Abstract
Background
Rapid accurate diagnosis followed by effective treatment is very important for malaria control. Light microscopy remains the “golden standard” method for malaria diagnosis. Diagnostic test method must have sufficient level of accuracy for detecting malaria parasites. Therefore, this study aimed to investigate the diagnostic accuracy of rapid diagnostic tests (RDTs), microscopy, loop-mediated isothermal amplification (LAMP) and/or polymerase chain reaction (PCR) for the malaria diagnosis in Ethiopia.
Methods
Data bases such as PubMed, PubMed central, Science direct databases, Google scholar, and Scopus were searched from September to October, 2020 for studies assessing the diagnostic accuracy of RDTs, microscopy, LAMP and PCR methods for malaria diagnosis.
Results
A total of 29 studies published between 2001 and 2020 were analysed using review manager, Midas (Stata) and Meta-disc. The sensitivity and specificity of studies comparing RDT with microscopy varies from 79%–100% to 80%–100%, respectively. The sensitivity of LAMP (731 tests) was 100% and its specificity was varies from 85 to 99% when compared with microscopy and PCR. Considerable heterogeneity was observed between studies included in this meta-analysis. Meta-regression showed that blinding status and target antigens were the major sources of heterogeneity (P < 0.05). RDT had an excellent diagnostic accuracy (Area under the ROC Curve = 0.99) when compared with microscopy. Its specificity was quite good (93%–100%) except for one outlier (28%), but lower “sensitivity” was observed when PCR is a reference test. This indicates RDT had a good diagnostic accuracy (AUC = 0.83). Microscopy showed a very good diagnostic accuracy when compared with PCR.
Conclusions
The present study showed that microscopy and RDTs had high efficiency for diagnosing febrile malaria patients. The diagnostic accuracy of RDT was excellent when compared with microscopy. This indicates RDTs have acceptable sensitivities and specificities to be used in resource poor settings as an alternative for microscopy. In this study, LAMP showed an excellent sensitivities and specificities. Furthermore, the need of minimum equipment and relatively short time for obtaining results can made LAMP one of the best alternatives especially for accurate diagnosis of asymptomatic malaria.
Similar content being viewed by others
Background
Malaria is a major public health problem that still causes significant morbidity and mortality in developing countries [1, 2]. In 2019, an estimated 228 million cases and 405,000 deaths were recorded annually [3]. Most malaria cases were in the World Health Organization (WHO) African region (213 million or 93%) [3]. An estimated 90% of all global malaria mortality is also in sub-Saharan Africa, mainly in children aged under 5 years [4].
In Ethiopia, approximately 75% of the land mass is estimated to be malarious with about 52 million people being at risk of malaria. Plasmodium falciparum (70%) and Plasmodium vivax (30%) were the dominant Plasmodium species in Ethiopia as measured by microscopy [5]. Malaria transmission in Ethiopia is seasonal and unstable with the peak transmission season from September to December, following the main rainy season from June/July to September [5, 6].
Rapid accurate diagnosis followed by effective treatment is very important for malaria control. For decades, light microscopy remains the “golden standard” method for detecting and identifying malaria parasites although it requires training and experience [7]. The use of rapid diagnostic test (RDT) that detects malarial antigen is vital especially in resource-poor settings; it is simple to use and needs a little expertise, it doesn’t require electricity and results can be obtained in few minutes [7,8,9]. Rapid diagnostic tests are immunochromatographic lateral flow devices detect parasite antigens, such as histidine rich protein 2 (HRP2) that detect only P. falciparum and Plasmodium lactate dehydrogenase (pLDH) or aldolase that detect all Plasmodium species [7]. Histidine rich protein 2 (HRP-2) and pLDH are the most commonly detected malarial antigens [7, 9].
