Abstract
Prostate cancer (PCa) is a non-cutaneous malignancy in males with wide variation in incidence rates across the globe. It is the second most reported cause of cancer death. Its etiology may have been linked to genetic polymorphisms, which are not only dominating cause of malignancy casualties but also exerts significant effects on pharmacotherapy outcomes. Although many therapeutic options are available, but suitable candidates identified by useful biomarkers can exhibit maximum therapeutic efficacy. The single-nucleotide polymorphisms (SNPs) reported in androgen receptor signaling genes influence the effectiveness of androgen receptor pathway inhibitors and androgen deprivation therapy. Furthermore, SNPs located in genes involved in transport, drug metabolism, and efflux pumps also influence the efficacy of pharmacotherapy. Hence, SNPs biomarkers provide the basis for individualized pharmacotherapy. The pharmacotherapeutic options for PCa include hormonal therapy, chemotherapy (Docetaxel, Mitoxantrone, Cabazitaxel, and Estramustine, etc.), and radiotherapy. Here, we overview the impact of SNPs reported in various genes on the pharmacotherapy for PCa and evaluate current genetic biomarkers with an emphasis on early diagnosis and individualized treatment strategy in PCa.
Similar content being viewed by others
Introduction
Prostate cancer is a frequently diagnosed malignancy, estimated 1.3 million newly diagnosed cases worldwide annually. It has surpassed breast cancer and become the most prevalent, increasingly crucial medical issue in males. Among 10 million clinically diagnosed PCa men, approximately 0.7 million are living with metastatic PCa, and more than 0.4 million deaths occur annually. This mortality rate is expected to double by 2040 [1]. Despite improvements in metastatic PCa treatment managing this disease remains challenging [2]. Prostate cancer cells are increasingly resistant to various treatments, which can affect the course of the disease and survival [3]. The mortality rate will be high if the development of resistance continues to outpace the development of new treatment options. Physicians can evaluate the chance of PCa recovery using different types of statistics called survival statistics [4]. According to the survival rate statistic, only a percentage of patients survive cancer [5, 6]. Since 2014, incidence rates for prostate cancer in its advanced stages have increased by 5% annually. Overall incidence rates have increased by 3% annually [7,8,9]. The above finding is not surprising due to the limited resources for prostate cancer screening and detection [10,11,12].
Almost 98% of PCa cases originated from the organ's glandular part, and their microscopic examination is based on certain glandular patterns. The Gleason score is a commonly used assessment technique to grade prostate adenocarcinoma and has remarkable prognostic value [13]. Most malignancies arise in the peripheral glandular zone, which results in asymptomatic prostatic cancer at earlier stages, whereas symptomatic presentation occurs at the metastatic state of the disease [14]. Despite advanced ages, suggestive evidence provided by family history data reported that the critical risk factors for PCa are genetic factors that may lead to the progression of abnormal prostatic cell growth and are responsible for developing cancerous cells [15]. The initial emergence of PCa in the majority of men population is due to hereditary factors, having a family member’s history, and the chance of its occurrence in first-rank relatives is increased by two to three-fold [16]. However, the findings of segregation analysis of multi-case families supported an autosomal dominant inheritance mode, but it is estimated that this inherited form causes only 9% of all PCa. A multigenic etiology has also been proposed for the majority of PCa cases. In intraepithelial neoplasia lesions, the multilayered luminal epithelium is observed, which serves as a promising biomarker of adenocarcinoma, such as loss of cytokeratin-5 and cytokeratin-14 (basal markers), the gain of cytokeratin-8 and cytokeratin-18 (luminal markers), and altered expression of α-methyl acyl-CoA racemase [17].
In current clinical practice, inadequate diagnostic investigations are involved in screening PCa patients that are usually based on blood prostate-specific antigen (PSA) levels and the tumor stage. The classification of tumor stages is based on the blood PSA level, progression of PCa, and Gleason score of tumor grading. Though PSA is a commonly used diagnostic and prognostic marker of PCa, but numerous studies highlighted their poor correlation with survival outcomes [18]. For early prediction and prognosis of PCa, recent studies published evidence focused on the clinical importance of a genetic feature called Single Nucleotide polymorphisms (SNPs). Single nucleotide polymorphism (SNP) is the substitution, insertion, or deletion of a single nucleotide at a specific genomic position. It is the most prevalent type of genetic variation in people. A single base pair difference in the DNA sequence at a specific location in the genome causes the difference. SNPs may affect several aspects of an individual's biology, including disease susceptibility, drug response, and phenotypic traits [19]. Many SNPs in the human genome appear roughly every 300 nucleotides [20]. Specific SNPs also impact susceptibility to disease and treatment response. For instance, a specific SNP may increase an individual's risk of developing a specific disease or alter the response to a specific drug. These SNPs associated with certain traits or diseases are identified through genome-wide association studies (GWAS) [21]. Researchers identified phenotypic-related genetic markers by comparing SNP profiles of patients with healthy controls. The function of genes relating to particular pathways is altered by genetic variations that may have significant implications in clinical practice for personalized medicine [22].
These studies have evaluated the coding sequences and assessed long noncoding RNAs (LncRNAs) having more than 200 nucleotides. Although LncRNAa does not translate, they interact with DNA, RNA, and proteins to perform their regulatory effects for differentiating, migrating, and proliferating cells and inducing apoptosis [23]. A polymorphism in the promoter region of LncRNA also modulates the expression pattern. Recently, a GAS5 gene encodes tumor suppressor LncRNA (Growth arrest-specific 5) reported to be involved in developing many cancers, such as lung, prostate, colorectal, and breast [24]. GAS5 is considered to cause the invasion, proliferation, migration, and metastasis of PCa cells, but its exact expression level is still controversial [25]. Numerous studies highlighted that the various genetic polymorphisms are linked with the risk level, grading, and mortality of PCa. In the promoter region of GAS5, a 5-bp indel polymorphism is reported as variant rs145204276, shown as “-/AGGCA”, alters the gene expression pattern, which results in increased susceptibility to cancers. This SNP also significantly affects prognosis, disease stage, and the Gleason score of PCa [26].
An oncogenic transcription factor (TMPRSS2 and ERG fusion) is the most frequently reported chromosomal aberration in PCa, which causes carcinogenesis in > 50% of patients. In the prostate tumor-permissive inflammatory microenvironment, epithelial transformation is followed by a phenotypic and genotypic series of changes [27]. Up till now, about 5000 somatic mutations have been detected in prostate growth, and among these, the highly reported mutated genes are MED12, SCN11A, CDKN1B, SPOP, PIK3CA, PTEN, THSD7B, C14orf49, NIPA2, TP53, FOXA1, and ZNF595. Almost 15–25% risk of PCa is found in individuals having mutations in the BRCA gene, and life-threatening prostate cancer is reported to be linked with the mutations in BRCA1, BRCA2, and HOXB13 [28].
Recent molecular genetic studies on the pathogenesis of the tumor, including the inactivation of tumor suppressor genes and activation of oncogenes, have explained the multiple genetic alterations. The loss of heterozygosity causes chromosomal instability that inactivates the tumor suppressor genes, which can serve as an indicator to identify these genes containing chromosomal regions for selective growth and are found as the primary source of tumorigenesis [29]. The high-frequency loss of heterozygosity is a form of allelic loss observed in tumor suppressor genes located on chromosomes 16q and 10q and are involved in the pathogenesis of human PCa [30]. As an alternative to curative PCa therapy, active surveillance (measuring cancer progression) is a strategy for monitoring old-age patients when their low life expectancy is anticipated. However, there is a very high chance of PCa diagnosis at an older age. A reduction of 46% in mortality risk has been observed in older men treated with local therapy compared to patients treated conservatively [31]. This review presents an overview of the influence of the SNPs reported in different genes on the pharmacotherapy for PCa and assesses present genetic biomarkers with a focus on early diagnosis and personalized therapeutic approach in PCa.
Prostate cancer biology
There are three human prostate structural zones; central, transition, and peripheral. Mainly prostate tumors arise in the outermost peripheral zone, either with luminal or basal cancer-initiating epithelial cells, which give rise to lesions indicative of adenocarcinomas [32,33,34]. The PCa oncogenesis is linked with a series of interactions between various factors, including somatic acquired genetic mutations, germline susceptibility, macro-environment, and microenvironment [35,36,37]. Tumors are complex tissues of multiple distinct cell types that undergo collaborative interactions during tumorigenesis. To maintain the tumor growth, invasion, or metastasis, the tumor cells are highly selective to shape their microenvironment by allowing the critical supportive interaction among tumor cells via soluble factors and extracellular matrix (ECM) [38]. The multiple foci forms in localized prostate cancer have many genetic alterations, diverse metastatic seeding capacities, and inherent resistance to the treatment. It is also well established that prostate carcinogenesis is also promoted by urinary microbes-induced chronic inflammation and infections, which leads to the generation of oxidative stress by free radicals to damage the DNA [39]. The proliferative inflammatory atrophy increases the number of proliferative luminal epithelial cells in the prostate, which are highly susceptible to epigenetic and genomic chromatin alterations that initiate malignant transformation and intraepithelial neoplasia [40].
There are several diagnostic tests to determine PCa staging, including prostate-specific antigen (PSA) blood tests, a digital rectal exam, imaging tests, and biopsies [41]. The specific stage of PCa plays a crucial role in determining treatment options and prognosis. PCa is characterized by three main terms: initiation, progression, and advancement. Imitation occurs when normal prostate gland cells are genetically mutated to cause PCa's development [42]. The combination of genetic predisposition and environmental factors can cause these mutations. Although the exact cause of PCa initiation is still unknown and is being studied, reported risk factors include age, family history, race, and certain genetic abnormalities [43]. PCa that occurs in distant parts of the body is called metastatic PCa. At this stage, treatment options may include hormone therapy, chemotherapy, targeted therapies, immunotherapy, and participation in clinical trials [44]. Figure 1 shows the different stages of PCA.
