Abstract
Background
The ESR1 gene suffers methylation changes in many types of cancers, including breast cancer (BC), the most frequently diagnosed cancer in women that is also present in men. Methylation at promoter A of ESR1 is the worse prognosis in terms of overall survival; thus, the early detection, prognostic, and prediction of therapy involve some methylation biomarkers.
Methods
Therefore, our study aimed to examine the methylation levels at the ESR1 gene in samples from Mexican BC patients and its possible association with menopausal status.
Results
We identified a novel 151-bp CpG island in the promoter A of the ESR1 gene. Interestingly, methylation levels at this CpG island in positive ERα tumors were approximately 50% less than negative ERα or control samples. Furthermore, methylation levels at ESR1 were associated with menopausal status. In postmenopausal patients, the methylation levels were 1.5-fold higher than in premenopausal patients. Finally, according to tumor malignancy, triple-negative cancer subtypes had higher ESR1 methylation levels than luminal/HER2+ or luminal A subtypes.
Conclusions
Our findings suggest that methylation at this novel CpG island might be a promising prognosis marker
Similar content being viewed by others
Introduction
One of the most common cancers among women is breast cancer (BC), the second leading cause of cancer mortality in women [1]. Although this cancer occurs in men and women, statistics show that only 1 of every 100 cases of BC is diagnosed in men. In premenopausal women (younger than 50 years), BC is more frequent in low-income and middle-income countries than in high-income countries. In contrast, BC in postmenopausal women (50 years and older) is more common in higher-income countries [2].
Different risk factors for BC have been identified in premenopausal and postmenopausal women. For instance, excessive body weight and abdominal adiposity are important risk factors in postmenopausal ages [3]. On the other hand, risk factors for premenopausal BC are mainly reproductive. For instance, having few or no children or having children later in life reduces the risk of triple-negative BC (TNBC) but increases the risk of hormone-receptor-positive tumors [4]. Remarkably, BC therapeutic management and prognosis are different in premenopausal and postmenopausal patients. For example, the breast density in premenopausal women hinders early detection of BC. Furthermore, the BC subtype in young and older patients has important implications in survival and prognosis. In this regard, estrogen receptor-positive (ER+) tumors have a better prognosis in postmenopausal ages [2].
Epigenetic regulation in cancer, such as DNA methylation of cytosine-guanine dinucleotides (CpG), histone post-translational modifications, alterations in non-coding RNA expression, and recruitment of chromatin modifications have crucial roles in cancer initiation and progression [5]. In BC, hypermethylation at specific gene sites, mainly at CpG islands, is involved in oncogenes expression [6, 7]. Hypermethylation in BC includes genes encoding for BRCA1 protein [8], TIMP metallopeptidase inhibitor 3 (TIMP3) [9], Ras association domain family 1 isoform A (RASSF1A) [10], retinoic acid receptor β (RARβ) [11], and estrogen receptor-alpha (ERα) [12], among others. DNA methyltransferases 1 (DNMT1) and 3A (DNMT3A) levels are associated with hypermethylation of the ESR1 gene and decreased expression of ERα, its encoded protein [12].
Molecules expressed in BC, such as estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2), help to categorize the tumors into five subtypes such as luminal A and B, HER2 enriched, TNBC, or basal-like, and normal-like BC [13]. ER-negative cases seem to be more aggressive and confer a worse prognosis than ER-positive [14]. Thus, ERα is considered a prognostic biomarker suitable for predicting endocrine therapy response because 60% of ER-positive and 8% of ER-negative tumors showed an objective response. ESR1 methylation was observed in 80% of TNBC, 60% of HER2-enriched, 28% of luminal A, and 36% of luminal B cases [15]. A worse prognosis in overall survival correlated with methylation of the ESR1 promoter [16]. Likewise, metastatic tumors contained lower levels of ERα compared to primary tumors [17]. Moreover, 40–85% of BC patients presented hypermethylation of the ESR1 gene [18] that highly correlated with ER-negative/progesterone receptor (PR) negative conditions, suggesting that ESR1 methylation status strongly contributes to tumor phenotypes [19]. On the other hand, in free circulating DNA samples, the ESR1 methylation profile is correlated with ER-negative status and may be associated with resistance to hormonal treatment in BC patients [15]. Besides, the lack of response to exemestane treatment in BC patients related to the hypermethylation of ESR1 [20] and ERα's in vitro reactivation reveals sensitivity to tamoxifen in hypermethylated ESR1 samples.
Located in chromosome 6 (chr6), the ESR1 gene contains approximately 475,472 bases, including the 140 kb section containing the eight protein-coding exons and eight regulatory elements utilized in a tissue-specific manner [21]. ER-positive BC primarily operates the proximal promoter (A promoter), whereas promoters B and C reported less activity [22,23,24,25]. Overlapping in the A promoter is more frequent than C promoter's overlapping in ER-positive primary patient samples [23].