Molecular detection of DNA/RNA using polymerase chain reaction (PCR) is another alternative method for malaria diagnosis. The PCR techniques includes conventional PCR, nPCR, qPCR and multiplex PCR [2]. It is more sensitive than microscopy and RDTs for malaria detection, but requires well-trained staff, sophisticated laboratory equipments and a good quality assurance system [10, 11]. The loop-mediated isothermal amplification (LAMP) is a recently developed molecular technique to that is simpler and faster with an excellent diagnostic accuracy [2].
Selection of diagnostic tests should consider affordability, number of tests to be performed, equipment required, trained staff, besides diagnostic method accuracy. However, a test method must have sufficient level of accuracy although the test method is practical and affordable and perhaps the only possibility in a certain situation. Therefore, this study aimed to investigate the published studies of diagnostic accuracy of RDTs, microscopy, LAMP and PCR for the malaria diagnosis in Ethiopia.
Methods
Search strategy and eligible studies
The preferred reporting items for systematic review and meta-analysis’ (PRISMA) guidelines was used to report this systematic review and meta-analysis. Studies that assessed the diagnostic accuracy of RDTs, microscopy, LAMP and PCR for the detection of malaria parasites were eligible for this review. Case–control studies were excluded to overcome an over-estimation of the sensitivity and specificity of tests [12]. RDTs detecting any type of antigens in any format and from any manufacturer were eligible. Molecular diagnostic tests (PCR) in any format using Plasmodium DNA and/or RNA amplification were also eligible to be included.
Studies were searched on electronic databases such as PubMed, PubMed central, Science direct databases, Google scholar, Scopus, proceedings of health professional associations [such as Ethiopian medical laboratory association (EMLA), and Ethiopian public health laboratory associations’ (EPHLAs)]. The search was performed from September to October, 2020 using key words including “malaria”, “P. falciparum”, “P. vivax”, “P. ovale”, “P. malariae”,“diagnosis”, “RDT”, “HRP-2”, “pLDH” “PCR”, “microscopy” “diagnostic test accuracy”, “systematic review”, “meta-analysis” and “Ethiopia”. These search terms were also combined with each other using Boolean operators (AND, OR) to retrieve all relevant studies. Overlapped studies found in more than one databases were excluded. The reference lists of included studies were searched to retrieve additional studies.
Selection of studies
Studies were selected based on their title and abstract by two authors (DGF and YA) independently. Duplicated, studies published before the year 2000 and studies without reference test methods were removed. Studies considered relevant by at least one of the two authors were considered in further review. Disagreements about the eligibility of studies were solved by all authors after the detailed discussions on the pre-set inclusion and exclusion criteria. Studies found only in abstract form were included only after obtaining full article by communicating the authors whenever their contact is available.
Data extraction and management
Pre-designed data extraction form was developed on Microsoft excel 2010 by authors based on the objective of this review. Two authors (DGF and YA) collected the required data from the included studies independently. Information about studies (title, authors, journal), study design, descriptions of reference and index tests and data for 2 × 2 tables were collected. Methodological quality of the included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies QUADAS-2 tool [13] by two reviewers independently (DGF, NY). This tool assesses bias in patient selection, index test, reference standards, and flow/timing areas. A risk of bias summary and graph was generated in Review Manager 5 (RevMan version 5.4.1). When a study compares more than two tests, multiple 2 × 2 tables were extracted from a single study. In this case for each test comparisons, separate quality items were considered within a single study.
Statistical analysis
The estimates of sensitivity and specificity and their 95% confidence interval were plotted in forest plots using Review Manager 5.4.1 [14]. Review manager plots do not provide summary points and heterogeneity measures are not sufficiently provided. Therefore, Midas in Stata 14.0 was used to calculate the summary estimates of the sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio. Diagnostic accuracy tests are expected to show considerable heterogeneity. As a result, hierarchical summary receiver operating characteristic curve (HSROC) was used [15]. Midas was also used to assess heterogeneity of the included studies. When the heterogeneity was significant or I2 > 50%, Meta-disc 1.4.0 software was used to explore whether a threshold effect existed. Meta-regression was performed to investigate the potential sources of heterogeneity. The covariates investigated for possible source of heterogeneity were sample size, sampling method, blinding status, study population and target antigens.
