition and fabric integrity monitoring masterlist (A-list) and (2) destructive sampling for bio-efficacy and chemical retention monitoring masterlist (B-list). These are separate because bio-efficacy and chemical retention monitoring will require ITNs to be removed from the cohort and replaced with new nets. For attrition and fabric integrity monitoring, the same ITNs will be followed up to the end of the study period unless the household is lost to follow-up or withdraws consent.
Follow-up surveys
Study ITNs per household visited during attrition monitoring at 6, 12, 24 and 36 months will be inspected for fabric integrity. All study ITNs listed in the master list provided will be inspected while non-study nets found in the household will only be recorded. In the households selected for bio-efficacy testing, the first ITN listed will be withdrawn, and if that ITN is not present in the household, the second ITN on the list provided will be sampled. If no study ITNs are present in the household, another household with the same net product will be visited as a replacement.
Adverse effects and ITN usage
One month after distribution, 250 households for each ITNs product will be selected randomly from the household master list for the assessment of ITN usage and adverse events. The head of the selected households will be interviewed on perceived adverse effects from all members using the adverse effect questionnaire adapted from the WHO guideline [8].
Monitoring attrition
Attrition (the proportion of ITNs no longer found in their respective households) will be assessed at 6, 12, 24, and at 36 months after distribution. After 6 months, 2712 individual households (678 per product per location) will be selected randomly from the A-list prepared for each country separately. The same households will be followed up longitudinally at 12, 24 and 36 months. The presence or absence of all the ITNs that were distributed will be recorded and if absent, the reasons for absence in the household will be provided by the head of the household. ITNs missing due to wear and tear will be considered as lost due to attrition, and those missing because they were stolen, sold or given away to others, will be considered as lost to follow-up.
Monitoring fabric integrity
Each ITN found during the attrition monitoring will be draped over a collapsible net frame [41] and the number of holes of four different sizes categories [8] will be counted by their locations on the net, recorded in a hole tally sheet and entered in the follow-up questionnaire. The locations of the net are obtained by dividing the side panels into four equal zones from top to the bottom and a roof as a fifth location of the net [41]. The four-size categories of the holes are 0.5–2 cm, 2–10 cm, 10–25 cm, and above 25 cm for sizes 1, 2, 3 and 4, respectively approximated using a thumb, fist, head and larger than a head [44]. The overall physical condition of the net will be obtained by weighting the number of holes of each size by 1, 23, 196 and 576 for the four-size categories of holes (based on the average assumed surface area for each size category) to obtain a proportionate hole index (pHI) following WHO guidelines [8]. The pHI value obtained will be used to classify the net as serviceable if the pHI value is less or equal to 642 and too torn if the pHI value obtained is greater than 642 [44].
Additionally, three new nets per product from the same production batch used in this study will be assessed for the Resistance to Damage (RD) score [31] at CITEVE (Centro Tecnológico das Indústrias Têxtil e do Vestuário de Portugal). The RD score is a quantitative metric based on four standardized textile tests taking into account different mechanisms of damage to ITNs including both human factors for damaging the net and laboratory testing data [45, 46]. The average value of standardized laboratory textile test data for snag strength, bursting strength, abrasion and hole enlargement [47] is divided by four so that each laboratory parameter contribute equally to the overall RD value which is expressed in percentage with a value ranging between 0 and 100 [31].
Monitoring bio-efficacy
Mosquito species
Pyrethroid susceptible and pyrethroid-resistant strains will be used for bio-efficacy testing in each country. Mosquitoes are maintained following MR4 guidance [48] by feeding larvae on Tetramin fish food and adults on blood meal between 3 and 6 days after emergence and 10% glucose solution ad libitum. All mosquitoes are maintained inside cages in different rooms to prevent cross-contamination. Temperature and humidity within insectaries are between 27 °C ± 5 °C and 70% ± 20% relative humidity, respectively.
In Tanzania, metabolic pyrethroid-resistant Anopheles arabiensis Kingani strain and a full pyrethroid susceptible Anopheles gambiae sensu stricto (s.s.) Ifakara strain will be used. In Côte d’Ivoire, metabolically resistant An. gambiae s.s. Tiassalé strain and a fully pyrethroid susceptible An. gambiae s.s. Kisumu strain will be used. In India, a pyrethroid-resistant Anopheles culicifacies strain and a fully pyrethroid susceptible Anopheles stephensi strain will be used. The bio-efficacy outcomes will be reported separately for each strain in each country. The susceptibility status of the susceptible and resistant strains will be confirmed at least twice per year in each country following standard procedures [49, 50] during the study period. If the susceptibility status changes during the study, a new colony will be established by either selecting resistant mosquitoes from a mixed colony or re-establishing a new colony from the original source.