Since endocrine therapy resistance in patients involves an ER dysregulated expression, the investigation about ESR1 will allow a better knowledge of the treatment effectiveness for BC. Since genetic modifications might contribute to the incidence of BC, this study aimed to investigate the methylation levels at the ESR1 gene in samples from Mexican BC patients and correlate these findings with the menopausal status. We found that the proximal promoter of the ESR1 gene contains a 151-bp CpG island located between the transcription start site (+ 1) and the ATG codon. Moreover, our findings indicated that methylation levels at 151-bp CpG island of the ESR1 gene are associated with menopausal status. Finally, according to tumor malignancy, TNBC subtypes had higher methylation levels than luminal/HER2+ or luminal A types, indicating that methylation at this novel CpG island might be a prognosis marker.
Materials and methods
Study population and ethics statement
This study enrolled a cohort of 20 women controls and 38 women with BC diagnosis between 2018 and 2019. All women who participated in this research had at least two generations born and lived in Mexico.
Participant women attended the Hospital Juárez de México (Mexico City, Mexico) for a breast biopsy because they felt a lump when performing a breast self-examination. The biopsies were analyzed immunohistochemically, and samples diagnosed with hyperplasia were considered as controls. All patients included in this research received no treatment at the time of sampling. Participants were included in this protocol after signing a written informed consent. We subdivided BC samples into ER+ or negative (ER−) groups, resulting in 19 patients per group. The immunohistochemical analysis performed by the Oncology and Pathology Services at the Hospital Juárez de México supported the categorization. The medical records endorsed the clinicopathological characteristics, such as menopausal state, cancer family history, and age at diagnosis time. All procedures performed in this research were performed according to the Code of Ethics of the Helsinki Declaration. The Ethics Committee from the Hospital Juárez de México approved this study (HMJ 2231/13-B).
Nucleic acids isolation from tumor samples
Breast biopsies were stored at − 70 °C until their analysis. For DNA extraction, 200 mg of tissue was disrupted with mortar in the presence of liquid nitrogen. The frozen powder was transferred to a 1.5 ml tube, and 750 μl of lysis buffer (50 mM Tris–HCL, pH8.0; 25 mM EDTA, 400 mM NaCl, 10% SDS and, 10 mg/mL Proteinase K) was added and incubated for 1 h at 60 °C with agitation. Then, RNase (3 μl) was added to each sample, mixed carefully, and set at 37 °C for 30 min. Samples were kept at room temperature for 5 min, and then 200 μl of protein precipitation solution (6 M NaCl, 8 M guanidine hydrochloride (pH 8.0), and 0.49 M potassium acetate solution) were added. Samples were vortexed vigorously and kept on ice for 5 min. Samples were centrifugated at 16,000×g for 4 min to pellet debris and proteins. Supernatants were separated and collected in a clean tube. Then, 600 μl of 100% isopropyl alcohol was added and mixed carefully. Samples were centrifugated as described above. DNA pellet was washed three times with 70% ethanol. Finally, ethanol residues were eliminated, 100 μl of resuspension buffer (10 mM Tris–HCl, pH 8.0; 1 mM EDTA) was added, and DNA samples were kept at − 20 °C until further analysis.
RNA isolation from tissues was performed using the TRIzol Reagent (Invitrogen). Briefly, 20 mg of the biopsy was disrupted with mortar in the presence of liquid nitrogen. The frozen powder was transferred to a 1.5 ml tube, mixed with 1 ml of TRIzol reagent, and incubated at − 70 °C for 20 h. Then, samples were kept on ice until defrosting, and 200 μl of samples were transferred to a new tube and gently mixed with 40 μl chloroform. Samples were chilled ice for 4 min and centrifugated at 11,000 rpm for 12 min at 4 °C. The aqueous layer was transferred to a 1.5 ml tube, mixed with 500 μl isopropyl alcohol, and incubated at − 70 °C for 24 h. Samples were kept on ice until defrosting, centrifugated at 11,000 rpm for 5 min, and the pellet was washed with 200 μl ethanol and centrifugated at the same conditions. Pellet was resuspended in RNase-free water. DNA and RNA quantity and quality were determined using a spectrophotometer and gel electrophoresis, respectively.
Analysis of ESR1 methylation
Bisulfite modification of genomic DNA was performed using the MethylCode Bisulfite Conversion Kit (Invitrogen), and methylation in ESR1 was measured from BC patients' biopsies. Briefly, 500 ng of genomic DNA were denatured by incubation with 130 μl of CT Conversion Reagent for 10 min at 98 °C, followed by 150 min at 65 °C, and finally, samples were kept at 4 °C for 20 h. Modified DNA was purified using a spin column following the manufacturer's instructions and eluted with 10 μl of elution buffer. Samples were stored at − 20 °C until their use.