Prostatic intraepithelial neoplasia (PIN)
A premalignant condition of epithelial cells that occurs due to neoplastic growth in benign prostatic acini or ducts is called prostatic intraepithelial neoplasia. The reduction or loss of basal epithelium by hyper-proliferation of luminal epithelial cells is linked with a malignancy precursor called prostatic intraepithelial neoplasia [45]. Transformation into a malignant tumor has multiple steps, such as intraepithelial neoplasia origination, localized PCa followed by advanced adenocarcinoma, and culmination with metastatic cancer [46]. A Gleason grading system defined by Donald Gleason is now widely used in clinical settings to grade the aggressiveness of prostate cancers. The prostatic intraepithelial neoplasia can be categorized as high or low grade based on the extent of intraepithelial neoplasia [47]. The prostatic intraepithelial neoplasia is considered a high grade if lesions are produced by the multilayered luminal epithelium, which can serve as transformation-related biomarkers, such as the absence of basal markers [(KRT5), (KRT14) and TP63], gaining of luminal markers [(KRT18) and (KRT8)], and overexpression of α-methylacyl-CoA racemase (AMACR). The most common chromosomal aberration is an oncogenic transcription factor resulting from the fusion of TMPRSS2 and ERG genes [48].
Metastatic prostate cancer
In metastatic cancer, invasion of tumor cells occurs in surrounding tissues where they undergo a series of inter and intracellular complex remodeling process, which has been classified into five stages. Each stage is highly energy-demanding for the cancer cells [49]. Prostate cancer-associated mortality generally causes by a metastatic disease, which primarily metastases in the primary tumor adjacent lymph nodes, followed by the lungs, liver, and bone cancer. Bone metastases produce osteoblastic lesions that cause bone pain, frequent fractures, and hypercalcemia [50]. Among other cancers, Epithelial-mesenchymal transition (EMT) has been reported to be involved in the metastasis of prostate cancer cells by disseminating as circulating tumor cells (CTCs) into systemic circulation that easily crosses physical barriers to develop bone metastasis [51].
In a mechanistic design study, molecular and phenotypic characteristics of CTCs were focused on understanding the dissemination of cancer cells to distant organs and detecting novel prognostic biomarkers. It was observed that the metastatic tumor cell invasion of the bones is caused by stromal cell-derived factor-1 (SDF-1) and its receptor (CXCR4) [52]. SDF-1 anchor, Annexin A2, directs the binding of hematopoietic stem cells to the niche to enhance expression levels for proliferation in prostate cancer cells and apoptosis resistance during patient chemotherapy [53]. Bone metastasis is a major clinical condition of PCa. Previous studies showed a comparison between non-metastatic and progressive castration-resistance human samples and reported that more than 80% of bone lesions were found in all men who die with PCa, and the mechanism behind the prevalence of PCa in bone is not well understood yet. However, the highest mortality rate was found in the patients diagnosed with skeletal metastasis [54]. In PCa-induced mortality, Ras and other GTP-binding proteins perform several important cellular functions, such as intracellular signaling and cytoskeletal assembly. Ras is a glycosylated transmembrane protein that acts as a membrane transducer and regulates the various downstream cellular events such as proliferation, apoptosis, and invasion [55]. The Ras family consists of h-ras, k-ras, m-ras, n-ras, and r-ras, associated with 30% of solid tumors. As the tumor load grows, the invasion of malignant cells also upsurges in the systemic circulation. The dissemination of Latrogenic cells occurs during clinical procedures such as prostate biopsy, transurethral resection of the prostate (TURP), and brachytherapy [56].
A prostate biopsy involves the removal of small samples of tissue from the prostate gland using a needle. It is generally safe to perform this procedure; however, there is a small risk that the cells may be displaced and spread to other body parts, such as the bloodstream or nearby tissues [57]. The risk of significant complications from a prostate biopsy is relatively low. However, factors such as the needle traversing different areas of the prostate and possible bleeding at the biopsy site can contribute to cell dissemination during a biopsy [58]. Transurethral prostate resection (TURP) is a surgical procedure used to treat benign prostatic hyperplasia (BPH) by removing excess prostate tissue through the urethra with a resectoscope. Although the procedure aims to remove prostate tissue, iatrogenic cell dissemination is possible, particularly if the procedure involves cutting or manipulating tissue near the prostate [59]. The possibility of iatrogenic cell dissemination exists in both cases. Healthcare professionals must take the appropriate precautions during these procedures to minimize complications and risks and monitor patients for adverse reactions [60]. Previous studies explained that tumor growth is linked with the cellular clearance process of the circulation, which may take almost 4 weeks. This cellular clearance is mainly related to the arrest of cellular clumps in the first capillary and other factors affecting the cellular motility of differential PCa cells and differences in the chemo-attraction [61].
Castration-resistant prostate cancer (CRPC) and ADT
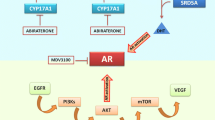
PCa progresses despite androgen deprivation therapy (ADT) or hormone therapy known as castration-resistant prostate cancer (CRPC). It is the primary treatment for advanced prostate cancer and reduces male hormones, specifically testosterone. These hormones fuel prostate cancer cell growth [62]. It is established that ADT is a cornerstone in PCa management, both as a primary treatment and in combination with other treatments. ADT can be achieved by using a variety of approaches, such as surgical castration (the removal of the testicles) or medical castration (the use of medications that suppress testosterone production [63]. The application of ADT initially controls the growth and spread of prostate cancer, which leads to the shrinkage of tumors and the relief of symptoms, but in some cases, the cancer cells continue to grow despite low testosterone levels and lead to the development of CRPC [64]. ADT alleviates symptoms by reducing cancer cell growth in locally advanced and metastatic PCa conditions. It can also be used as adjuvant therapy to eliminate residual cancer cells after primary treatment to reduce the risk of disease recurrence. This is done in combination with other treatments. In cases where PCa has spread to other body parts, ADT can improve symptoms, such as bone pain, by shrinking the tumors and decreasing their activity [65]. It is a vital component of palliative care and integral to improving the quality of life for patients with advanced disease. Along with other targeted therapies, such as abiraterone acetate or enzalutamide, ADT can further suppress androgen signaling pathways and inhibit cancer cell growth. These combinations of treatments have improved CRPC outcomes. Castration-resistant prostate cancer cells develop mechanisms to survive and grow despite low testosterone levels. These mechanisms include mutations in the androgen receptor, an increase in androgen synthesis, the amplification of the androgen receptor, and activating alternative signaling pathways [66] (Fig. 2).
Signal transmission through AR is the main pathway for prostate cancer cell growth and spread. Therefore, regulating androgen receptors (ARs) in cells is the key to many cancer-related genes. Testosterone or dehydroepiandrosterone is converted to dehydroepiandrosterone by 5 α reductase. DHT then dissociates HSP and AR to form a complex, which is transferred to the nucleus and activates cancer-associated genes. Androgens and androgen receptors (AR) in cells regulate cancer-related genes. It is possible to control human androgen-related malignant tumors by targeting androgen signaling pathways in tumor cells with anti-androgen, 5-α reductase inhibitors, heat shock protein 90 inhibitors, androgen receptor agonists, and serotonin inhibitors
Genetic biomarkers for early prostate cancer detection
Numerous cancer research studies have validated tumor-associated genetic aberration-based biomarkers, which can help predict the risks, early diagnosis, and prediction of therapeutic outcomes. An aggressive tumor cannot be distinguished only by biopsy and blood PSA tests [79]. The dysregulation of LncRNAs controls the critical cancer hallmarks that can serve as an attractive biomarker for diagnosing PCa. Several cancer-specific LncRNAs are upregulated in PCa, such as PCATs, PCA3, SPRY4-IT1, SChLAP1, and TRPM2-AS. The altered expression pattern of LncRNA promotes the progression of tumors and metastasis [80]. Several investigative studies on determining prostate cancer antigen 3 (PCA3) level in urine have confirmed the specificity and sensitivity of this non-invasive test [81]. Noncoding RNAs (ncRNAs) have gained significant importance in tumor biology and can potentially act as cancer biomarkers. PCAT-1 is a prostate cancer-associated ncRNA transcript that acts as a prostate-specific regulator for cancer cell proliferation and is a suitable PCa marker [82]. α-methyl acyl-CoA racemase (AMACR) is a mitochondrial and peroxisomal enzyme overexpressed in prostate cancer, while its low expression level was observed in benign prostatic tissue. Hence, AMACR is a promising prostate tumor marker for early diagnosis [83] (Table 1).
PCa markers are also assessed in urine samples that are a favorable alternative to serum-based biomarkers. Golgi phosphoprotein-2, a Golgi membrane antigen encoded by GOLPH2, is reported to be overexpressed in almost 90% of PCa patients, it does not only serve as a suitable biomarker for early diagnosis but also helps in distinguishing normal cells from cancerous cells [84]. The present studies have successfully established the relationship between aggressive PCa phenotype and TMPRSS2-ERG fusion because the overexpression of the TMPRSS2-ERG gene is linked with shorter survival of PCa patients. It also possesses prognostic significance as a tumor cell marker [85]. A protein kinase encoding the PIM1 gene is not expressed in the benign prostatic epithelium, but its expression level is elevated significantly in advanced PCa cases. Therefore PIM1 is a promising target for developing PIM1 inhibitor drugs [86]. Another useful prognostic biomarker is PTEN, a tumor suppressor usually deleted in prostate cancer and independently linked with the risk of lethal prostate cancer progression [87]. Hypermethylation of the PDLIM4 gene is also used as a marker for cancer detection because, in prostate cancerous cells, up-regulation of the expression level of mRNA of PDLIM4 and its protein was found, and it acts as a tumor suppressor [88] (Table 1).