Mosquito net sampling and preparation
Cone bioassays will be conducted at baseline using 30 nets per product from B-list to ensure nets are of sufficient quality before distribution. At baseline, five net pieces (25 cm × 25 cm) will be cut from position 1 to position 5 of the net as per WHO guidelines [8] and all net pieces will be tested in cone bioassay [38]. For each sampled net from the field, four net pieces (25 cm × 25 cm) will be cut from position 2 to position 5 of the net and tested in cone bioassay. A piece from position 1 (the bottom of the net) will not be cut, as it may be exposed to excessive abrasion due to tucking under the bed [38].
Bio-efficacy testing will be conducted on a total of 30 nets (one from each of 30 households) sampled at random from the B-list for each product at 6, 12, 18, 24, and 30 months post-distribution survey. Only at 36 months, 50 nets per product will be sampled for bio-efficacy and chemical testing estimates. However, 60 (30 selected for bio-efficacy testing and a further 30) nets per product will be sampled at 12 and 24 months for additional testing in the IACT [51]. If the listed ITN is not present, another net (of the same product) within the same household will be selected. After a net is withdrawn for bio-efficacy testing, the household will not be eligible for a next round of bio-efficacy sample collection from the B-master list. The withdrawn net samples will be replaced. The head of the household will be offered any of the ITN products in the trial and allowed to select their replacement based on preference, and this choice will be recorded.
WHO cone test procedure
The WHO cone bioassays will be conducted separately at the (1) Vector Control Product Testing Unit (VCPTU) in Bagamoyo, Tanzania, (2) Swiss Centre for Scientific Research (CSRS) in Tiassalé district, Côte d’Ivoire, and 3) ICMR-National Institute of Malaria Research (NIMR) field unit in Bengaluru, Karnataka state, India. Four cones will be attached to each net piece and 5 mosquitoes exposed per cone, 100 mosquitoes per strain at baseline and 80 mosquitoes per strain at each post-distribution survey to each ITN [8]. Non-blood-fed, 2–5 days old, female mosquitoes each of the susceptible and resistant strains for the PBO nets and susceptible strain for the pyrethroid only net will be exposed to the ITN for 3 min [8]. After the exposure, mosquitoes will be removed and kept in holding cups provided with 10% glucose or sucrose solution. Mosquito knockdown will be recorded after 60 min (KD60) and mortality after 24-h (M24). ITNs that do not meet WHO bio-efficacy thresholds of KD60 ≥ 95% or M24 ≥ 80% with the susceptible mosquito strains will be tested in the WHO tunnel test [8].
WHO tunnel test
Each site will perform tunnel tests independently on each ITN that did not meet WHO efficacy criteria (i.e., ≥ 95% KD60 or ≥ 80% M24) in the cone test against the susceptible strains of mosquitoes, only one out of four net pieces will be selected for the tunnel test. This is the piece that gave mortality closest to the average mortality in the cone test of the four pieces (i.e., average for that net). The selected piece will be fixed in the tunnel for testing. One tunnel with untreated netting will be used as a negative control. Fifty non-blood fed female susceptible Anopheles mosquitoes aged 5–8 days sugar starved for 6–8 h will be released in a tunnel (square section 25 cm × 25 cm) made of glass, 60 cm in length [8]. For pyrethroid only nets, susceptible mosquitoes will be used, while for PBO nets, both susceptible and resistant strains will be used.
At one-third of the length, the netting sample is fixed, with the surface of netting available to mosquitoes of 400 cm2 (20 cm × 20 cm) with nine holes each 1 cm in diameter: one hole is located at the centre of the square; the other eight are equidistant and located at 5 cm from the border. In the shorter section of the tunnel, a small rabbit, which will be restrained and unable to move but available to mosquitoes, will be placed as bait. To minimize discomfort to the rabbits, all applicable experimental animal welfare procedures will be adhered to following specific country regulations. In the cage at the end of the longer section of the tunnel, 50 female mosquitoes [52] will be introduced at 18:00. The following morning from 09:00, the mosquitoes will be removed using a mouth aspirator and counted separately from each section of the tunnel and mortality and blood-feeding rates will be recorded. During the test, ambient conditions will be maintained at 27 °C ± 5 °C and 60–100% relative humidity. Acceptable feeding success and mortality in controls will be ≥ 50% and ≤ 10%, respectively.