Results
Search results and eligible studies
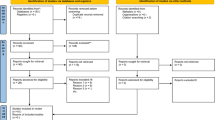
The literature search identified 109 records from different sources (Fig. 1). Seventy-nine studies were excluded from the meta-analysis due to different reasons. Some of the reasons for exclusions were duplicate records, studies that were not related to the objective of this meta-analysis, incomplete data for extracting 2 × 2 tables and studies that were not directly comparing the malaria diagnostic tests. In addition, one study was excluded due to high risk of bias after quality assessment. Finally, twenty-nine studies were included in this systematic review and meta-analysis (Table 1).
Studies characteristics
Twenty nine studies published between 2001 and 2020 were included in the analysis, leading to 29,419 individuals tested to evaluate the performance of RDTs, microscopy, LAMP and PCR. All of the included studies were cross-sectional studies. Microscopy was used as a reference method for RDT in 26 studies (22,450 tests) and for LAMP in 3 studies (731 tests). Loop-mediated isothermal amplification (LAMP) was a reference test for RDT (435 test) and microscopy (435 tests). The reference method for 6 RDT studies (2095 tests), 6 microscopy studies (2542 tests) and 3 LAMP studies (731 tests) were PCR. PCR was used as reference method for microscopy in 6 studies. There was one study that compared RDT and microscopy using LAMP as a reference method (870 tests) (Table 1).
Data quality assessment and heterogeneity of included studies
Risk of bias for patient selection was considered low in 85% of the diagnostic studies and high in 5%. There was no high risk of bias for patent selection in the applicability concern domain. The quality of verification with a flow and timing was good in more than 95% of the studies (Fig. 2).
Considerable heterogeneity was observed between studies included for comparing RDT with microscopy (Q = 285.586, I2 = 99%, P < 0.01), RDT with PCR (Q = 135.765, I2 = 99%, P < 0.01) and microscopy with PCR (Q = 36.925, I2 = 95%, P < 0.01). The source of heterogeneity was explored through the threshold effect analysis and meta-regression. The results suggested that there was no threshold effect between studies (P = 0.88), RDT with PCR (P = 0.46) and microscopy with PCR (P = 0.54). Meta-regression of these studies showed that blinding status and target antigens were the major sources of heterogeneity (P < 0.05) (Table 2).
Subgroup analyses of studies that compare RDT with microscopy showed that HRP-2 based RDTs demonstrated higher sensitivity (94%–100%) than HRP2/pLDH antigen based kits. While the specificity of HRP-2 was a little lower than HRP-2/pLDH based RDTs. There were no enough studies that used HRP-2 based RDT for subgroup analyses in the RDT with PCR comparison group.
RDT and LAMP compared with microscopy as reference
Twenty-six studies (22,450 tests) that compare RDT with microscopy were included in this systematic review and meta-analysis [6, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. The sensitivity and specificity of these studies varies from 79%–100% to 80%–100%, respectively. Fifteen (57.7%, 15/26) and eighteen (69.2%, 18/26) studies showed a sensitivity and specificity ≥ 95%, respectively. The sensitivity of 20 (20/26, 76.9%) studies and specificity of 21 (21/26, 80.8%) studies were greater than 90%. The three included LAMP studies (731 tests) had a sensitivity of 100% (Fig. 3).
The summary estimate of sensitivity and specificity of RDT using microscopy as a golden standard method were 95.05% (95% CI 92.95–96.55) and 96.47% (95% CI 94.69–97.67), respectively. It showed diagnostic odds ratio of 525.67 (95% CI 299.89–924.41), positive likelihood ratio (LR+) of 26.67 (95% CI 17.85–40.73) and negative likelihood ratio (LR–) of 0.05 (95% CI 0.03–0.07). The area under the curve (AUC) was 0.99 (95% CI 0.98–1.00) that indicated the test had an excellent diagnostic accuracy (Fig. 4).
