Abstract
GAS41, a member of the human YEATS domain family, plays a pivotal role in human cancer development. It serves as a highly promising epigenetic reader, facilitating precise regulation of cell growth and development by recognizing essential histone modifications, including histone acetylation, benzoylation, succinylation, and crotonylation. Functional readouts of these histone modifications often coincide with cancer progression. In addition, GAS41 functions as a novel oncogene, participating in numerous signaling pathways. Here, we summarize the epigenetic functions of GAS41 and its role in the carcinoma progression. Moving forward, elucidating the downstream target oncogenes regulated by GAS41 and the developing small molecule inhibitors based on the distinctive YEATS recognition properties will be pivotal in advancing this research field.
Similar content being viewed by others
Introduction
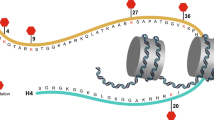
The human YEATS family comprises four members: all1-fused genes from chromosome 9 (AF9), eleven-nineteen-leukemia (ENL), glioma amplified sequence 41 (GAS41), and YEATS2 (Fig. 1). The acronym "YEATS" stems from the initial letters of five related domain proteins (Yaf9, ENL, AF9, Taf14, and Sas5) [1]. The family exhibits a conserved YEATS domain at the N-terminus (Fig. 1) and plays diverse roles in chromatin dynamics, histone modifications, and gene regulation [2]. YEATS primarily governs transcriptional elongation, histone modification, histone variant (H2A.Z) deposition, and chromatin remodeling in epigenetics (Fig. 2). AF9 and ENL act as fusion chaperones for human mixed lineage leukemia proteins (MLL) resulting from chromosomal translocations and contribute to acute myeloid leukemia [3, 4]. At the molecular level, AF9 recruits transcription factors such as the super elongation complex (SEC) [5] and polymerase-associated factor 1 (PAF1) [6] to recognize acetylation modifications and regulate downstream transcriptional elongation (Fig. 2). Acetylation is one of diverse acylation. The type of acylation depends primarily on the acyl groups attached to the lysine residue, including acetyl-, succinyl-, malonyl-, crotonyl-, β-hydroxybutyryl-, lactyl-, myristoyl-, and palmitoyl-CoA [7]. Furthermore, AF9 is involved in histone methylation through AF10 [8] and histone-lysine N-methyltransferase DOT1L [9] (Fig. 2). ENL, in combination with the monocytic leukemia zinc (MOZ) complex [10], and the BRG1-associated factor (BAF) [11] facilitates histone acetylation reading and chromatin remodeling (Fig. 2). Extensive research has unveiled MLL as a classical downstream target gene of AF9 and ENL [12, 13]. Additionally, AF9 targets a crucial set of genes associated with epithelial-to-mesenchymal transition (EMT) [14]. Apart from MLL, the well-known proto-oncogene MYC is also targeted by ENL [14], and it holds great potential as a candidate for cancer therapy [15]. YEATS2 acts as a distinct reader of histone crotonylation [16] and serves as a novel oncogene in various cancers [17,18,19]. YEATS2 has also been found to activate the TAK1/NF-κB and PI3K/AKT signaling pathways, influencing cancer cell survival [20, 21]. To delve into the underlying mechanisms, YEATS2 is known to recruit the Ada-two-A-containing (ATAC) complex [22] to identify specific histone modifications and facilitate histone modifications (Fig. 2). It is noteworthy that ATAC functions as a transcriptionally active complex involved in chromosome remodeling [23], comprising transcription factors such as general control non-depressible protein 5 (GCN5) [24], ATAC2, alternation/deficiency in activation-3 (ADA3) [25] and zinc finger ZZ-type containing 3 (ZZZ3) [26]. YEATS2 is highly expressed in human pancreatic ductal adenocarcinoma (PDAC) and can positively regulate the growth, survival, and tumorigenesis of PDAC cells [21]. The binding of YEATS2 is crucial for maintaining transforming growth factor beta-activated kinase 1 (TAK1) activation and NF-κB transcriptional activity. Of importance, YEATS2 promotes NF-κB transcriptional activity through modulating TAK1 abundance and directly interacting with NF-κB as a co-transcriptional factor [21]. GAS41, a polymorphic protein, plays a crucial role in recognizing lysine-acylated histones through its YEATS domain [27]. GAS41 selectively recognizes histone modifications that are frequently associated with downstream diseases, including cancer [28]. Complex structural studies have revealed that the YEATS domain utilizes a common binding pocket to interpret distinct lysine acylation modifications [29]. The acylated lysine side chain extends into the YEATS domain, where it interacts with a set of aromatic residues, forming the well-known aromatic sandwich model (also referred to as ‘π-π-π’) [28]. Notably, the aromatic amino acids W93, F96, and Y74 play a central role in the representative structure of the GAS41-YEATS-H3K27ac complex [30, 31]. Intriguingly, GAS41 has been characterized as a novel oncogene with indications of aberrant amplification in various cancers [32]. Importantly, GAS41 has been found to target downstream regulators such as Zinc finger E-box-binding homeobox 1 (ZEB1) [33], transforming acidic coiled-coil 1 (TACC) [34], and (Transcription elongation factor A protein 1) TCEA1 [35] to modulate cancer progression (Fig. 2). In conclusion, GAS41 exhibits dual identities in the organism, functioning either as a transcription factor involved in epigenetic regulation or as a signal transduction protein participating in intracellular signal transduction within cancer cells.
Structure of the human YEATS family. A Schematic diagram of the one-dimensional structure of the YEATS family. GAS41 is the shortest one, comprising 227 amino acids and a CC region at the C-terminus, whereas YEATS2 is the longest amino acid sequence and contains a histone fold at the end. B PDB structure of the YEATS domain. File from PBD: 7eic, 5j9s, 7eif, 7eie. ‘aa’: amino acid, ‘CC’: coiled-coil, ‘α/β’: α helix and β hairpin motif, 'HF': histone fold
The oncogenic potential of GAS41
GAS41, originating from the chromosome 12q13-15 region of glioma cells, is frequently amplified in gliomas [36]. Notably, most gene amplifications in gliomas occur in advanced tumor stages. However, the early-stage amplification of GAS41 suggests its significant oncogenic activity during early tumor progression [37]. Overexpression of GAS41 has been observed in various cancer types, such as breast cancer [38], non-small cell lung cancer [31, 39], hepatocellular carcinoma [35, 40], pancreatic cancer [41, 42], gastric cancer [43, 44], colorectal cancer [45, 46], atypical adipose carcinoma [47], ovarian cancer [48], and uterine fibroids [49, 50]. These findings have prompted researchers to reevaluate the oncogenic properties of GAS41.
GAS41 as a transcription factor
Initially identified in the nucleus, GAS41 demonstrates transcription factor properties [51]. Notably, GAS41 shares significant sequence homology with the human mixed spectrum leukemia translocation protein (MLLT1/MLLT3), commonly referred to as ENL and AF9 [13, 52]. Studies have revealed that GAS41 participates in the assembly of two multi-subunit complexes, namely tat-interactive protein 60 / e1a-binding protein p400 / transcription/transformation domain-associated protein (TIP60/p400/TRRAP) and SNF2-related CBP activator protein (SRCAP) [53]. The involvement of activating enhancer-binding protein 2-beta (AP-2β) [54] and RNA polymerase-associated proteins 30(RAP30) [55] is essential for the recognition of histone acylation modifications by GAS41 (Fig. 2). By recognizing histone modifications, GAS41 facilitates the participation of histones in nucleosome remodeling, thereby influencing gene transcription [1, 28, 56].