Chemical residual analysis
The residual chemical concentration will be estimated for all products at baseline and after every 12 months using nets allocated in B-list. At baseline, five net pieces (25 cm × 25 cm) will be cut from 30 different mosquito nets (position 1 to 5 of each net) per product and at 12, 24 and 36 months, four net pieces will be cut from position 2 to 5 from the nets sampled for bio-efficacy testing of each product [8]. These pieces will be rolled up and placed in a labelled aluminium foil and stored at 4 °C until they are shipped for chemical analysis following CIPAC methods as described in the WHO specifications for each ITN to check if the chemical concentrations are within the tolerance limit as per the manufacturer’s specifications [Tsara Boost: 333/LN/(M)/3, 33/LN/(M)/3 [53]; Veeralin: 454/LN/(M)/3, 33/LN/(M)/3 [54]; Olyset Plus 331/LN/(M)/3, 33/LN/(M)/3 [55]; MAGNet 454/LN/(M)/3] and within range after operational use [56].
Mortality and blood-feeding for PBO ITNs in Ifakara Ambient Chamber Test
Whole ITNs returned from the field at 12 and 24 months for bio-efficacy testing in Tanzania and unwashed and 20 times laboratory washed nets of each product will be evaluated in 18 chambers of the Ifakara Ambient Chamber Test (IACT) in Tanzania [41, 57]. Overall, the 18-arm study will include 4 arms of field-aged nets, 4 arms of unwashed and 4 arms of twenty-times laboratory-washed nets of the brands: Olyset® Plus, Veeralin®, Tsara® Boost and MAGNet® and two untreated nets (negative controls) and two additional positive control nets: PermaNet® 3.0 and standard Olyset® nets both unwashed and twenty-times washed. Unwashed, twenty-times washed nets, positive and negative control nets will be deliberately holed with six holes (4 × 4 cm) as per guidance [8]. Eighteen volunteers will rotate sequentially among the 18 chambers of the IACT nightly based on a prepared roster to allow equal sleeping under nets per product among volunteers. Each chamber of the IACT will have a bed net frame over which the ITN will be draped and a foam mattress upon which one volunteer will sleep. The study will run for seventy-two experimental nights per time point (12 months and 24 months). After 54 experimental nights, each unwashed, 20 times washed nets and positive controls will be replaced with field aged nets for the last 18 nights from the IACT to allow four field aged nets of each product tested for each of the remaining 18 experimental nights, leading to 126 replicates per field-aged product in total. This is done to allow a sufficient sample size for the non-inferiority comparison of field aged nets against Olyset® Plus field-aged net.
IACT allows the testing of multiple strains of mosquitoes at the same time. To understand the bio-efficacy of ITNs on the susceptible and resistant mosquitoes, fifteen nulliparous, sugar starved for 6–8 h, female susceptible An. gambiae s.s. Ifakara strain and a resistant An. arabiensis Kingani strain mosquitoes will be released at 21.00 h in each of the compartments occupied by one volunteer sleeping under one of the tests ITNs or a negative control net. These species are morphologically identical; thus, one strain will be marked with a fluorescent dye that does not affect their survival or behaviour [58]. At 06.00 h, each volunteer will collect mosquitoes inside the compartment using a mouth aspirator in paper cups. Recaptured mosquitoes will be sorted by species and recorded as fed alive, fed dead, unfed alive and unfed dead, then will be provided with 10% sucrose solution, and held under standard laboratory conditions to assess delayed mortality at 24-h.
Sample size
The sample size for the ITN surveys
The sample size was calculated based on the primary outcome measure of net attrition together with additional nets for the bio-efficacy components. Assuming an average of 2.7 nets per household and a coefficient of variation of 0.25, the formula on page 110 of Hayes and Moulton [59] gives a sample size of 678 households per arm to detect the difference in attrition between two products assuming 3-year attrition rates of 47.5% and 52.5% with 80% power. An additional 260 households for bio-efficacy, chemical analysis and IACT have been added to 678 making a total number of 938 households. Since the median lifespan of ITNs products from durability studies in Tanzania is less than 3 years [29, 60, 61], a 41% loss to follow-up of households is assumed making addition of 657 households to the 938 households. The final total number of households that will be recruited at baseline per product per country is 1595. With an average of 2.7 nets per household [62], the total number of ITNs required per product per country will be 4521 including 5% for error due to poor quality or nets with open seam prior to distribution, Table 5.
The sample size for the entomological outcomes in the IACT
Simulation was used to determine the sample size for the bio-efficacy testing of ITNs in the IACT. Each PBO net product will be assessed for non-inferiority against Olyset® Plus on mortality at 24 h. Each product will have sixty nets some tested more than one times for 72 nights with 15 mosquitoes per strain per chamber per night. From a previous study (author, pers.commun.), the variation between individual field-aged nets per product was assumed at a standard deviation of 0.1 and between chamber-nights at 0.15, both on the log-odds scale. Assuming mortality of 70% for the PBO nets and Olyset Plus, 1000 simulation trials were conducted to assess non-inferiority for each trial and the power estimated by the proportion of trials that showed non-inferiority where the product was indeed non-inferior, was over 80%.