RDT, microscopy and LAMP compared with PCR
Six studies (2 095 tests) that compare RDT with PCR were analysed [19, 32, 34, 36, 37]. These studies showed a quite good “specificity” (93%–100%) except for one outlier (28%), but a low “sensitivity” was observed, which varies from 37 to 88%. Two studies [32, 36] showing lower sensitivity (37% and 51%) were done on asymptomatic individuals and sub-clinical subjects to detect submicroscopic infections. Similarly, six studies (2542 tests) comparing microscopy with PCR were included in the analysis [19, 34, 36, 38,39,40]. Microscopy showed a quite good specificity that varies between 80 and 100%. LAMP was compared in three studies (731 tests) with PCR [38, 41, 42]. It showed excellent sensitivity (100%). Its specificity was also quite good varying between 86 and 99%. In the present study, there was a single study that compared RDT and microscopy using LAMP as a reference test (870 tests) [43]. RDT showed 67% sensitivity and 100% of specificity. The sensitivity and specificity of microscopy were 56% and 100%, respectively (Fig. 5).
Summary estimate of sensitivity and specificity of microscopy using PCR as a reference method was 75.20% (95% CI 57.12–87.35) and 97.12% (95% CI 91.47–99.07), respectively. Microscopy revealed that the diagnostic odds ratio was 102.57 (20.50–513.04). It also showed that the positive and negative likelihood ratio was 26.18 (95% CI 7.93–86.50) and 0.26 (95% CI 0.14–0.05), respectively. The summary receiver operating characteristic plot showed that the area under the curve (AUC) was 0.95 (95% CI 0.93–0.97). Microscopy had a very good diagnostic accuracy (Fig. 6).
Summary estimates of sensitivity and specificity were 66.18% (95% CI 50.29–79.10), and 95.36% (95% CI 74.78–99.30), respectively. The summary estimates for diagnostic odds ratio, the positive and negative likelihood ratio (95% CI) were 40.22 (9.23–175.28), 14.26 (2.67–76.21) and 0.35 (0.25–0.51), respectively. The AUC was 0.83 (95% CI 0.79–0.86) which showed a good accuracy of rapid diagnostic test (Fig. 7).
Discussion
There are several strategies used to achieve malaria control and elimination. These are accurate and prompt diagnosis, measuring the impact of intervention and effective treatment [2]. In malaria endemic areas, there are a significant proportion of asymptomatic malaria carriers due to the decrease in a patient’s parasitaemia. Microscopy is an appropriate method for detecting and identifying malaria parasites and has been the golden standard method for malaria diagnosis for decades. However, it requires training and experience of microscopist.
Rapid diagnostic tests that detect malarial antigens (HRP-2, pLDH, Aldolaes) are an alternative malaria diagnosis method especially in resource-poor settings; it needs a little expertise and doesn’t require electricity. Molecular detection of malaria using PCR is another advanced diagnostic method. Although this method requires well-trained staff and well-structured laboratory infrastructure, it is more sensitive than microscopy and RDTs. The aim of this study was to compare and analyse the performance of RDTs, microscopy, LAMP and PCR. An extensive search was performed for all the available studies regardless of study areas and clinical presentation of tested individuals in Ethiopia. In the present study, 26 studies were analysed to estimate the accuracy of RDTs for diagnosing malaria. With the exception of one study, all the included studies were done between 2009 and 2020.
Statistical heterogeneity was tested using the I2 statistic, which measures the variation across studies due to inter-study heterogeneity. Heterogeneity was expected to be related to the method of test reading, the patients levels of parasitaemia, and the comparators. There was significant heterogeneity among the studies included in this meta-analysis. Meta-regression showed variables such as blinding status and target antigens were the source of heterogeneity. Subgroup analyses of studies based on target antigen showed that HRP2 based RDTs demonstrated higher sensitivity than HRP2/pLDH antigen based kits. This was in agreement with reports of previous studies that showed HRP2-based RDTs are more sensitive compared with pLDH-based RDTs [44, 45].