In multiple cancers, hypermethylation has been observed to indicate the earliest somatic genome alterations. Several studies on cancer have also highlighted the aberrant methylation patterns at specific genes. The hypermethylation at GSTP1 is used to detect PCa and has been correlated significantly with the tumor stage. It also allows the early detection of more than 82% of PCa [89]. Several tumor suppressors (PTEN, RB1, and TP53) undergo mutations or allelic loss in an advanced stage of PCa, while the rare mutations are found in the RAS family (proto-oncogenes) [90]. After the surgical procedure, the DNA copy number alteration (CNA) burden across the genome of PCa patients is linked with metastasis. The CNA burden is an independent prostate-specific biomarker with a significant prognostic impact in conservative treatment [91]. The initial localized prostate cancer management is very complex. Three commonly available commercial tests (the Cell Cycle Progression score, the Genomic Prostate score, and Genomic Classifier) provide the maximum supporting information to manage and treat localized prostate cancer. Among the prognostic markers, 12 genes-based prostate markers help clinicians in the early diagnosis of PCa [92]. For appropriate assessment of pharmacotherapy, robust prognostic markers can be used, which are based on the altered expression pattern of 31 reported genes (ASPM, ASF1B, BUB1B, BIRC5, CENPF, CDC20, CDCA8, CDC2, CDCA3, CDKN3, CEP55, C18orf24, DLGAP5, DTL, FOXM1, PLK1, MCM10, NUSAP1, KIF11, KIF20A, KIAA0101, PRC1, RRM2, PBK, TOP2A, TK1, RAD51, RAD54L, PTTG1, CENPM, and ORC6L) [93]. Hypermethylation in CpG islands of DNA of cancer tissues is used as a diagnostic marker of prostate cancer. DNA methylation occurs at the specific promoter of CpG islands that can cause gene repression, such as in the case of GSTP-1 and DAB2IP, while DNA hypermethylation does not occur in normal cells [94]. In the postoperative setting, biochemical recurrence causes the progression of the disease, which can lead to lethal prostate cancer. Several studies have explained that metastasis development after biochemical recurrence is linked with the validated differential gene expression that can be used as metastatic biomarkers [95] (Table 1).
Association between single nucleotide polymorphisms (snps) and prostate cancer
The inter-individual germline DNA differences are called genetic polymorphisms, which are differences in genomic sequences that occur between individuals at the frequency of about 1% of the general population. The most commonly reported polymorphisms in the repeated sequences (microsatellites) are Single-nucleotide polymorphisms (SNPs) [67]. A genome base pair variation in the DNA sequence is called SNP, with a frequency of about 1 out of 800 base pairs. These SNPs induce clinically significant changes in cellular proteins and enzymatic machinery. Several studies suggested that SNPs are not only important in the inheritance of genes within families but exert a strong influence on the susceptibility or risk of prostate cancer in certain individuals than others [68]. It has been reported that the entire human genome contains almost 2 million SNPs which are classified based on their functions into the following types; promoter regions SNPs are called regulatory SNPs (rSNPs); A SNP region at which nucleotide substitution causes the substitution of amino acid/ affects a protein is called as coding SNP (cSNP). Silent SNPs (sSNPs) are not involved in amino acid substitution and are present in the exon region; in the intronic region, (iSNPs) are located; and intergenic regions SNPs are called genome SNPs (gSNPs) (Fig. 1) [69].
SNPs are also responsible for altering the gene expression pattern and protein function. Regulatory SNPs and amino acid-substituting SNPs cause differences in the functional and phenotypic traits, respectively. Moreover, the gene expression level is also affected significantly by sSNPs and iSNPs [70]. The rearrangements that occur in the genomic structure or copy number alterations are usually involved in the early development of prostate cancer, however, SNPs are less commonly involved in the early development of PCa. In 40–60% of early prostate cancer patients, genomic aberrations are observed, such as fusion in TMPRSS2-ERG, whereas 5–15% of patients exhibited loss of function mutation in SPOP genes, and 3–5% of patients showed gain-of-function mutations in FOXA1. The androgen receptor gene (AR) alterations are also rarely observed in early prostate cancer [71]. Most prostate tumors in Asian men are caused by recurrent hotspot mutations in CHD1, FOXA1, and ZNF292, while only a few cases showed TMPRSS2-ERG fusions. In localized PCa, few deletions in PTEN and mutations in the TP53 gene have been detected, and the frequency of occurrence of these deletions increases in patients with advanced disease states [72]. It has been well established by fine-mapping and genome-wide association studies (GWAS) that the susceptibility of PCa is associated with more than 100 commonly reported SNPs. The 8q24 polymorphisms are reportedly strongly linked with prostate cancer susceptibility, representing a promising biological marker for diagnosis and pharmacotherapy [73]. The variants of genes involved in oxidative stress, steroid metabolism, angiogenesis, cell adhesion, DNA repair, and cell cycle can also serve as suitable candidates for the disease state. An association analysis has reported 63 susceptibility loci for PCa in more than 140,000 men [74].
Previously, the clinical diagnosis was based on the digital examination or detection of blood levels of prostate-specific antigen (PSA) for prostate cancer screening. While the risk of PCa progression was also co-related to PSA, tumor stage, and Gleason score [75]. Recently, many studies highlighted the importance of SNPs and genomic alterations in the prediction, prognosis, and outcomes of pharmacotherapy of PCa. Apart from the coding sequence's role, tumor biology has assessed the effects of almost 200 nucleotides long noncoding RNAs (LncRNAs) in the development of PCa, which do not translate into proteins [76]. By interacting with macromolecules, LncRNAs perform several important cellular regulatory functions such as differentiation, migration, proliferation, and apoptosis [77]. The LncRNA promoter region containing genetic variants modulates gene expression patterns of methylation. Recently, the GAS5 gene encoded LncRNA termed Growth arrest-specific 5 (GAS5) was found to act as a tumor suppressor in prostate, breast, lung, and colorectal cancers. It is considered that GAS5 may be involved in the migration, invasion, proliferation, and metastasis of PCa cells; however, the exact GAS5 expression level is still controversial in PCa cells [78]. Several studies have evidence that the down-regulation of microRNA-21/microRNA-1284 and up-regulation of PTEN/ PCDC4/AKT are linked with the expression of GAS5 to induce apoptosis and reduce the proliferation rate of prostate cancer cells (Fig. 1) [47]. Figure 2 represents the functions and locations of SNP genes in translated and untranslated regions.
Pharmacogenomics and pharmacogenetics of prostate cancer
The phenotypic variations occur due to an alteration of expression level or activity in the corresponding genes, which are not only linked with vulnerability to disease but also significantly affect pharmacotherapy outcomes [133]. Pharmacogenetics is the variability in drug response due to heredity or polymorphism in a single gene. This term is used to study the genes involved in the metabolism of drugs, whereas ‘Pharmacogenomics’ is the study of all genes in the DNA, which may help determine the drug's response [134]. Irinotecan treats prostate cancer by exerting cytotoxic effects by 7-ethyl-10-hydroxycamptothecin (SN-38), an active metabolite. The irinotecan-induced toxicity is associated with the polymorphisms in the genes involved in the irinotecan metabolic pathway. A polymorphism in the UDP-glucuronosyltransferase encoding gene UGT1A1 (UGT1A1*28 and UGT1A1*6) causes a decrease in metabolic enzyme activities, which results in delay in SN-38 metabolism and a higher incidence rate of adverse events [135]. Pharmacotherapy is linked with genetic background and interactions with several factors, such as acquired somatic genetic alterations, inherent germline susceptibility, and micro-environmental (immune cells) and macro-environmental (blood, lymph vessels) conditions [136]. For PCa patients, excessive advancement has been made in the therapeutic landscape of pharmacotherapy, such as Androgen-deprivation therapy (ADT) which is considered the gold standard for the primary pharmacotherapy of PCa [137]. Recently, second-generation anti-androgen agents have been developed for castration-resistant prostate cancer (CRPC) patients, such as CYP17 inhibitor abiraterone, apalutamide, enzalutamide, and darolutamide [138]. These drugs are also used for hormone-sensitive prostate cancer (HSPC) patients; however, initially, these were developed for treating CRPC patients. Nowadays, various therapeutic options are available for CRPC and HSPC patients. Among taxane chemotherapy, docetaxel and cabazitaxel have been used for CRPC treatment, whereas docetaxel is used to treat HSPC [139]. Also, there is a need to identify useful, suitable candidates for maximum efficacy and individualized pharmacotherapy regimen. The aberrant activation of androgen receptor signaling pathways (AR) is linked with castrate-resistant prostate cancer. Therefore, polymorphisms in AR pathway-related genes significantly impact the therapeutic efficacy of primary androgen deprivation therapy (PADT) by influencing the AR signaling activity [140]. The SNPs of various other genes have also significantly impacted the therapeutic outcome of primary ADT for the treatment of prostate cancer, as shown in (Table 2).