Role of GAS41 in the context of epigenetic signaling
Epigenetic signaling is the biological process that leads to changes in epigenetic marks such as DNA methylation, histone modifications (Kac, Kcr, Klac, etc.), non-coding RNAs (miRNAs and siRNAs), and chromatin accessibility [57,58,59,60]. Functional readouts of histone modifications serve as an important mechanism for epigenetic signaling. GAS41 is involved in epigenetic signaling primarily as a reader recognizing histone modifications [61], including histone acetylation (H3K27ac and H3K14ac) [30, 31], benzoylation (H3K27bz) [62], crotonylation (H3K27cr) [63], and succinylation (H3K122suc) [64]. Histone modifications typically affect nucleosome stability and serve as anchors for chromatin-associated protein complexes [28]. GAS41 likely establishes a signaling axis that links histone modification readouts to H2A.Z deposition. For example, the readouts of H3K27ac and H3K14ac by GAS41 could recruit Tip60/p400 or SRCAP complexes to deposit H2A.Z into specific chromatin regions [30, 31]. GAS41 could recruit the Dot1l-RNA polymerase (Pol) II complex to the gene promoter by recognizing H3K27ac, thereby initiating gene transcription [65]. By binding to H3K27cr, GAS41 can be recruited by MYC to the SIN3A-HDAC1 co-repressor to repress the transcription of p21-related genes [63]. H3K122suc is recognized by GAS41 in a pH-dependent manner and is co-enriched with GAS41 at the p21 promoter [64]. In parallel to histone modifications, GAS41 can also participate in epigenetic signaling through non-coding RNAs. lncAKHE, a long non-coding RNA highly expressed in hepatocellular carcinoma, was found to cooperate with GAS41 to enhance the expression of NOTCH2-related genes [40]. Additionally, GAS41 is a pivotal component of RNAi and has been identified as a potential epigenetic regulator of miR-203, miR-218, and miR-10b [46, 66, 67]. A recent study indicated that HDAC3 mediates transcriptional repression through GAS41 and the co-repressor DMAP1 [68]. Such evidence provides additional context for the epigenetic regulatory functions of GAS41 beyond the well-described mechanisms primarily associated with histone modification.
GAS41: Advancing cancer research
The exploration of the role of GAS41 in cancer has been a gradual process, with significant attention being directed to this field only in recent years. Initially, Park et al. identified that loss of GAS41 function resulted in the upregulation of two tumor suppressors, p14ARF and p53 [53]. Building upon these findings, Llanos et al. proposed GAS41 as a robust negative regulator of p53, independent of the chromatin removal or modification complexes [69]. Subsequent investigations revealed a novel oncogenic mechanism of p53 dephosphorylation by GAS41 through the phosphatase specificity of the GAS41-PP2Cβ complex, specifically targeting phosphorylated serine at position 366 of p53 [37]. Studies have shown that increased GAS41 inhibits apoptosis. The collaboration between GAS41 and lncAKHE activates the NOTCH2 pathway, which plays a critical role in controlling the apoptosis of hepatocellular carcinoma cells [40]. The coexistence of GAS41 and lncRNA implies a complex regulatory mechanism for GAS41. Fu et.al uncovered that miR-218 sensitized HCT-116/L-OHP (Oxaliplatin) cells to L-OHP-induced cell apoptosis via inhibition of cytoprotective autophagy by targeting GAS41 expression. Xian et.al indicated that the upregulation of GAS41 promotes DNA damage repair and prevents cell death, whereas its downregulation inhibits DNA replication and induces apoptosis [70]. Moreover, GAS41 enhances the proliferation of gastric cancer cells by upregulating its expression to activate the Wnt/β-catenin signaling pathway [43]. However, while many studies have focused on the role of GAS41 in promoting cancer cells, there is still a pressing need to understand its contributions to cancer invasion and metastasis. In this context, we present the existing reported pathways involving GAS41 in cancer to lay the foundation for further investigations on GAS41 (Fig. 3 and Additional file 1: Fig. S1).
An overview of cancer-associated roles of GAS41. An overview of the molecular mechanisms of GAS41 in numerous cancers. HCC hepatocellular carcinoma, BC breast cancer, NSCLC non-small cell lung cancer, PC pancreatic cancer, GC gastric cancer, CRC colorectal cancer, ULs uterine fibroids, LPS liposarcoma, OC ovarian cancer
GAS41 in Hepatocellular Carcinoma (HCC)
Hepatocellular carcinoma (HCC) is a highly lethal liver malignancy with a rising global incidence [71]. The challenging aspects of early diagnosis and the aggressive, metastatic, and recurrent nature of HCC contribute to its poor prognosis [72]. Therefore, investigating the key pathways involved in HCC development is crucial, as it may provide valuable insights into identifying early biomarkers and potential therapeutic targets. Previous studies show that TCEA1 is significantly upregulated in HCC [73]. DDX3 was first known for its role in the proliferation and transformation of eternalized human breast cancer epithelial cells [74]. Studies have hinted at DEAD box protein 3 (DDX3) growth-regulatory functions in hepato-carcinogenesis and progression [75, 76]. Upregulation of TCEA1 increased the stability of DDX3 protein and enhanced the proliferation and colony formation of HCC cells [35]. You et al. uncover that GAS41 is significantly upregulated in HCC and correlates with poor prognosis, tumor size, differentiation, and metastasis [35]. GAS41 enhances the transcription of TCEA1 by binding to the TCEA1 promoter, resulting in the upregulation of TCEA1 expression, which stabilizes DDX3 protein and promotes proliferation and colony formation of HCC cells [35]. Furthermore, recent findings highlight the strong expression of lncAKHE in HCC tissues, and its interaction with GAS41 activates the NOTCH2 pathway [40]. These observations strongly support the potential role of GAS41 in HCC (Fig. 3). Consequently, GAS41 emerges as a promising therapeutic target and prognostic indicator for HCC.
GAS41 in breast cancer (BC)
Breast cancer (BC) is a prevalent malignancy affecting the epithelial cells of the breast, and it ranks as the second most common cancer in women, leading to significant morbidity and mortality [77]. Although BC is often curable in the early stages, its metastatic nature poses significant challenges for treatment. Therefore, there is an urgent need to identify biomarkers associated with metastatic BC [78]. Previous research has established a strong correlation between GAS41 and the breast cancer suppressor TACC [79], with GAS41 shown to interact with TACC1 and TACC2 of the TACC family [80]. However, the exact role of this interaction in the oncogenic process is currently unknown. TACC has been instrumental in BC cell proliferation, and the immunohistochemical status of TACC2 has emerged as a potential prognostic marker of poor prognosis of BC patients [81]. TACC proteins have recently emerged as important players in the complex process of regulating microtubule dynamics during cell division [82, 83]. TACC proteins are usually localized to centrosomes [84] and all phenotypes of altered TACC expression are associated with defects in microtubule stability [85, 86]. Therefore, it is reasonable to speculate that the binding of GAS41 and TACC may affect the cytokinesis process in cancer cells. Notably, overexpression of GAS41 in BC reinforces the malignant features, especially inducing epithelial-mesenchymal transition (EMT), which contributes to an aggressive phenotype in both vitro and vivo models [38]. In contrast, the knockdown of GAS41 suppresses cell growth, promotes mesenchymal-epithelial transformation (MET), and inhibits BC metastasis [38]. The positive regulatory impact of GAS41 on ZEB1 transcription through the recognition of histone H3K27 acetylation (H3K27ac) underlies these biological behaviors [38]. In summary, GAS41 can influence breast cancer progression either through its interaction with the TACC pathway or by regulating ZEB1 expression (Fig. 3). The documented significance of GAS41 in BC progression and metastasis underscores the potential therapeutic value of targeting GAS41 expression in BC treatment.