In this study there was a study that target HRP-2 & pan-aldolase. It showed a sensitivity of 95% and specificity of 89%. This finding was lower than a study that reported a sensitivity of 97.4%, and a specificity of 100% that target P. vivax-specific aldolase [46].
In the present study, the summary estimate of sensitivity and specificity of RDTs using microscopy as a golden standard method were 95.05% (95% CI 92.95–96.55%) and 96.47% (95% CI 94.69–97.67%), respectively. In this study the sensitivity was lower than a systematic review and meta-analysis study conducted in India that reported a sensitivity of 97.0% (95% CI 95.0–98.0%). On the other hand, the specificity of this study was almost similar with a study conducted in India (specificity = 96.0% (95% CI 93.0–97.0%) [47]. In contrast to the summary estimate of sensitivity, the summary estimate of specificity of this study (96.47%) was almost similar with a report from a study that compared RDTs with microscopy and PCR among pregnant women (94%) [15].
The AUC of RDT using microscopy as a reference method was 0.99 (95% CI 0.98–1.00). This indicates that RDTs are diagnostic test methods with an excellent specificity and sensitivity. Therefore, RDTs can be used as an alternative malaria diagnosis method for microscopy especially in resource poor settings.
The PCR techniques used as a reference test for evaluating RDTs includes qPCR, nested PCR, qRT-PCR and Semi-nested Multiplex PCR. Rapid diagnostic tests had an AUC of 0.83 (95% CI 0.79–0.86). However, RDT showed lower sensitivity (37%) in a study conducted among mixed population of symptomatic and asymptomatic individuals. Similarly the sensitivity of RDT was 51% in a study conducted among clinical and sub-clinical patients. This might be due to the reason that among asymptomatic individuals and sub-clinical patients the parasitaemia level expected to be low and usually submicroscopic. However, the specificity of RDT in these study groups (asymptomatic individuals and sub-clinical patients) was 100% and 94%, respectively. This indicates RDT still has a good diagnostic accuracy for malaria parasite detection.
In this study, the performance of microscopy was also evaluated using PCR as a reference test. Microscopy showed a sensitivity that varies between 39% and 97% and a specificity between 80% and 100%. However, two of the studies conducted among asymptomatic individuals and clinical and sub-clinical patients were showed lower sensitivities. Overall, microscopy demonstrated a very good diagnostic accuracy for the malaria parasite detection (AUC = 0.95 (95% CI 0.93–0.97).
Rapid diagnostic test (RDT) has lower sensitivity (37%–88%) but had good specificity (93%–100%) with the exception of one outlier (28%) using PCR as a reference test methods. A decrease in sensitivity might be due to low parasitaemia of individuals that may influences the detection ability of RDTs.
Rapid diagnostic tests showed higher diagnostic odds ratio when compared with microscopy than PCR. It had 525.67 (95% CI 299.89–924.41) times higher odds of obtaining positive result in diseased individuals than in non‐diseased. On the other hand, it showed 40.22 (9.23–175.28) times higher odds of positive test in positive individuals than negative individuals. This can be explained by the fact that performance of RDT is much closer to microscopy. This was supported by the present study finding as it showed an excellent diagnostic accuracy (AUC = 0.99).
The present study showed that LAMP had an excellent sensitivity and quite good specificity when PCR is used as a reference test. This was in line with a systematic review and meta-analysis of diagnostic accuracy of LAMP methods which showed a sensitivity and specificity of > 95% in majority of the studies [15].
Conclusion
The effort of malaria elimination should target transmission in the community by accurate identification of asymptomatic infections. In the present study, microscopy and RDTs showed high efficiency for diagnosing febrile malaria patients. The diagnostic accuracy of RDT was excellent when compared with microscopy. This indicates RDTs have acceptable sensitivities and specificities to be used in resource poor settings as an alternative malaria diagnostic method for microscopy. In this study, although there was limited number of studies comparing LAMP with other diagnostic methods, LAMP showed excellent sensitivities and specificities. Furthermore, the need of minimum equipment and relatively short time for obtaining results can made LAMP one of the best alternatives especially for accurate diagnosis of asymptomatic malaria.
