Abstract
Background
A deeper understanding of the ecology and small-scale heterogeneity of malaria transmission is essential for the design of effective prevention, control and elimination interventions. The spatial and temporal distribution of malaria vectors was investigated in five villages in close proximity to a hydro-agricultural system in Côte d’Ivoire over the course of construction and the early phase of irrigated rice farming.
Methods
The study was carried out in five villages (Raffierkro, N’Douakro, Ahougui, Kpokahankro, Koffikro) near Bouaké, central Côte d’Ivoire, between early 2007 and late 2009. In each village, mosquitoes were collected by human landing catches and identified morphologically at genus and species level, and entomological parameters were determined. Plasmodium infection was assessed by dissection and an enzyme-linked immunosorbent assay.
Results
A total of 19,404 mosquitoes belonging to the genus Anopheles were sampled during 328 human-night catches. Before the construction of the hydro-agricultural system, comparable densities of Anopheles gambiae were observed in all villages. In subsequent years, densities in Raffierkro and Ahougui were significantly higher than the other villages [Kruskal–Wallis (KW) test = 31.13, p < 0.001]. The density of Anopheles funestus in the five villages was comparable in the early stage of the project, while a high density was reported in Koffikro at the end (KW test = 11.91, p = 0.018). Transmission of Plasmodium falciparum is perennial in the study area. Over the course of the study, high entomological inoculation rates (EIRs) were found: 219–328 infectious bites per person per year with An. gambiae. For An. funestus considerably lower EIRs were observed (5.7–39.4). Changing patterns of An. gambiae were not correlated with malaria transmission.
Conclusion
In this study setting, located in the bioclimatic transition zone of Côte d’Ivoire, rice cultivation was not observed to increase malaria transmission. The entomological parameters recorded until the onset of rice-growing activities in a hydro-agricultural system presented considerable heterogeneity both in space and time; a strong increase of Anopheles mosquitoes was observed in two of the five villages located in close proximity to the dam and irrigated rice fields. Malaria still is a main public health problem in all villages that require adequate control measures.
Similar content being viewed by others
Background
As a result of major droughts in sub-Saharan Africa in the 1960s and 1970s, and in order to intensify agricultural production, many Africans countries engaged in the construction of small and large dams [1, 2]. Indeed, hydro-agricultural and aquaculture developments have been realized across Africa with the aim to reduce hunger and poverty. On the one hand, this commitment lead to an improvement of agricultural production and inland fish cultivation, but on the other hand, these water resources development and management projects altered the risk of water-based and vector-borne diseases, such as schistosomiasis and malaria [3, 4].
In spite of the huge efforts across Africa to prevent, control and eliminate malaria, the disease remains a serious and complex public health issue with a heavy burden [5, 6]. Previous research has shown that ecological transformations consequential to rice developments considerably influenced the diversity and density of the culicidae fauna and sometimes malaria transmission. For example, studies carried out in Ethiopia [7, 8], Kenya [9] and Madagascar [10] suggested that irrigation enhances malaria transmission. In Mali [11] and Senegal [12] higher densities of Anopheles in rice area did not influence malaria transmission. The same divergent observations were made in irrigated rice farming areas in different setting in Côte d’Ivoire. Studies in the savannah area have shown that an increased density of Anopheles did not influence malaria transmission [13]. However, in the western forest area of Côte d’Ivoire, the high aggressive density of Anopheles funestus resulted in an increase of malaria transmission in villages performing one rice crop per year [14]. The three main malaria vector species in Côte d’Ivoire are Anopheles gambiae s.s., An. funestus s.s. and Anopheles nili s.s. [15–18]. Plasmodium falciparum is the predominant malaria species encountered (80–95 % of infections), followed by Plasmodium malariae (7–10 %) and Plasmodium ovale (1–3 %) [19].
For the current study setting in central Côte d’Ivoire, located in the bioclimatic transition between the savannah in the north and tropical rainforest in the south [20], it was important to clarify the following issues. What is the effect of dam construction and irrigated rice farming on malaria transmission? Do mosquito dynamics change over the course of dam construction, including the first cycle of irrigated rice farming? Are there differences in malaria transmission parameters from one village to another in function of distance to the main dam and irrigation site? A deeper understanding of these questions at this small-scale of investigation will assist in tailoring malaria control interventions and preventive measures to the prevailing social-ecological systems.