GAS41 in non-small cell lung cancer (NSCLC)
Non-small cell lung cancer (NSCLC) comprises approximately 80–85% of all lung cancers [87]. Unfortunately, only a small fraction of NSCLC patients are diagnosed at an early stage (stage I/II) when surgical resection is a viable treatment option. The majority of lung cancer patients (more than 60%) present with locally advanced or metastatic disease (stage III/IV) at the time of diagnosis [88]. Pikor et al., through their gene expression analysis, identified GAS41 as a novel candidate oncogene for NSCLC. Moreover, they revealed that GAS41 is an important negative regulator of the p21-p53 pathway [39]. Frequent amplification of GAS41 in NSCLC has been observed, and its presence is crucial for the survival and transformation of NSCLC cells [31]. Intriguingly, ChIP-seq results have shown that GAS41 co-localizes with H3K27ac and H3K14ac at the promoters of actively transcribed genes. The knockdown of GAS41 or disruption of the interaction between the YEATS domain and acetylated histones impairs the association of the histone variant H2A.Z with chromatin, thereby inhibiting the growth and survival of NSCLC cells both in vitro and in vivo [31]. These findings suggest that GAS41 influences the deposition of H2A.Z on chromatin by recognizing histone acetylation modifications, ultimately regulating the promotion of NSCLC (Fig. 3). Recent studies have reported the development of a new dimeric analog with a nanomolar activity that targets lung cancer cells by blocking the interaction between GAS41 and acetylated histone H3. This analog effectively inhibits the growth of NSCLC cells [27]. The investigation of the regulatory mechanisms of GAS41 in NSCLC and the identification of small molecule inhibitors could offer a promising framework for the treatment of NSCLC.
GAS41 in pancreatic cancer (PC)
Pancreatic cancer (PC) is a highly aggressive malignancy with a discouraging 5-year overall survival rate of only 11% [89]. In the early stages, PC often presents with no noticeable symptoms, and clinical manifestations typically appear once the tumor invades surrounding tissues or metastasizes to distant organs. Remarkably, epigenetic alterations, including DNA methylation, histone modifications, and alterations in non-coding RNAs, can profoundly impact gene function in PC [90]. Elevated expression of GAS41 has been observed in clinical PC specimens and mouse models, and the expression level of GAS41 is associated with PC cell growth, migration, and invasion [41]. Mechanistic investigations have revealed that GAS41 interacts with β-catenin and acts as a positive regulator to activate β-catenin/TCF signaling to promote PC cell growth and metastasis [41, 91, 92]. It is important to note that GAS41 has previously been shown to promote H2A.Z deposition via recognition of histone acetylation [31]. Recent studies have reported that H2A.Z.2 is overexpressed in human PC tissues and cell lines, and exogenous expression of GAS41 or H2A.Z.2 promotes NOTCH and NOTCH-mediated cancer cell stemness and GEM resistance [42]. However, a number of questions remain to be addressed. For example, what is the mechanism by which GAS41 promotes the deposition of ac H2A.Z.2. In summary, the association of GAS41 with PC is mediated through the β-catenin/TCF pathway and the NOTCH pathway (Fig. 3). Understanding the intricate mechanisms involving GAS41 in PC progression provides valuable insights for developing targeted therapeutic strategies against this devastating disease.
GAS41 in gastric cancer (GC)
Gastric cancer (GC) is a significant global concern, with the majority of cases being diagnosed at stage IV of the disease, and poor prognosis [93]. Late-stage diagnosis and high mortality rates highlight the urgent need for novel therapeutic targets in GC. Claudin-18.2 [94], inhibitors of the fibroblast growth factor receptor 2 (FGF2) pathway [95], and combinations of anti-angiogenesis with immune checkpoint blockade are three recognized therapeutic targets for GC [96]. It has been established that GAS41 is highly expressed in GC tissues and cell lines, and its increased expression has been linked to enhanced cell proliferation and attenuated apoptosis through activation of the Wnt/β-catenin signaling pathway [43, 70]. Furthermore, an analysis of five GC cell lines and 135 GC primary tumor samples revealed that patients with GAS41 overexpressing tumors have lower overall survival rates, and cell lines with GAS41 knockouts exhibit significantly increased chemosensitivity to CDDP (Cisplatin) and L-OHP [44]. In summary, GAS41 regulates the survival of GC cells by activating the Wnt/β-catenin signaling pathway (Fig. 3). The role of GAS41 as a prognostic factor and potential therapeutic target highlights its contribution to tumor malignancy in GC. Therefore, modulating GAS41 expression holds promise as an effective therapeutic approach for GC.
GAS41 in colorectal cancer (CRC)
Colorectal cancer (CRC) ranks as the second most common cancer in women and the third most common cancer in men [97]. Globally, approximately 10% of cancer cases and cancer-related deaths can be attributed to CRC [98]. The majority of CRC cases arise from stem cells or stem cell-like cells [99], resulting from the accumulation of genetic and epigenetic alterations. An analysis of GAS41 expression in 85 pairs of CRC and paracancerous tissues reveals that inhibition of GAS41 expression leads to cell cycle arrest in the G0/G1 phase and a significant increase in apoptotic cell numbers [45]. Additionally, a recent discovery unveiled that miR-218 inhibits cytoprotective autophagy by targeting GAS41, thereby sensitizing CRC cells to apoptosis induced by Oxaliplatin (L-OHP) [46]. MiRNAs have been extensively implicated in tumor proliferation, invasion, angiogenesis, and drug resistance [100], and dysregulation of several miRNAs has been reported in CRC [101, 102]. These findings represent a potential breakthrough in the diagnosis and treatment of colon cancer. Collectively, these studies indicate that GAS41 may serve as a critical modulator of proliferation and apoptosis in CRC cells and could regulate drug sensitivity in CRC through miRNAs (Fig. 3).
GAS41 in uterine leiomyomas (ULs)
Uterine leiomyomas (ULs) represent the most prevalent benign gynecological tumors observed in women of reproductive age and postmenopausal women [103]. Abnormalities in various epigenomes have been identified in ULs, suggesting their involvement in the development and growth of these tumors [104]. In a recent clinical study utilizing genome-wide datasets to analyze UL origins, somatic mutations in six genes encoding the SRCAP histone loading complex were identified as biomarkers [50]. Notably, germline mutations in GAS41 and ZNHIT1 were found to predispose women to ULs. Tumors harboring these mutations exhibited impaired deposition of the histone variant H2A.Z [50]. Therefore, GAS41 may regulate the formation of uterine fibroids by influencing the deposition of the histone variant H2A.Z (Fig. 3). Additionally, a comprehensive evaluation of protein-coding genes in an extended exome sequencing cohort of 233,614 white European women further confirmed GAS41 as a significant contributor to UL susceptibility [49]. However, the biological function of GAS41 in ULs remains largely unknown, as current investigations are predominantly limited to bioinformatic analyses. Further research is needed to elucidate the precise role of GAS41 in ULs.
GAS41 in liposarcoma (LPS)
Liposarcoma (LPS) is a rare malignant tumor characterized by adipocytic differentiation [105]. It is classified into four major subtypes: highly differentiated LPS (WDLPS, also known as atypical lipomatous tumors), dedifferentiated LPS (DDLPS), mucinous-like LPS (MLPS), and pleomorphic LPS (PLPS) [47]. Previous studies have suggested that GAS41 may serve as a critical oncogene in atypical LPS [106]. Barretina et al. showed that the knockdown of GAS41 significantly reduced cell proliferation in DDLPS [107]. Moreover, recent literature has reported aberrant amplification of GAS41 in atypical LPS [108]. Collectively, these findings suggest a potential active role for GAS41 in liposarcoma. However, the precise function of GAS41 in LPS and the underlying signaling pathways involved remain unclear.
GAS41 in ovarian cancer (OC)
Ovarian cancer (OC) is a highly life-threatening malignancy affecting women worldwide, necessitating the urgent discovery of effective biomarkers [109]. This rapidly proliferating cancer exhibits temporary chemosensitivity, imposes pressure on internal organs, and exhibits a cure rate of only 30% [110]. An inherent challenge in OC treatment is that most patients are diagnosed with advanced-stage disease, and long-term chemotherapy often leads to drug resistance [111]. In a study by Kim et al., the analysis of drug resistance-associated transcription factors (TFs) in OC highlighted GAS41 as a key transcription factor that induces chemoresistance through an intrinsic apoptosis-related pathway [48] (Fig. 3). To future elucidate the role and mechanism of GAS41 in the pathogenesis of OC, comprehensive investigations are warranted.