Primer sequences for the ESR1 gene for methylated sequences (M) were the following: forward primer 5′-TGCACTTGCTCCCGTCGGGTC-3′ and reverse primer 5′-AACCGGCGGGCCACCTGGAA-3′. The primer sequences for the ESR1 unmethylated sequences (U) were the following: forward primer 5′-GATTGTATTTGTTTTTGTTGGGTT-3′ and reverse primer 5′-AACCAACAAACCACCTAAAAAAA-3′. The cycling conditions were as follows: initial denaturation at 94 °C for 5 min, followed by 40 cycles at 94 °C for 30 s, at 58 °C for 45 s, and 72 °C for 60 s, the final extension was at 72 °C for 8 min.
Genomic DNA isolation allows the obtention of positive and negative methylation controls from healthy women's whole blood. The positive control was artificially methylated. Briefly, genomic DNAs (1 µg) were mixed with 2 µl of 10× NEBuffer2, 2 µl of SAM, and 1 µl of methyltransferase M.SssI enzyme. Samples were incubated 60 min at 37 °C, followed by incubation at 65 °C for 20 min to stop the reaction. Then bisulfite modification was performed as mentioned above. For negative controls, genomic DNA was subjected to bisulfite conversion without pretreatment. Finally, bisulfite sequencing PCR reactions were analyzed by densitometry, and normalized data were used to create all graphs.
ESR1 expression analysis
For cDNA synthesis, 2 µg of total RNA were reverse-transcribed using the Superscript II (Invitrogen) and the oligo-dT primer (500 µg/ml) according to instructions of the manufacturer. PCRs were performed using an ABI-PRISM 7000 Sequence Detector System (Applied Biosystems, Branchburg, NJ). Measurements of the relative amount of ESR1 in tumor samples were conducted in a single PCR reaction to normalize the number of target copies to that of the 18S rRNA gene using the critical threshold cycle (Ct). Reaction mixture consisted of cDNA (2 ml), 1× TaqMan Universal Master Mix (Applied Biosystems, USA) and 0.9 mM primers and 0.25 mM of TaqMan probes (Thermo Scientific) for ESR1 (Hs00174860_ml) and 18S-ribosomal RNA (Hs99999901_sl). We used the conditions indicated by the manufacturer for the RT-PCR reactions.
Statistical analysis
Pathological characteristics were summarized through descriptive analysis. Categorical variables were described through frequency distribution, whereas continuous variables were reported through the median and standard deviation (SD). Associations between promoter methylation, pathological characteristics, and transcriptional expression level were explored through χ2 or ANOVA tests by Prism software (GraphPad, San Diego, CA, USA).
Results
The study population comprised 38 BC patients and 20 controls. For premenopausal women, the mean of age in cases was 41.33 ± 5.97, whereas in controls was 34.80 ± 12.42. For postmenopausal women, the mean of age in cases was 57.58 ± 12.56 and in controls was 49.60 ± 20.21. Table 1 represents the descriptive statistics of variables in the groups. According to the cancer family history, cases and controls were statistically different (P = 0.0410). Furthermore, we observed a difference in the premenopausal stage between BC and controls (P = 0.0016). When we analyzed the immunohistochemical profile, we found a statistically significant difference in the expression of HER2+ (P = 0.0457) (Table 1).
According to our analysis, the proximal promoter of the ESR1 gene contains a putative CpG island located between the transcription start site (+ 1) and the ATG codon (+ 235) (Fig. 1A, B). In order to determine its methylation status, we designed a primers pair that allowed us to amplify a single 151-bp amplicon (Fig. 1C) using genomic DNA from samples of patients diagnosed with fibroadenoma. Furthermore, a band of approximately 400 bp was detected in negative methylated controls, which is attributed to the non-specificity of the primers used in our study.
The sequence of the CpG island in the ESR1 gene. A ESR1 promoter region scheme in chromosome 6 (Chr6). The 300-kilobases ESR1 gene contains seven promoters (color boxes) utilized in a tissue-specific manner and eight exons (only exon 1 is shown). B Assessed DNA sequence in the proximal promoter. A 151-bp sequence (blue box) located in the A promoter between the transcription start site (+ 1) and the ATG codon (+ 235) was evaluated. Underlined sequences corresponded to the primers used for methylation analysis. Nomenclature and numbering are based on previous reports [21, 54]. C Methylation-specific PCR analysis of ESR1. Electrophoretic profile of methylated (M) (lanes 2 and 4) and unmethylated (U) (lanes 3 and 5) samples from positive (lanes 2 and 3) and negative (lanes 4 and 5) controls using genomic DNA from patients with fibroadenoma. 50-bp DNA Ladder (lane 1). Arrowhead indicates the amplicon size. Genomic coordinates of the 151-bp island in the ESR1 gene: GRCh38: Chr6: 151,500,579–151,500,730
We compared ESR1 methylation levels in controls with BC samples classified into ERα+ or ERα− (Fig. 2A). Controls had no difference with ERα− samples. In contrast, methylation in ERα+ samples was half that controls or ERα− samples (p < 0.05) (Fig. 2A). As expected, ESR1 mRNA expression was inversely proportional to the methylation status (Fig. 2B). Controls and ERα− samples with the higher methylation levels exposed a minimal amount of ESR1 mRNA, while the higher mRNA quantity corresponded to ERα+ samples. Interestingly, in BC samples, we found a difference between the ERα+ and ERα− samples in terms of ESR1 mRNA expression levels, the quantity of ESR1 mRNA in ERα+ samples was threefold higher than in ERα− samples (p < 0.05) (Fig. 2B). Representative immunohistochemical images of control patients and case ERα– and ERα+ patients are shown in Additional file 1: Fig. S1.