Methods
Study site and characteristics
The study was carried out near Bouaké, the second largest town of Côte d’Ivoire located in the central part of the country (geographical coordinates: 7°44′N latitude, 5°41′W longitude). The mean daily temperature ranges between 23.7 and 33.8 °C. The mean annual rainfall in the years 2007–2009 ranged between 1229 and 1334 mm. The climate is tropical humid, characterized by two well-defined seasons: a dry season from November to February and a long rainy season from March to October. The vegetation is typical for the transition zone from the tropical rainforest in the south and savannah in the north.
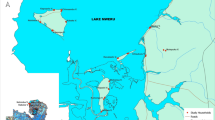
The study was conducted in five villages that were all located within a maximum distance of 5 km from the Raffiekro dam site (i.e. Ahougui, Koffikro, Kpokahankro, N’Douakro and Raffierkro) located about 15 km from Bouaké. The villages are located in proximity to a small multipurpose dam in Raffierkro (Fig. 1). The five villages represent typical ecological features for central Côte d’Ivoire and show slight differences in their agricultural practices, as follows. Raffierkro formerly was a leprosy-village with a hospital. It is the most developed among the five villages with key commodities, such as electricity, water and a school with informatics class. The water availability allows for irrigation with two rice growing cycle per year. The dam was constructed on Balloba River about 100 m from the closest houses of the village. Ahougui is located at 2 km downstream of the water reservoir and at 400 m to the surface extended for the rice and fish farming. It is a traditional rice-growing village with a non-functional dam, which had previously been used for rice farming. Kpokahankro is located at 3.5 km upstream. The village is still densely forested and characterized by many streams. The predominant agricultural activities in this area are vegetable faming (e.g. tomatoes and aubergines). N’Douakro is at 1.6 km far from the dam and is a non-rice-growing village. The land is marshy and the population is mainly engaged in subsistence farming with sweetcorn, yams and cassava. Koffikro, located in close proximity to Raffierkro, is situated at 500 m away. This village has no open surface water apart from the dam. Traditionally, the main activity of the populations was the cultivation of yams and cassava. Due to the construction of the dam, people have become involved in rice and vegetable crop production.
Study design and procedures
This study was designed as a repeated cross-sectional survey. There were three main study periods: (1) the construction of the dam; (2) the construction of irrigation canals, rice fields and fish ponds; and (3) the onset of rice-growing activities. The entomological surveys were done from June 2007 to November 2009, while the construction of the dam started in February 2007 (Fig. 2).
Mosquitoes were collected in four sentinel sites per village, using human landing catches [21]. Sampling was carried out in sentinel sites every month by two teams of four collectors, two indoors and two outdoors, once a month over a 9-month period. Collections were made from 18:00 to 06:00 h by the volunteers over two or three consecutive nights.
Mosquitoes caught were kept individually in haemolysis tubes plugged with cotton. All mosquitoes sampled were morphologically identified [22], sorted according to sampling site, house, date and species. An. gambiae, An. funestus and An. nili females were dissected to determine the degree of ovarian tracheoles coiling [23]. Salivary glands of parous mosquitoes were examined for malaria parasites using standard dissection techniques [24].
A sample of female Anopheles mosquitoes kept individually in 1.5 ml Eppendorf tubes and stored over silica gel were tested by enzyme-linked immunosorbent assay (ELISA) for P. falciparum circumsporozoite protein [25]. This test was conducted to assess the proportion of infected specimens among the main vectors species. It is assessing the transmission rate in Raffierkro’s dam site and nearby villages.
Statistical analysis
Data were analysed using Statistica version 7.1 (Tulsa, USA). The human biting rate (HBR) was expressed as the number of female anopheline bites per person per night (b/p/n). The result was obtained by the number of anophelines captured at each sampling point divided by the total number of sampling days and the average number of collectors. Parous females percentages and survival rates using 2 days for the gonotrophic cycle were calculated [17].