To assess the equivalence of field nets to 20-times washed nets on mortality, each product consisting of sixty replicates of field-aged nets as well as four replicates of twenty times laboratory washed and unwashed nets will be tested 54 times (three rounds with 18 replicates each). Assuming 70% mortality for each product of field and washed nets, and assuming 0.1 standard deviation between individual replicate nets per product and a chamber-night standard deviation of 0.15 on the log-odds scale, using 15 mosquitoes per strain per chamber per night, the study is powered at 80%.
Samples size for chemical analysis
The baseline quality assurance of the ITNs used in the durability trial is conducted by chemical analysis and biological efficacy testing. A problem with the batch is detected if one or more of the sampled nets fails to meet the acceptance criteria. Since the same batch of nets are tested in three study countries, 10 ITNs are evaluated per site to give a total sample size of 30 ITNs tested at baseline as per WHO guidelines [8]. During each of the subsequent survey time point, 30 net samples will be sampled for chemical analysis as per WHO guidelines [8].
Data management
Field data will be collected using electronic data capture format in Open Data Kit (ODK) Collect. The data will be sent to the secure server located at Ifakara Health Institute in Tanzania. Cleaned data sets will be returned to CSRS and NIMR India. The information from the baseline questionnaire will be linked to the follow-up surveys using the unique household and country identification number as well as unique net identification numbers. Data collected on paper forms in the laboratory and in the IACT will be entered in excel using double-entry to facilitate cross-referencing and validation. The cleaned excel file of the data will be uploaded to the IHI server. Access to the data on the server will be limited to the data manager.
Data analysis
All data analyses will be conducted using STATA software (Stata Corp LLC, College Station TX, USA). The durability outcomes; attrition, fabric integrity, bio-efficacy and insecticide content will be estimated (Table 4). These outcomes will be estimated for each net product by survey period and country. A pooled estimate from three countries will also be presented, if appropriate.
The non-inferiority of Veeralin® and Tsara® Boost nets to Olyset® Plus will be carried out using logistic regression for the binary outcome of mosquito mortality with ITN product, compartment, volunteer and day as covariates. A random effect for chamber-night will be included. The 95% confidence interval for the estimated odds ratio for the effect of Tsara Boost® and Veeralin® compared to Olyset® Plus will be presented. Non-inferiority is shown if the confidence interval excludes an unacceptably worse performance. The bound of the margin of non-inferiority for mortality will be set at 0.7. If the lower bound of the confidence interval for the effect of the candidate net compared to Olyset® Plus is greater than 0.7, then we will conclude that the net product is non-inferior [36].
The utility of washed nets as a proxy for field nets will be assessed on the mortality and blood-feeding inhibition endpoints using logistic regression. The estimated odds ratios for the effect of washed nets compared to the negative control, and field-aged nets compared to the negative control will be presented. The ratio of these odds ratios will be estimated along with the 95% confidence interval using interaction terms in the logistic regression model.
Discussion
This study is the first large-scale prospective household randomized controlled trial of PBO ITNs in three different countries simultaneously. It will allow a pooled estimate of ITN durability from three countries, enabling precise estimates of product performance over time.
In a cluster randomized trial that assessed the effectiveness of Olyset® plus, there was a high washout of PBO concentration within 21 months of use under operational conditions [19] and more than 97% washout after 3 years [63]. Similarly, Olyset® Plus lost more than 87% of PBO concentration after 3 years in Kenya [64]. In Uganda, more than 55% of PBO concentration in Olyset® Plus was washed out within 25 month [65]. These publications highlight the importance of describing the estimated period of performance of PBO ITNs in different contexts. This study will report the persistence of PBO and pyrethroid concentrations in Olyset® Plus, Veeralin®, and Tsara Boost® beside pyrethroid concentration in MAGNet® for the period of 3 years of use in the field in three locations. It will also monitor the added value of PBO against resistant strains of mosquitoes over time as recommended for durability monitoring of products designed to kill pyrethroid-resistant malaria vectors [66].
PBO ITNs including Veeralin® and Tsara® Boost are currently recommended as pyrethroid only ITNs [67, 68] and are required to demonstrate non-inferiority using 24-h mortality and feeding inhibition endpoints compared to the first-in-class Olyset® Plus [69]. This study will measure the non-inferiority of Veeralin® and Tsara® Boost compared to the Olyset® Plus, collected from the field operational settings i.e. the non-inferiority as part of product durability evaluation. Currently, twenty times washed, deliberately holed ITN is used as a reasonable proxy for a field aged net. This study will explore whether this assumption is acceptable, or if it should be revised.
The results of this trial will provide robust and rigorous evidence of the likely replenishment interval, quality and additional bio-efficacy of PBO ITNs compared to pyrethroid only ITNs up to 3 years after the distribution.