Continued research on GAS41
Cells employ a diverse repertoire of transcriptional regulatory proteins to finely modulate gene expression [32]. Studies have established the crucial role of recognizing post-translational modifications of histones in transcriptional regulation [112]. A relatively recent discovery, the YEATS domain proteins, constitute a family of epigenetic reader proteins [1, 2, 62, 113]. Dysregulation of epigenetic reader proteins is frequently observed in cancer, making them attractive targets for the development of small molecule inhibitors [27, 114,115,116]. Listunov et al. have developed a nanomolar active dimeric analog that disrupts the interaction between GAS41 and acetylated histone H3 [27]. Subsequently, Londregan et al. have identified selective small molecule inhibitors with a bias toward the YEATS domain [117]. Biochemical investigations have shown that the YEATS domain of GAS41 recognizes histone acetylation, benzoylation, succinylation, and crotonylation [31, 62,63,64, 118] (Table 1). Notably, Liu et al. assume a three-phase traffic-light system model describing three different H3K27 modifications as distinct chromatin states for gene transcription [63]. H3K27me3 (stop) marks for transcriptional silencing, H3K27cr (pause) for transcriptional repression, and H3K27ac for transcriptional activation (go) [63]. Aberrant patterns of histone acylation are closely linked to human cancers [119], influencing gene expression and cell signaling processes within tumors [7, 120]. Understanding the mechanisms underlying histone acylation recognition and deposition is vital for developing effective anti-cancer strategies [7]. While the functional aspects of acetylation are relatively well-established and comprehensive, studying the functional disparities between non-acetylated and acetylated histones remains challenging. Histone Kcr levels are reduced in prostate cancer, HCC, GC, and kidney cancer [121, 122], while they are increased in intestinal cancer, thyroid cancer, esophagus cancer, PC, and NSCLC [122, 123]. Thus, the level of histone Kcr directly or indirectly affects the characteristics of cancer cells. Histone Ksucc is implicated in PC [124], esophageal squamous cell carcinoma [125], GC [126], renal cell carcinoma [127], HCC [128], CRC [129], and glial blastoma [130]; however, its role in tumor development is context-dependent. All the evidence suggests that GAS41 holds significant potential for association with various cancers through the recognition of acylated modifications (Fig. 4, Additional file 2: Fig. S2).
Conclusions and outlooks
Cancer cells rely on chromatin regulatory pathways and transcriptional mechanisms to maintain an oncogenic state, making these processes attractive targets for drug development. Metabolic remodeling is a hallmark of cancer cells, leading to abnormal accumulation of metabolites. Covalent modification of proteins through lysine acylation by various metabolites contributes to epigenetic remodeling. Aberrant epigenetic landscapes in cancer cells often exploit chromatin mechanisms to activate oncogenic gene expression programs. The recognition of histone modifications by "reader" proteins is a key process in these events. As a representative of the reader module for short-chain lysine acylation, the YEATS domain plays a critical role in lysine acylation biology, serving as a link between metabolism and gene regulation. GAS41, a novel epigenetic reader of acylated modifications, holds significant potential for association with pathophysiological processes in relevant cancers through its recognition of acylated modifications (Additional file 2: Fig. S2). Histone lactylation (Klac) is a recently discovered component of the human cellular epigenetic landscape (Fig. 5B), sensitive to both exogenous and endogenous lactate levels [131]. Elevated lactate levels in the tumor microenvironment (TME) lead to increased intracellular lactylation, and both lactylation and lactate have been considered for cancer therapy [132]. Lactylation has been implicated in tumor immune escape mediated by tumor-infiltrating myeloid cells (TIMs) [133]. Lactate enhances the stemness of CD8+ T cells and improves anti-tumor capacity [134]. Controlling the glycolytic switch marked by lactylation presents therapeutic opportunities for cancer [135]. Lysine glutarylation (Kglu), another newly characterized protein lysine modification (Fig. 5C), exhibits diverse functions in eukaryotic cells [136,137,138,139]. However, the role of Kglu as a reader in cells and its contribution to cancer remains unclear. Given its chemical structural properties resembling Kac and Ksucc modifications, GAS41 is likely to act as a reader for Klac and Kglu (Fig. 5). Currently, there are limited studies on GAS41’s recognition of acylated modifications in cancer cells. Therefore, establishing a comprehensive framework to understand the complexity and specificity of GAS41 is crucial. Moving forward, investigating GAS41’s recognition of histone acylation modifications to target downstream oncogenes and developing small molecule inhibitors to disrupt this process will be promising areas of research. In summary, further in-depth exploration is required to enhance our understanding of GAS41 as a signaling transduction protein and transcription factor.
GAS41 plays a role in various cancers by reading novel modifications. Schematic representation of the hypothesis that GAS41 recognizes novel histone acylation modifications. A GAS41 recognizes the histone modifications through the YEATS domain. B Chemical structural formulae of some lysine acylation modifications. Lac lactylation modification; Glu glutarylation modification
Availability of data and materials
Not applicable.
Abbreviations
- ADA3:
-
Alternation/deficiency in activation-3
- AF9:
-
ALL1-fused gene from chromosome 9
- AP-2β:
-
Activating enhancer-binding protein 2-beta
- ATAC:
-
Ada-two-A-containing
- BAF:
-
BRG1-associated factor
- BC:
-
Breast cancer
- CRC:
-
Colorectal cancer
- CDDP:
-
Cisplatin
- DDX3:
-
DEAD-box protein 3
- DDLPS:
-
Dedifferentiated LPS
- DOT1L:
-
Histone-lysine N-methyltransferase
- EMT:
-
Epithelial-mesenchymal transition
- ENL:
-
Eleven-nineteen-leukemia
- FGF2:
-
Fibroblast growth receptor 2
- GAS41:
-
Glioma amplified sequence 41
- GC:
-
Gastric cancer
- GCN5:
-
General control non-repressed protein 5
- GEM:
-
Gemcitabine
- HCC:
-
Hepatocellular carcinoma
- Kac:
-
Lysine acetylation
- Kbz:
-
Benzoylation
- Kcr:
-
Lysine crotonylation Klac Lysine lactylation
- Kglu:
-
Lysine glutarylation
- Ksucc:
-
Lysine succinylation
- L-OHP:
-
Oxaliplatin
- LPS:
-
Liposarcoma
- Me:
-
Methylation
- MLL:
-
Mixed lineage leukemia
- MLLT1:
-
Mixed-lineage leukemia translocated to 1
- MLLT3:
-
Mixed-lineage leukemia translocated to 3
- MLPS:
-
Mucinous-like LPS
- MOZ:
-
Monocytic leukemia zinc
- NSCLC:
-
Non-small cell lung cancer
- OC:
-
Ovarian cancer
- PAF1:
-
Polymerase-associated factor 1
- PC:
-
Pancreatic cancer
- PLPS:
-
Pleomorphic LPS
- PP2Cβ:
-
Protein phosphatase 2C protein phosphatase 2C β
- p400:
-
E1A-binding protein p400
- RAP30:
-
RNA polymerase-associated proteins 30
- SEC:
-
Super elongation complex
- SRCAP:
-
SNF2-related CBP activator protein
- TACC:
-
Transforming acidic coiled-coil 1
- TAK1:
-
Transforming growth factor beta-activated kinase 1
- TCEA1:
-
Transcription elongation factor A protein 1
- TFs:
-
Transcription factors
- TIP60:
-
Tat-interactive protein 60
- TME:
-
Tumor microenvironment
- TRRAP:
-
Transcription/transformation domain-associated protein
- ULs:
-
Uterine leiomyomas
- WDLPS:
-
Highly differentiated LPS
- ZEB1:
-
Zinc finger E-box-binding homeobox 1
- ZZZ3:
-
Zinc finger ZZ-type containing 3
References
Schulze JM, Wang AY, Kobor MS. YEATS domain proteins: a diverse family with many links to chromatin modification and transcription. Biochem Cell Biol. 2009;87:65–75.