The sporozoite index for a given species was calculated as the proportion of females carrying infective sporozoites in the head–thorax of the total number tested. The entomological inoculation rate (EIR) was defined as the product of human biting rate and sporozoite index. This parameter expresses the number of infectious b/p/n, and is a widely used measure of malaria transmission [26]. The Kruskal–Wallis (KW) test was used to compare the differences in mosquito densities, and HBR among villages. The χ2 test was used to compare sporozoite rates. For all statistics, a p value below 0.05 was considered as statistically significant.
Ethical considerations
This study was approved by the institutional research commission of the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire (Abidjan, Côte d’Ivoire) and, as stated before, received approval by Bouaké’s health authorities. In each village permission to work was granted by chiefs. Community members were informed in detail about the objectives, procedures, and potential risks and benefits related to the study. People who were illiterate were informed in their local language. Written informed consent was obtained at the beginning of the study. Free and informed consent was obtained from volunteer collectors and heads of household of sentinel houses. For the volunteers who served as night baits, measures had been taken to prevent malaria (i.e. prophylactic treatment). Insecticide-treated nets (ITNs) were made available for the entire population in the five villages.
Results
Relative importance of vector species in anopheline fauna
A total of 19,404 mosquitoes belonging to the genus Anopheles were sampled during 328 human-night catches. Among the entire mosquito fauna, An. gambiae, An. funestus and An. nili accounted for 89.7 %, while other mosquito species accounted for the remaining 10.3 %. In Ahougui, Kpokahankro and Raffierkro, the three Anopheles species together accounted for 92.6, 91.7 and 90.2 %, respectively. Considerably lower proportions were observed in Koffikro and N’Douakro 82.4 and 85.8 %, respectively. As shown in Table 1, the predominant Anopheles species in all villages was An. gambiae with particularly high proportions found in Raffierkro (84.6 %) and Koffikro (72.3 %).
In the first period of the study, 2864 Anopheles were collected. Among them, 2696 (94.2 %) were main malaria vector species; An. gambiae (68.1 %), and An. funestus (31.9 %). No An. nili were caught in the first period. In the second period, 4533 Anopheles were collected and 3599 (79.4 %) were malaria vectors. An. gambiae was the predominant species (89.0 %), followed by An. funestus (9.2 %) and An. nili (1.8 %). In the third period, 12,007 specimens were collected; among them 11,103 (92.5 %) were malaria vectors. An. gambiae was the predominant species (94.9 %), followed by An. funestus (3.4 %) and An. nili (1.6 %). Over the entire study period, An. gambiae was by far the most important species in Ahougui and Raffierkro, while the relative importance of An. funestus increased in Ahougui and Kpokahankro over the course of the current study (Table 2).
Human biting rate
The average specific aggressive density found throughout the study area was estimated at 47.5 b/p/n with a [95 % confidential interval (CI) of 42.0–53.0 b/p/n] for An. gambiae, 4.8 b/p/n (95 % CI 3.9–5.6) for An. funestus and 0.7 b/p/n (95 % CI 0.5–1.0 b/p/n) for An. nili. The difference in the average specific aggressive density was highly significant between the three species (KW test = 540.22, p < 0.001). The An. gambiae aggressive density recorded over the three periods was, respectively, 19.1 b/p/n (95 % CI 14.0–23.7 b/p/n), 28.6 b/p/n (95 % CI 22.9–34.3 b/p/n) and 87.8 b/p/n (95 % CI 77.6–98.0 b/p/n). A significant difference was found in the aggressive density over the three periods (KW test = 136.48, p < 0.001).
The average aggressive densities evaluated for An. funestus were, 9.0 b/p/n (95 % CI 6.5–11.4 b/p/n), 2.9 b/p/n (95 % CI, 2.2–3.6 b/p/n) and 3.1 b/p/n (95 % CI 2.3–4.01), respectively (KW test = 7.75, p = 0.020). For An. nili, the average aggressive density, although low, increased significantly over the course of the study (KW test = 63.51, p < 0.001).