Methylation levels at the ESR1 CpG island among Mexican BC patients. A The methylation levels were significantly lower in ERα+ than in controls or ERα− samples (*p < 0.05). B ESR1 transcript levels. Relative ESR1 mRNA amount was determined by qRT-PCR and normalized with 18S RNA gene expression. Data represented the mean ± SD of three independent experiments (**p < 0.05)
Moreover, we analyzed the methylation levels taking together all samples (Fig. 3A) and separately by menopausal stage and controls (Fig. 3B). According to ANOVA analysis, methylation in the postmenopausal women subgroup was twofold higher than in premenopausal women (p < 0.05) (Fig. 3A). Furthermore, the methylation levels of pre and postmenopausal women were analyzed separately (Fig. 3B). We observed significant differences in the methylation levels of the novel CpG island in controls and ERα− samples. Data showed that methylation levels in controls and ERα− samples belonging to the postmenopausal subgroup were significantly higher than in premenopausal women of those subgroups (*p < 0.05). Likewise, in tumor phenotype subgroup analyses, samples classified as HER2+ and TNBC phenotypes revealed higher relative methylation levels (Fig. 3C). The pairwise comparisons among the HER2+ and all subgroups indicated that methylation level in the HER2+ subgroup was higher than in mixed phenotype (p = 0.0237), luminal/HER2+ (p = 0.0002), and luminal A phenotypes (p = 0.0035). Additionally, methylation levels in TNBC phenotype samples were significantly higher than in luminal/HER2+ (p = 0.0054) and luminal A (p = 0.0131) phenotypes (Fig. 3C). Finally, statistical correlation tests suggested that the studied population's other clinicopathological characteristics were not associated with methylation levels at this region of the ESR1 gene. However, in the immunohistochemical profile analysis, the expression of HER2+ was statistically different (P = 0.0457) (Table 1).
Methylation levels at the ESR1 CpG island between patients’ subgroups. A Premenopausal and postmenopausal subgroups. Methylation levels were significantly higher in postmenopausal than in premenopausal samples (*p < 0.05). B Methylation level for pre and postmenopausal women. Controls and BC subgroups were analyzed separately by methylation state, showing that methylation levels of controls and ERα− samples belonging to the postmenopausal subgroup (black), were significantly higher than in premenopausal (white) (*p < 0.05). C Tumor phenotype subgroups. Methylation levels at ESR1 were higher in HER2+ and triple-negative phenotypes and lowered in luminal A and luminal/HER2+ phenotypes. HER2+ samples were significantly different from luminal A, luminal/HER2+, and mixed phenotypes (*p < 0.05), while triple-negative phenotype was statistically different than luminal A and luminal/HER2+ phenotypes (*p < 0.05)
Discussion
Estrogens exert a vast range of biological effects in menopausal women, and their receptors, such as ERα, are a critical BC prognostic biomarker [26]. ESR1 gene has a complex regulation due to its regulatory elements, such as promoters with tissue-specificity [27].
We analyzed the promoter A of ESR1 to find sequences that undergo epigenetic modifications. We identified a 151-bp sequence located from + 29 to + 180, considering the transcription start site of ESR1 that begins from chr6:152,128,814–152,129,050 based on the genomic coordinates previously described [28]. Interestingly, the CpG island reported here is located between two different functional islands, one situated at promoter A (− 403 to − 266) and the other one located at exon 1 (+ 356 to + 549) [29].
We investigated the association of the methylation at the 151-bp CpG island with the expression of ERα. Our results suggested that methylation of this novel island depends on ER but not on the estrogen binding. As previously reported, methylation of ESR1 in proximal regulatory regions was not associated with estradiol levels [30, 31]. Since the 151-bp island is in a proximal promoter, its degree of methylation might not be associated with estradiol levels.
Our results suggested that postmenopausal women have a higher susceptibility to BC than premenopausal women (P = 0.0016). In these patients, the methylation levels of 151-bp island were higher in comparison with premenopausal ones. Therefore, we suggested that the low methylation level of this island is associated with ER-. Furthermore, the methylation level of the identified CpG island in postmenopausal women could be linked with a predisposition to developing BC.
According to statistics, most BC cases are classified as ER-positive, and the ER-negative tumors are declining [32]. Our findings indicated that methylation in ERα+ samples are half that found in controls or ERα− samples, suggesting that the high expression of ESR1 mRNA might be related to the rising number of ER-positive BC cases. ESR1 expression in ER-positive tumors is associated with longer relapse-free survival time [33].
Gene expression is regulated through several mechanisms, such as epigenetic modifications and post-translational modifications. Furthermore, transcriptional regulation of ESR1 is critical in controlling ER expression [34]. Our results showed evidence that methylation at the novel CpG island might be associated with mRNA expression.
