Abstract
Sestrin 2, a highly conserved stress-induced protein, participates in the pathological processes of metabolic and age-related diseases. This p53-inducible protein also regulates cell growth and metabolism, which is closely related to malignant tumorigenesis. Sestrin 2 was reported to regulate various cellular processes, such as tumor cell proliferation, invasion and metastasis, apoptosis, anoikis resistance, and drug resistance. Although sestrin 2 is associated with colorectal, lung, liver, and other cancers, sestrin 2 expression varies among different types of cancer, and the effects and mechanisms of action of this protein are also different. Sestrin 2 was considered a tumor suppressor gene in most studies, whereas conflicting reports considered sestrin 2 an oncogene. Thus, this review aims to examine the literature regarding sestrin 2 in various cancers, summarize its roles in suppression and tumorigenesis, discuss potential mechanisms in the regulation of cancer, and provide a basis for follow-up research and potential cancer treatment development.
Similar content being viewed by others
Introduction
Cancer is a category of malignant diseases where cell proliferation and survival are uncontrolled [1]. Cancer is the second leading cause of death worldwide following cardiovascular disease [2]. Emerging evidence has shown that the molecular mechanisms of cancer progression and suppression are quite complex. Recent studies demonstrated that the processes of tumor development and progression involved transcription factors [3], microRNAs [4, 5], long noncoding RNAs [6], circular RNAs [7], cytokines [8, 9], exosomes [10], inflammasomes [11], immune microenvironment [12], hormones [13], and other potential therapeutic protein targets [14, 15]. Various molecular targeted therapies have been developed to treat malignancies because of their specificity, efficacy, improved patient tolerance, and lower toxicity [16]. Therefore, the exploration and discovery of effective potential molecule targets are important to significantly improve cancer treatment.
The sestrin proteins comprise three subtypes: sestrin 1, sestrin 2, and sestrin 3 [14]. Sestrin 2, a homolog of the p53-activated gene 26, is encoded by the hypoxia-inducible gene 95 [17]. Sestrin 2, a highly conserved stress-induced protein, is mainly expressed in mammals and secreted by various immune and non-immune cells, such as macrophages, T lymphocytes, and epithelial cells [18,19,20,21]. Human sestrin 2 has three sub-domains (Fig. 1A): sestrin-A, sestrin-B, and sestrin-C [22]. The physiological function of sestrin 2 is mainly dependent on the symmetrical structures of the sestrin-A and sestrin-C domains, which have similar spherical structures but different functions that are regulated by various transcription regulators (Fig. 1B) [23]. The structured domain of sestrin-A is regarded as an alkyl hydroperoxide reductase, which is responsible for antioxidant activity. Sestrin-B is a linker that connects A and C. Sestrin-C is a leucine binding site that acts as a leucine sensor in the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) pathway. The interaction between sestrin-C and the GTPase-activated RAG protein complex is essential for regulating 5ʹ-adenosine monophosphate-activated protein kinase (AMPK) and mTORC1 signaling by sestrin 2 [18, 24]. Under the conditions of DNA damage, oxidative stress, hypoxia and nutritional deficiency, sestrin2 can maintain cell homeostasis by activating AMPK and inhibiting mTOR signaling pathways [14]. Furthermore, sestrin 2 plays roles in maintaining cellular activity, antioxidation, mitochondrial homeostasis and in regulating autophagy [14]. Besides, studies have demonstrated that sestrin 2 is associated with immune system diseases, liver diseases, ischemic-reperfusion lesions, neurodegenerative diseases, cardiovascular disorders, aging, and cancer [14, 18, 25,26,27].