Current study status
This study has finished baseline household data collection and the distribution of ITNs in the study areas in all countries. Currently, follow-up households' data collection and ITNs durability monitoring is ongoing in all study countries. The last follow-up household data collection and ITNs monitoring is expected to end in October 2023.
Availability of data and materials
Not applicable.
Abbreviations
- CIPAC:
-
Collaborative International Pesticides Analytical Council
- GPS:
-
Global positioning system
- IACT:
-
Ifakara Ambient Chamber Test
- ICF:
-
Informed consent form
- IRB:
-
Institutional Review Board
- ITNs:
-
Insecticide-treated nets
- KD60 :
-
Knockdown after 60 min
- LLINs:
-
Long-lasting insecticidal nets
- M24 :
-
Mortality after 24 h
- ODK:
-
Open Data Kit
- PBO:
-
Piperonyl-butoxide
- pHI:
-
Proportionate hole index
- UID:
-
Unique identifiers
- WHO:
-
World Health Organization
References
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Karl S, Katusele M, Freeman TW, Moore SJ. Quality control of long-lasting insecticidal nets: are we neglecting it? Trends Parasitol. 2021;37:610–21.
Bradley AK, Greenwood BM, Greenwood AM, Marsh K, Byass P, Tulloch S, et al. Bed-nets (mosquito-nets) and morbidity from malaria. Lancet. 1986;328:204–7.
MacCormack CP, Snow RW. What do people think of bednets? Parasitol Today. 1985;1:147–8.
Zaim M, Aitio A, Nakashima N. Safety of pyrethroid-treated mosquito nets. Med Vet Entomol. 2000;14:1–5.
WHO. Safety of pyrethroids for public health use. Geneva: World Health Organization; 2005.
Le Menach A, Takala S, McKenzie FE, Perisse A, Harris A, Flahault A, et al. An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets. Malar J. 2007;6:10.
WHOPES. Guidelines for laboratory and field testing of long-lasting insecticidal nets. Geneva: World Health Organization; 2013.
Carnevale P, Bitsindou P, Diomande L, Robert V. Insecticide impregnation can restore the efficiency of torn bed nets and reduce man-vector contact in malaria endemic areas. Trans R Soc Trop Med Hyg. 1992;86:362–4.
Randriamaherijaona S, Briet OJ, Boyer S, Bouraima A, N’Guessan R, Rogier C, et al. Do holes in long-lasting insecticidal nets compromise their efficacy against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus? Results from a release-recapture study in experimental huts. Malar J. 2015;14:332.
Hawley WA, Phillips-Howard PA, Ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, et al. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in Western Kenya. Am J Trop Med Hyg. 2003;68:121–7.
WHO. Guidelines for malaria. Geneva: World Health Organization; 2022.
WHO. World malaria report. Geneva: World Health Organization; 2018.
WHO. Guideline for malaria vector control. Geneva: World Health Organization; 2019.
Dadzie SK, Chabi J, Asafu-Adjaye A, Owusu-Akrofi O, Baffoe-Wilmot A, Malm K, et al. Evaluation of piperonyl butoxide in enhancing the efficacy of pyrethroid insecticides against resistant Anopheles gambiae s.l. in Ghana. Malar J. 2017;16:342.
Gleave K, Lissenden N, Richardson M, Choi L, Ranson H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database Syst Rev. 2018;11:CD012776.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84.
Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653–65.
Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, Mwalimu CD, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet. 2018;391:1577–88.
Gleave K, Lissenden N, Chaplin M, Choi L, Ranson H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database Syst Rev. 2021;5:CD012776.
WHO. Determination of fabric strength of long-lasting insecticidal nets. Geneva: World Health Organization; 2015.
Koenker H, Kilian A, ZegersdeBeyl C, Onyefunafoa EO, Selby RA, Abeku T, et al. What happens to lost nets: a multi-country analysis of reasons for LLIN attrition using 14 household surveys in four countries. Malar J. 2014;13:464.
Hakizimana E, Cyubahiro B, Rukundo A, Kabayiza A, Mutabazi A, Beach R, et al. Monitoring long-lasting insecticidal net (LLIN) durability to validate net serviceable life assumptions, in Rwanda. Malar J. 2014;13:344.
Tami A, Mbati J, Nathan R, Mponda H, Lengeler C, Schellenberg JR. Use and misuse of a discount voucher scheme as a subsidy for insecticide-treated nets for malaria control in southern Tanzania. Health Policy Plan. 2006;21:1–9.
Birhanu A, Asale A, Yewhalaw D. Bio-efficacy and physical integrity of piperonylbutoxide coated combination net (PermaNet 3.0) against pyrethroid resistant population of Anopheles gambiae s.l. and Culex quinquefasciatus mosquitoes in Ethiopia. Malar J. 2019;18:224.