Availability of data and materials
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the manuscript.
References
Kendie FA, Nibret Semegn E, Ferede MW. Prevalence of malaria among adults in Ethiopia: a systematic review and meta-analysis. J Trop Med. 2021;2021:8863002.
Ocker R, Prompunjai Y, Chutipongvivate S, Karanis P. Malaria diagnosis by loop-mediated isothermal amplification (LAMP) in Thailand. Rev Inst Med Trop Sao Paulo. 2016;58:27.
WHO. World Malaria Report. 2019. Geneva, World Health Organization, 2019. https://www.who.int/publications/i/item/9789241565721. Accessed 4 Dec 2019.
WHO. World Malaria Report. Geneva, World Health Organization, 2014. https://www.who.int/malaria/publications/world_malaria_report_2014/en/. Accessed 9 Dec 2014.
EMIS. Ethiopian national malaria indicator survey, Ethiopian Public Health Institute 2016. https://www.ephi.gov.et/images/pictures/download2009/Ethiopia-2015-Malaria-Indicator-Survey-Summary-after-----implication-meeting_V6_9_02_2016.pdf.
Feleke DG, Tarko S, Hadush H. Performance comparison of CareStartTM HRP2/pLDH combo rapid malaria test with light microscopy in north-western Tigray, Ethiopia: a cross-sectional study. BMC Infect Dis. 2017;17:399.
WHO. New Perspectives: Malaria Diagnosis. Geneva, World Health Organization, 1999. https://www.who.int/tdr/publications/documents/malaria-diagnosis.pdf. Accessed 25–27 Oct 1999.
WHO. Malaria rapid diagnostic test performance. 2010–2011. Geneva, World Health Organization. https://www.who.int/tdr/publications/documents/rdt3.pdf?ua=1. Accessed Dec 2011.
Mouatcho JC, Goldring JD. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62:1491–505.
Zheng Z, Cheng Z. Advances in molecular diagnosis of malaria. Adv Clin Chem. 2017;80:155–92.
Berzosa P, de Lucio A, Romay-Barja M, Herrador Z, González V, García L, et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malar J. 2018;17:333.
Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, Van Der Meulen JH, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999;282:1061–6.
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25.
Review Manager (RevMan) (5.4.1). The Nordic Cochrane Centre, The Cochrane Collaboration; 2020. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman.
Kattenberg JH, Ochodo EA, Boer KR, Schallig HD, Mens PF, Leeflang MM. Systematic review and meta-analysis: rapid diagnostic tests versus placental histology, microscopy and PCR for malaria in pregnant women. Malar J. 2011;10:321.
Hailu T, Kebede T. Assessing the performance of CareStart Malaria Pf/Pv Combo test against thick blood film in the diagnosis of malaria in northwest Ethiopia. Am J Trop Med Hyg. 2014;90:1109–12.
Moges B, Amare B, Belyhun Y, Tekeste Z, Gizachew M, Workineh M, et al. Comparison of CareStartTM HRP2/pLDH COMBO rapid malaria test with light microscopy in north-west Ethiopia. Malar J. 2012;11:234.
Woyessa A, Deressa W, Ali A, Lindtjørn B. Evaluation of CareStartTM malaria Pf/Pv combo test for Plasmodium falciparum and Plasmodium vivax malaria diagnosis in Butajira area, south-central Ethiopia. Malar J. 2013;12:218.
Alemayehu GS, Lopez K, Dieng C, Lo E, Janies D, Golassa L. Evaluation of PfHRP2 and PfPLDH malaria rapid diagnostic test performance in Assosa Zone, Ethiopia. Am J Trop Med Hyg. 2020;103:1902–9.
Abebe A, Gebretsadik A, Nega D. Evaluation of GENEDIA® malaria pf/pan antigen test relative to blood smear microscopy in a malaria diagnosis in Ethiopia. J Parasit Dis Diagn Ther. 2018;3:20–35.
