Abstract
Background
Artemisinin-based combination therapy (ACT) was deployed in 2005 as an alternative to chloroquine and is considered the most efficacious treatment currently available for uncomplicated falciparum malaria. While widespread artemisinin resistance has not been reported to date in Africa, recent studies have reported partial resistance in Rwanda. The purpose of this study is to provide a current systematic review and meta-analysis on ACT at Mali study sites, where falciparum malaria is highly endemic.
Methods
A systematic review of the literature maintained in the bibliographic databases accessible through the PubMed, ScienceDirect and Web of Science search engines was performed to identify research studies on ACT occurring at Mali study sites. Selected studies included trials occurring at Mali study sites with reported polymerase chain reaction (PCR)-corrected adequate clinical and parasite response rates (ACPRcs) at 28 days. Data were stratified by treatment arm (artemether–lumefantrine (AL), the first-line treatment for falciparum malaria in Mali and non-AL arms) and analysed using random-effects, meta-analysis approaches.
Results
A total of 11 studies met the inclusion criteria, and a risk of bias assessment carried out by two independent reviewers determined low risk of bias among all assessed criteria. The ACPRc for the first-line AL at Mali sites was 99.0% (95% CI (98.3%, 99.8%)), while the ACPRc among non-AL treatment arms was 98.9% (95% CI (98.3%, 99.5%)). The difference in ACPRcs between non-AL treatment arms and AL treatment arms was not statistically significant (p = .752), suggesting that there are potential treatment alternatives beyond the first-line of AL in Mali.
Conclusions
ACT remains highly efficacious in treating uncomplicated falciparum malaria in Mali. Country-specific meta-analyses on ACT are needed on an ongoing basis for monitoring and evaluating drug efficacy patterns to guide local malaria treatment policies, particularly in the wake of observed artemisinin resistance in Southeast Asia and partial resistance in Rwanda.
Similar content being viewed by others
Background
Falciparum malaria is the most deadly type of malaria and causes most cases of malaria in sub-Saharan Africa [1, 2]. For over four decades, chloroquine was the first-line treatment of uncomplicated falciparum malaria, and molecular markers for its resistance were first observed in 2001 [3]. Artemisinin-based combination therapy (ACT) has since replaced chloroquine due to widespread resistance [4, 5]. Between 2001 and 2004, ACT was launched in 20 African countries in response to World Health Organization (WHO) recommendations [6]. ACT is currently used in Africa, Asia and South America [7] and is recommended as first-line treatment of uncomplicated falciparum malaria [8]. Since 2005, 81.5% (25.5 of 31.3 million) of ACT courses deployed globally were distributed in Africa [9]. ACT is deployed as a combination therapy as the use of artemisinin monotherapy has been shown to promote the development of artemisinin resistance [10]. Most ACT failures are a result of re-infections [11], and the most widely used approach for assessing ACT efficacy is the in vivo approach at 28 days following treatment with molecular correction for re-infection [12]. While ACT is intended for malaria treatment, it has also been efficacious in preventing transmission [13].
WHO currently recommends five artemisinin-based combinations: artesunate-amodiaquine (AS + AQ); artesunate–mefloquine (AS + MQ); artesunate–sulfadoxine–pyrimethamine (AS + SP); artemether–lumefantrine (AL); and, dihydroartemisinin–piperaquine (DHA + PQ) [8]. A newer ACT, artesunate–pyronaridine (AS + Pyr) is being considered for use where others are failing [8]. Artesunate–atovaquone–proguanil (AS + AP) is not usually used in endemic areas due to the high cost of atovaquone [14]. Artesunate–chlorproguanil–dapsone (AS + CD) is no longer used in African settings due to its haemolytic potential in glucose-6-phosphate dehydrogenase (G6PD)-deficient patients [15]. Artesunate–sulfamethoxypyrazine–pyrimethamine (AS + SMP) is widely used in Central African markets but is not recommended by the WHO for treating falciparum malaria [16].
Artemisinin combination therapy in Mali
Malaria remains a substantial burden in Mali and is its leading cause of morbidity and mortality in children aged under 5 years [17, 18]. In 2005, a pilot campaign for free ACT (AS + AQ) was introduced in Mali by Doctors Without Borders (abbreviated as MSF for its French translation: Médecins Sans Frontières) [19]. Later in 2005, Mali officially adopted AL ACT as a replacement for chloroquine [20]. AL remains the recommended first-line treatment for uncomplicated falciparum malaria in Mali, with AS + AQ as second-line treatment [20, 21].