Kilian A, Koenker H, Obi E, Selby RA, Fotheringham M, Lynch M. Field durability of the same type of long-lasting insecticidal net varies between regions in Nigeria due to differences in household behaviour and living conditions. Malar J. 2015;14:123.
Allan R, O’Reilly L, Gilbos V, Kilian A. An observational study of material durability of three World Health Organization-recommended long-lasting insecticidal nets in eastern Chad. Am J Trop Med Hyg. 2012;87:407–11.
Kilian A, Byamukama W, Pigeon O, Gimnig J, Atieli F, Koekemoer L, et al. Evidence for a useful life of more than three years for a polyester-based long-lasting insecticidal mosquito net in Western Uganda. Malar J. 2011;10:299.
Haji KA, Khatib BO, Obi E, Dimoso K, Koenker H, Babalola S, et al. Monitoring the durability of the long-lasting insecticidal nets Olyset® and PermaNet® 2.0 in similar use environments in Zanzibar. Malar J. 2020;19:187.
Briet O, Koenker H, Norris L, Wiegand R, Vanden Eng J, Thackeray A, et al. Attrition, physical integrity and insecticidal activity of long-lasting insecticidal nets in sub-Saharan Africa and modelling of their impact on vectorial capacity. Malar J. 2020;19:310.
Wheldrake A, Guillemois E, Chetty V, Kilian A, Russell SJ. Development of a single resistance to damage metric for mosquito nets related to physical integrity in the field. Malar J. 2021;20:46.
Mboma ZM, Festo C, Lorenz LM, Massue DJ, Kisinza WN, Bradley J, et al. The consequences of declining population access to insecticide-treated nets (ITNs) on net use patterns and physical degradation of nets after 22 months of ownership. Malar J. 2021;20:171.
Tsuang A, Lines J, Hanson K. Which family members use the best nets? An analysis of the condition of mosquito nets and their distribution within households in Tanzania. Malar J. 2010;9:211.
Azondekon R, Gnanguenon V, Oke-Agbo F, Houevoessa S, Green M, Akogbeto M. A tracking tool for long-lasting insecticidal (mosquito) net intervention following a 2011 national distribution in Benin. Parasites Vectors. 2014;7:6.
WHO. Determining non-inferiority of insecticide-treated nets and indoor residual spray products within an established product class: evidence review group meeting report, 5–6 July 2018. Geneva: World Health Organization; 2018.
WHO. Data requirements and protocol for determining non-inferiority of insecticide-treated net and indoor residual spraying products within an established WHO policy class. Geneva: World Health Organization; 2018.
Koenker H, Taylor C, Burgert-Brucker CR, Thwing J, Fish T, Kilian A. Quantifying seasonal variation in insecticide-treated net use among those with access. Am J Trop Med Hyg. 2019;101:371–82.
WHO. Guidelines for monitoring the durability of long-lasting insecticidal mosquito nets under operational conditions. Geneva: World Health Organization; 2011.
WHO. List of WHO prequalified vector control product. Geneva: World Health Organization; 2020. https://extranet.who.int/pqweb/vector-control-products/prequalified-product-list?field_product_type_tid=100&field_pqt_vc_ref_number_value=&title=&field_applicant_tid=&field_active_ingredient_synergis_tid=. Accessed 28 Dec 2022.
Lees RS, Armistead JS, Azizi S, Constant E, Fornadel C, Gimnig JE, et al. Strain characterisation for measuring bioefficacy of ITNs treated with two active ingredients (Dual-AI ITNs): developing a robust protocol by building consensus. Insects. 2022;13:434.
Lorenz LM, Overgaard HJ, Massue DJ, Mageni ZD, Bradley J, Moore JD, et al. Investigating mosquito net durability for malaria control in Tanzania—attrition, bioefficacy, chemistry, degradation and insecticide resistance (ABCDR): study protocol. BMC Public Health. 2014;14:1266.
Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–68.
Kilian A, Obi E, Mansiangi P, Abílio AP, Haji KA, Blaufuss S, et al. Variation of physical durability between LLIN products and net use environments: summary of findings from four African countries. Malar J. 2021;20:26.
WHO. Estimating functional survival of long-lasting insecticidal nets from field data. Geneva: World Health Organization; 2013.
Kilian A, Obi E, Mansiangi P, Abilio AP, Haji KA, Guillemois E, et al. Correlation of textile ‘resistance to damage’ scores with actual physical survival of long-lasting insecticidal nets in the field. Malar J. 2021;20:29.
Wheldrake A, Guillemois E, Arouni H, Chetty V, Russell SJ. The causes of holes and loss of physical integrity in long-lasting insecticidal nets. Malar J. 2021;20:45.