Liu Y, Li Q, Alikarami F, Barrett DR, Mahdavi L, Li H, Tang S, Khan TA, Michino M, Hill C, et al. Small-molecule inhibition of the Acyl-lysine reader ENL as a strategy against acute myeloid leukemia. Cancer Discov. 2022;12:2684–709.
Zhu XN, Wei YS, Yang Q, Liu HR, Zhi Z, Zhu D, Xia L, Hong DL, Yu Y, Chen GQ. FBXO22 promotes leukemogenesis by targeting BACH1 in MLL-rearranged acute myeloid leukemia. J Hematol Oncol. 2023;16:9.
Hu H, Saha N, Yang Y, Ahmad E, Lachowski L, Shrestha U, Premkumar V, Ropa J, Chen L, Teahan B, et al. The ENL YEATS epigenetic reader domain critically links MLL-ENL to leukemic stem cell frequency in t(11;19) Leukemia. Leukemia. 2023;37:190–201.
Pal S, Yadav D, Biswas D. ATM-mediated ELL phosphorylation enhances its self-association through increased EAF1 interaction and inhibits global transcription during genotoxic stress. Nucleic Acids Res. 2022;50:10995–1012.
Rauth S, Ganguly K, Atri P, Parte S, Nimmakayala RK, Varadharaj V, Nallasamy P, Vengoji R, Ogunleye AO, Lakshmanan I, et al. Elevated PAF1-RAD52 axis confers chemoresistance to human cancers. Cell Rep. 2023;42: 112043.
Shang S, Liu J, Hua F. Protein acylation: mechanisms, biological functions and therapeutic targets. Signal Transduct Target Ther. 2022;7:396.
Chen BR, Deshpande A, Barbosa K, Kleppe M, Lei X, Yeddula N, Vela PS, Campos AR, Wechsler-Reya RJ, Bagchi A, et al. A JAK/STAT-mediated inflammatory signaling cascade drives oncogenesis in AF10-rearranged AML. Blood. 2021;137:3403–15.
Valencia-Sánchez MI, De Ioannes P, Wang M, Truong DM, Lee R, Armache JP, Boeke JD, Armache KJ. Regulation of the Dot1 histone H3K79 methyltransferase by histone H4K16 acetylation. Science. 2021. https://doi.org/10.1126/science.abc6663.
Komata Y, Kanai A, Maeda T, Inaba T, Yokoyama A. MOZ/ENL complex is a recruiting factor of leukemic AF10 fusion proteins. Nat Commun. 1979;2023:14.
Jiménez C, Antonelli R, Nadal-Ribelles M, Devis-Jauregui L, Latorre P, Solé C, Masanas M, Molero-Valenzuela A, Soriano A, Sánchez de Toledo J, et al. Structural disruption of BAF chromatin remodeller impairs neuroblastoma metastasis by reverting an invasiveness epigenomic program. Mol Cancer. 2022;21:175.
Olsen SN, Godfrey L, Healy JP, Choi YA, Kai Y, Hatton C, Perner F, Haarer EL, Nabet B, Yuan GC, Armstrong SA. MLL::AF9 degradation induces rapid changes in transcriptional elongation and subsequent loss of an active chromatin landscape. Mol Cell. 2022;82:1140-1155.e1111.
Kabra A, Bushweller J. The intrinsically disordered proteins MLLT3 (AF9) and MLLT1 (ENL)—multimodal transcriptional switches with roles in normal hematopoiesis, MLL fusion leukemia, and kidney cancer. J Mol Biol. 2022;434: 167117.
Tian X, Yu H, Li D, Jin G, Dai S, Gong P, Kong C, Wang X. The miR-5694/AF9/Snail axis provides metastatic advantages and a therapeutic target in basal-like breast cancer. Mol Ther. 2021;29:1239–57.
Duffy MJ, O’Grady S, Tang M, Crown J. MYC as a target for cancer treatment. Cancer Treat Rev. 2021;94: 102154.
Zhao D, Guan H, Zhao S, Mi W, Wen H, Li Y, Zhao Y, Allis CD, Shi X, Li H. YEATS2 is a selective histone crotonylation reader. Cell Res. 2016;26:629–32.
Sha T, Li J, Sun S, Li J, Zhao X, Li Z, Cui Z. YEATS domain-containing 2 (YEATS2), targeted by microRNA miR-378a-5p, regulates growth and metastasis in head and neck squamous cell carcinoma. Bioengineered. 2021;12:7286–96.
Zeng Z, Lei S, He Z, Chen T, Jiang J. YEATS2 is a target of HIF1α and promotes pancreatic cancer cell proliferation and migration. J Cell Physiol. 2021;236:2087–98.
Mi W, Guan H, Lyu J, Zhao D, Xi Y, Jiang S, Andrews FH, Wang X, Gagea M, Wen H, et al. YEATS2 links histone acetylation to tumorigenesis of non-small cell lung cancer. Nat Commun. 2017;8:1088.
Liu X, Hu Y, Li C, Chen J, Liu X, Shen Y, Xu Y, Chen W, Xu X. Overexpression of YEATS2 remodels the extracellular matrix to promote hepatocellular carcinoma progression via the PI3K/AKT pathway. Cancers. 2023. https://doi.org/10.3390/cancers15061850.
Sheng H, Zheng F, Lan T, Chen HF, Xu CY, Wang SW, Weng YY, Xu LF, Zhang F. YEATS2 regulates the activation of TAK1/NF-κB pathway and is critical for pancreatic ductal adenocarcinoma cell survival. Cell Biol Toxicol. 2021. https://doi.org/10.1007/s10565-021-09671-4.
Suganuma T, Gutiérrez JL, Li B, Florens L, Swanson SK, Washburn MP, Abmayr SM, Workman JL. ATAC is a double histone acetyltransferase complex that stimulates nucleosome sliding. Nat Struct Mol Biol. 2008;15:364–72.
Merritt N, Garcia K, Rajendran D, Lin ZY, Zhang X, Mitchell KA, Borcherding N, Fullenkamp C, Chimenti MS, Gingras AC, et al. TAZ-CAMTA1 and YAP-TFE3 alter the TAZ/YAP transcriptome by recruiting the ATAC histone acetyltransferase complex. elife. 2021;10:62857.
Haque ME, Jakaria M, Akther M, Cho DY, Kim IS, Choi DK. The GCN5: its biological functions and therapeutic potentials. Clin Sci (Lond). 2021;135:231–57.
Espinola-Lopez JM, Tan S. The Ada2/Ada3/Gcn5/Sgf29 histone acetyltransferase module. Biochim Biophys Acta Gene Regul Mech. 2021;1864: 194629.
Mi W, Zhang Y, Lyu J, Wang X, Tong Q, Peng D, Xue Y, Tencer AH, Wen H, Li W, et al. The ZZ-type zinc finger of ZZZ3 modulates the ATAC complex-mediated histone acetylation and gene activation. Nat Commun. 2018;9:3759.
Listunov D, Linhares BM, Kim E, Winkler A, Simes ML, Weaver S, Cho HJ, Rizo A, Zolov S, Keshamouni VG, et al. Development of potent dimeric inhibitors of GAS41 YEATS domain. Cell Chem Biol. 2021;28:1716-1727.e1716.
Zhao D, Li Y, Xiong X, Chen Z, Li H. YEATS domain-A histone acylation reader in health and disease. J Mol Biol. 2017;429:1994–2002.
Li X, Liu S, Li X, Li XD. YEATS Domains as Novel Epigenetic Readers: Structures, Functions, and Inhibitor Development. ACS Chem Biol. 2022;18(4):1013.