Genome-wide association studies reported that androgen metabolism and pharmacotherapy outcomes are related to multiple SNPs reported in various genes. For example, a variant cSNP (rs1047303) of 3β-hydroxysteroid dehydrogenase 1 (3β-HSD1) encodes by HSD3B1 influences the enzymatic activity significantly, so carriers of this variant are the poor drug metabolizer [141]. The prognostic impact of this variant was observed in patients in the USA and confirmed in an Asian cohort study, which reported that this variant is rare in Asian patients but successfully validated as a prognostic marker in primary ADT plus docetaxel for HSPC [142]. There are four reported SNPs (rs6162, rs743572, rs1004467, and rs6163) in the CYP17A1 genes influencing prostate cancer progression to CRPC after ADT. Furthermore, the risk of development of CRPC is also linked with dehydroepiandrosterone (DHEA), a steroidal hormone that acts as a precursor of intratumoral androgen biosynthesis that controls the progression of cancer and is an important target for novel therapies [143]. Enzymes encoded by CYP19A1 catalyze the conversion of androgens to estrogen. Three SNPs (rs10459592, rs2470152, rs4775936) reported in this gene are related to the risk of development of prostate cancer [144]. In HSD3B1, the validation of prognostic values of cSNP (rs1047303 and rs1856888) was performed in ADT plus docetaxel therapy for HSPC, and it was found that the low risk of progression of the disease is linked with (rs1856888) which is located in the variant G allele [145]. Another study on iSNP in CYP19A1 described that a high risk of progression of the disease is associated with the variant C allele in rs1870050 [146]. The function and expression pattern of HSD17B2 is reported to reduce prostate cancer, as it suppresses AR signaling and cell growth by blocking androgen synthesis. Various studies on gene expression profiling have explained that the disease progression is caused by altering expression patterns of specific genes (HSD17B2, HSD17B3, SRD5A1, and SHBG) [147]. SLCO2B1 and SLCO1B3 are involved in the steroidal hormone uptake, and thrombotic thrombocytopenic purpura (TTP) is linked with three SNPs present in SLCO2B1 expressed in various tissues. These SNPs transport steroid conjugates, such as estrone-3-sulfate and DHEAS. SLCO1B3 expresses in different types of cancer cells and is responsible for the uptake of several hormones [148]. In-vivo studies confirmed that tumor growth is enhanced by HIF1a signaling, whereas its stable expression is linked with the restoration of tumor growth. After evaluating SNPs in the binding sites of estrogen and androgen receptors, it was found that the 5 SNPs localized on ARRDC3, TACC2, SKAP1, FLT1, and BNC2 are specifically associated with prostate cancer mortality [149]. It is also observed that BNC2 (rs16934641) is linked with the progression of the disease, while ALPK1 (rs2051778) is associated with ACM. SNP in the TACC2 (rs3763763) is involved in ACM and prostate cancer-specific mortality. The less significant associations of SKAP1 (rs7209855) and KLHL14 (rs12970312) were observed with PCSM [150]. Similarly, NR4A2 (rs2691786), FBXO32 (rs7830622), AATF (rs 9330247), and KLHL14 (rs12970312) were found to be less significantly associated with ACM. The high expression level of BGLAP is responsible for the survival of bone metastasized tumor cells in prostate cancer [151] (Table 2).
The survival rate of CRPC patients has been improved with the use of novel androgen receptor pathway inhibitors (ARPIs) (Enzalutamide, Apalutamide, Darolutamide, and Abiraterone) because their therapeutic effects depend upon the activity of molecules involved in their uptake and metabolism. For instance, SLCO2B1 encodes OATP2B1, which is responsible for the uptake of abiraterone into cells metabolized by 3-HSD and 5-reductase [195]. It is reported that the therapeutic effect of abiraterone depends on SNPs in the genes involved in the transport and metabolism of androgen. Few SNPs are associated with prognosis after ARPI treatment, such as (rs2486758) in CYP17A1 and (rs1047303) in HSD3B1. The overlapping of several genes has been observed in the prognosis because ARPIs and primary ADT outcomes depend on the SNPs located in different genes (CYP17A1 and YB-1) [61]. Similarly, SNPs in various genes [(rs1789693, rs1077858, and rs12422149 in SLCO2B1), (rs523349 in SRD5A2) and (rs1047303 in HSD3B1)] are acting as prognostic markers in ARPIs for CRPC and primary ADT for HSPC. It has been found that a variant allele in HSD3B1 (rs1047303) is a prognostic marker for patients treated with abiraterone [196]. Docetaxel is used to treat various types of cancers. Several studies have shown a correlation between efficacy and adverse effects of docetaxel with genetic polymorphism in transport genes (ABCC2, ABCB1, ABCG1, SLCO1B3, ABCG2) and metabolizing genes (CYP1B1, CYP3A4, CYP2C8, and CYP3A5). In CYP1B1, reported cSNP (rs1056836, 4326C>G, L432V) is linked with the poor therapeutic response of the drug and prognosis [197]. The patient's response to taxane chemotherapy depends on SNPs found in various positions in estrogen receptor-1 (ESR1). These SNPs serve as potential predictive biomarkers for taxane chemotherapy. The resistance to Taxane therapy is induced by the OATP1B3 transport protein in prostate cancer cells encoded by SLCO1B3 [198]. Another predictive marker for PCa that affects the efficacy of taxane therapy is cSNP (rs4149117), located in SLCO1B3. Although SNPs are influencing pharmacotherapy, but still there are only a few genetic markers that have been used in pharmacotherapy or individualized treatment strategy for cancer patients [199]. In markers validation studies, the reproducibility of some SNPs has occurred successfully. In contrast, other studies failed to produce consistent results because of racial differences, and there are variations in the frequency of genetic polymorphisms [200]. Figure 3 exhibits the signals transduction through AR.
Conclusion
Disease risk is associated with genetic variations. Most PCa research focuses on a limited number of genetic markers commonly used in clinical practice. These markers include PSA, TMPRSS2-ERG Gene Fusion, PTEN Loss, and mutations in BRCA1 and BRCA2. However, many novel genetic markers have been identified in recent years. Genome-wide association studies (GWASs) provide valuable information on identifying SNP groups that accurately predict prostate cancer risk, development, and pharmacotherapy response. Clinically, multiple drugs are available to treat Prostate Cancer, but Individualized treatment regimens for patients with advanced-stage prostate cancer are largely determined by the availability of suitable genetic biomarkers (SNPs). Combining SNPs with traditional clinicopathological parameters will lead to earlier diagnosis, better prognoses, and more effective pharmacotherapy. Additionally, SNP-based personalized medicine will reduce the need for ineffective pharmacotherapy trials in prostate cancer patients. Further studies are needed to validate these SNPs in PCa progression and to identify biomarker inter-individual variations. In terms of the future perspective of this field, integrating multiple genetic markers, along with clinical and pathological parameters, may enhance risk stratification, prognosis prediction, and treatment selection. This will also help tailor interventions and healthcare decisions based on individual genetic makeup.
Availability of data and materials
Not applicable.
References
Franlund M. Prostate cancer screening: outcomes and risk prediction. Doctoral thesis, Gothenburg University. 2018.
Wang L, Lu B, He M, Wang Y, Wang Z, Du L. Prostate cancer incidence and mortality: global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health. 2022;10:811044.
Withrow D, Pilleron S, Nikita N, Ferlay J, Sharma S, Nicholson B, Lu-Yao G. Current and projected number of years of life lost due to prostate cancer: a global study. Prostate. 2022;82(11):1088–97.
Roubaud G, Liaw BC, Oh WK, Mulholland DJ. Strategies to avoid treatment-induced lineage crisis in advanced prostate cancer. Nat Rev Clin Oncol. 2017;14(5):269–83.
Xia C, Yu XQ, Chen W. Measuring population-level cure patterns for cancer patients in the United States. Int J Cancer. 2023;152(4):738–48.
Nduma BN, Ambe S, Ekhator C, Fonkem E, Ekhator C. Geographical distribution of pancreatic cancer in the state of mississippi by incidence and mortality from 2003 to 2019. Cureus. 2022;14(11):e31605.
Yuan Y, Ahn E, Feng D, Khadra M, Kim J. Z-SSMNet: A Zonal-aware Self-Supervised Mesh Network for Prostate Cancer Detection and Diagnosis in bpMRI. arXiv Prepr. 2022. https://doi.org/10.48550/arXiv.2212.05808.
Zhang H, Huang D, Zhang Y, Wang X, Wu J, Hong D. Global burden of prostate cancer attributable to smoking among males in 204 countries and territories, 1990–2019. BMC Cancer. 2023;23(1):92.
Pathirana T, Sequeira R, Del Mar C, Dickinson JA, Armstrong BK, Bell KJ, Glasziou P. Trends in prostate specific antigen (PSA) testing and prostate cancer incidence and mortality in Australia: a critical analysis. Cancer Epidemiol. 2022;77:102093.
Ferlay JEM, Lam F, Colombet M, Mery L, Pineros M, Znaor A, Soerjomataram I. et al. Global cancer observatory: cancer tomorrow. Lyon, France: International Agency for Research on Cancer. 2019. https://gco.iarc.fr/tomorrow, Accessed 02 Feb 2019.
Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. 2009;53(2):171–84. https://doi.org/10.1002/mnfr.200700511.
Bosland MC, Shittu OB, Ikpi EE, Akinloye O. Potential new approaches for prostate cancer management in resource-limited countries in Africa. Annals of Global Health. 2023;89(1):14.
McKenney JK, Wei W, Hawley S, Auman H, Newcomb LF, Boyer HD, Fazli L, Simko J, Hurtado-Coll A, Troyer DA, Tretiakova MS. Histologic grading of prostatic adenocarcinoma can be further optimized. Am J Surg Pathol. 2016;40(11):1439–56.
Humphrey PA. Histopathology of prostate cancer. Cold Spring Harb Perspect Med. 2017;7(10):a030411.
Rawla P. Epidemiology of prostate cancer. World journal of oncology. 2019;10(2):63.
Habib A, Jaffar G, Khalid MS, Hussain Z, Zainab SW, Ashraf Z, Haroon A, Javed R, Khalid B, Habib P. Risk Factors Associated with Prostate Cancer. Journal of Drug Delivery and Therapeutics. 2021;11(2):188–93.
Barros-Silva JD, Linn DE, Steiner I, Guo G, Ali A, Pakula H, Ashton G, Peset I, Brown M, Clarke NW, Bronson RT. Single-cell analysis identifies LY6D as a marker linking castration-resistant prostate luminal cells to prostate progenitors and cancer. Cell Rep. 2018;25(12):3504–18.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22.
Sameer AS, Banday MZ, Nissar S. Mutations and polymorphisms: what is the difference? Genet Polymorph Cancer Susceptibility. 2021. https://doi.org/10.1007/978-981-33-6699-2_1.
Mullins VA, Bresette W, Johnstone L, Hallmark B, Chilton FH. Genomics in personalized nutrition: can you “eat for your genes”? Nutrients. 2020;12(10):3118.
Ahmed Z, Zeeshan S, Mendhe D, Dong X. Human gene and disease associations for clinical-genomics and precision medicine research. Clin Transl Med. 2020;10(1):297–318.