Methylation levels in BC Mexican patients classified as ERα− were twofold higher than in ERα+ samples, while ESR1 mRNA in ERα+ tumors were threefold higher than in ERα− samples. Interestingly, 20–25% of ER-negative BC samples express detectable levels of ESR1 mRNA [35]. Here, we reported a similar result; we found a considerable amount of ESR1 mRNA in ERα− samples. According to previous reports, the ER expression might be regulated at different levels in these tumors, such as posttranscriptional or post-translational mechanisms [35,36,37]. Our findings suggested that in ERα− tumors, methylations levels in the 151-bp island are higher compared to ERα+. Inversely, the ESR1 mRNA expression is lower in ERα− compared with ERα+. These data suggested that ESR1 gene expression in BC is a complex process regulated at several levels that might include methylation at 151-bp island in the proximal promoter region and its chromatin environment [34]. In addition to the high methylation status of ESR1 in samples classified as ERα− and controls, we observed a minimal amount of ESR1 mRNA in those. Then, approximately 3% of BC patients contain amplifications of the ESR1 gene [38, 39], suggesting the importance of regulation of ESR1 expression. Methylation of ER genes revealed a decrease in the levels of ERs proteins [40]. Since ERα protein expression diminished with increases in the methylation at ESR1 [41], we might suggest that decreased levels of mRNA ESR1 in ERα− samples and controls were due to methylation of the 151-bp CpG island and chromatin components involved in the basal activity of the ESR1 gene.
Interestingly, we found an association between ESR1 methylation at 151-bp island and postmenopausal BC patients. This CpG island is located in the middle of two neighboring CpG islands, which showed a high methylation level in obese postmenopausal healthy women [29]. There might be an association between methylation at the region close to the transcription start site in the ESR1 gene and postmenopausal stage regardless of the BC's presence. Since methylation might occur as an early initiation event or even BC development, quantifying methylation levels at 151-bp island might be a crucial tool for better stratification of tumor subtype in the Mexican population from the country's center. It is noteworthy to mention that Mexico shows genetic differences mainly due to the Amerindian and European contributions; however, in the center of the country, the principal origin of the patients included in this study is mestizo [42].
For the reasons stated, we reported an association between the methylation at 151-bp island of the ESR1 gene and immunohistochemical tumor characterization. Previous reports suggested that ER-positive tumors, such as luminal A, are linked with long-term survival, whereas ER-negative subtypes such as TNBC and HER2+ had poor prognoses [43].
Remarkably, luminal A and luminal A/HER2+ subtypes presented diminished ESR1 methylation levels, whereas TNBC and HER2+ tumors had increased levels, suggesting a possible relationship between high methylation levels at 151-bp island in the ESR1 gene and poor prognosis. Therefore, methylation levels at the novel CpG island might be related to tumor subtype resulting in an additional tool for better stratification of BC.
Although BC subtypes are commonly determined by molecular expression and hormone indicators (among others), their stratification is still challenging due to the heterogenicity of BC at histological and molecular levels. In order to differentiate ER+ from luminal A or B tumors, a gene expression profiling (GEP) of about 500 genes is used [44].
Methylation signatures in BC subtypes have been used for tumor stratification [45,46,47]. Since methylation levels at the 151-bp CpG island in HER2+ and TNBC are higher in comparison with luminal ones, the quantification of methylation status at this novel CpG island might be used as an additional screening assay to better BC stratification in Mexican women.
Until now, 87 distinct DNA methylation biomarkers had been reported; among them, 68 markers were analyzed once in a single population [48], exposing the need for biomarkers for a specific community as Mexican women. Some reports indicate that methylation at ESR1 has no statistically significant correlation with BC outcome [15, 41, 49,50,51,52]. However, our findings suggested that methylation levels in the 151-bp island from that gene might be implemented to assist BC stratification. Nowadays, one of the challenges facing the use of DNA methylation as a potential biomarker is to define the precise genomic location, the effect of DNA methylation on gene expression and its ability to change throughout the patient's life [53]. Therefore, our evidences could be the first step for an epigenetic biomarker proposal, which has been a growing field in clinical research.
Conclusion
DNA methylation biomarkers together with histochemical characterization are helpful tools for BC prognosis. Since high methylation levels at the 151-bp CpG island into the ESR1 gene are associated with the postmenopausal stage and poor prognosis in BC Mexican patients, methylation at this island might be a potential prognostic biomarker in the Mexican population. In BC, molecular biomarkers predict the effectiveness of therapies and prognosis. Until now, the biomarkers used for this purpose include ER, PR, and HER2, which are tested by immunohistochemical analysis. However, these results might be subject to interpretation.
Our results suggest that the use of molecular tools such as the methylation status of 151-bp on the proximal promoter of ESR1 gene could contribute to a better understanding of the BC stratification, allowing to go one step further in the search for useful prognosis biomarkers for breast cancer patients.