In the first period, similar aggressive densities were observed in all localities (KW test = 6.65, p = 0.080) with an exception in Koffikro, where a considerably lower density was observed (KW test = 12.05, p = 0.016). In Ahougui, Kpokahankro and Raffierkro, in the second period, comparable aggressive densities were found, which were significantly higher than those of N’Douakro and Koffikro (KW test = 31.13, p < 0.001). In the third period, the highest aggressive densities were observed in Ahougui (125.8 b/p/n) and Raffierkro (108.6 b/p/n), while the lowest were recorded in N’Douakro and Koffikro (Fig. 3). An. funestus aggressive densities within the five localities were comparable in 2007 and 2008. However, in the third period in 2009, the highest density was recorded in Koffikro (5.0 b/p/n) (KW test = 11.91, p = 0.018) (Fig. 4). For An. nili, the highest aggressive density was observed in Ahougui (KW test = 21.12, p < 0.001) (Fig. 5).
Parous and daily survival rates
During the 3-year-period, the parity rates showed a slight variation from 97.2 % (n = 1640) in Ahougui to 99.7 % (n = 668) in Koffikro. In each village, the monthly values did not vary significantly, and hence, parity rates were homogeneous. The overall parity rates for An.s gambiae and An. funestus are shown in Figs. 6 and 7. Higher parity rates of An. gambiae and An. funestus were measured in all five localities. The daily survival rate for the major malaria vector (An. gambiae) was very high; the values varied between 0.98 in Ahougui and 0.99 in Koffikro, Kpokahankro, N’Douakro and Raffierkro. Assuming a P. falciparum gonotrophic cycle of 12 days, the proportion of An. gambiae population able to transmit malaria were 82.4 % in Ahougui, 93.0 % in Koffikro and 87.6 % in Kpokahankro, N’Douakro and Raffierkro.
Infection rate
Table 3 summarizes annual sporozoite infection rates over the course of the study. Overall, An. gambiae and An. funestus had comparable sporozoite infection rates (χ2 = 3.56, p = 0.059) with an exception of the first year when An. gambiae showed a higher infection rate than An. funestus (χ2 = 11.86, p = 0.001). The average sporozoite infection rates in An. gambiae changed significantly over the three-year period. These average rates were 4.7 % (95 % CI 3.4–6.2 %), 2.1 % (95 % CI 1.4–2.8 %) and 0.8 % (95 % CI 0.5–1. 2 %) in the first, second and third period, respectively. An. funestus infection rates during the three study periods were 1.2 % (95 % CI 0.4–2.5 %), 2.2 % (95 % CI 1–3.9 %) and 0.5 % (95 % CI 0.1–1.2 %), respectively.
Transmission
A random sample of 2411 An. gambiae mosquitoes were subjected to ELISA for P. falciparum sporozoites and identified 24 as infected. All carried P. falciparum. The average rates of P. falciparum infection varied from 3.6 % in Ahougui to 6.1 % in N’Douakro. These average rates obtained by ELISA were significantly higher than those obtained by dissection, but the two methods presented the same tendencies. The sub-sample tested by ELISA covered the entire period of the study. In all study villages, values obtained by ELISA testing were comparable by dissection and showed the same temporal trend.
Malaria transmission is perennial in all study villages. An. gambiae mosquitoes were present during the three periods with high EIRs ranging from 219 to 328 infectious bites per person per year (ib/p/y) in the three periods. During the first and third period, the peak was observed in Ahougui [459.9 (ib/p/y) and 321.2 ib/p/y]. In the second period, the highest EIR was identified at Kpokahankro (401.5 ib/p/y). In all study villages, malaria transmission estimated by An. gambiae is consistently higher than An. funestus (Z = 4.86, p < 0.001). It is provided intermittently by the two vector species (Table 4).
Discussion
An important aspect of malaria vector control programmes is to take into account the ecology and small-scale heterogeneity of environmental factors, in order to put forward setting-specific control measures. Studies were conducted in five villages in close proximity to a small hydro-agricultural system in an area of high malaria transmission in central Côte d’Ivoire and the dynamics of entomological parameters were studied over a three-year period.