Ashton RA, Kefyalew T, Tesfaye G, Counihan H, Yadeta D, Cundill B, et al. Performance of three multi-species rapid diagnostic tests for diagnosis of Plasmodium falciparum and Plasmodium vivax malaria in Oromia Regional State, Ethiopia. Malar J. 2010;9:297.
Beyene BB, Yalew WG, Demilew E, Abie G, Tewabe T, Abera B. Performance evaluation of rapid diagnostic test for malaria in high malarious districts of Amhara region, Ethiopia. J Vector Borne Dis. 2016;53:63–9.
Bayisa S, Kebede T, Bugssa G, Alemu M. Performance of SO Bioline FK80 test kit in diagnosis of malaria at Adama Malaria Center, southeast Oromia, Ethiopia. Sci J Public Health. 2015;3:31–6.
Chanie M, Erko B, Animut A, Legesse M. Performance of CareStartTM malaria Pf/Pv combo test for the diagnosis of Plasmodium falciparum and Plasmodium vivax infections in the Afar region, North East Ethiopia. Ethiop J Health Dev. 2011;25:206–11.
Eticha T, Tamire T, Bati T. Performance evaluation of malaria Pf/Pv Combo test kit at highly malaria-endemic area, Southern Ethiopia: a cross-sectional study. J Trop Med. 2020;2020:1807608.
Endeshaw T, Graves PM, Ayele B, Mosher AW, Gebre T, Ayalew F, et al. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centers in Northwest Ethiopia. PLoS One. 2012;7:e33014.
Mekonnen Z, Ali S, Belay G, Suleman S, Chatterjee S. Evaluation of the performance of CareStartTM malaria Pf/Pv Combo rapid diagnostic test for the diagnosis of malaria in Jimma, southwestern Ethiopia. Acta Trop. 2010;113:285–8.
Morankar S, Tegene A, Kassahun W, Sulueiman S, Negatu YA, Yazachew M, et al. Validity and reliability of RDT for diagnosis of malaria among febrile children in Jimma Town: southwest Ethiopia. Ethiop Med J. 2011;49:131–8.
Bekele S, Mengistu L, Abebe A, Daddi J, Girmay M, Berhanu E. Evaluation of the performance of CareStartTM Malaria Pf/Pv Combo and Paracheck Pf® tests for the diagnosis of malaria in Wondo Genet, southern Ethiopia. Acta Trop. 2009;111:321–4.
Mohammed H, Kassa M, Kebede A, Endeshaw T. Paracheck-PF® test versus microscopy in the diagnosis of falciparum malaria in Arbaminch. Ethiop J Health Sci. 2012;22:93–8.
Tekeste Z, Workineh M, Petros B. Comparison of Paracheck Pf® test with conventional light microscopy for the diagnosis of malaria in Ethiopia. Asian Pac J Trop Dis. 2012;2:1–3.
Tegegne G. Comparative assessment of microscopy, malaria rapid diagnostic test and polymerase chain reaction as diagnostic test tools in Adama Woreda, East Shoa zone of Ethiopia: Dissertation, Addis Ababa University, 2017. http://etd.aau.edu.et/handle/123456789/2598.
Tadesse G, Mekete G. Evaluation of ICT Malaria Pf/Pv immunochromatographic test for rapid diagnosis of Plasmodium falciparum and Plasmodium vivax infections at Jimma malaria sector, Southwestern Ethiopia. Ethiop J Health Sci. 2001;11:5–16.
Golassa L, Baliraine FN, Enweji N, Erko B, Swedberg G, Aseffa A. Microscopic and molecular evidence of the presence of asymptomatic Plasmodium falciparum and Plasmodium vivax infections in an area with low, seasonal and unstable malaria transmission in Ethiopia. BMC Infect Dis. 2015;15:310.