A schematic diagram showing the structure and transcription regulators of sestrin 2. A The functional domains of human sestrin 2 (sestrin-A, sestrin-B, and sestrin-C). In sestrin-A, the cysteine residue (C125) and conserved residues of the proton relay system (Y127 and H132) are important for its antioxidant activity. In sestrin-C, the two surface-exposed aspartates (D406 and D407, DD motif) are responsible for interacting with GATOR2 to inhibit mTORC1 signaling. B Sestrin2 expression plays a central role in cancer regulated by multiple transcription factors and miRNAs
Recent studies have shown that sestrin 2 is a cancer biomarker and potential therapeutic target that is critical for cancer occurrence and development [14, 18, 28]. Sestrin 2 is involved in many processes, such as tumor cell proliferation; apoptosis; autophagy; anoikis resistance; drug resistance; sensitivity to radiotherapy; tumor differentiation; tumor, node, and metastasis (TNM) staging; lymphatic metastasis; and patient survival [29,30,31,32]. Alterations in sestrin 2 expression have been found in a majority of cancers (Table 1). Moreover, sestrin 2 function is different in several cancers. Sestrin 2 was verified as a tumor suppressor gene in some studies, while other reports regarded sestrin 2 as an oncogene. As a novel diagnostic and therapeutic target, the paradoxical role of sestrin 2 in cancers may hinder clinical application in the future. Therefore, it is of great significance to investigate the possible reasons behind these contradictions. In this review, we will examine the state of research on sestrin 2 in different types of cancers and demonstrate its role in inhibition and promotion of cancer by focusing on related mechanisms.
The alteration of sestrin 2 gene expression in various cancers
Changes in sestrin 2 expression have been observed in numerous cancer tissues and cell lines and play a significant role in cell proliferation, invasion, metastasis, apoptosis, autophagy, anoikis resistance, drug resistance, oxidative stress, and endoplasmic reticulum (ER) stress. Sestrin 2 appears to be a novel prognostic marker for cancers (Table 1). In colorectal cancer (CRC) tissues and cell lines [30, 33], sebaceous gland carcinoma [34], neuroblastomas [35], bladder cancer tissues [36] and prostate cancer cells [37], sestrin 2 was downregulated in comparison with related non-cancerous tissues and cells. The lower expression of sestrin 2 was associated with advanced tumor stage, lymphatic and vascular invasion, liver metastasis, shorter disease-free and overall survival [30], upper eyelid involvement in sebaceous gland carcinoma [34], and radiosensitization [37]. Sestrin 2 was deemed a potential tumor suppressor that repressed cell proliferation, enhanced apoptosis, and induced autophagy [30, 34, 35]. Interestingly, multiple studies revealed higher expression levels of sestrin 2 in several cancers, including squamous cell carcinoma (SCC) and melanoma [38], metastatic melanoma tissues and cell lines [39], and endometrial cancer [40] compared with non-cancerous tissues or cell lines, and the increased expression of sestrin 2 was associated with poor prognosis [39, 40]. In these cancers, sestrin 2 was verified as an oncogene, which suppressed apoptosis and promoted drug [38] and anoikis resistance [39].
Notably, there have been conflicting reports regarding sestrin 2 alterations in lung and liver cancer. Primary lung cancer has the highest mortality worldwide, and non-small cell lung cancer (NSCLC) accounts for approximately 80% of cases [41]. Chen et al. found that the expression of sestrin 2 in NSCLC was markedly lower than that in corresponding non-cancerous lung tissues in 210 patients with NSCLC. Lower expression of sestrin 2 was correlated with poor differentiation, advanced TNM stage, and lymph node metastasis [42]. Lung cancer patients with a high expression of sestrin 2 had a longer overall survival rate than those with low expression of this protein [42, 43]. Interestingly, in contrast to this study, Chae et al. demonstrated that the expression of sestrin 2 in lung cancer was higher than in normal lung tissue by using the Oncomine and Human Protein Atlas databases. Furthermore, these authors showed that overexpressed sestrin 2 was a marker for poor prognosis in lung cancer by analyzing the PrognoScan database and Kaplan–Meier Plotter [44]. Additionally, by analyzing data from the Gene Expression Omnibus and Cancer Genome Atlas analysis, Lin et al. revealed that the survival time for lung cancer patients with lower sestrin 2 expression was significantly prolonged [45].