Owen KA, Bell KA, Price A, Bachali P, Ainsworth H, Marion MC, Lipsky PE. Molecular pathways identified from single nucleotide polymorphisms demonstrate mechanistic differences in systemic lupus erythematosus patients of Asian and European ancestry. Sci Rep. 2023;13(1):1–17.
Yang PJ, Hsieh MJ, Hung TW, Wang SS, Chen SC, Lee MC, Yang SF, Chou YE. Effects of long noncoding RNA H19 polymorphisms on urothelial cell carcinoma development. Int J Environ Res Public Health. 2019;16(8):1322.
Yu X, Li Z. Long non-coding RNA growth arrest-specific transcript 5 in tumor biology. Oncol Lett. 2015;10(4):1953–8.
Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, Ashrafizadeh M. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. 2022;15(1):1–39.
Zhu L, Zhu Q, Wen H, Huang X, Zheng G. Mutations in GAS5 affect the transformation from benign prostate proliferation to aggressive prostate cancer by affecting the transcription efficiency of GAS5. J Cell Physiol. 2019;234(6):8928–40.
Kang J, La Manna F, Bonollo F, Sampson N, Alberts IL, Mingels C, Afshar-Oromieh A, Thalmann GN, Karkampouna S. Tumor microenvironment mechanisms and bone metastatic disease progression of prostate cancer. Cancer Lett. 2022;1(530):156–69.
Ewing CM, Ray AM, Lange EM, Zuhlke KA, Robbins CM, Tembe WD, Wiley KE, Isaacs SD, Johng D, Wang Y, Bizon C. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366(2):141–9.
Mundade R, Imperiale TF, Prabhu L, Loehrer PJ, Lu T. Genetic pathways, prevention, and treatment of sporadic colorectal cancer. Oncoscience. 2014;1(6):400.
Carter BS, Ewing CM, Ward WS, Treiger BF, Aalders TW, Schalken JA, Epstein JI, Isaacs WB. Allelic loss of chromosomes 16q and 10q in human prostate cancer. Proc Natl Acad Sci. 1990;87(22):8751–5.
Borza T, Kaufman SR, Shahinian VB, Yan P, Miller DC, Skolarus TA, Hollenbeck BK. Sharp decline in prostate cancer treatment among men in the general population, but not among diagnosed men. Health Aff. 2017;36(1):108–15.
Klusa D, Lohaus F, Furesi G, Rauner M, Benešová M, Krause M, Peitzsch C. Metastatic spread in prostate cancer patients influencing radiotherapy response. Front Oncol. 2021;10:627379.
Tonry C, Finn S, Armstrong J, Pennington SR. Clinical proteomics for prostate cancer: understanding prostate cancer pathology and protein biomarkers for improved disease management. Clin Proteomics. 2020;17:1–31.
Giovannelli P, Di Donato M, Galasso G, Monaco A, Licitra F, Perillo B, Castoria G. Communication between cells: exosomes as a delivery system in prostate cancer. Cell Commun Signal. 2021;19(1):1–12.
Sengupta S, Asha Krishnan M, Chattopadhyay S, Chelvam V. Comparison of prostate-specific membrane antigen ligands in clinical translation research for diagnosis of prostate cancer. Cancer Reports. 2019;2(4):e1169.
Macaya Erro I. Role of myosin VI in colorectal cancer. Barcelona: Universitat Autònoma de Barcelona; 2018.
Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014;33(19):2413–22.
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):1–9.
Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52(1):55–81.
Yadav SS, Stockert JA, Hackert V, Yadav KK, Tewari AK. Intratumor heterogeneity in prostate cancer. Urol Oncol. 2018;36(8):349–60.
Gogola S, Rejzer M, Bahmad HF, Alloush F, Omarzai Y, Poppiti R. Anti-cancer stem-cell-targeted therapies in prostate cancer. Cancers. 2023;15(5):1621.
Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules. 2022;27(17):5730.
Seibert TM, Garraway IP, Plym A, Mahal BA, Giri V, Jacobs MF, Morgan TM. Genetic risk prediction for prostate cancer: implications for early detection and prevention. Eur Urol. 2023;83(3):241–8.
Crocetto F, Barone B, Ferro M, Busetto GM, La Civita E, Buonerba C, Schalken JA. Liquid biopsy in bladder cancer: state of the art and future perspectives. Crit Rev Oncol Hematol. 2022;170:103577.
Giacomini A, Grillo E, Rezzola S, Ribatti D, Rusnati M, Ronca R, Presta M. The FGF/FGFR system in the physiopathology of the prostate gland. Physiol Rev. 2021;101(2):569–610.
Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24(18):1967–2000.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2016;40(2):244–52.
El-Deen FE, Muhammad EM, Zaki M, Saleem MD, Mohammed RA. Basal cell hyperplasia (BCH) versus high grade prostatic intraepithelial neoplasia (HGPIN) in tiny prostatic needle biopsies: Unusual diagnostic dilemma. J Egypt Natl Canc Inst. 2014;26(1):15–22.
Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metas. 2019;36(3):171–98.
Datta K, Muders M, Zhang H, Tindall DJ. Mechanism of lymph node metastasis in prostate cancer. Future Oncol. 2010;6(5):823–36.
Body JJ, Casimiro S, Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol. 2015;12(6):340–56.
Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Can Res. 2002;62(6):1832–7.
Jung KU, Kim HC, Park JO, Park YS, Park HC, Choi DH, Cho YB, Yun SH, Lee WY, Chun HK. Adjuvant chemotherapy after neoadjuvant chemoradiation and curative resection for rectal cancer: is it necessary for all patients? J Surg Oncol. 2015;111(4):439–44.
Wong SK, Mohamad NV, Giaze TR, Chin KY, Mohamed N, Ima-Nirwana S. Prostate cancer and bone metastases: the underlying mechanisms. Int J Mol Sci. 2019;20(10):2587.
Zinatizadeh MR, Momeni SA, Zarandi PK, Chalbatani GM, Dana H, Mirzaei HR, Akbari ME, Miri SR. The role and function of ras-association domain family in cancer: a review. Genes Dis. 2019;6(4):378–84.
Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–43.
Gholizadeh N, Pundavela J, Nagarajan R, Dona A, Quadrelli S, Biswas T, Ramadan S. Nuclear magnetic resonance spectroscopy of human body fluids and in vivo magnetic resonance spectroscopy: potential role in the diagnosis and management of prostate cancer. Urol Oncol. 2020;38(4):150–73.
Lomas DJ, Ahmed HU. All change in the prostate cancer diagnostic pathway. Nat Rev Clin Oncol. 2020;17(6):372–81.
Cabañas JGR, Fernando GP, Islas GC. Holmium laser enucleation of the prostate (HOLEP) technique: a safe and effective option in transurethral prostate resection. Int J Med Sci Clin Res Stud. 2023;3(05):854–6.
Chapman CG, Waxman I. EUS-guided portal venous sampling of circulating tumor cells. Curr Gastroenterol Rep. 2019;21:1–7.
Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72.
Beck J, Rouleau M, Lemire F, Neveu B, Déry M, Thériault B, Pouliot F. Mass spectrometry redefines optimal testosterone thresholds in prostate cancer patients undergoing androgen deprivation therapy. Prostate. 2023;83(7):670–7.
Terzic J, Abu el Maaty MA, Lutzing R, Vincent A, El Bizri R, Jung M, Metzger D. Hypoxia-inducible factor 1A inhibition overcomes castration resistance of prostate tumors. EMBO Mol Med. 2023;15(6):e17209.
Archer M, Dogra N, Kyprianou N. Inflammation as a driver of prostate cancer metastasis and therapeutic resistance. Cancers. 2020;12(10):2984.
Fang D, Zhou L. Androgen deprivation therapy in nonmetastatic prostate cancer patients: Indications, treatment effects, and new predictive biomarkers. Asia Pac J Clin Oncol. 2019;15(3):108–20.
Swami U, McFarland TR, Nussenzveig R, Agarwal N. Advanced prostate cancer: treatment advances and future directions. Trends in cancer. 2020;6(8):702–15.
Shiota M, Akamatsu S, Narita S, Terada N, Fujimoto N, Eto M. Genetic polymorphisms and pharmacotherapy for prostate cancer. JMA J. 2021;4(2):99–111.
Allemailem KS, Almatroudi A, Alrumaihi F, Almansour NM, Aldakheel FM, Rather RA, Rah B. Single nucleotide polymorphisms (SNPs) in prostate cancer: its implications in diagnostics and therapeutics. American Journal of Translational Research. 2021;13(4):3868.
Frazer KA, Murray SS, Schork NJ, Topol EJ. Human genetic variation and its contribution to complex traits. Nat Rev Genet. 2009;10(4):241–51.
Deng NA, Zhou H, Fan H, Yuan Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget. 2017;8(66):110635.
Teles Alves I, Hartjes T, McClellan E, Hiltemann S, Böttcher R, Dits N, Temanni MR, Janssen B, Van Workum W, van der Spek P, Stubbs A. Next-generation sequencing reveals novel rare fusion events with functional implication in prostate cancer. Oncogene. 2015;34(5):568–77.
Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, Syndikus I, Ralph C, Jain S, Varughese M, Parikh O. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):162–74.
Matejcic M, Saunders EJ, Dadaev T, Brook MN, Wang K, Sheng X, Olama AA, Schumacher FR, Ingles SA, Govindasami K, Benlloch S. Germline variation at 8q24 and prostate cancer risk in men of European ancestry. Nat Commun. 2018;9(1):1–1.
Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, Dadaev T, Leongamornlert D, Anokian E, Cieza-Borrella C, Goh C. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–36.
Li J, Xu C, Lee HJ, Ren S, Zi X, Zhang Z, Wang H, Yu Y, Yang C, Gao X, Hou J. A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature. 2020;580(7801):93–9.