The results shows as previously known in central Côte d’Ivoire [15, 18] that An. gambiae s.s, and An. funestus s.s. were the main malaria vectors driving the high level of transmission in this region. It was found that An. gambiae was the predominant species at the onset of irrigated rice farming activities in Raffierkro and Ahougui. The high density of An. gambiae might result from the presence of suitable larval sites, especially irrigation canals, aquaculture reservoirs and streams that were constructed during the second period. These artificial reservoirs provide suitable breeding sites for An. gambiae larvae and were linked to the exploitation of rice fields. The study site was sunlit and the irrigation water in the dug-out wells and furrows were clean, conditions which support the breeding of An. gambiae [27].
These observations are in agreement with previous studies done in different parts of Côte d’Ivoire, where irrigated rice farming is pursued, such as in the North, South [28, 29], and in the West of the country [30]. A study conducted in Malawi [31] also showed the proliferation of malaria vectors in rice fields.
The results of the study presented here are important for the control of mosquito larval stages in the Raffierkro area. Knowledge of larval habitat productivity could help in the planning and implementation of mosquito larval control interventions in such irrigated areas [32]. Interestingly, the density of An. funestus was low throughout the study. This observation could indicate that rice paddies in the current context were not favourable to larval breeding and could be the result of loss of habitats after transformation of natural habitats. Prior studies across the African continent have reported lowest densities of An. funestus s.l. in rice-cultivating areas [33–35].
Another aspect of the current study is that An. gambiae had an important implication in the transmission of malaria, in contrast to An. funestus, which was of lesser relevance. In Côte d’Ivoire, the three malaria vectors are An. gambiae s.s., An. funestus s.s. and An. nili s.s. The current results confirm prior studies conducted in the central part of Côte d’Ivoire that revealed An. gambiae s.s. and An funestus s.s. as the dominant malaria vectors [36]. An. nili was recorded during the two last periods of the current study, but at very low abundance, and hence it is unlikely to play a major role in malaria transmission. Anopheles pharoensis was not reported as vector in central Côte d’Ivoire [37], but previous studies in Senegal and Cameroon reported this vectors species there [12, 38]. Sporozoite rates recorded in the first period, before implementation of the hydro-agricultural project, were higher than in later time points. However, aggressive densities were considerably higher in all study villages during the third period, while EIR remained at the same level. High EIRs have also been reported before and might reflect the natural history of transmission during the early stage of operation of a hydro-agricultural system. This high transmission may be due to the age of female Anopheles mosquitoes and their capacity to transmit Plasmodium due to the longevity of the malaria vectors. The non-correlation of the high aggressive density with EIR in the third period indicates that in the Raffierkro area, irrigated rice farming did not result in an increase of malaria transmission. A possible explanation of this observation is that the area is in a perennial transmission zone with high longevity of malaria vectors. These results are similar to those observed in the savannah [39, 40]. However, contrasting findings were made in central Ethiopia and in the forest area of western Côte d’Ivoire, where irrigation canals, non-functional canal pools and extensive cultivation of rice resulted in favourable conditions to vector breeding sites, thus facilitating high malaria transmission [41–44]. The low transmission of malaria parasites by An. funestus in the five villages studied here can be justified by their low abundance. This observation underscores that An. funestus is a relatively rare species in irrigated rice agro-ecosystems. This study confirm previous findings that rice areas are unsuitable for An. funestus [45, 46]. Interestingly though, the current observations are in contrast with those made elsewhere in Africa [47, 48] where, after deforestation and installation of irrigated rice farming, An. funestus became the main malaria vector species with an EIR estimated at 172 ib/p/y over the study period and a high abundance (91 %); an order of magnitude higher than An. gambiae (7 %). During the dam construction period, insecticide-treated nets have been offered to all households in the five study villages largely subsidized. In addition, a community health worker was trained in each village and malaria treatments were made available at the village level according to a contribution equivalent to the prices observed in the public health pharmacies. The current study showed-at a scale of about 5 km around a new, multipurpose small dam that malaria transmission is characterized by small-scale heterogeneity, partially influenced by this water resource development and management system. The findings assisted in designing and prioritizing malaria control and prevention measures that are tailored to the specific social-ecological contexts. For example, villages in close proximity to the new dam require particular attention, such as high coverage with long-lasting insecticidal nets and other preventive measures particularly during the rice growing period. The investigations were performed during the construction of a dam until the first cycle of irrigated rice. It will be interesting to study patterns of transmission over a longer period to determine whether or not the results here after stabilization of rice cycles remain.