In 2018, liver cancer had the sixth highest incidence and the fourth highest mortality rate for cancers worldwide, and hepatocellular carcinoma (HCC) accounted for approximately 90% of cases [46, 47]. The expression of sestrin 2 in HCC was lower than that in non-cancerous tissues [48, 49], and lower sestrin 2 expression was associated with hepatitis B/hepatitis C viral infections, lymph node metastasis, tumor progression, and poor prognosis in HCC patients [48]. However, Dai et al. found that the levels of sestrin 2 were remarkably upregulated in HCC tissues and cell lines compared with the corresponding adjacent liver tissue and normal hepatocytes, and increased sestrin 2 expression was positively correlated with the proliferation marker Ki-67 [31].
Inhibitory effects of sestrin 2 in cancer
Sestrin 2 inhibits cell proliferation, migration, and invasion and induces apoptosis
The highly conserved serine-threonine kinase mTOR is frequently activated in cancer. Activated mTOR is involved in cell proliferation, migration and invasion, anti-apoptosis, and inhibition of autophagy [50, 51]. Two different protein complexes contain mTOR: mTORC1 and mTORC2 [52, 53]. The phosphoinositide 3-kinase (PI3K)/AKT and extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) pathways activate mTORC1, whereas mTORC1 is inhibited by the AMPK pathway. Activated mTORC1 activates the downstream p70 ribosomal protein S6 kinase (p70S6K) and inhibits eucaryotic translation initiation factor 4E binding protein, which results in increased protein synthesis and tumor progression [54, 55]. Sestrin 2 was reported to participate in inhibiting tumor cell proliferation, migration, and invasion and inducing apoptosis. The anti-cancer functions of sestrin 2 are closely related to the inhibition of mTORC1 activity in endometrial cancer, CRC, and lung cancer.
Knockdown of sestrin 2 with short hairpin RNA (shRNA) induced migration and promoted proliferation by increasing mRNA expression of the proliferation marker Ki-67 and decreasing mRNA expression of the cyclin-dependent kinase inhibitors 1A and 1B (cell cycle-associated genes) in HEC-1A and Ishikawa endometrial cancer cell lines, which are dependent on the mTORC1 pathway [40]. Ro et al. revealed that sestrin 2 suppressed the development of colitis and CRC tumor growth by inhibiting ER stress and mTORC1, respectively [33]. Wei et al. found that sestrin 2 overexpression inhibited proliferation and activated apoptosis in CRC cells (SW620 and LoVo lines) by activating AMPK and inhibiting the mTORC1 pathway, which downregulated proliferating cell nuclear antigen and survivin proteins and upregulated caspase proteins 3, 7, and 9 [56]. Silencing sestrin 2 with shRNA facilitated proliferation and migration of lung epithelial cells. Moreover, this inhibitory role of sestrin 2 in lung epithelial cells was related to the regulation of the AKT-mTOR-p70S6K pathway [43]. In addition, sestrin 2 knockdown accelerated the xenograft tumor growth of HEC-1A [40], the CRC line SW620 [56], and bronchial epithelial line BEAS-2B [43] transplanted in Balb/c nude mice by targeting the mTORC1 mechanism. These studies indicated that sestrin 2 has potential anti-tumor effects in human endometrial cancer, CRC, and lung cancer cells by negatively regulating the mTORC1 pathway. Sestrin 2 may be a potential tumor suppressor in these types of cancer.
Interestingly, sestrin 2 also plays a significant role in growth inhibition of CRC, human head and neck cancer, medullary thyroid cancer, and breast cancer cells induced by the chemical drugs quercetin and 5-fluorouracil (5-FU), fisetin, 2-imino-6-methoxy-2H-chromene-3-carbothioamide (IMCA), and adiponectin, respectively. Furthermore, this growth inhibitory effect is closely related to the regulation of the AMPK/mTOR pathway. Quercetin is a flavonoid that exists in many fruits and vegetables and exerts anticancer effects [57]. Kim et al. demonstrated that quercetin suppressed proliferation and induced apoptosis in the CRC lines HCT116 and HT29 by targeting the sestrin 2/AMPK/mTOR pathway [58]. Seo and colleagues verified that 5-FU treatment significantly increased the expression of sestrin 2 in HCT116 and HT29 CRC cells by targeting p53, which consequently repressed CRC cell migration [59]. This latter study provides new evidence for the anti-tumor mechanism of 5-FU [59]. Fisetin, another key flavonoid, induced apoptosis in human head and neck cancer cells via upregulation of sestrin 2 expression and downregulation of phospho-mTOR and myeloid cell leukemia-1 protein [60]. IMCA inhibited proliferation and induced apoptosis by promoting the translocation of the orphan nuclear receptor 4A1 from the nucleus to the cytoplasm, further increasing sestrin 2 expression and phosphorylated-AMPK levels, and decreasing p70S6K in the TT thyroid cancer cell line [61].