Systematic reviews and meta-analyses as evaluation tools for artemisinin-based combination therapy
Systematic reviews and meta-analyses have routinely been performed over the past decade to monitor and assess ACT efficacy, tolerability and adherence for African regions. In 2004, a meta-analysis of 16 clinical trials evaluated the effects of adding artesunate to standard anti-malarial treatments, such as amodiaquine [22]. In 2009, a review of 50 ACT studies in Asia and Africa revealed that all five ACT in use at the time yielded treatment failure rates of under 10% at most study sites, which met WHO guidelines [23]. Another meta-analysis focusing on sub-Saharan Africa study sites showed that ACT yielded lower failure rates (relative to oral quinine) in second and third-trimester pregnancies [24]. A review of 11 studies on the repeated dosages of DHA + PQ in pregnant women showed the regimen to be well tolerated as an intermittent preventive treatment (IPT) [25]. A larger meta-analysis of 78 studies focusing on drug resistance to falciparum malaria revealed that ACT was less prone to drug resistance than non-ACT (NACT) [26]. Another review of 76 studies conducted in sub-Saharan Africa published between 2002 and 2016 revealed that, among the WHO-recommended ACT, DHA + PQ was the most efficacious [27].
Recent country-specific meta-analyses in African countries
Country-specific meta-analyses for ACT have recently been performed for several African countries. A review of 13 studies conducted in Uganda between 2002 and 2010 showed that AL was highly efficacious in Uganda, with efficacy rates of 98% [28]. In 2017, a meta-analysis including study sites in Ethiopia showed that anti-malarial treatment success rates were 92.9%, and standard regimens showed high success rates against both Plasmodium falciparum (98.1%) and Plasmodium vivax (94.7%) infections [29]. In 2018, a meta-analysis showed that ACT remains highly efficacious in Sudan, where overall malaria treatment success rates were 98.0%, and the AL regimen showed higher efficacy compared to AS + SP [30]. In 2019, a network meta-analysis of six studies in Cameroon showed anti-malarial success rates were between 88.2 and 100% [31]. In a recent review of ACT efficacy at Guinea sites, each of the three included studies reported ACT efficacy rates over 95% [32].
Artemisinin resistance has recently been observed in Southeast Asia, where, in 2020, a network meta-analysis of 82 studies reported artemisinin resistance [33]. While widespread artemisinin resistance has not been reported in Africa, at least one case study has reported resistance [34]. A recent review study in Burkina Faso did not reveal ACT resistance, but one of the reviewed studies raised concern about the possibility [35, 36]. Evidence of emerging artemisinin partial resistance has been observed in Rwanda, and the evaluation of additional anti-malarials in Rwanda has been recommended [37]. A recent study has shown evidence for the de novo emergence of Pfkelch13-mediated resistance in Rwanda, potentially compromising the continued success of anti-malarial chemotherapy in Africa [38]. Additionally, recent studies in Mali have also shown an increased frequency of recurrent parasitaemia following AL treatment [39]. Recent studies such as these suggest that African countries may be on the verge of meaningful artemisinin resistance, as previously observed in Southeast Asia [40].
While country-specific meta-analyses have recently been carried out for Sudan, Ethiopia, Cameroon, Burkina Faso, and Guinea, an analogue study has yet to be performed for Mali, where falciparum malaria rates are among the most prevalent and burdensome in the world. Recent reports showing the emergence of artemisinin resistance illustrate the need for monitoring and evaluating the efficacy of ACT to guide local health policy and decision making. This systematic review aims to accentuate the current efficacy of ACT in Mali and provide much-needed information on potential artemisinin resistance, which is currently lacking in Mali. The aim of this study, therefore, is to fill this gap through a systematic review and meta-analysis of ACT trials carried out at Mali study locations. More specifically, the study aims to evaluate the current efficacy of first-line ACT in Mali and determine whether other treatments are equally efficacious for alternative candidate therapy. To our knowledge, this is the first systematic review of ACT focused exclusively on Mali study locations.
Methods
A systematic review and meta-analysis of ACT trials was performed according to the established Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [41]. The review focused on evaluating the overall efficacy of first-line AL ACT and other ACT deployed at Mali study sites. A systematic review of the literature was performed by querying bibliographic databases indexed by the PubMed, ScienceDirect and Web of Science search engines. These queries were performed without any time or language restrictions. The search terms were input as: (“Mali” and “artemisinin” and ACPR”) or (“Mali” and “artemisinin-based and (“trial” or “randomized”)) or (“Mali” and “artemether” and “lumefantrine” and (“trial” or “randomized”)). “Therapies” was purposely omitted as its plurality varied across studies. The latter search component (“Mali” and “artemether” and “lumefantrine” and (“trial” or “randomized”)) was used because AL is currently the first-line treatment for uncomplicated falciparum malaria in Mali. Further inclusion criteria required at least one ACT comparison arm, the ability to disaggregate study results for Mali locations, and inclusion of the primary outcome (absence of parasitaemia at day 28 irrespective of axillary temperature and without early or late treatment failure or late parasitological failure corrected by PCR (adequate clinical and parasite response (ACPRc)) [42]. Publications on editorials, guidelines and theoretical articles were excluded from the final selected studies.
Study outcomes and data extraction
The primary outcome was ACPRc at 28 days following treatment. ACPRc data were abstracted by study arm for each of the selected studies. Other data abstracted included author names, year of publication, journal name and type, treatment arm type, treatment regimen, study design type, study location, age range inclusion criteria, analysis type (intention to treat or per protocol), parasitaemia at day 28 irrespective of axillary temperature and without early or late treatment failure or late parasitological failure (uncorrected ACPR, referred to here as ACPR) and their respective confidence intervals, and ACPRcs and their respective confidence intervals. Study locations were geocoded and mapped.