Conclusion
During the installation of a small hydro-agricultural system until the first cycle of irrigated rice farming, heterogeneities were observed in five villages situated in close proximity to a dam. An. gambiae and An. funestus were the two prominent vectors of malaria and high transmission was primarily due to An. gambiae. The hydro-agricultural development is the likely cause of a large aggressive mosquito density, which did not influence the transmission of malaria in Raffierkro’s environment. However, malaria still is a main public health problem in all villages. In this setting, malaria transmission is perennial with high survival of vectors and EIRs rates. Hence adequate control measures are required. First, the future hydro-agricultural dam must be constructed far from villages. Second, malaria control can be optimized taking in account the spatial and temporal variability. Third, the vectors control in these areas with preventive measures combining long-lasting insecticidal nets (LLINs), indoor residual spraying (IRS) and larval control must be undertaken.
Abbreviations
- b/p/n:
-
bites per person per night
- CI:
-
confidence interval
- EIR:
-
entomological inoculation rate
- ELISA:
-
enzyme-linked immunosorbent assay
- HBR:
-
human biting rate
- ib/p/n:
-
infectious bites per person per night
- ib/p/y:
-
infectious bites per person per year
- ITN:
-
insecticide-treated nets
- KW:
-
Kruskal–Wallis
- LLINs:
-
long-lasting insecticidal nets
- IRS:
-
indoor residual spraying
References
WHO. Water Sanitation and Health Team, World Commission on Dams. Human health and dams: the World Health Organization’s submission to the World Commission on Dams (WCD). Geneva: World Health Organization; 2000.
ICOLD. World Register of Dams, 2003. International Commission on Large Dams. http://www.icold-cigb.org/registre/index.php?lang=en. Accessed 22 March 2015.
Hunter JM, Rey L, Chu KY, Adekolu-John EO, Mott KE. Parasitic diseases in water resources development: the need for intersectoral negotiation. Geneva: World Health Organization; 1993.
Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25.
WHO. World Malaria Report 2008. Geneva: World Health Organization; 2008.
Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223.
Kassahun TJ, Sharon RH, Emiru S, Meshesha B, Teshome G-M, Rickard I, et al. Agro-ecosystems impact malaria prevalence: large-scale irrigation drives vector population in western Ethiopia. Malar J. 2013;12:350.
Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes AM, Yohannes M, et al. Incidence of malaria among children living near dams in northern Ethiopia: community based incidence survey. BMJ. 1999;319:663–6.
Khaemba BM, Mutani A, Bett MK. Studies of anopheline mosquitoes transmitting malaria in a newly developed highland urban area: a case study of Moi University and its environs. East Afr Med J. 1994;71:159–64.
Marrama L, Jambou R, Rakotoarivony I, Leong Pock Tsi JM, Duchemin JB, Laventure S, et al. Malaria transmission in southern Madagascar: influence of the environment and hydro-agricultural works in sub-arid and humid regions: part 1. entomological investigations. Acta Trop. 2004;89:193–203.
Dolo G, Briët OJT, Dao A, Traoré SF, Bouaré M, Sogoba N, et al. Malaria transmission in relation to rice cultivation in the irrigated Sahel, Mali. Acta Trop. 2004;89:147–59.
Dia I, Konate L, Samb B, Sarr JB, Diop A, Rogerie F, et al. Bionomics of malaria vectors and relationship with malaria transmission and epidemiology in three physiographic zones in the Senegal River Basin. Acta Trop. 2008;105:145–53.
Henry MC, Rogier C, Nzeyimana I, Dossou-Yovo J, Assy SB, Audibert M, et al. Inland valley rice production systems and malaria infection and disease in the savannah of Côte d’Ivoire. Trop Med Int Health. 2003;8:449–58.
Betsi N, Koua H, Foua Bi K. Anopheles funestus (Giles, 1900), la riziculture et le paludisme dans la région forestière ouest de la Côte d’Ivoire. Cah Agri. 2003;12:341–6.