Lin CY, Wang SS, Yang CK, Li JR, Chen CS, Hung SC, Chiu KY, Cheng CL, Ou YC, Yang SF. Genetic polymorphism and carbonic anhydrase 9 expression can predict nodal metastatic prostate cancer risk in patients with prostate-specific antigen levels≤ 10 ng/ml at initial biopsy. Urol Oncol. 2019;37(11):814-e9.
Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22(2):96–118.
Yu S, Han K. Taking others’ perspectives enhances situation awareness in the smart home interface. Front Psychol. 2019;10(10):2761.
Couñago F, López-Campos F, Díaz-Gavela AA, Almagro E, Fenández-Pascual E, Henríquez I, Lozano R, Linares Espinós E, Gómez-Iturriaga A, de Velasco G, Quintana Franco LM. Clinical applications of molecular biomarkers in prostate cancer. Cancers. 2020;12(6):1550.
Mouraviev V, Lee B, Patel V, Albala D, Johansen TE, Partin A, Ross A, Perera RJ. Clinical prospects of long noncoding RNAs as novel biomarkers and therapeutic targets in prostate cancer. Prostate Cancer Prostatic Dis. 2016;19(1):14–20.
Cui Y, Cao W, Li Q, Shen H, Liu C, Deng J, Xu J, Shao Q. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6(1):1–9.
Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, Cao X. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29(8):742–9.
Jiang N, Zhu S, Chen J, Niu Y, Zhou L. A-methylacyl-CoA racemase (AMACR) and prostate-cancer risk: a meta-analysis of 4385 participants. PLoS ONE. 2013;8(10):e74386.
Kojima S, Enokida H, Yoshino H, Itesako T, Chiyomaru T, Kinoshita T, Fuse M, Nishikawa R, Goto Y, Naya Y, Nakagawa M. The tumor-suppressive microRNA-143/145 cluster inhibits cell migration and invasion by targeting GOLM1 in prostate cancer. J Hum Genet. 2014;59(2):78–87.
Hägglöf C, Hammarsten P, Strömvall K, Egevad L, Josefsson A, Stattin P, Granfors T, Bergh A. TMPRSS2-ERG expression predicts prostate cancer survival and associates with stromal biomarkers. PLoS ONE. 2014;9(2):e86824.
Holder SL, Abdulkadir SA. PIM1 kinase as a target in prostate cancer: roles in tumorigenesis, castration resistance, and docetaxel resistance. Curr Cancer Drug Targets. 2014;14(2):105–14.
Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL, Hicks JL, Wilson KM, Rider JR, Sesso HD, Fiorentino M. A prospective investigation of PTEN loss and ERG expression in lethal prostate cancer. J Natl Cancer Inst. 2016;108(2):346.
Vanaja DK, Ballman KV, Morlan BW, Cheville JC, Neumann RM, Lieber MM, Tindall DJ, Young CY. PDLIM4 repression by hypermethylation as a potential biomarker for prostate cancer clinical cancer research: an official. J Am Assoc Cancer Res. 2006;12(4):1128–36.
Florl AR, Steinhoff C, Müller M, Seifert HH, Hader C, Engers R, Ackermann R, Schulz WA. Coordinate hypermethylation at specific genes in prostate carcinoma precedes LINE-1 hypomethylation. Br J Cancer. 2004;91(5):985–94.
Xu Z, Bensen JT, Smith GJ, Mohler JL, Taylor JA. GWAS SNP replication among African American and European American men in the North Carolina-Louisiana prostate cancer project (PCaP). Prostate. 2011;71(8):881–91.
Hieronymus H, Schultz N, Gopalan A, Carver BS, Chang MT, Xiao Y, Heguy A, Huberman K, Bernstein M, Assel M, Murali R. Copy number alteration burden predicts prostate cancer relapse. Proc Natl Acad Sci. 2014;111(30):11139–44.
Martin NE. New developments in prostate cancer biomarkers. Curr Opin Oncol. 2016;28(3):248–52.
Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, Mesher D, Speights VO, Stankiewicz E, Foster CS, Møller H. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–55.
Phé V, Cussenot O, Rouprêt M. Methylated genes as potential biomarkers in prostate cancer. BJU Int. 2010;105(10):1364–70.
Alshalalfa M, Crisan A, Vergara IA, Ghadessi M, Buerki C, Erho N, Yousefi K, Sierocinski T, Haddad Z, Black PC, Karnes RJ. Clinical and genomic analysis of metastatic prostate cancer progression with a background of postoperative biochemical recurrence. BJU Int. 2015;116(4):556–67.
Deng J, Tang J, Wang G, Zhu YS. Long non-coding RNA as potential biomarker for prostate cancer: is it making a difference? Int J Environ Res Public Health. 2017;14(3):270.
Rodríguez SVM, García-Perdomo HA. Diagnostic accuracy of prostate cancer antigen 3 (PCA3) prior to first prostate biopsy: a systematic review and meta-analysis. Can Urol Assoc J. 2020;14(5):E214.
Xiong T, Li J, Chen F, Zhang F. PCAT-1: a novel oncogenic long non-coding RNA in human cancers. Int J Biol Sci. 2019;15(4):847.
Alinezhad S, Väänänen RM, Ochoa NT, Vertosick EA, Bjartell A, Boström PJ, Pettersson K. Global expression of AMACR transcripts predicts risk for prostate cancer–a systematic comparison of AMACR protein and mRNA expression in cancerous and noncancerous prostate. BMC Urol. 2016;16(1):1–10.
Jerónimo C, Henrique R, Hoque MO, Mambo E, Ribeiro FR, Varzim G, Sidransky D. A quantitative promoter methylation profile of prostate cancer. Clin Cancer Res. 2004;10(24):8472–8.
Yamoah K, Johnson MH, Choeurng V, Faisal FA, Yousefi K, Haddad Z, Schaeffer EM. Novel biomarker signature that may predict aggressive disease in African American men with prostate cancer. J Clin Oncol. 2015;33(25):2789.
Wang Z, Wang Y, Zhang J, Hu Q, Zhi F, Zhang S, Liang H. Significance of the TMPRSS2: ERG gene fusion in prostate cancer. Molecular Med Rep. 2017;16(4):5450–8.
Padar A, Sathyanarayana UG, Suzuki M, Maruyama R, Hsieh JT, Frenkel EP, Gazdar AF. Inactivation of cyclin D2 gene in prostate cancers by aberrant promoter methylation. Clin Cancer Res. 2003;9(13):4730–4.
Holder SL, Abdulkadir SA. PIM1 kinase as a target in prostate cancer: roles in tumorigenesis, castration resistance, and docetaxel resistance. Curr Cancer Drug Targets. 2014;14(2):105–14.
Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, Lotan TL. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15(4):222–34.
Ploussard G, De La Taille A. Urine biomarkers in prostate cancer. Nat Rev Urol. 2010;7(2):101–9.
Vanaja DK, Ehrich M, Van den Boom D, Cheville JC, Karnes RJ, Tindall DJ, Young CY. Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest. 2009;27(5):549–60.
Schulz WA, Burchardt M, Cronauer MV. Molecular biology of prostate cancer. Mol Hum Reprod. 2003;9(8):437–48.
Woodson K, O’Reilly KJ, Hanson JC, Nelson D, Walk EL, Tangrea JA. The usefulness of the detection of GSTP1 methylation in urine as a biomarker in the diagnosis of prostate cancer. J Urol. 2008;179(2):508–12.
Ye J, Wu M, He L, Chen P, Liu H, Yang H. Glutathione-s-transferase gene promoter methylation in cell-free dna as a diagnostic and prognostic tool for prostate cancer: a systematic review and meta-analysis. Int J Endocrinol. 2023. https://doi.org/10.1155/2023/7279243.
Han W, Liu M, Han D, Li M, Toure AA, Wang Z, Cai C. RB1 loss in castration-resistant prostate cancer confers vulnerability to LSD1 inhibition. Oncogene. 2022;41(6):852–64.
Hieronymus H, Murali R, Tin A, Yadav K, Abida W, Moller H, Sawyers CL. Tumor copy number alteration burden is a pan-cancer prognostic factor associated with recurrence and death. Elife. 2018;7:e37294.
Khemlina G, Ikeda S, Kurzrock R. Molecular landscape of prostate cancer: implications for current clinical trials. Cancer Treat Rev. 2015;41(9):761–6.
Collard RL, Harya NS, Monzon FA, Maier CE, O’Keefe DS. Methylation of the ASC gene promoter is associated with aggressive prostate cancer. Prostate. 2006;66(7):687–95.
Colicchia M, Morlacco A, Cheville JC, Karnes RJ. Genomic tests to guide prostate cancer management following diagnosis. Expert Rev Mol Diagn. 2017;17(4):367–77.
Vasiljević N, Ahmad AS, Carter PD, Fisher G, Berney DM, Foster CS, Lorincz AT. DNA methylation of PITX2 predicts poor survival in men with prostate cancer. Biomarkers Med. 2014;8(9):1143–50.
Bastian PJ, Palapattu GS, Yegnasubramanian S, Rogers CG, Lin X, Mangold LA, Nelson WG. CpG island hypermethylation profile in the serum of men with clinically localized and hormone refractory metastatic prostate cancer. The Journal of urology. 2008;179(2):529–35.
Van Neste L, Herman JG, Otto G, Bigley JW, Epstein JI, Van Criekinge W. The epigenetic promise for prostate cancer diagnosis. Prostate. 2012;72(11):1248–61.
Friedemann M, Horn F, Gutewort K, Tautz L, Jandeck C, Bechmann N, Menschikowski M. Increased Sensitivity of Detection of RASSF1A and GSTP1 DNA Fragments in Serum of Prostate Cancer Patients: Optimisation of Diagnostics Using OBBPA-ddPCR. Cancers. 2021;13(17):4459.
Meyer MS, Penney KL, Stark JR, Schumacher FR, Sesso HD, Loda M, Mucci LA. Genetic variation in RNASEL associated with prostate cancer risk and progression. Carcinogenesis. 2010;31(9):1597–603.
Verma M, Patel P, Verma M. Biomarkers in prostate cancer epidemiology. Cancers. 2011;3(4):3773–98.