Wheldrake A, Guillemois E, Arouni H, Chetty V, Russell SJ. Textile testing to assess the resistance to damage of long-lasting insecticidal nets for malaria control and prevention. Malar J. 2021;20:47.
MR4. Methods in Anopheles research manual. https://www.beiresources.org/Publications/MethodsinAnophelesResearch.aspx. 2009.
WHO. Standard operating procedure for determining the ability of PBO to restore susceptibility of adult mosquitoes to pyrethroid insecticides in WHO tube tests. PBO-insecticide synergist bioassay/01/14 January 2022 edition. Geneva: World Health Organization; 2022. https://apps.who.int/iris/handle/10665/352314.
WHO. Standard operating procedure for testing insecticide susceptibility of adult mosquitoes in WHO tube tests. WHO Tube test/01/14 January 2022 edition. Geneva: World Health Organization; 2022. https://apps.who.int/iris/handle/10665/352316.
Kibondo UA, Odufuwa OG, Ngonyani SH, Mpelepele AB, Matanilla I, Ngonyani H, et al. Influence of testing modality on bioefficacy for the evaluation of Interceptor(®) G2 mosquito nets to combat malaria mosquitoes in Tanzania. Parasites Vectors. 2022;15:124.
Kamande DS, Odufuwa OG, Mbuba E, Hofer L, Moore SJ. Modified World Health Organization (WHO) Tunnel test for higher throughput evaluation of insecticide-treated nets (ITNs) considering the effect of alternative hosts, exposure time, and mosquito density. Insects. 2022;13:562.
WHO. Specifications and Evaluations for publichHealth pesticides: Deltamethrin + Piperonyl butoxide long-lasting (incorporated into Filaments) insecticidal net. Geneva: World Health Organization; 2019.
WHO. Specifications and evaluations for public health pesticides: Alpha-cypermethrin + piperonyl butoxide long-lasting (incorporated into filaments) insecticidal net. Geneva: World Health Organization; 2015.
WHO. Specifications and evaluations for public health pesticides: permethrin (40:60 cis: trans isomer ratio) + piperonyl butoxide long-lasting (incorporated into filaments) insecticidal net. Geneva: World Health Organization; 2013.
WHO. Specifications and evaluations for public health pesticides: alpha-cypermethrin long-lasting (incorporated into filaments) insecticidal net. Geneva: World Health Organization; 2020.
Massue DJ, Lorenz LM, Moore JD, Ntabaliba WS, Ackerman S, Mboma ZM, et al. Comparing the new Ifakara Ambient Chamber Test with WHO cone and tunnel tests for bioefficacy and non-inferiority testing of insecticide-treated nets. Malar J. 2019;18:153.
Saddler A, Kreppel KS, Chitnis N, Smith TA, Denz A, Moore JD, et al. The development and evaluation of a self-marking unit to estimate malaria vector survival and dispersal distance. Malar J. 2019;18:441.
Hayes RJ, Moulton LH. Cluster randomised trials. Chapman & Hall/CRC Interdisciplinary Statistics; 2009.
Lorenz LM, Bradley J, Yukich J, Massue DJ, Mageni Mboma Z, Pigeon O, et al. Comparative functional survival and equivalent annual cost of 3 long-lasting insecticidal net (LLIN) products in Tanzania: a randomised trial with 3-year follow up. PLoS Med. 2020;17: e1003248.
Bertozzi-Villa A, Bever CA, Koenker H, Weiss DJ, Vargas-Ruiz C, Nandi AK, et al. Maps and metrics of insecticide-treated net access, use, and nets-per-capita in Africa from 2000–2020. Nat Commun. 2021;12:3589.
Odufuwa OG, Ross A, Mlacha YP, Juma O, Mmbaga S, Msellemu D, et al. Household factors associated with access to insecticide-treated nets and house modification in Bagamoyo and Ulanga districts, Tanzania. Malar J. 2020;19:220.
Lukole E, Cook J, Mosha JF, Messenger LA, Rowland M, Kleinschmidt I, et al. Protective efficacy of holed and aging PBO-pyrethroid synergist-treated nets on malaria infection prevalence in north-western Tanzania. PLOS Glob Public Health. 2022;2: e0000453.
Gichuki PM, Kamau L, Njagi K, Karoki S, Muigai N, Matoke-Muhia D, et al. Bioefficacy and durability of Olyset® Plus, a permethrin and piperonyl butoxide-treated insecticidal net in a 3-year long trial in Kenya. Infect Dis Poverty. 2021;10:135.
Mechan F, Katureebe A, Tuhaise V, Mugote M, Oruni A, Onyige I, et al. LLIN evaluation in Uganda project (LLINEUP): the fabric integrity, chemical content and bioefficacy of long-lasting insecticidal nets treated with and without piperonyl butoxide across two years of operational use in Uganda. Curr Res Parasitol Vector Borne Dis. 2022;2: 100092.