Adja AM, N’Goran EK, Koudou BG, Dia I, Kengne P, Fontenille D, et al. Contribution of Anopheles funestus, An. gambiae and An. nili (Diptera: Culicidae) to the perennial malaria transmission in the southern and western forest areas of Côte d’Ivoire. Ann Trop Med Parasitol. 2011;105:13–24.
Elissa N, Mouchet J, Rivière F, Meunier JY, Yao K. Resistance of Anopheles gambiae s.s to pyrethroids in Côte d’Ivoire. Ann Soc Belg Méd Trop. 1993;73:291–4.
Adja MA, N’Goran KE, Kengne P, Koudou GB, Touré M, Koffi AA, et al. Transmission vectorielle du paludisme en savane arborée à Gansé Côte d’Ivoire. Med Trop. 2006;66:445–55.
Tia E, Akogbeto M, koffi A, Touré M, Adja MA, Moussa K, et al. Situation de la résistance d’Anopheles gambiae s.s (Diptera :Culicidae) aus pyréthrinoides at au DDT dans Cinq écosystèmes agricoles de Côte d’Ivoire. Bull soc Pathol Exot. 2006;99:278–82.
MSHP. Plan national de développement sanitaire. Abidjan: Ministère de la Santé et de l’Hygiène Publique; 2012.
Diakité NR, Adja AM, von Stamm T, Utzinger J, N’Goran EK. Situation épidémiologique avant la mise en eau du barrage hydroagricole de cinq villages de Bouaké, centre Côte-d’Ivoire. Bull Soc Pathol Exot. 2010;103:22–8.
WHO. Manual on practical entomology in malaria. Part II. Methods and techniques. Geneva: World Health Organization; 1975.
Gillies MT, de Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian Region). Johannesburg: The South African Institute for Medical Research; 1968.
Detinova TS. Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monograph series. World Health Organ. 1962;47:13–191.
Strome CPA, DeSantis PL, Leef JL, Beaudoin RL. A convenient technique for the dissection of mosquito salivary gland. J Tissue Cult Methods. 1980;6:9–11.
Burkot TR, Zavala F, Gwadz RW, Collins FH, Nussenzweig RS, Robert DR. Identification of malaria infected mosquitoes by a two-site enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1984;33:227–31.
Beier JC. Vector incrimination and entomological inoculation rates. Methods Mol Med. 2002;72:3–11.
Afrane YA, Lawson BW, Brenya R, Kruppa T, Yan G. The ecology of mosquitoes in an irrigated vegetable farm in Kumasi, Ghana: abundance, productivity and survivorship. Parasit Vectors. 2012;5:233.
Doannio JMC, Dossou-Yovo J, Diarrassouba S, Rakotondraibé ME, Chauvancy G, Chandre F, et al. La dynamique de la transmission du paludisme à Kafiné, un village rizicole en zone de savane humide de Côte d’Ivoire. Bull Soc Pathol Exot. 2002;95:11–6.
Briet J, Dossou-Yovo J, Akodo E, Van de Giesen N, Teucher M. The relationship between Anopheles gambiae density and rice cultivation in the savannah and forest zone of Côte d’Ivoire. Trop Med Int Health. 2003;8:439–48.
Betsi AN, Tchicaya SE, Koudou GB. Forte prolifération de larves de An. gambiae et An. funestus en milieux rizicoles irrigués et non irrigués dans la région forestière ouest de la Côte-d’Ivoire. Bull Soc Pathol Exot. 2012;105:220–9.
Mzilahowa T, Hastings IM, Molyneux ME, McCall JP. Entomological indices of malaria transmission in Chikwawa district, Southern Malawi. Malar J. 2012;11:380.
Fillinger U, Sonye G, Killeen GF, Knols BG, Becker N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Trop Med Int Health. 2004;9:1274–89.
Lindsay SW, Wilkins HA, Zieler HA, Daly RJ, Petraca V, Byass P. Ability of Anopheles gambiae mosquitoes to transmit malaria during the dry and wet seasons in an area of irrigated rice cultivation in the Gambia. J Trop Med Hyg. 1990;94:313–24.