Availability of data and materials
Contact the corresponding author to have access to the data that served to support the results found in this study.
Abbreviations
- BC:
-
Breast cancer
- CpG:
-
DNA methylation of cytosine-guanine dinucleotides
- ERα:
-
Estrogen receptor-alpha
- ESR1:
-
Estrogen receptor 1
- TIMP3:
-
TIMP metallopeptidase inhibitor 3
- RASSF1A:
-
Ras association domain family 1 isoform A
- RARβ:
-
Retinoic acid receptor β
- DNMT1:
-
DNA methyltransferases 1
- DNMT3A:
-
DNA methyltransferases 3A
- TNBC:
-
Triple-negative breast cancer
- PR:
-
Progesterone receptor
- chr6:
-
Chromosome 6
- ER+:
-
Estrogen receptor-positive
- ER−:
-
Estrogen receptor-negative
References
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–51.
Waks AG, Winer EP. Breast cancer treatment: A review. JAMA. 2019;321(3):288–300.
Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan BJ, Tinker LF. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1(5):611–21.
Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–37.
Kanwal R, Gupta K, Gupta S. Cancer epigenetics: an introduction. In: Cancer epigenetics. Berlin: Springer; 2015. p. 3–25.
Pasculli B, Barbano R, Parrella P. Epigenetics of breast cancer: Biology and clinical implication in the era of precision medicine. In: Seminars in cancer biology. Amsterdam: Elsevier; 2018. p. 22–35.
Stolzenburg S, Beltran A, Swift-Scanlan T, Rivenbark A, Rashwan R, Blancafort P. Stable oncogenic silencing in vivo by programmable and targeted de novo DNA methylation in breast cancer. Oncogene. 2015;34(43):5427–35.
Zhang L, Long X. Association of BRCA1 promoter methylation with sporadic breast cancers: evidence from 40 studies. Sci Rep. 2015;5(1):1–12.
Kostovska IM, Jakimovska M, Popovska-Jankovic K, Kubelka-Sabit K, Karagjozov M, Plaseska-Karanfilska D. TIMP3 promoter methylation represents an epigenetic marker of BRCA1ness breast cancer tumours. Pathol Oncol Res. 2018;24(4):937–40.
Yadav P, Masroor M, Nandi K, Kaza R, Jain S, Khurana N, Saxena A. Promoter methylation of BRCA1, DAPK1 and RASSF1A is associated with increased mortality among indian women with breast cancer. Asian Pacific J Cancer Prev. 2018;19(2):443.
Xu X-C. Tumor-suppressive activity of retinoic acid receptor-β in cancer. Cancer Lett. 2007;253(1):14–24.
Yu Z, Xiao Q, Zhao L, Ren J, Bai X, Sun M, Wu H, Liu X, Song Z, Yan Y. DNA methyltransferase 1/3a overexpression in sporadic breast cancer is associated with reduced expression of estrogen receptor-alpha/breast cancer susceptibility gene 1 and poor prognosis. Mol Carcinog. 2015;54(9):707–19.
Zubair M, Wang S, Ali N. Advanced approaches to breast cancer classification and diagnosis. Front Pharmacol. 2021;11:2487.
Li Y, Meeran SM, Patel SN, Chen H, Hardy TM, Tollefsbol TO. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol Cancer. 2013;12(1):1–17.
Martínez-Galán J, Torres-Torres B, Núñez MI, López-Peñalver J, Del Moral R, De Almodóvar JMR, Menjón S, Concha Á, Chamorro C, Ríos S. ESR1gene promoter region methylation in free circulating DNA and its correlation with estrogen receptor protein expression in tumor tissue in breast cancer patients. BMC Cancer. 2014;14(1):59.
Sheng X, Guo Y, Lu Y. Prognostic role of methylated GSTP1, p16, ESR1 and PITX2 in patients with breast cancer: a systematic meta-analysis under the guideline of PRISMA. Medicine. 2017;96(28):e7476.
Cejalvo JM, de Dueñas EM, Galván P, García-Recio S, Gasión OB, Paré L, Antolin S, Martinello R, Blancas I, Adamo B. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Can Res. 2017;77(9):2213–21.
Donovan MG, Wren SN, Cenker M, Selmin OI, Romagnolo DF. Dietary fat and obesity as modulators of breast cancer risk: focus on DNA methylation. Br J Pharmacol. 2020;177(6):1331–50.
Pirouzpanah S, Taleban F-A, Mehdipour P, Sabour S, Atri M. Hypermethylation pattern of ESR and PgR genes and lacking estrogen and progesterone receptors in human breast cancer tumors: ER/PR subtypes. Cancer Biomark. 2018;21(3):621–38.
Mastoraki S, Strati A, Tzanikou E, Chimonidou M, Politaki E, Voutsina A, Psyrri A, Georgoulias V, Lianidou E. ESR1 methylation: A liquid biopsy-based epigenetic assay for the follow-up of patients with metastatic breast cancer receiving endocrine treatment. Clin Cancer Res. 2018;24(6):1500–10.