Adiponectin, a cytokine primarily secreted by adipocytes, is well correlated with the occurrence and development of malignant tumors [62, 63]. Globular adiponectin suppressed the growth of breast cancer cells by inhibiting the activation of inflammasomes [64]. Adiponectin was reported to bind to receptors on the cell membrane, activate the downstream sestrin 2/AMPK pathway, inhibit ER stress, suppress inflammatory activity, inhibit proliferation, and promote apoptosis in breast cancer cell lines MCF-7, MDA-MB-231, and T47D [64].
Sestrin 2 has also been implicated in cold atmospheric plasma (CAP)-induced apoptosis of melanoma cells by activating the sestrin 2/nitric oxide synthase (iNOS) pathway [65]. Using CAP to treat cancer is a promising new technology in plasma medicine [66]. Xia et al. found that CAP induced caspase 3/8-mediated apoptosis in A375 and A875 melanoma cell lines by increasing sestrin 2 expression, which activated p38 MAPK signaling and increased the expression of downstream Fas, Fas ligand, and iNOS; this process was reversed by using short interfering RNA targeting sestrin 2 [65].
Sestrin 2 expression was upregulated by the different treatment factors described above, but the mechanism of upregulation was not consistent. Upregulation of sestrin 2 protein by quercetin was related to the increase in reactive oxygen species (ROS) production [58]. Sestrin 2 mRNA upregulation by ICMA and increased mRNA and protein levels by 5-FU were dependent on the tumor-suppressor protein p53 [59, 61]. The specific mechanisms remain unclear for upregulation of sestrin 2 protein by globular adiponectin [64] and increased sestrin 2 mRNA and protein levels by fisetin and CAP [60, 65]. In conclusion, the upregulation of sestrin 2 expression is an essential mediator for inhibiting cancer cell growth through the AMPK/mTOR or iNOS pathway and may relate to ROS induction and p53 activation.
In addition to regulating mTOR and iNOS pathways, sestrin 2 has been shown to promote cancer cell apoptosis by targeting X-linked inhibitor of apoptosis protein (XIAP) and suppress cell migration and invasion by targeting hypoxia inducible factor-1α (HIF-1α). XIAP is a powerful cellular inhibitor of apoptosis that suppresses caspase 3, 7, and 9 activity [67]. Ding et al. reported that sestrin 2 stimulated the degradation of XIAP through the lysosomal pathway and promoted death receptor-induced apoptosis of lung adenocarcinoma cell lines H460 and A549 [68]. In hypoxic microenvironments, tumor cells overproduce HIF-1α, which is conducive to survival [69]. Decreasing HIF-1α expression is an efficient strategy for inhibiting tumor cell survival and invasion [70]. Seo et al. revealed that sestrin 2 suppressed HCT116 and HT29 CRC cell migration and invasion in vitro and inhibited tumor growth in vivo by promoting the degradation of HIF-1α through the AMPK-prolyl hydroxylase pathway [71].
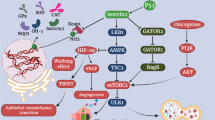
Overall, sestrin 2 plays a crucial role in suppressing tumor growth by inhibiting cellular proliferation, migration, and invasion, and inducing apoptosis. The signaling pathways involved in sestrin 2-mediated suppression of different cancers are summarized in Fig. 2.