Hsu CC, Zhao D, Shi J, Peng D, Guan H, Li Y, Huang Y, Wen H, Li W, Li H, Shi X. Gas41 links histone acetylation to H2AZ deposition and maintenance of embryonic stem cell identity. Cell Discov. 2018;4:28.
Hsu CC, Shi J, Yuan C, Zhao D, Jiang S, Lyu J, Wang X, Li H, Wen H, Li W, Shi X. Recognition of histone acetylation by the GAS41 YEATS domain promotes H2A.Z deposition in non-small cell lung cancer. Genes Dev. 2018;32:58–69.
Yeewa R, Chaiya P, Jantrapirom S, Shotelersuk V, Lo Piccolo L. Multifaceted roles of YEATS domain-containing proteins and novel links to neurological diseases. Cell Mol Life Sci. 2022;79:183.
Liu W, Zheng L, Zhang R, Hou P, Wang J, Wu L, Li J. Circ-ZEB1 promotes PIK3CA expression by silencing miR-199a-3p and affects the proliferation and apoptosis of hepatocellular carcinoma. Mol Cancer. 2022;21:72.
Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P, Pellegatta S, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–5.
You S, Wang F, Hu Q, Li P, Zhang C, Yu Y, Zhang Y, Li Q, Bao Q, Liu P, Li J. Abnormal expression of YEATS4 associates with poor prognosis and promotes cell proliferation of hepatic carcinoma cell by regulation the TCEA1/DDX3 axis. Am J Cancer Res. 2018;8:2076–87.
Fischer U, Meltzer P, Meese E. Twelve amplified and expressed genes localized in a single domain in glioma. Hum Genet. 1996;98:625–8.
Park JH, Smith RJ, Shieh SY, Roeder RG. The GAS41-PP2Cbeta complex dephosphorylates p53 at serine 366 and regulates its stability. J Biol Chem. 2011;286:10911–7.
Li Y, Li L, Wu J, Qin J, Dai X, Jin T, Xu J. YEATS4 is associated with poor prognosis and promotes epithelial-to-mesenchymal transition and metastasis by regulating ZEB1 expression in breast cancer. Am J Cancer Res. 2021;11:416–40.
Pikor LA, Lockwood WW, Thu KL, Vucic EA, Chari R, Gazdar AF, Lam S, Lam WL. YEATS4 is a novel oncogene amplified in non-small cell lung cancer that regulates the p53 pathway. Cancer Res. 2013;73:7301–12.
Huang G, Jiang H, Lin Y, Wu Y, Cai W, Shi B, Luo Y, Jian Z, Zhou X. lncAKHE enhances cell growth and migration in hepatocellular carcinoma via activation of NOTCH2 signaling. Cell Death Dis. 2018;9:487.
Jixiang C, Shengchun D, Jianguo Q, Zhengfa M, Xin F, Xuqing W, Jianxin Z, Lei C. YEATS4 promotes the tumorigenesis of pancreatic cancer by activating beta-catenin/TCF signaling. Oncotarget. 2017;8:25200–10.
Han S, Cao C, Liu R, Yuan Y, Pan L, Xu M, Hu C, Zhang X, Li M, Zhang X. GAS41 mediates proliferation and GEM chemoresistance via H2A.Z.2 and Notch1 in pancreatic cancer. Cell Oncol. 2022;45:429–46.
Ji S, Zhang Y, Yang B. YEATS domain containing 4 promotes gastric cancer cell proliferation and mediates tumor progression via activating the Wnt/β-catenin signaling pathway. Oncol Res. 2017;25:1633–41.
Kiuchi J, Komatsu S, Imamura T, Nishibeppu K, Shoda K, Arita T, Kosuga T, Konishi H, Shiozaki A, Kubota T, et al. Overexpression of YEATS4 contributes to malignant outcomes in gastric carcinoma. Am J Cancer Res. 2018;8:2436–52.
Tao K, Yang J, Hu Y, Deng A. Knockdown of YEATS4 inhibits colorectal cancer cell proliferation and induces apoptosis. Am J Transl Res. 2015;7:616–23.
Fu Q, Cheng J, Zhang J, Zhang Y, Chen X, Xie J, Luo S. Downregulation of YEATS4 by miR-218 sensitizes colorectal cancer cells to L-OHP-induced cell apoptosis by inhibiting cytoprotective autophagy. Oncol Rep. 2016;36:3682–90.
Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and molecular spectrum of liposarcoma. J Clin Oncol. 2018;36:151–9.
Kim YR, Park MS, Eum KH, Kim J, Lee JW, Bae T, Lee DH, Choi JW. Transcriptome analysis indicates TFEB1 and YEATS4 as regulatory transcription factors for drug resistance of ovarian cancer. Oncotarget. 2015;6:31030–8.
Välimäki N, Jokinen V, Cajuso T, Kuisma H, Taira A, Dagnaud O, Ilves S, Kaukomaa J, Pasanen A, Palin K, et al. Inherited mutations affecting the SRCAP complex are central in moderate-penetrance predisposition to uterine leiomyomas. Am J Hum Genet. 2023;110:460–74.
Berta DG, Kuisma H, Välimäki N, Räisänen M, Jäntti M, Pasanen A, Karhu A, Kaukomaa J, Taira A, Cajuso T, et al. Deficient H2A.Z deposition is associated with genesis of uterine leiomyoma. Nature. 2021;596:398–403.
Fischer U, Heckel D, Michel A, Janka M, Hulsebos T, Meese E. Cloning of a novel transcription factor-like gene amplified in human glioma including astrocytoma grade I. Hum Mol Genet. 1997;6:1817–22.
Debernardi S, Bassini A, Jones LK, Chaplin T, Linder B, de Bruijn DR, Meese E, Young BD. The MLL fusion partner AF10 binds GAS41, a protein that interacts with the human SWI/SNF complex. Blood. 2002;99:275–81.
Park JH, Roeder RG. GAS41 is required for repression of the p53 tumor suppressor pathway during normal cellular proliferation. Mol Cell Biol. 2006;26:4006–16.
Raap M, Gierendt L, Kreipe HH, Christgen M. Transcription factor AP-2beta in development, differentiation and tumorigenesis. Int J Cancer. 2021;149:1221–7.
Taylor NM, Baudin F, von Scheven G, Müller CW. RNA polymerase III-specific general transcription factor IIIC contains a heterodimer resembling TFIIF Rap30/Rap74. Nucleic Acids Res. 2013;41:9183–96.
Cai Y, Jin J, Florens L, Swanson SK, Kusch T, Li B, Workman JL, Washburn MP, Conaway RC, Conaway JW. The mammalian YL1 protein is a shared subunit of the TRRAP/TIP60 histone acetyltransferase and SRCAP complexes. J Biol Chem. 2005;280:13665–70.
Lempiäinen JK, Garcia BA. Characterizing crosstalk in epigenetic signaling to understand disease physiology. Biochem J. 2023;480:57–85.
Manna S, Mishra J, Baral T, Kirtana R, Nandi P, Roy A, Chakraborty S. Epigenetic signaling and crosstalk in regulation of gene expression and disease progression. Epigenomics. 2023;15:723–40.
Wilson KD, Porter EG, Garcia BA. Reprogramming of the epigenome in neurodevelopmental disorders. Crit Rev Biochem Mol Biol. 2022;57:73–112.
Kirtana R, Manna S, Patra SK. KDM5A noncanonically binds antagonists MLL1/2 to mediate gene regulation and promotes epithelial to mesenchymal transition. Biochim Biophys Acta Gene Regul Mech. 2023;1866: 194986.
Li X, Liu S, Li X, Li XD. YEATS domains as novel epigenetic readers: structures, functions, and inhibitor development. ACS Chem Biol. 2023;18:994–1013.