Meta-statistical analysis
Data were expressed as frequencies, percentages and standard errors (SEs). Outcomes (ACPRs and ACPRcs) were rounded to one decimal place as it was the most consistent reporting method for the primary outcome in the selected studies. Confidence intervals and SEs were calculated according to the normal approximation formula for a single proportion \(\left( {SE = \sqrt {{\raise0.7ex\hbox{${p\left( {1 - p} \right)}$} \!\mathord{\left/ {\vphantom {{p\left( {1 - p} \right)} n}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$n$}}} } \right)\), where p and n represent the reported proportions and sample sizes, respectively. Sample sizes were considered as the total number of subjects evaluated (number of enrolled subjects excluding withdrawals) by study arm. Confidence intervals were based on a standard normal distribution with a 5% type I error rate and calculated as 1.96 times the standard error for each reported proportion. Heterogeneity was assessed according to Cochran’s Q and I2 tests [43]. Evidence of heterogeneity was considered as justification for using random-effects over fixed-effects models, and the threshold for meeting statistically significant heterogeneity was set at I2 > 50% and p < 0.05. Forest plots were generated using the STATA Meta-analysis workflow and Metaprop command (version 16, StataCorp LLC, College Station, TX, USA) [44, 45]. Results from individual studies were weighted according to their standard errors. Comparisons of ACPRs between AL and non-AL treatment arms were performed using sub-group meta-analyses considering the AL and non-AL arms as comparison groups and testing hypotheses for heterogeneity. The type I error threshold for all hypothesis tests was set at 5%.
Quality assessment
Publication bias was assessed for selected studies using the Cochrane Risk-of-Bias tool [46]. Bias was classified according to randomization processes, deviations from intended interventions, missing outcome data, outcome measurements, and selection of the primary outcome. Risk of bias was classified as ‘low’, ‘unclear’, and ‘high’. Bias assessments were conducted independently by two assessors, and the results were graphed according to the percentage of low concern.
Results
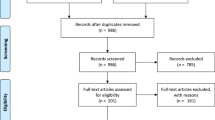
A total of 43 publications were identified from bibliographic databases using the PubMed, ScienceDirect and Web of Science search engines. Among these publications, 32 were excluded for the following reasons: protocol studies (one study); replicate results from other selected studies (two studies); ACT was studied as a preventive treatment therapy, the study did not include an ACT arm or did not include reported ACPRcs (20 studies); study did not include Mali study sites (three studies); and, Mali sites could not be de-aggregated from multi-country studies or included replicate results from previously selected studies (six studies). A total of 11 studies met the inclusion criteria [47,48,49,50,51,52,53,54,55,56,57,58,59] (Fig. 1). ACPRcs at day 28 was considered in this review as the primary outcome. Characteristics for the 11 selected studies are listed in Table 1.
The 11 selected studies included a total of 28 study arms, including 26 ACT arms and two NACT arms. Ten of the studies included an AL arm, and both of the two NACT arms were partial ACT (non-combinations AS and SP). The total number of subjects evaluated in the selected studies was 5,578, with 1,357 subjects in AL arms and 4,221 subjects in non-AL arms. For the non-AL arms, the number of evaluated subjects were: AS + SP (n = 1,139); AS + AQ (n = 747); AS + Pyr (n = 533); AS + SMP (n = 464); SP + AQ (n = 423); SP (n = 294); AS (n = 234); AS + MQ (n = 232); and, DHA-PQ (n = 155). All of the results were based on per protocol analyses, save for the study by Ndiaye et al. [51] as results were only available for intention-to-treat analyses. The majority of the field study sites for the selected studies were situated in rural or semi-rural southern parts of Mali, where most of its population resides. The locations of the field study sites are shown in Fig. 2.
Because AL was the first-line ACT in Mali and thus was the most common treatment arm in the selected trials, a stratified meta-analysis was performed for the AL arm (Fig. 3).
Forest plot of artemether–lumefantrine polymerase chain reaction-corrected adequate clinical and parasite responses for included studies. The pooled ACPRc for AL treatment arms was 99.0% (95% CI 98.3%, 99.8%). ACPRc: polymerase chain reaction-corrected adequate clinical and parasite response for falciparum malaria at 28 days; AL: artemether–lumefantrine; PCR: polymerase chain reaction
Eight studies were included in the stratified analysis for the AL arm (the other three selected studies did not include an AL arm). The ACPRs ranged from 94 (Ndiaye et al. [51]) to 100% (Kayentao et al. [58]). The hypothesis test for heterogeneity was significant (p = 0.001), and therefore random-effects meta-analyses approaches were used to assess the AL arm. The overall ACPRc for AL was 99.0% (95% CI 98.3%, 99.8%). An analogue, stratified meta-analysis was carried out for non-AL treatment arms (Fig. 4).