Koš M, Reid G, Denger S, Gannon F. Minireview: genomic organization of the human ERα gene promoter region. Mol Endocrinol. 2001;15(12):2057–63.
Grandien K. Determination of transcription start sites in the human estrogen receptor gene and identification of a novel, tissue-specific, estrogen receptor-mRNA isoform. Mol Cell Endocrinol. 1996;116(2):207–12.
Higuchi T, Gohno T, Nagatomo T, Tokiniwa H, Niwa T, Horiguchi J, Oyama T, Takeyoshi I, Hayashi S-I. Variation in use of estrogen receptor-α gene promoters in breast cancer compared by quantification of promoter-specific messenger RNA. Clin Breast Cancer. 2014;14(4):249-257.e242.
Inoue A, Hayashi S-I, Aoyagi K, Nishigaki M, Sasaki H, Kiyama R. A reporter gene assay for evaluation of tissue-specific responses to estrogens based on the differential use of promoters A to F of the human estrogen receptor α gene. J Pharmacol Toxicol Methods. 2002;47(3):129–35.
Reid G, Denger S, Kos M, Gannon F. Human estrogen receptor-α: regulation by synthesis, modification and degradation. Cell Mol Life Sci. 2002;59(5):821–31.
Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–81.
Brand H, Kos M, Denger S, Flouriot G, Gromoll J, Gannon F, Reid G. A novel promoter is involved in the expression of estrogen receptor α in human testis and epididymis. Endocrinology. 2002;143(9):3397–404.
Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006.
Daraei A, Izadi P, Khorasani G, Nafissi N, Naghizadeh MM, Younosi N, Meysamie A, Mansoori Y, Bastami M, Tavakkoly-Bazzaz J. Epigenetic changes of the ESR1 gene in breast tissue of healthy women: a missing link with breast cancer risk factors? Genet Test Mol Biomarkers. 2017;21(8):464–70.
Tsuboi K, Nagatomo T, Gohno T, Higuchi T, Sasaki S, Fujiki N, Kurosumi M, Takei H, Yamaguchi Y, Niwa T, et al. Single CpG site methylation controls estrogen receptor gene transcription and correlates with hormone therapy resistance. J Steroid Biochem Mol Biol. 2017;171:209–17.
Gardini ES, Chen GG, Fiacco S, Mernone L, Willi J, Turecki G, Ehlert U. Differential ESR1 promoter methylation in the peripheral blood—findings from the women 40+ healthy aging study. Int J Mol Sci. 2020;21(10):3654.
Rosenberg PS, Barker KA, Anderson WF. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J Natl Cancer Inst. 2015;107(9):djv159.
Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–31.
Ellison-Zelski SJ, Solodin NM, Alarid ET. Repression of ESR1 through actions of estrogen receptor alpha and Sin3A at the proximal promoter. Mol Cell Biol. 2009;29(18):4949–58.
Itoh M, Iwamoto T, Matsuoka J, Nogami T, Motoki T, Shien T, Taira N, Niikura N, Hayashi N, Ohtani S. Estrogen receptor (ER) mRNA expression and molecular subtype distribution in ER-negative/progesterone receptor-positive breast cancers. Breast Cancer Res Treat. 2014;143(2):403–9.
Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-α (ERα) and represses ERα messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21(5):1132–47.
Tian D, Solodin NM, Rajbhandari P, Bjorklund K, Alarid ET, Kreeger PK. A kinetic model identifies phosphorylated estrogen receptor-α (ERα) as a critical regulator of ERα dynamics in breast cancer. FASEB J. 2015;29(5):2022–31.
Gao M-Q, Kim BG, Kang S, Choi YP, Park H, Kang KS, Cho NH. Stromal fibroblasts from the interface zone of human breast carcinomas induce an epithelial–mesenchymal transition-like state in breast cancer cells in vitro. J Cell Sci. 2010;123(20):3507–14.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. In: AACR. 2012.
Tang Z-R, Zhang R, Lian Z-X, Deng S-L, Yu K. Estrogen-receptor expression and function in female reproductive disease. Cells. 2019;8(10):1123.
Ramos EA, Camargo AA, Braun K, Slowik R, Cavalli IJ, Ribeiro EM, de O Pedrosa F, de Souza EM, Costa FF, Klassen G. Simultaneous CXCL12 and ESR1 CpG island hypermethylation correlates with poor prognosis in sporadic breast cancer. BMC Cancer. 2010;10(1):1–13.
Silva-Zolezzi I, Hidalgo-Miranda A, Estrada-Gil J, Fernandez-Lopez JC, Uribe-Figueroa L, Contreras A, Balam-Ortiz E, del Bosque-Plata L, Velazquez-Fernandez D, Lara C, et al. Analysis of genomic diversity in Mexican Mestizo populations to develop genomic medicine in Mexico. Proc Natl Acad Sci. 2009;106(21):8611.