Ren X, Zhou Y, Xue Z, Hao N, Li Y, Guo X, Wang D, Shi X, Li H. Histone benzoylation serves as an epigenetic mark for DPF and YEATS family proteins. Nucleic Acids Res. 2021;49:114–26.
Liu N, Konuma T, Sharma R, Wang D, Zhao N, Cao L, Ju Y, Liu D, Wang S, Bosch A, et al. Histone H3 lysine 27 crotonylation mediates gene transcriptional repression in chromatin. Mol Cell. 2023;83:2206-2221.e2211.
Wang Y, Jin J, Chung MWH, Feng L, Sun H, Hao Q. Identification of the YEATS domain of GAS41 as a pH-dependent reader of histone succinylation. Proc Natl Acad Sci U S A. 2018;115:2365–70.
Liu B, Yang L, Zhu X, Li H, Zhu P, Wu J, Lu T, He L, Liu N, Meng S, et al. Yeats4 drives ILC lineage commitment via activation of Lmo4 transcription. J Exp Med. 2019;216:2653–68.
Pal D, Mukhopadhyay D, Ramaiah MJ, Sarma P, Bhadra U, Bhadra MP. Regulation of cell proliferation and migration by miR-203 via GAS41/miR-10b axis in human glioblastoma cells. PLoS ONE. 2016;11: e0159092.
Gandhi SG, Bag I, Sengupta S, Pal-Bhadra M, Bhadra U. Drosophila oncogene Gas41 is an RNA interference modulator that intersects heterochromatin and the small interfering RNA pathway. Febs j. 2015;282:153–73.
Ukey S, Ramteke A, Choudhury C, Purohit P, Sharma P. Differential expression of zinc-dependent hdac subtypes and their involvement in unique pathways associated with carcinogenesis. Asian Pac J Cancer Prev. 2022;23:877–83.
Llanos S, Efeyan A, Monsech J, Dominguez O, Serrano M. A high-throughput loss-of-function screening identifies novel p53 regulators. Cell Cycle. 2006;5:1880–5.
Xian Q, Song Y, Gui C, Zhou Y. Mechanistic insights into genomic structure and functions of a novel oncogene YEATS4. Front Cell Dev Biol. 2023;11:1192139.
Foerster F, Gairing SJ, Müller L, Galle PR. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J Hepatol. 2022;76:446–57.
Hasegawa K, Kokudo T, Kokudo N. Current evidence and topics of diagnosis and treatment for hepatocellular carcinoma. Nihon Shokakibyo Gakkai Zasshi. 2017;114:1585–92.
Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y, Nakamura Y. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001;61:2129–37.
Botlagunta M, Vesuna F, Mironchik Y, Raman A, Lisok A, Winnard P Jr, Mukadam S, Van Diest P, Chen JH, Farabaugh P, et al. Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene. 2008;27:3912–22.
Chang PC, Chi CW, Chau GY, Li FY, Tsai YH, Wu JC, Wu Lee YH. DDX3, a DEAD box RNA helicase, is deregulated in hepatitis virus-associated hepatocellular carcinoma and is involved in cell growth control. Oncogene. 2006;25:1991–2003.
Huang JS, Chao CC, Su TL, Yeh SH, Chen DS, Chen CT, Chen PJ, Jou YS. Diverse cellular transformation capability of overexpressed genes in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2004;315:950–8.
Kolak A, Kamińska M, Sygit K, Budny A, Surdyka D, Kukiełka-Budny B, Burdan F. Primary and secondary prevention of breast cancer. Ann Agric Environ Med. 2017;24:549–53.
Kim HY, Lee KM, Kim SH, Kwon YJ, Chun YJ, Choi HK. Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget. 2016;7:67111–28.
Lauffart B, Howell SJ, Tasch JE, Cowell JK, Still IH. Interaction of the transforming acidic coiled-coil 1 (TACC1) protein with ch-TOG and GAS41/NuBI1 suggests multiple TACC1-containing protein complexes in human cells. Biochem J. 2002;363:195–200.
Lauffart B, Gangisetty O, Still IH. Molecular cloning, genomic structure and interactions of the putative breast tumor suppressor TACC2. Genomics. 2003;81:192–201.
Onodera Y, Takagi K, Miki Y, Takayama K, Shibahara Y, Watanabe M, Ishida T, Inoue S, Sasano H, Suzuki T. TACC2 (transforming acidic coiled-coil protein 2) in breast carcinoma as a potent prognostic predictor associated with cell proliferation. Cancer Med. 2016;5:1973–82.
Peset I, Vernos I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol. 2008;18:379–88.
Thakur HC, Singh M, Nagel-Steger L, Prumbaum D, Fansa EK, Gremer L, Ezzahoini H, Abts A, Schmitt L, Raunser S, et al. Role of centrosomal adaptor proteins of the TACC family in the regulation of microtubule dynamics during mitotic cell division. Biol Chem. 2013;394:1411–23.
Barros TP, Kinoshita K, Hyman AA, Raff JW. Aurora a activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J Cell Biol. 2005;170:1039–46.
Raff JW. Centrosomes and cancer: lessons from a TACC. Trends Cell Biol. 2002;12:222–5.
Gómez-Baldó L, Schmidt S, Maxwell CA, Bonifaci N, Gabaldón T, Vidalain PO, Senapedis W, Kletke A, Rosing M, Barnekow A, et al. TACC3-TSC2 maintains nuclear envelope structure and controls cell division. Cell Cycle. 2010;9:1143–55.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240–2.
Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10–27.
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–20.
Ji M, Fan D, Yuan L, Zhang Y, Dong W, Peng X. EBP50 inhibits pancreatic cancer cell growth and invasion by targeting the β-catenin/E-cadherin pathway. Exp Ther Med. 2015;10:1311–6.
Jiang JX, Sun CY, Tian S, Yu C, Chen MY, Zhang H. Tumor suppressor Fbxw7 antagonizes WNT signaling by targeting β-catenin for degradation in pancreatic cancer. Tumour Biol. 2016;37:13893–902.
Van Herpe F, Van Cutsem E. The Role of cMET in gastric cancer-a review of the literature. Cancers. 2023. https://doi.org/10.3390/cancers15071976.
Ooki A, Yamaguchi K. The dawn of precision medicine in diffuse-type gastric cancer. Ther Adv Med Oncol. 2022;14:17588359221083048.
Zeng J, Ran K, Li X, Tao L, Wang Q, Ren J, Hu R, Zhu Y, Liu Z, Yu L. A novel small molecule RK-019 inhibits FGFR2-amplification gastric cancer cell proliferation and induces apoptosis in vitro and in vivo. Front Pharmacol. 2022;13: 998199.
Herbst RS, Arkenau HT, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, Bendell J, Penel N, Krebs MG, Martin-Liberal J, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20:1109–23.
Luo Y, Deng X, Liao W, Huang Y, Lu C. Prognostic value of autophagy-related genes based on single-cell RNA-sequencing in colorectal cancer. Front Genet. 2023;14:1109683.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Xie L, Jing R, Qi J, Lin Z, Ju S. Drug resistance-related microRNAs in hematological malignancies: translating basic evidence into therapeutic strategies. Blood Rev. 2015;29:33–44.
Taniguchi K, Sugito N, Kumazaki M, Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama K, Akao Y. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363:17–27.
Zhong X, Xiao Y, Chen C, Wei X, Hu C, Ling X, Liu X. MicroRNA-203-mediated posttranscriptional deregulation of CPEB4 contributes to colorectal cancer progression. Biochem Biophys Res Commun. 2015;466:206–13.
Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet. 2020;149:3–9.
Mlodawska OW, Saini P, Parker JB, Wei JJ, Bulun SE, Simon MA, Chakravarti D. Epigenomic and enhancer dysregulation in uterine leiomyomas. Hum Reprod Update. 2022;28:518–47.