Forest plot of polymerase chain reaction-corrected adequate clinical and parasite responses for studies with non- artemether–lumefantrine treatment arms. Among non-AL treatment arms including at least two studies, AS + AQ and AS + Pyr were the only treatment arms with pooled ACPRcs less than 100.0% (97.7% (95% CI 95.9%, 99.4%) and 97.6% (95% CI 92.7%, 100.0%), respectively. The overall ACPRc for non-AL treatment arms was 98.9% (95% CI 98.3%, 99.5%). Only treatment arms with at least two representative studies are shown. ACPRc: polymerase chain reaction-corrected adequate clinical and parasite response for falciparum malaria at 28 days; AL: artemether–lumefantrine; PCR: polymerase chain reaction
The hypothesis test for heterogeneity was also significant for the non-AL meta-analyses (p < 0.001), and therefore random-effects meta-analyses approaches were applied to analyse the ACPRcs for the non-AL arms. The pooled ACPRc for non-AL treatment arms was 98.9% (95% CI (98.3%, 99.5%)). Only one study included a lower 95% confidence bound of less than 95% (Ndiaye et al. [51]; 95% CI (88.0%, 100.0%)), which is potentially attributable to its intent-to-treat analyses. ACPRs for all three studies in the AS + SMP arm were 100% with no reported treatment failures.
Comparison of AL and non-AL treatment arms
Figure 5 shows treatment success rates according to ACPR and ACPRc 28 days following treatment by treatment type (classified as AL and non-AL arms).
Uncorrected versus corrected malaria polymerase chain reaction-corrected adequate clinical and parasite responses for artemisinin-based combination therapy arms in Mali trials. AL and non-AL treatment arms did not significantly differ according to ACPRs or ACPRcs (p = .120 and p = .752, respectively). The error bars denote the 95% confidence intervals for each combination of treatment arm (AL or non-AL) and outcome (ACPR or ACPRc). ACPR: adequate clinical and parasite response; ACPRc: polymerase chain reaction-corrected adequate clinical and parasite response for falciparum malaria at 28 days; ACT: artemisinin-based combination therapy
The overall ACPRs and 95% confidence intervals for the AL and non-AL groups were 77.7% (67.2%, 88.1%) and 87.4% (81.0%, 93.8%), respectively. Sub-group meta-analysis tests of heterogeneity revealed that the AL and non-AL treatment arms were not statistically different with respect to ACPRc or ACPR outcomes (p = 0.752 and p = 0.120, respectively). However, the ACPRs were significantly lower than the ACPRcs for both the AL and non-AL treatment arms (p < 0.001 and p < 0.001, respectively).
Publication bias assessment
Bias assessments were conducted independently by two of the co-authors for this review paper, and six major assessment criteria were evaluated: selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessment; attrition bias (incomplete outcome data); and, reporting bias (selective reporting; and a general category for other types of bias. The publication bias assessment results were graphed as the proportion of reported low risk of bias according to the guidelines in the Cochrane Review Manager application (Fig. 6).
The majority of the included studies showed high quality. Six out of the 11 studies (54.5%) were scored as having no bias according to the six criteria. For those cases where bias was reported, it was most commonly observed for the random sequence generation and blinding of participants' criteria (each of these criteria included two out of the 11 studies (18.2%) with an unclear risk of bias). No selective reporting bias or high risk of bias was observed in any of the studies.
Discussion
ACT remains highly efficacious in treating uncomplicated falciparum malaria in Mali. The extremely high efficacy in both AL and non-AL treatment arms reported here suggests that non-AL therapy lines are available as potential alternatives to AL in the event of drug resistance or supply shortage. While the AL and non-AL treatment arms did not significantly differ according to ACPRcs 28 days following treatment, the lack of difference may suggest that non-AL treatment arms are at least equally efficacious as AL in Mali for treating uncomplicated falciparum malaria. Artemisinin resistance has recently been observed in Southeast Asia [60] and on at least one occasion in Africa [34], and recent studies for Rwanda have shown partial artemisinin resistance [37]. Together, these findings of potential artemisinin resistance suggest that country-specific review studies will play a key role in monitoring anti-malarial drug resistance patterns. Additionally, country-specific review studies on ACT are needed to complement more extensive multi-country meta-analyses to evaluate potential aggregation bias introduced in multi-country studies. Carrying out this strategy, however, is contingent upon regularly carrying out clinical trials or high-quality observational studies that would ideally adequately represent both rural and urban locations. It is worth adding that several available treatment arms were either not previously tested at Mali locations or were not available based on the selection criteria, including AS + CD, and artesunate–pyronaridine (AP). AS + SMP was last evaluated in Mali approximately 11 years ago [2009], so the results here may not reflect its current efficacy.