Robert V, Dieng H, Lochouarn L. La transmission du paludisme dans la zone de Niakhar. Sénégal. Trop Med Int Health. 1998;3:667–77.
Muturi EJ, Muriu S, Shililu J, Mwangangi J, Jacob BG, Mbogo C, et al. Effect of rice cultivation on malaria transmission in central Kenya. Am J Trop Med Hyg. 2008;78:270–5.
Dossou-yovo J. Etude éthologique des moustiques vecteurs du paludisme en rapport avec les aspects parasitologiques de la transmission du Plasmodium dans la région de Bouaké. Thèse de doctorat d’Etat en Entomologie Médicale. 2000.
Koudou BG, Ouattara FA, Edi AVC, Nsanzabana C, Tia E, Tchicaya ES, et al. Transmission du paludisme en zone de haute couverture en moustiquaires imprégnées d’insecticide de longue durée, au centre de la Côte d’Ivoire. Med Trop. 2010;70:479–84.
Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, Tchuinkam T, et al. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol. 2006;43:1215–21.
Sissoko MS, Dicko A, Briët OJ, Sissoko M, Sagara I, Keita HD, et al. Malaria incidence in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop. 2004;89:161–70.
Dolo G, Briet OJ, Dao A, Traore SF, Bouare M, Sogoba N, et al. Malaria transmission in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop. 2004;89:147–59.
Nzeyimana I, Henry MC, Dossou-Yovo J, Doannio JMC, Diawara L, Carnevale P. Épidémiologie du paludisme dans le Sud-Ouest forestier de la Côte-d’Ivoire (Région de Taï). Bull Soc Pathol Exot. 2002;95:89–94.
Kibret S, Wilson GG, Tekie H, Petros B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar J. 2014;13:360.
Klinkenberg E, Takken W, Huibers F, Toure Y. The phenology of malaria mosquitoes in irrigated rice fields in Mali. Acta Trop. 2003;85:71–82.
Mutero C, Kabutha C, Kimani V, Kabuage L, Gitau G, Ssennyonga J, et al. A trans-disciplinary perspective on the links between malaria and agroecosystems in Kenya. Acta Trop. 2004;89:171–86.
Marrama L, Rajaonarivelo E, Laventure S, Rabarison P. Anopheles funestus and rice culture on the Plateau of Madagascar. Cahi Etu Recher Franc Sante. 1995;5:415–9.
Githeko A, Service MW, Mbogo CM, Atieli FK, Juma FO. Plasmodium falciparum sporozoite and entomological inoculation rates at the Ahero rice irrigation scheme and the Miwani sugar-belt in western Kenya. Ann Trop Med Parasitol. 1993;87:379–91.
Dadzie SK, Brenyah R, Appawu MA. Role of species composition in malaria transmission by the Anopheles funestus group (Diptera: Culicidae) in Ghana. J Vector Ecol. 2013;38:105–10.
Cohuet A, Simard F, Toto JC, Kengne P, Coetzee M, Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am J Trop Med Hyg. 2003;69:200–5.
Authors’ contribution
NRD, JU, and EKN designed the study; NRD, MO, and EKN implemented the study; NRD, NG-C, AMA, MO, and JTC managed the data; NRD, AMA, JU, and EKN analysed and interpreted the data; NRD wrote the first draft of the paper, NG-C, AMA, MO, JTC, JU, and EKN revised the paper. All authors read and approved the final manuscript.
Acknowledgements
We thank Dr. Gilbert Raffier, the collector’s team, and all the population of the five villages. Thanks are addressed to Assamoi J Baptiste and N’Dri Louis for their technical assistance during the laboratory analysis. We are also grateful to Coulibaly Bamoro for generating the map of the study site, to Fofana Diakaridia, Koné Moussa and Koffi Bernard for their excellent collaboration during the mosquito collections. This study was financially supported by FAIRMED (Bern, Switzerland).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Diakité, N.R., Guindo-Coulibaly, N., Adja, A.M. et al. Spatial and temporal variation of malaria entomological parameters at the onset of a hydro-agricultural development in central Côte d’Ivoire. Malar J 14, 340 (2015). https://doi.org/10.1186/s12936-015-0871-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-015-0871-4