Willard SS, Koochekpour S. Regulators of gene expression as biomarkers for prostate cancer. Am J Cancer Res. 2012;2(6):620.
Liu X, Pan YJ, Zheng JN, Pei DS. The role of tumor suppressor DLC-1: Far from clear. Anticancer Agents Med Chem. 2017;17(7):896–901.
Rodic N. LINE-1 retrotransposons as neoplastic biomarkers human retrotransposons in health and disease. Berlin: Springer; 2017. p. 275–95.
Singh AN, Sharma N. Identification of key pathways and genes with aberrant methylation in prostate cancer using bioinformatics analysis. Onco Targets Ther. 2017;10:4925.
Madu CO, Lu Y. Novel diagnostic biomarkers for prostate cancer. J Cancer. 2010;1:150–77.
Taeb J, Asgari M, Abolhasani M, Farajollahi MM, Madjd Z. Expression of prostate stem cell antigen (PSCA) in prostate cancer: a tissue microarray study of Iranian patients. PatholResearch Pract. 2014;210(1):18–23.
Schagdarsurengin U, Lammert A, Schunk N, Sheridan D, Gattenloehner S, Steger K, Dansranjavin T. Impairment of IGF2 gene expression in prostate cancer is triggered by epigenetic dysregulation of IGF2-DMR0 and its interaction with KLF4. Cell Commun Signal. 2017;15(1):1–14.
Voutsadakis IA, Vlachostergios PJ, Daliani DD, Karasavvidou F, Kakkas G, Moutzouris G, Melekos MD, Papandreou CN. CD10 is inversely associated with nuclear factor-kappa B and predicts biochemical recurrence after radical prostatectomy. Urol Int. 2012;88:158–64.
Pang J, Yang YW, Huang Y, Yang J, Zhang H, Chen R, Li B. P110β inhibition reduces histone H3K4 Di-Methylation in prostate cancer. Prostate. 2017;77(3):299–308.
Damodaran S, Damaschke N, Gawdzik J, Yang B, Shi C, Allen GO, Jarrard D. Dysregulation of Sirtuin 2 (SIRT2) and histone H3K18 acetylation pathways associates with adverse prostate cancer outcomes. BMC Cancer. 2017;17:1–8.
Idrissou M, Daures M, Jemia AB, Judes G, Rifaï K, Penault-Llorca F, Bernard-Gallon D. EZH2 histone methyltransferase and JMJD3 histone demethylase implications in prostate cancer. OMICS. 2017;21(12):751–3.
Ieiri I, Higuchi S, Sugiyama Y. Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol. 2009;5(7):703–29.
Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286(5439):487–91.
Yu Q, Li Z, Nie X, Wang L, Gong C, Liu B, Liao X, Zhao B, Li Q, Zhang M, Qiu H. Predictive Value of UGT1A1 Polymorphisms in Irinotecan-Induced Toxicity and Therapeutic Efficacy in Colorectal Cancer Patients. J Cancer Treat Diagn. 2020;4(2):39–46.
Ventura S, Oliver VL, White CW, Xie JH, Haynes JM, Exintaris B. Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH). Br J Pharmacol. 2011;163(5):891–907.
Yip HY, Papa A. Signaling pathways in cancer: therapeutic targets, combinatorial treatments, and new developments. Cells. 2021;10(3):659.
Moussa M, Papatsoris A, Abou Chakra M, Sryropoulou D, Dellis A. Pharmacotherapeutic strategies for castrate-resistant prostate cancer. Expert Opin Pharmacother. 2020;21(12):1431–48.
Sathianathen NJ, Koschel S, Thangasamy IA, Teh J, Alghazo O, Butcher G, Howard H, Kapoor J, Lawrentschuk N, Siva S, Azad A. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2020;77(3):365–72.
Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15(12):701–11.
Shiota M, Narita S, Akamatsu S, Fujimoto N, Sumiyoshi T, Fujiwara M, Uchiumi T, Habuchi T, Ogawa O, Eto M. Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Netw Open. 2019;2(2):e190115.
Hearn JW, Sweeney CJ, Almassi N, Reichard CA, Reddy CA, Li H, Hobbs B, Jarrard DF, Chen YH, Dreicer R, Garcia JA. HSD3B1 genotype and clinical outcomes in metastatic castration-sensitive prostate cancer. JAMA oncology. 2020;6(4):e196496.
Robles-Fernandez I, Martinez-Gonzalez LJ, Pascual-Geler M, Cozar JM, Puche-Sanz I, Serrano MJ, Lorente JA, Alvarez-Cubero MJ. Association between polymorphisms in sex hormones synthesis and metabolism and prostate cancer aggressiveness. PLoS ONE. 2017;12(10):e0185447.
Kanda S, Tsuchiya N, Narita S, Inoue T, Huang M, Chiba S, Akihama S, Saito M, Numakura K, Tsuruta H, Satoh S. Effects of functional genetic polymorphisms in the CYP19A1 gene on prostate cancer risk and survival. Int J Cancer. 2015;136(1):74–82.
Ross RW, Oh WK, Xie W, Pomerantz M, Nakabayashi M, Sartor O, Taplin ME, Regan MM, Kantoff PW, Freedman M. Inherited variation in the androgen pathway is associated with the efficacy of androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2008;26(6):842–7.
Yamada T, Nakayama M, Shimizu T, Nonen S, Nakai Y, Nishimura K, Fujio Y, Okuyama A, Azuma J, Nonomura N. Genetic polymorphisms of CYP17A1 in steroidogenesis pathway are associated with risk of progression to castration-resistant prostate cancer in Japanese men receiving androgen deprivation therapy. Int J Clin Oncol. 2013;18(4):711–7.
Bamodu OA, Tzou KY, Lin CD, Hu SW, Wang YH, Wu WL, Chen KC, Wu CC. Differential but concerted expression of HSD17B2, HSD17B3, SHBG and SRD5A1 testosterone tetrad modulate therapy response and susceptibility to disease relapse in patients with prostate cancer. Cancers. 2021;13(14):3478.
Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N, Venzon D, Maeda K, Nagao K, Sparreboom A, Mitsuya H. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14(11):3312–8.
Huang CN, Huang SP, Pao JB, Chang TY, Lan YH, Lu TL, Lee HZ, Juang SH, Wu PP, Pu YS, Hsieh CJ. Genetic polymorphisms in androgen receptor-binding sites predict survival in prostate cancer patients receiving androgen-deprivation therapy. Ann Oncol. 2012;23(3):707–13.
Sullivan J, Kopp R, Stratton K, Manschreck C, Corines M, Rau-Murthy R, Hayes J, Lincon A, Ashraf A, Thomas T, Schrader K. An analysis of the association between prostate cancer risk loci, PSA levels, disease aggressiveness and disease-specific mortality. Br J Cancer. 2015;113(1):166–72.
Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39(4):246–61.
Lévesque É, Huang SP, Audet-Walsh É, et al. Molecular markers in key steroidogenic pathways, circulating steroid levels, and prostate cancer progression. Clin Cancer Res. 2013;19(3):699–709.
Hamada A, Danesi R, Price DK, et al. Association of a CYP17 polymorphism with overall survival in Caucasian patients with androgen-independent prostate cancer. Urology. 2007;70(2):217–20.
Ross RW, Oh WK, Xie W, et al. Inherited variation in the androgen pathway is associated with the efficacy of androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2008;26(6):842–7.
Pastina I, Giovannetti E, Chioni A, et al. Cytochrome 450 1B1 (CYP1B1) polymorphisms associated with response to docetaxel in castration-resistant prostate cancer (CRPC) patients. BMC Cancer. 2010;10:511.
Kanda S, Tsuchiya N, Narita S, et al. Effects of functional genetic polymorphisms in the CYP19A1 gene on prostate cancer risk and survival. Int J Cancer. 2015;136(1):74–82.
Shiota M, Endo S, Fujimoto N, et al. Polymorphisms in androgen metabolism genes with serum testosterone levels and prognosis in androgen-deprivation therapy. Urol Oncol. 2020;38(11):849.e11-8.
Sissung TM, Baum CE, Deeken J, et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res. 2008;14(14):4543–9.
Sissung TM, Deeken J, Leibrand CR, et al. Identification of novel SNPs associated with risk and prognosis in patients with castration-resistant prostate cancer. Pharmacogenomics. 2016;17(18):1979–86.
Hahn NM, Marsh S, Fisher W, et al. Hoosier Oncology Group randomized phase II study of docetaxel, vinorelbine, and estramustine in combination in hormone-refractory prostate cancer with pharmacogenetic survival analysis. Clin Cancer Res. 2006;12(20 Pt 1):6094.
Shiota M, Fujimoto N, Yokomizo A, et al. SRD5A gene polymorphism in Japanese men predicts prognosis of metastatic prostate cancer with androgen-deprivation therapy. Eur J Cancer. 2015;51(14):1962–9.
Wang X, Harshman LC, Xie W, et al. Association of SLCO2B1 genotypes with time to progression and overall survival in patients receiving androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2016;34(4):352–9.
Yang M, Xie W, Mostaghel E, et al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2011;29(18):2565–73.
Fujimoto N, Kubo T, Inatomi H, et al. Polymorphisms of the androgen transporting gene SLCO2B1 may influence the castration resistance of prostate cancer and the racial differences in response to androgen deprivation. Prostate Cancer Prostatic Dis. 2013;16(4):336–40.
Shiota M, Fujimoto N, Takeuchi A, et al. The association of polymorphisms in the gene encoding gonadotropin-releasing hormone with serum testosterone level during androgen deprivation therapy and prognosis of metastatic prostate cancer. J Urol. 2018;199(3):734–40.
Yu CC, Huang SP, Lee YC, et al. Molecular markers in sex hormone pathway genes associated with the efficacy of androgen-deprivation therapy for prostate cancer. PLoS ONE. 2013;8(1):e54627.