Reported artemisinin resistance calls for increased evaluation of ACT
Companion studies on alleles known to affect anti-malarial drug resistance patterns provide additional context for the results presented in this work. Regular monitoring of alleles such as kelch13 has been shown to be the key reasons for artemisinin resistance in Southeast Asia, and more recently, in Uganda and are needed to be assessed through field studies such as those by Diakité et al. [61]. While drug resistance has been reported in Southeast Asia, such resistance has not been reported in Mali [61], which is consistent with the high efficacy rates reported here. However, the recent reports in Rwanda regarding partial artemisinin resistance may suggest the emergence of artemisinin resistance in Africa [40]. It is worth mentioning that the introduction of ACT may, in turn, lead to reduced resistance in earlier first-line regimens, such as chloroquine, due to their decreased usage.
Also worth mentioning is that parasitological responses shortly following treatment are important measures for early detection of resistance. The early manifestation of resistance may become evident from slow parasitological responses according to measures such as parasite clearance half-lives. While data on early parasitological response were unavailable here, such data would have great utility for fully assessing drug resistance patterns. The assessment of early parasitological responses is perhaps most useful when efficacy rates are high, as was observed here for Mali study locations before resistance becomes widespread.
Implementation of ACT differs outside of controlled settings
The studies analysed here were performed under controlled experimental settings and were based on per protocol analyses. The results focused on ACT efficacy in terms of ACPRcs and did not consider potential confounding factors such as treatment compliance, side effects, drug cost, and drug quality. Also, malaria is often an assumed illness for symptomatic febrile subjects in malaria-endemic countries, and symptomatic subjects commonly receive anti-malarial treatment therapy for unconfirmed malaria or unknown febrile illnesses in the absence of diagnostic testing. Delivery approaches (fixed-point delivery at public health units or door-to-door delivery) may also directly impact patient compliance to drug instructions and recommended usage. For these reasons, the ability to implement anti-malarial treatment strategies in practical terms should be considered along with their performance under controlled settings. These types of limitations have recently been noted for Burkina Faso as limiting factors for investigating current artemisinin resistance [35].
Non-artemisinin-based combination therapy such as aminoquinoline-13 may provide plausible alternative therapy
Aminoquinoline-13, or AQ-13 is an analogue of chloroquine that is active against chloroquine-resistant Plasmodium species [62]. Phase II trials have shown the regimen to be non-inferior to the AL ACT according to per-protocol analyses with no treatment failures at 28 days following treatment [55]. The regimen is gaining considerable support for phase III clinical trials, specifically as a candidate treatment of uncomplicated falciparum malaria as a partner drug in combination therapy [63].
Utility of pharmacy data sources in monitoring ACT efficacy
Pharmacy consultations and inventories offer potentially valuable data sources for monitoring ACT efficacy spatial and temporal patterns. Pharmacies maintain a wealth of information on ACT and are often the first point of contact for febrile subjects. However, pharmacy data sources are rarely adequately monitored or considered as a means for measuring community health. The cost reduction of smartphones presents opportunities for building automated systems for capturing pharmacy data to monitor ACT and improve associated adherence and compliance. Data platforms are needed to capture data within the pharmacies and link them with their associated public health units. Through such platforms, pharmacy supply and clinical presentation data sources provide a potential approach for carrying out syndromic surveillance activities through surveillance of pharmacy presentations and monitoring drug supplies and inventories.
Study strengths and potential limitations
A particular strength of this work is its direct focus on Mali study sites. This work was made possible through the numerous field studies carried out in Mali over the past several decades. However, the study had several limitations. First, the selection approach for this study included only published works maintained in bibliographic databases available through the PubMed, ScienceDirect and Web of Science search engines, which did not capture unpublished studies or studies indexed through those bibliographic databases. Also, the studies contributing to this review paper were published between 2006 and 2018, and lag periods between the time of the field study to publication usually exceeded 1 year. Several studies contributed multiple comparison arms, and intra-trial dependence was not accounted for in the meta-analyses. Finally, several of the studies here occurred prior to the 2009 WHO protocol guidelines [64] for determining ACT efficacy that was applied in the studies following the establishment of these guidelines.
Conclusions
ACT remains highly efficacious in Mali, and the results here suggest that AL will continue to be a viable treatment option in Mali for the foreseeable future. These findings also suggest that potential ACT alternatives may be at least equally efficacious as first-line AL therapy. Country-specific meta-analyses on ACT play a central role in monitoring and evaluating drug efficacy patterns and for guiding local malaria treatment policies, particularly in the wake of reported partial artemisinin resistance in Rwanda.
Availability of data and materials
All data generated or analyzed during this study are included in this article and its supplemental materials.
Abbreviations
- ACE:
-
African Centres of Excellence in Bioinformatics
- ACPR:
-
Adequate clinical and parasite response
- ACT:
-
Artemisinin-based combination therapy
- AL:
-
Artemether–lumefantrine
- AP:
-
Artesunate–pyronaridine
- AQ:
-
Amodiaquine
- AQ-13:
-
Aminoquinoline-13
- AS:
-
Artesunate
- AS + MQ:
-
Artesunate–mefloquine
- CD:
-
Chlorproguanil–dapsone
- CQ:
-
Chloroquine
- DHA:
-
Dihydroartemisinin
- GPS:
-
Global positioning system
- IPT:
-
Intermittent preventive treatment
- ITT:
-
Intention to treat
- NACT:
-
Non-artemisinin-based combination therapy
- P.:
-
Plasmodium
- PCR:
-
Polymerase chain reaction
- PP:
-
Per protocol
- PQ:
-
Piperaquine
- Pyr:
-
Pyronaridine
- RCT:
-
Randomized controlled trial
- SE:
-
Standard error
- SMP:
-
Sulfamethoxypyrazine–pyrimethamine
- SP:
-
Sulfadoxine–pyrimethamine
- USTTB:
-
University of Sciences, Techniques and Technologies of Bamako, Mali
- WHO:
-
World Health Organization
References
WHO. Malaria: key facts. Geneva, World Health Organization, 2020. https://www.who.int/news-room/fact-sheets/detail/malaria.