Assi T, Kattan J, Rassy E, Nassereddine H, Farhat F, Honore C, Le Cesne A, Adam J, Mir O. Targeting CDK4 (cyclin-dependent kinase) amplification in liposarcoma: a comprehensive review. Crit Rev Oncol Hematol. 2020;153: 103029.
Italiano A, Bianchini L, Keslair F, Bonnafous S, Cardot-Leccia N, Coindre JM, Dumollard JM, Hofman P, Leroux A, Mainguené C, et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int J Cancer. 2008;122:2233–41.
Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho A, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–21.
Mashima E, Sawada Y, Nakamura M. Recent Advancement in Atypical Lipomatous Tumor Research. Int J Mol Sci. 2021;22(3):994.
Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131:909–27.
Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64.
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M, Eisenhauer EL, et al. Ovarian cancer, version 2 2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:191–226.
Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17:487–500.
Klein BJ, Vann KR, Andrews FH, Wang WW, Zhang J, Zhang Y, Beloglazkina AA, Mi W, Li Y, Li H, et al. Structural insights into the π-π-π stacking mechanism and DNA-binding activity of the YEATS domain. Nat Commun. 2018;9:4574.
Garnar-Wortzel L, Bishop TR, Kitamura S, Milosevich N, Asiaban JN, Zhang X, Zheng Q, Chen E, Ramos AR, Ackerman CJ, et al. Chemical Inhibition of ENL/AF9 YEATS domains in acute leukemia. ACS Cent Sci. 2021;7:815–30.
Moustakim M, Christott T, Monteiro OP, Bennett J, Giroud C, Ward J, Rogers CM, Smith P, Panagakou I, Díaz-Sáez L, et al. Discovery of an MLLT1/3 YEATS domain chemical probe. Angew Chem Int Ed Engl. 2018;57:16302–7.
Jiang Y, Chen G, Li XM, Liu S, Tian G, Li Y, Li X, Li H, Li XD. Selective targeting of AF9 YEATS domain by cyclopeptide inhibitors with preorganized conformation. J Am Chem Soc. 2020;142:21450–9.
Londregan AT, Aitmakhanova K, Bennett J, Byrnes LJ, Canterbury DP, Cheng X, Christott T, Clemens J, Coffey SB, Dias JM, et al. Discovery of high-affinity small-molecule binders of the epigenetic reader YEATS4. J Med Chem. 2023;66:460–72.
Cho HJ, Li H, Linhares BM, Kim E, Ndoj J, Miao H, Grembecka J, Cierpicki T. GAS41 recognizes diacetylated histone h3 through a bivalent binding mode. ACS Chem Biol. 2018;13:2739–46.
Wang M, Lin H. Understanding the function of mammalian sirtuins and protein lysine acylation. Annu Rev Biochem. 2021;90:245–85.
Fu Y, Yu J, Li F, Ge S. Oncometabolites drive tumorigenesis by enhancing protein acylation: from chromosomal remodelling to nonhistone modification. J Exp Clin Cancer Res. 2022;41:144.
Xu X, Zhu X, Liu F, Lu W, Wang Y, Yu J. The effects of histone crotonylation and bromodomain protein 4 on prostate cancer cell lines. Transl Androl Urol. 2021;10:900–14.
Wan J, Liu H, Ming L. Lysine crotonylation is involved in hepatocellular carcinoma progression. Biomed Pharmacother. 2019;111:976–82.
Fellows R, Denizot J, Stellato C, Cuomo A, Jain P, Stoyanova E, Balázsi S, Hajnády Z, Liebert A, Kazakevych J, et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat Commun. 2018;9:105.
Tong Y, Guo D, Yan D, Ma C, Shao F, Wang Y, Luo S, Lin L, Tao J, Jiang Y, et al. KAT2A succinyltransferase activity-mediated 14-3-3ζ upregulation promotes β-catenin stabilization-dependent glycolysis and proliferation of pancreatic carcinoma cells. Cancer Lett. 2020;469:1–10.
Guo Z, Pan F, Peng L, Tian S, Jiao J, Liao L, Lu C, Zhai G, Wu Z, Dong H, et al. Systematic proteome and lysine succinylome analysis reveals enhanced cell migration by hyposuccinylation in esophageal squamous cell carcinoma. Mol Cell Proteom. 2021;20: 100053.
Li X, Zhang C, Zhao T, Su Z, Li M, Hu J, Wen J, Shen J, Wang C, Pan J, et al. Lysine-222 succinylation reduces lysosomal degradation of lactate dehydrogenase a and is increased in gastric cancer. J Exp Clin Cancer Res. 2020;39:172.
Ma Y, Qi Y, Wang L, Zheng Z, Zhang Y, Zheng J. SIRT5-mediated SDHA desuccinylation promotes clear cell renal cell carcinoma tumorigenesis. Free Radic Biol Med. 2019;134:458–67.
Yuan Y, Yuan H, Yang G, Yun H, Zhao M, Liu Z, Zhao L, Geng Y, Liu L, Wang J, et al. IFN-α confers epigenetic regulation of HBV cccDNA minichromosome by modulating GCN5-mediated succinylation of histone H3K79 to clear HBV cccDNA. Clin Epigenetics. 2020;12:135.
Ren M, Yang X, Bie J, Wang Z, Liu M, Li Y, Shao G, Luo J. Citrate synthase desuccinylation by SIRT5 promotes colon cancer cell proliferation and migration. Biol Chem. 2020;401:1031–9.
Wang Y, Guo YR, Liu K, Yin Z, Liu R, Xia Y, Tan L, Yang P, Lee JH, Li XJ, et al. KAT2A coupled with the α-KGDH complex acts as a histone H3 succinyltransferase. Nature. 2017;552:273–7.
Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–80.
Taddei ML, Pietrovito L, Leo A, Chiarugi P. Lactate in sarcoma microenvironment: much more than just a waste product. Cells. 2020;9:510.
Xiong J, He J, Zhu J, Pan J, Liao W, Ye H, Wang H, Song Y, Du Y, Cui B, et al. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. 2022;82:1660-1677.e1610.
Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang J, Wilhelm J, Li S, Song J, Li W, et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat Commun. 2022;13:4981.
Dai X, Lv X, Thompson EW, Ostrikov KK. Histone lactylation: epigenetic mark of glycolytic switch. Trends Genet. 2022;38:124–7.
Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–17.
Hirschey MD, Zhao Y. Metabolic regulation by lysine malonylation, succinylation, and glutarylation. Mol Cell Proteomics. 2015;14:2308–15.
Cheng YM, Hu XN, Peng Z, Pan TT, Wang F, Chen HY, Chen WQ, Zhang Y, Zeng XH, Luo T. Lysine glutarylation in human sperm is associated with progressive motility. Hum Reprod. 2019;34:1186–94.
Bao X, Liu Z, Zhang W, Gladysz K, Fung YME, Tian G, Xiong Y, Wong JWH, Yuen KWY, Li XD. Glutarylation of histone H4 lysine 91 regulates chromatin dynamics. Mol Cell. 2019;76:660-675.e669.
Acknowledgements
We are very grateful to Sanger for their excellent service. We thank Binhai County People's Hospital for its support and guidance in writing this paper.
Funding
This study was supported by the Binhai County People's Hospital and the Jiangsu Provincial Key Laboratory of Integrative Medicine of Yangzhou University (Z2018027).
Author information
Authors and Affiliations
Contributions
All the authors made substantial, direct, and intellectual contributions to the review. KJ and LL organized and wrote the review. HL, YS, and Jian Jiang collected the data, MZ and HT revised the description of the article’s specialist vocabulary, and XY and YZ provided editorial assistance. YC provided help with the framework and logic of the paper. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to the publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
. An overview of the GAS41 involved in cancer.
Additional file 2: Figure S2
. A working model for GAS41.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ji, K., Li, L., Liu, H. et al. Unveiling the role of GAS41 in cancer progression. Cancer Cell Int 23, 245 (2023). https://doi.org/10.1186/s12935-023-03098-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-023-03098-z