Tadesse E, Workalemahu B, Shimelis T. Diagnostic performance evaluation of the SD Bioline malaria antigen Ag Pf/Pan test (05fk60) in a malaria endemic area of southern Ethiopia. Rev Inst Med Trop Sao Paulo. 2016;58:59.
Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G. Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J. 2013;12:352.
Getnet G, Getie S, Srivastava M, Birhan W, Fola AA, Noedl H. Diagnostic performance of rapid diagnostic tests for the diagnosis of malaria at public health facilities in north-west Ethiopia. Trop Med Int Health. 2015;20:1564–8.
Sema M, Alemu A, Bayih AG, Getie S, Getnet G, Guelig D, et al. Evaluation of non-instrumented nucleic acid amplification by loop-mediated isothermal amplification (NINA-LAMP) for the diagnosis of malaria in Northwest Ethiopia. Malar J. 2015;14:44.
Alemu A, Fuehrer H-P, Getnet G, Kassu A, Getie S, Noedl H. Comparison of Giemsa microscopy with nested PCR for the diagnosis of malaria in North Gondar, north-west Ethiopia. Malar J. 2014;13:174.
Díaz PB, Lozano PM, Rincón JMR, García L, Reyes F, Llanes AB. Quality of malaria diagnosis and molecular confirmation of Plasmodium ovale curtisi in a rural area of the southeastern region of Ethiopia. Malar J. 2015;14:357.
Tegegne B, Getie S, Lemma W, Mohon AN, Pillai DR. Performance of loop-mediated isothermal amplification (LAMP) for the diagnosis of malaria among malaria suspected pregnant women in Northwest Ethiopia. Malar J. 2017;16:34.
Girma S, Cheaveau J, Mohon AN, Marasinghe D, Legese R, Balasingam N, et al. Prevalence and epidemiological characteristics of asymptomatic malaria based on ultrasensitive diagnostics: a cross-sectional study. Clin Infect Dis. 2019;69:1003–10.
Tadesse G, Kamaliddin C, Doolan C, Amarasekara R, Legese R, Mohon AN, et al. Active case detection of malaria in pregnancy using loop-mediated amplification (LAMP): a pilot outcomes study in South West Ethiopia. Malar J. 2020;19:305.
Hopkins H, Kambale W, Kamya MR, Staedke SG, Dorsey G, Rosenthal PJ. Comparison of HRP2-and pLDH-based rapid diagnostic tests for malaria with longitudinal follow-up in Kampala Uganda. Am J Trop Med Hyg. 2007;76:1092–7.
Hendriksen IC, Mtove G, Pedro AJ, Gomes E, Silamut K, Lee SJ, et al. Evaluation of a PfHRP2 and a pLDH-based rapid diagnostic test for the diagnosis of severe malaria in 2 populations of African children. Clin Infect Dis. 2011;52:1100–7.
Dzakah EE, Kang K, Ni C, Tang S, Wang J, Wang J. Comparative performance of aldolase and lactate dehydrogenase rapid diagnostic tests in Plasmodium vivax detection. Malar J. 2014;13:2725.
Kojom Foko LP, Pande V, Singh V. Field performances of rapid diagnostic tests detecting human Plasmodium species: a systematic review and meta-analysis in India, 1990–2020. Diagnostics. 2021;11:590.
Acknowledgements
We deeply express our gratefulness to authors helped as to access the full text of their works.
Funding
This study was not funded by any organization.
Author information
Authors and Affiliations
Contributions
DGF and YA were involved in the design of the study and participated in all implementation stages. DGF, YA and NY analysed the data and finalized the write up of the manuscript. DGF and YA critically revised the manuscript. DGF, YA and NY were responsible for searching studies and drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Feleke, D.G., Alemu, Y. & Yemanebirhane, N. Performance of rapid diagnostic tests, microscopy, loop-mediated isothermal amplification (LAMP) and PCR for malaria diagnosis in Ethiopia: a systematic review and meta-analysis. Malar J 20, 384 (2021). https://doi.org/10.1186/s12936-021-03923-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-03923-8