WHO. World Malaria Report 2018. Geneva, World Health Organization, 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/.
Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourté Y, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–63.
Diallo MA, Yade MS, Ndiaye YD, Diallo I, Diongue K, Sy SA, et al. Efficacy and safety of artemisinin-based combination therapy and the implications of Pfkelch13 and Pfcoronin molecular markers in treatment failure in Senegal. Sci Rep. 2020;10:8907.
Frosch AE, Venkatesan M, Laufer MK. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar J. 2011;10:116.
WHO. Surge in demand leads to shortage of artemisinin-based combination therapy for malaria. Geneva, World Health Organization, 2004. https://www.who.int/mediacentre/news/releases/2004/pr77/en/.
Travassos MA, Laufer MK. Resistance to antimalarial drugs: molecular, pharmacologic, and clinical considerations. Pediatr Res. 2009;65(5 Pt 2):64r–70r.
WHO. Malaria: Q&A on artemisinin resistance. Geneva: World Health Organization; 2019.
Bosman A, Mendis KN. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am J Trop Med Hyg. 2007;77(6 Suppl):193–7.
WHO. Overview of malaria treatment. Geneva: World Health Organization; 2018.
Mavoko HM, Nabasumba C, da Luz RI, Tinto H, D’Alessandro U, Kambugu A, et al. Efficacy and safety of re-treatment with the same artemisinin-based combination treatment (ACT) compared with an alternative ACT and quinine plus clindamycin after failure of first-line recommended ACT (QUINACT): a bicentre, open-label, phase 3, randomised controlled trial. Lancet Global Health. 2017;5:e60–8.
Dama S, Djimde AA, Doumbo OK. Methods for monitoring artemisinin-based combination therapies efficacy. Clin Rev Opin. 2017;8:1–13.
Pousibet-Puerto J, Salas-Coronas J, Sánchez-Crespo A, Molina-Arrebola MA, Soriano-Pérez MJ, Giménez-López MJ, et al. Impact of using artemisinin-based combination therapy (ACT) in the treatment of uncomplicated malaria from Plasmodium falciparum in a non-endemic zone. Malar J. 2016;15:339.
Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77(6 Suppl):181–92.
Premji Z, Umeh RE, Owusu-Agyei S, Esamai F, Ezedinachi EU, Oguche S, et al. Chlorproguanil–dapsone-artesunate versus artemether–lumefantrine: a randomized, double-blind phase III trial in African children and adolescents with uncomplicated Plasmodium falciparum malaria. PLoS ONE. 2009;4:e6682.
Djallé D, Njuimo SP, Manirakiza A, Laganier R, Le Faou A, Rogier C. Efficacy and safety of artemether + lumefantrine, artesunate + sulphamethoxypyrazine-pyrimethamine and artesunate + amodiaquine and sulphadoxine-pyrimethamine + amodiaquine in the treatment of uncomplicated falciparum malaria in Bangui, Central African Republic: a randomized trial. Malar J. 2014;13:9.
WHO. The "World malaria report 2019" at a glance. Geneva, World Health Organization, 2019. https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019.
President’s Malaria Initiative. Mali 2018. https://www.pmi.gov/docs/default-source/default-document-library/country-profiles/mali_profile.pdf?sfvrsn=24.
Kone D, Coulibaly D, Doumbo O, Fall IS, Kibuchi E, Mitto B, et al. An epidemiological profile of malaria in Mali. PNLP, MRTC and INFORM. 2015. http://www.inform-malaria.org/wp-content/uploads/2015/03/Mali-Malaria-Epi-Profile-Report_030315.pdf.
Ashton RA, Doumbia B, Diallo D, Druetz T, Florey L, Taylor C, et al. Measuring malaria diagnosis and treatment coverage in population-based surveys: a recall validation study in Mali among caregivers of febrile children under 5 years. Malar J. 2019;18:3.
WHO. Mali: Malaria country profile. Geneva, World Health Organization, 2017. https://www.who.int/gho/countries/mli/country_profiles/en/.
Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17.
Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev. 2009;2009:CD007483.
Burger RJ, van Eijk AM, Bussink M, Hill J, Ter Kuile FO. Artemisinin-based combination therapy versus quinine or other combinations for treatment of uncomplicated Plasmodium falciparum malaria in the second and third trimester of pregnancy: a systematic review and meta-analysis. Open Forum Infect Dis. 2016;3(1):ofv170.