Li T, Peng J, Zeng F, Zhang K, Liu J, Li X, Liu Y. Association between polymorphisms in CTR1, CTR2, ATP7A, and ATP7B and platinum resistance in epithelial ovarian cancer. Int J Clin Pharmacol Ther. 2017;55(10):774.
Varnai R, Koskinen LM, Mäntylä LE, Szabo I, FitzGerald LM, Sipeky C. Pharmacogenomic biomarkers in docetaxel treatment of prostate cancer: from discovery to implementation. Genes. 2019;10(8):599.
Sissung TM, Danesi R, Kirkland CT, et al. Estrogen receptor α and aromatase polymorphisms affect risk, prognosis, and therapeutic outcome in men with castration-resistant prostate cancer treated with docetaxel-based therapy. J Clin Endocrinol Metab. 2011;96(2):E368–72.
Fraga A, Ribeiro R, Príncipe P, et al. The HIF1A functional genetic polymorphism at locus +1772 associates with progression to metastatic prostate cancer and refractoriness to hormonal castration. Eur J Cancer. 2014;50(2):359–65.
Tran MG, Bibby BA, Yang L, Lo F, Warren AY, Shukla D, Neal DE. Independence of HIF1a and androgen signaling pathways in prostate cancer. BMC Cancer. 2020;20:1–12.
Huang CN, Huang SP, Pao JB, Hour TC, Chang TY, Lan YH, Bao BY. Genetic polymorphisms in oestrogen receptor-binding sites affect clinical outcomes in patients with prostate cancer receiving androgen-deprivation therapy. J Intern Med. 2012;271(5):499–509.
Huang SP, Lin VC, Lee YC, Yu CC, Huang CY, Chang TY, Bao BY. Genetic variants in nuclear factor-kappa B binding sites are associated with clinical outcomes in prostate cancer patients. Eur J Cancer. 2013;49(17):3729–37.
Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Alimonti A. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31(8):1040–5.
Hua X, Liu Z, Zhou M, Tian Y, Zhao PP, Pan WH, Yu LN. RETRACTED: LSAMP-AS1 binds to microRNA-183–5p to suppress the progression of prostate cancer by up-regulating the tumor suppressor DCN. EBioMedicine. 2019;50:178–90.
Deeken JF, Cormier T, Price DK, Sissung TM, Steinberg SM, Tran K, Figg WD. A pharmacogenetic study of docetaxel and thalidomide in patients with castration-resistant prostate cancer using the DMET genotyping platform. Pharmacogenomics J. 2010;10(3):191–9.
Yeon B, Ahn E, Kim KI, Kim IW, Oh JM, Park T. Analysis of pharmacogenomic variants associated with population differentiation. PLoS ONE. 2015;10(3):e0119994.
Liu Y, Colby JK, Zuo X, Jaoude J, Wei D, Shureiqi I. The role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int J Mol Sci. 2018;19(11):3339.
Hartley A, Ahmad I. The role of PPARγ in prostate cancer development and progression. Br J Cancer. 2022;128(6):940–5. https://doi.org/10.1038/s41416-022-02096-8.
Shiota M, Fujimoto N, Itsumi M, Takeuchi A, Inokuchi J, Tatsugami K, Eto M. Gene polymorphisms in antioxidant enzymes correlate with the efficacy of androgen-deprivation therapy for prostate cancer with implications of oxidative stress. Annals of Oncology. 2017;28(3):569–75.
Jo JK, Oh JJ, Kim YT, Moon HS, Choi HY, Park S, Byun SS. A genetic variant in SLC28A3, rs56350726, is associated with progression to castration-resistant prostate cancer in a Korean population with metastatic prostate cancer. Oncotarget. 2017;8(57):96893.
Holt SK, Karyadi DM, Kwon EM, Stanford JL, Nelson PS, Ostrander EA. Association of megalin genetic polymorphisms with prostate cancer risk and prognosis. Clin Cancer Res. 2008;14(12):3823–31.
Teixeira AL, Ribeiro R, Cardoso D, Pinto D, Lobo F, Fraga A, Medeiros R. Genetic polymorphism in EGf is associated with prostate cancer aggressiveness and progression-free interval in androgen blockade-treated patients. Clin Cancer Res. 2008;14(11):3367–71.
Teixeira AL, Gomes M, Nogueira A, Azevedo AS, Assis J, Dias F, Medeiros R. Improvement of a predictive model of castration-resistant prostate cancer: functional genetic variants in TGFβ1 signaling pathway modulation. PloS ONE. 2013;8(8):e72419.
Liu JM, Liu JN, Wei MT, He YZ, Zhou Y, Song XB, Huang J. Effect of IL-18 gene promoter polymorphisms on prostate cancer occurrence and prognosis in Han Chinese population. Genet Mol Res. 2013;12(1):820–9.
Geng JH, Lin VC, Yu CC, Huang CY, Yin HL, Chang TY, Bao BY. Inherited variants in Wnt pathway genes influence outcomes of prostate cancer patients receiving androgen deprivation therapy. Int J Mol Sci. 2016;17(12):1970.
Wu HC, Lin CC, Chen WC, Chen HY, Tsai FJ. Osteocalcin gene HindIII C/T polymorphism is a biomarker for prostate cancer and responsiveness to hormone therapy. Eur Urol. 2003;43(2):197–200.
Yin H, Wang L, Li F, Wang D, Zhang Z, Yu B, Liu Y. ET-1 promotes the growth and metastasis of esophageal squamous cell carcinoma via activating PI3K/Akt pathway. Translational Cancer Research. 2020;9(5):3282.
Kohli M, Riska SM, Mahoney DW, Chai HS, Hillman DW, Rider DN, Cerhan JR. Germline predictors of androgen deprivation therapy response in advanced prostate cancer. Mayo Clin Proc. 2012;87(3):240–6.
Suzuki M, Mamun MRI, Hara K, Ozeki T, Yamada Y, Kadowaki T, Kitamura T. The Val158Met polymorphism of the catechol-O-methyltransferase gene is associated with the PSA-progression-free survival in prostate cancer patients treated with estramustine phosphate. European Urol. 2005;48(5):752–9.
Bao BY, Pao JB, Huang CN, Pu YS, Chang TY, Lan YH, Huang SP. Polymorphisms inside microRNAs and microRNA target sites predict clinical outcomes in prostate cancer patients receiving androgen-deprivation therapy miRNA SNPs predict outcomes after ADT. Clin Cancer Res. 2011;17(4):928–36.
Yang Y, Liu KY, Liu Q, Cao Q. Androgen receptor-related non-coding RNAs in prostate cancer. Front Cell Dev Biol. 2021;9:660853.
Derosa L, Galli L, Orlandi P, Fioravanti A, Di Desidero T, Fontana A, Bocci G. Docetaxel plus oral metronomic cyclophosphamide: a phase II study with pharmacodynamic and pharmacogenetic analyses in castration-resistant prostate cancer patients. Cancer. 2014;120(24):3923–31.
Yuan Y, Weidhaas JB. Functional micro RNA binding site variants. Mol Oncol. 2019;13(1):4–8.
Hahn AW, Gill DM, Nussenzveig RH, Poole A, Farnham J, Cannon-Albright L, Agarwal N. Germline variant in HSD3B1 (1245 A> C) and response to abiraterone acetate plus prednisone in men with new-onset metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2018;16(4):288–92.
Aragon I, Khalaf DJ, Lozano R, Annala M, Taavitsainen S, Lorente D, Finch DL, Romero-Laorden N, Vergidis J, Cendon Y, Oja CD. HSD3B1 (1245A> C) polymorphism and clinical outcomes in metastatic castration-resistant prostate cancer (mCRPC) patients treated with abiraterone acetate (AA) and enzalutamide (ENZA): Results from two prospective studies. Ann Oncol. 2020;31(9):1186–97.
Hattinger CM, Vella S, Tavanti E, Fanelli M, Picci P, Serra M. Pharmacogenomics of second-line drugs used for treatment of unresponsive or relapsed osteosarcoma patients. Pharmacogenomics. 2016;17(18):2097–114.
Li J, Bluth MH. Pharmacogenomics of drug metabolizing enzymes and transporters: implications for cancer therapy. Pharmacogenomics Pers Med. 2011;4:11.
Sekino Y, Teishima J. Molecular mechanisms of docetaxel resistance in prostate cancer. Cancer Drug Resist. 2020;3(4):676.
Troutman SM, Sissung TM, Cropp CD, Venzon DJ, Spencer SD, Adesunloye BA, Huang X, Karzai FH, Price DK, Figg WD. Racial disparities in the association between variants on 8q24 and prostate cancer: a systematic review and meta-analysis. Oncologist. 2012;17(3):312–20.
Acknowledgements
Funding support was received from the Science and Technology Innovation Committee of Shenzhen (Grants JCYJ20200109150700942, JCYJ20200109150700942, JCYJ20180306170922163, SGDX20201103095800003,GJHZ20200731095606019), National Natural Science Foundation of China (Grants 81,972,116 and 81,772,394), the Guangdong Basic and Applied Basic Research Foundation (Grant 2021A1515010985, 2020A1515011581), Guangdong International Cooperation Project (Grant 2021A0505030011), the Key-Area Research and Development Program of Guangdong Province (Grant 2019B030335001), the Sanming Project of Medicine (Grant SZSM201612079), the Shenzhen Fund for Guangdong Provincial High Level Clinical Key Specialties (Grant SZGSP013), and the Shenzhen Key Medical Discipline Construction Fund (Grant SZXK042).
Author information
Authors and Affiliations
Contributions
Conceptualization and illustration drawing: Y. L. Supervision: D.L. Writing—original draft: ZI and KR. Writing support: KI, JX, MS, MZ, and JX Approval of final manuscript: all authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rehman, K., Iqbal, Z., Zhiqin, D. et al. Analysis of genetic biomarkers, polymorphisms in ADME-related genes and their impact on pharmacotherapy for prostate cancer. Cancer Cell Int 23, 247 (2023). https://doi.org/10.1186/s12935-023-03084-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-023-03084-5