Dwivedi S, Purohit P, Misra R, Lingeswaran M, Vishnoi JR, Pareek P, Sharma P, Misra S. Application of single-cell omics in breast cancer. In: Single-cell omics. Amsterdam: Elsevier; 2019. p. 69–103.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron J, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–23.
Holm K, Hegardt C, Staaf J, Vallon-Christersson J, Jönsson G, Olsson H, Borg Å, Ringnér M. Molecular subtypes of breast cancer are associated with characteristic DNA methylation patterns. Breast Cancer Res. 2010;12(3):1–16.
Fackler MJ, Umbricht CB, Williams D, Argani P, Cruz L-A, Merino VF, Teo WW, Zhang Z, Huang P, Visvananthan K. Genome-wide methylation analysis identifies genes specific to breast cancer hormone receptor status and risk of recurrence. Can Res. 2011;71(19):6195–207.
Fang F, Turcan S, Rimner A, Kaufman A, Giri D, Morris LG, Shen R, Seshan V, Mo Q, Heguy A. Breast cancer methylomes establish an epigenomic foundation for metastasis. Sci Transl Med. 2011;3(75):75ra25.
de Ruijter TC, van der Heide F, Smits KM, Aarts MJ, van Engeland M, Heijnen VC. Prognostic DNA methylation markers for hormone receptor breast cancer: a systematic review. Breast Cancer Res. 2020;22(1):1–12.
Müller HM, Widschwendter A, Fiegl H, Ivarsson L, Goebel G, Perkmann E, Marth C, Widschwendter M. DNA methylation in serum of breast cancer patients: an independent prognostic marker. Can Res. 2003;63(22):7641–5.
Widschwendter M, Siegmund KD, Müller HM, Fiegl H, Marth C, Müller-Holzner E, Jones PA, Laird PW. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Can Res. 2004;64(11):3807–13.
Li S, Rong M, Iacopetta B. DNA hypermethylation in breast cancer and its association with clinicopathological features. Cancer Lett. 2006;237(2):272–80.
Sharma G, Mirza S, Yang Y-H, Parshad R, Hazrah P, Datta Gupta S, Ralhan R. Prognostic relevance of promoter hypermethylation of multiple genes in breast cancer patients. Anal Cell Pathol. 2009;31(6):487–500.
Koch A, Joosten SC, Feng Z, de Ruijter TC, Draht MX, Melotte V, Smits KM, Veeck J, Herman JG, Van Neste L. Analysis of DNA methylation in cancer: location revisited. Nat Rev Clin Oncol. 2018;15(7):459–66.
Lung DK, Reese RM, Alarid ET. Intrinsic and extrinsic factors governing the transcriptional regulation of ESR1. Hormones Cancer. 2020;11(3–4):129–147.
Acknowledgements
We thank the Hospital Juárez de México for all the facilities granted to carry out this work. Thanks are due to Diana Casandra Rodriguez Ballesteros for her technical assistance and collection of the biological samples. Thanks to all co-authors and participants. We especially thank and dedicate this work to Leonardo Gabriel Reyes Figueroa for pushing us forward.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnología (grants CB-2015-258156 and CB-2016-284748) and by Hospital Juárez de México (HJM0012/15-I). We thank PAPIIT-IN222321 for academic and financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization, JS-R, GF-G and ODR-H; collection of patients samples, EYV-V; methodology, LIQ-G, HC, MG-DC, and GL-G; validation, HC, MG-DC, GL-G; formal analysis, LIQ-G, HC, EYV-V, LPB-M, MR-M, IL-R, SLA-E, ES-R, and ODR-H; investigation, LIQ-G, HC, JS-R, and ODR-H; resources, GF-G, and ODR-H; data curation, LIQ-G, HC, JS-B, and ODR-H; writing—original draft preparation, LIQ-G, and ODR-H; writing—review and editing, LIQ-G, JS-R, GF-G, ES-R, and ODR-H; visualization, ODR-H; supervision, JS-R, GF-G, and ODR-H; project administration, GF-G, and ODR-H; funding acquisition, ODR-H. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This protocol was evaluated by the Research Committee, in its ordinary session number five on May 7, 2015, in conjunction with the Research Ethics and Biosafety Committees of the Hospital Juárez de México, under registration number HJM0012/15-I. All the participants in this study were invited and signed a letter of consent from which they gave their approval for the use of biological samples for this work.
Consent for publication
Consent for publication was obtained from all co-authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
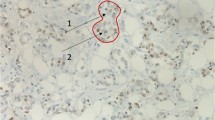
Expression of ERα in the mammary gland determined by immunodetection. Representative images correspond to control patients (a), case ERα− patients (b–d), and case ERα+ patients (e–g). Histological analysis was performed on paraffin-embedded mammary gland and imaged at 40×. Scale bar = 50 µm.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Quintas-Granados, L.I., Cortés, H., Carmen, M.GD. et al. The high methylation level of a novel 151-bp CpG island in the ESR1 gene promoter is associated with a poor breast cancer prognosis. Cancer Cell Int 21, 649 (2021). https://doi.org/10.1186/s12935-021-02343-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02343-7