Gutman J, Kovacs S, Dorsey G, Stergachis A, Ter Kuile FO. Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:184–93.
Zhou LJ, Xia J, Wei HX, Liu XJ, Peng HJ. Risk of drug resistance in Plasmodium falciparum malaria therapy-a systematic review and meta-analysis. Parasitol Res. 2017;116:781–8.
Whegang Youdom S, Tahar R, Basco LK. Comparison of anti-malarial drugs efficacy in the treatment of uncomplicated malaria in African children and adults using network meta-analysis. Malar J. 2017;16:311.
Talisuna A. The changing landscape of malaria case management in Uganda: decades of struggle with evolving malaria case management strategies and drug policies. Malar Chemother Control Elimin. 2014;3:1.
Gebreyohannes EA, Bhagavathula AS, Seid MA, Tegegn HG. Anti-malarial treatment outcomes in Ethiopia: a systematic review and meta-analysis. Malar J. 2017;16:269.
Adam I, Ibrahim Y, Gasim GI. Efficacy and safety of artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Sudan: a systematic review and meta-analysis. Malar J. 2018;17:110.
Whegang Youdom S, Chiabi A, Basco LK. Monitoring the efficacy and safety of artemisinin-based combination therapies: a review and network meta-analysis of antimalarial therapeutic efficacy trials in Cameroon. Drugs R D. 2019;19:1–14.
Cherif MS, Dahal P, Beavogui AH, Delamou A, Lama EK, Camara A, et al. Malaria epidemiology and anti-malarial drug efficacy in Guinea: a review of clinical and molecular studies. Malar J. 2021;20:272.
Rasmussen C, Ringwald P. Is there evidence of anti-malarial multidrug resistance in Burkina Faso? Malar J. 2021;20:320.
Gansané A, Moriarty LF, Ménard D, Yerbanga I, Ouedraogo E, Sondo P, et al. Anti-malarial efficacy and resistance monitoring of artemether–lumefantrine and dihydroartemisinin-piperaquine shows inadequate efficacy in children in Burkina Faso, 2017–2018. Malar J. 2021;20:48.
Mathenge PG, Low SK, Vuong NL, Mohamed MYF, Faraj HA, Alieldin GI, et al. Efficacy and resistance of different artemisinin-based combination therapies: a systematic review and network meta-analysis. Parasitol Int. 2020;74:101919.
Lu F, Culleton R, Zhang M, Ramaprasad A, von Seidlein L, Zhou H, et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med. 2017;376:991–3.
Dama S, Niangaly H, Ouattara A, Sagara I, Sissoko S, Traore OB, et al. Reduced ex vivo susceptibility of Plasmodium falciparum after oral artemether–lumefantrine treatment in Mali. Malar J. 2017;16:59.
Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis. 2021;21:1120–8.
Uwimana A, Legrand E, Stokes BH, Ndikumana JM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26:1602–8.
Rosenthal PJ. Has artemisinin resistance emerged in Africa? Lancet Infect Dis. 2021;21:1056–7.
PRISMA. Transparent reporting of systematic reviews and meta-analyses 2015. http://www.prisma-statement.org/PRISMAStatement/.
Taylor-Robinson D, Jones K, Garner P. Malaria: uncomplicated, caused by Plasmodium falciparum. BMJ Clin Evid. 2007;2007:0919.
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019)2019.
StataCorp LLC. Stata 16: Meta-analysis. 2020.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39.
Cochrane. RoB 2: A revised Cochrane risk-of-bias tool for randomized trials. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials.
Sagara I, Dicko A, Djimde A, Guindo O, Kone M, Tolo Y, et al. A randomized trial of artesunate–sulfamethoxypyrazine–pyrimethamine versus artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am J Trop Med Hyg. 2006;75:630–6.
Djimdé AA, Fofana B, Sagara I, Sidibe B, Toure S, Dembele D, et al. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am J Trop Med Hyg. 2008;78:455–61.
Sagara I, Diallo A, Kone M, Coulibaly M, Diawara SI, Guindo O, et al. A randomized trial of artesunate–mefloquine versus artemether–lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am J Trop Med Hyg. 2008;79:655–61.
Sagara I, Rulisa S, Mbacham W, Adam I, Sissoko K, Maiga H, et al. Efficacy and safety of a fixed dose artesunate-sulphamethoxypyrazine-pyrimethamine compared to artemether–lumefantrine for the treatment of uncomplicated falciparum malaria across Africa: a randomized multi-centre trial. Malar J. 2009;8:63.
Ndiaye JL, Randrianarivelojosia M, Sagara I, Brasseur P, Ndiaye I, Faye B, et al. Randomized, multicentre assessment of the efficacy and safety of ASAQ–a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:125.
Sagara I, Fofana B, Gaudart J, Sidibe B, Togo A, Toure S, et al. Repeated artemisinin-based combination therapies in a malaria hyperendemic area of Mali: efficacy, safety, and public health impact. Am J Trop Med Hyg. 2012;87:50–6.
Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini-Fuhrer I, Fofana B, et al. Safety and efficacy of re-treatments with pyronaridine-artesunate in African patients with malaria: a substudy of the WANECAM randomised trial. Lancet Infect Dis. 2016;16:189–98.
Niare K, Dara A, Sagara I, Sissoko MS, Guindo CO, Cisse NH, et al. In vivo efficacy and parasite clearance of artesunate + sulfadoxine–pyrimethamine versus artemether–lumefantrine in Mali. Am J Trop Med Hyg. 2016;94:634–9.
Koita OA, Sangaré L, Miller HD, Sissako A, Coulibaly M, Thompson TA, et al. AQ-13, an investigational antimalarial, versus artemether plus lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria: a randomised, phase 2, non-inferiority clinical trial. Lancet Infect Dis. 2017;17:1266–75.
Dama S, Niangaly H, Djimde M, Sagara I, Guindo CO, Zeguime A, et al. A randomized trial of dihydroartemisinin-piperaquine versus artemether–lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Malar J. 2018;17:347.
Kayentao K, Maiga H, Newman RD, McMorrow ML, Hoppe A, Yattara O, et al. Artemisinin-based combinations versus amodiaquine plus sulphadoxine-pyrimethamine for the treatment of uncomplicated malaria in Faladje, Mali. Malar J. 2009;8:5.
Kayentao K, Doumbo OK, Pénali LK, Offianan AT, Bhatt KM, Kimani J, et al. Pyronaridine-artesunate granules versus artemether–lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malar J. 2012;11:364.
Maiga H, Djimde AA, Beavogui AH, Toure O, Tekete M, Sangare CP, et al. Efficacy of sulphadoxine-pyrimethamine + artesunate, sulphadoxine-pyrimethamine + amodiaquine, and sulphadoxine-pyrimethamine alone in uncomplicated falciparum malaria in Mali. Malar J. 2015;14:64.
Takala-Harrison S, Laufer MK. Antimalarial drug resistance in Africa: key lessons for the future. Ann N Y Acad Sci. 2015;1342:62–7.
Diakité SAS, Traoré K, Sanogo I, Clark TG, Campino S, Sangaré M, et al. A comprehensive analysis of drug resistance molecular markers and Plasmodium falciparum genetic diversity in two malaria endemic sites in Mali. Malar J. 2019;18:361.
Ramanathan-Girish S, Catz P, Creek MR, Wu B, Thomas D, Krogstad DJ, et al. Pharmacokinetics of the antimalarial drug, AQ-13, in rats and cynomolgus macaques. Int J Toxicol. 2004;23:179–89.
Mengue JB, Held J, Kreidenweiss A. AQ-13 - an investigational antimalarial drug. Expert Opin Investig Drugs. 2019;28:217–22.
WHO. Methods for surveillance of antimalarial drug efficacy. Geneva, World Health Organization, 2009. https://www.who.int/malaria/publications/atoz/9789241597531/en/.
Acknowledgements
The authors are grateful for the financial support for our training programs by the Fogarty International Center of the National Institutes of Health to make this study possible. We also thank the African Centres of Excellence in Bioinformatics (ACE) for developing the teaching computer laboratories at the University of Sciences, Techniques and Technologies of Bamako for carrying out our training programs. We sincerely thank Dr. Donald J. Krogstad, who passed away in 2020. Dr. Krogstad was extremely influential in the careers of several of the co-authors on this paper and greatly influenced research efforts in Mali. We fully dedicate this work to Dr Krogstad. We are deeply saddened by his passing and hope that this work serves to continues his lifelong mission for improving health outcomes in West Africa.
Funding
This study was supported by the Fogarty International Center and National Institute of Allergy and Infectious Diseases sections of the National Institutes of Health under award numbers U2RTW010673 for the West African Center of Excellence for Global Health Bioinformatics Research Training and U19AI089696 and U19AI129387 for the West Africa International Center of Excellence for Malaria Research. FOM is supported by a fellowship through the DELTAS Africa Initiative (DELGEME Grant 107740/Z/15/Z). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS) Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (DELGEME Grant 107740/Z/15/Z) and the United Kingdom (UK) government. The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
FOM, SOD, FJM, JGS conceived and wrote the manuscript; FOM, JGS performed the initial reviews of the data; SMT, RRR carried out statistical and meta-analyses; FOM, JL, RRR, JGS carried out meta-analysis bias assessments; FOM, JGS, IS, performed the secondary reviews of the data; FOM, JL, MW, OT, KK, MD, AD, ADJ, SOD, JGS assisted in carrying out the training program; COT, FOM, JGS assisted in manuscript editing and consultation; FOM, MW, SOD, FJM, JGS, IS, AD prepared the final draft of the manuscript, which was then reviewed and approved by all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This work utilized publicly available data from published manuscripts from the publicly available bibliographic databases and did not require ethics approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maiga, F.O., Wele, M., Toure, S.M. et al. Artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Mali: a systematic review and meta-analysis. Malar J 20, 356 (2021). https://doi.org/10.1186/s12936-021-03890-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-03890-0