Lissenden N, Armistead JS, Gleave K, Irish SR, Martin JL, Messenger LA, et al. Developing consensus standard operating procedures (SOPs) to evaluate new types of insecticide-treated nets. Insects. 2022;13:7.
WHOPES. Report of the twentieth WHOPES working group meeting: review of Interceptor G2LN, DawaPlus 3.0 LN, DawaPlus 4.0 LN, SumiLarv 2 MR, Chlorfenapyr 240 SC. Geneva: World Health Organization; 2017.
WHOPES. Report of the nineteenth WHOPES working group meeting: review of Veeralin LN, VectoMax GR, Bactivec SC. Geneva: World Health Organization; 2016.
WHO. Determining non-inferiority of insecticide-treated nets and indoor residual spray products within an established product class. Evidence Review Group meeting report. Geneva: World Health Organization; 2018.
WHOPES. Report of the fifteenth WHOPES working group meeting: review of Olyset plus, Interceptor LN, Malathion 440 EW, Vectobac GR. Geneva: World Health Organization; 2012.
WHOPES. Report of the fourteenth WHOPES working group meeting: review of Spinosad EC, Lifenet LN, Magnet LN, Royal Sentry LN, Yahe LN. Geneva: World Health Organization; 2011.
Acknowledgements
We thank the Moon Netting FZCO, United Arab Emirates and the V.K.A. Polymers Pvt Ltd, India for funding this study. We acknowledge them for supplying all ITNs required for the conduct of this study.
Funding
This study is funded collaboratively by the Moon Netting FZCO from the United Arab Emirates and the V.K.A. Polymers Pvt Ltd from India as part of data generation for WHO Prequalification. The funders are not involved in the design of the study, collection and interpretation of data and in writing of this manuscript. Both funders accepted this protocol to be published.
Author information
Authors and Affiliations
Contributions
SJM conceived the study idea, designed the study, secured the funding and reviewed the manuscript. EM designed the study and wrote the manuscript. AR and OGO performed sample size calculations and reviewed the manuscript. JM, ET, EC, VC, SU conceived the study idea and reviewed the manuscript. SM, AS, AE, KR, MR, and HK reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In Tanzania, this study is approved by the Institutional Review Board of Ifakara Health Institute (IHI/IRB/No: 030-2018) and the National Institute for Medical Research (NIMR/HQ/R.8a/Vol. IX/2884). In Côte d’Ivoire, it is approved by the National Committee for Ethics of Life Sciences and Health (036-20/MSHP/CNESVS-km). In India, it is approved by the National Institute of Malaria Research (ECR/NIMR/EC/2018/99), the Institutional Scientific Advisory Committee of NIMR and the Health Minister’s Screening Committee of Indian Council of Medical Research, and the Ministry of Health and Family welfare. If the tunnel test will be required, necessary animal welfare will be adhered following specific country’ guidelines for the use of rabbits in this study. The informed consent form (ICF) is administered to all heads of households or older residents (aged 18 years old and above). The ICF is written in country-specific languages which are either Kiswahili, French or Telegu for Tanzania, Côte d’Ivoire and India, respectively. The ICF explains study objectives and participants’ rights including information that their participation in the study is voluntary and they are free to leave the study at any time they wish. If the household head is unable to read, field workers read out the informed consent form and explain it in clear language to the household heads who cannot read it. A witness from the village is invited to witness during the ICF administration while the respondent signs with their thumb. Also, a written ICF in Kiswahili will be obtained from all participants in the IACT after explaining the purpose, risks, benefits of the experiment, and their rights to free diagnosis and treatment are discussed, and free withdrawal from the experiment any time.
Consent for publication
The permission to publish this manuscript was granted by the National Institute for Medical Research in Tanzania with permission number: Ref. No: NIMR/HQ/P.12 VOL XXXIII/81.
Competing interests
SJM, EM, OGO, JM, SM, ET, and EC carry out evaluations of vector control products for a number of manufacturers of vector control interventions including ITNs. VC, SU, AS, AE, KR, MR, HK and AR have no conflict of interest. The Veeralin® and MAGNet® ITNs are manufactured by the V.K.A. Polymers Pvt Ltd, India and Tsara® Boost is manufactured by the Moon Netting FZCO from the United Arab Emirates.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mbuba, E., Odufuwa, O.G., Moore, J. et al. Multi-country evaluation of the durability of pyrethroid plus piperonyl-butoxide insecticide-treated nets: study protocol. Malar J 22, 30 (2023). https://doi.org/10.1186/s12936-023-04465-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-023-04465-x