Sestrin 2 participates in autophagy induction
Autophagy, or autophagocytosis, is another type of programmed cellular death, which is different from apoptosis. Autophagy maintains homeostasis by using lysosomes to degrade misfolded proteins and damaged organelles in the cytoplasm. Sestrin 2 is critical for the regulation of autophagy [72, 73]. Zhang et al. reported that c-Jun N-terminal kinase mediated the autophagy induced by excisanin A and serum deprivation through a sestrin 2-dependent mechanism in the CNE1 and CNE2 human nasopharyngeal carcinoma cell lines [29]. Additionally, the inhibition of lysine-specific demethylase 1 promoted autophagy through the sestrin 2-mTORC1 signaling pathway in the neuroblastoma cell lines SH-SY5Y, SHEP Tet-21/N, and SK-N-BE [35].
Sestrin 2-mediated autophagy is associated with antitumor activities of drugs [14]. Fangchinoline, a bisbenzylisoquinolin alkaloid from the dried roots of Stephania tetrandra S. Moore, has anti-inflammatory, antihyperglycemic, antioxidant, and anticancer activities [74,75,76]. Wang et al. elucidated a novel mechanism of action for fangchinoline-induced autophagy in HepG2 and PLC/PRF/5 HCC cells that used p53/sestrin 2/AMPK signaling [76]. Muscone, the main active ingredient in musk, promoted apoptosis of HepG2 hepatoma cells through the phosphorylated-protein kinase RNA-like endoplasmic reticulum kinase/activating transcription factor 4/DNA damage inducible transcript 3 mediated ER stress pathway and induced autophagy through the sestrin 2/AMPK/mTORC1 signaling pathway [49]. Apoptosis induced by muscone may be partially dependent on autophagy [49]. In vivo experiments with nude mice confirmed that muscone suppressed the growth of transplanted tumors. Furthermore, sestrin 2 was identified as a potential candidate gene for the diagnosis and therapy of liver cancer [49]. Isorhapontigenin (ISO), found in Chinese herbs, has anti-bladder cancer properties. After treatment of UMUC3 and T24T bladder cancer cells with ISO, sestrin 2 expression was elevated by activation of the MAPK8/JUN pathway, and activated sestrin 2 induced autophagy and inhibited the growth of bladder cancer cells [36]. Additionally, a new antitumor compound, known as ChlA-F, increased sestrin 2 transcription by activating transcription factor specificity protein 1. Ch1A-F enhanced sestrin 2 stability by inhibiting microRNA (miR)-27a-mediated degradation of sestrin 2 mRNA, which resulted in the increased expression of sestrin 2 protein, activation of the autophagy pathway, and inhibition of anchorage-independent growth of bladder cancer cell lines, including RT4, T24T, and UMUC3 cells [77]. The pathways of sestrin 2-mediated promotion of cell autophagy are summarized in Fig. 3.
Sestrin 2 enhances radiosensitivity
Radiation therapy is one of the critical methods for comprehensive treatment of cancer. It can improve a cancer patient’s quality of life and long-term survival rate by causing DNA damage, depriving cells of their reproductive capacity, and, ultimately, killing the tumor cells. Because of the different radiosensitivity between individuals and/or tumor cells, some patients will respond poorly to radiation therapy. Therefore, it is imperative to explore influential factors and mechanisms to reduce tumor radioprotection and enhance tumor radiosensitivity [37, 78]. One study showed that sestrin 2 increased the radiosensitivity of MCF-7 breast cancer cells. Sestrin 2 was implicated in radiation-induced death of breast cancer cells by stabilizing the AMPK complex and/or enhancing AMPK expression. Sestrin 2 associated with AMPK to inhibit mTOR signaling and enhanced radiation therapy-induced tumor cell death [78]. Another study found that sestrin 2 expression was low in prostate cancer cells lines, including PC3, LNCaP clone FGC, and DU145. Overexpression of sestrin 2 reduced the proliferation of PC3 cells and increased radiosensitivity [37].
Promotive effects of sestrin 2 in cancer
Sestrin 2 promotes tumor growth
Interestingly, the role and mechanisms of action of sestrin 2 in mediating cell growth and survival are still controversial. Some studies identified sestrin 2 as an oncogene, which caused accelerated tumor cell growth and migration and suppressed apoptosis. The tumor suppressor gene phosphatase and tensin homolog deleted on chromosome ten (PTEN) negatively regulates the PI3K/AKT pathway and participates in cell proliferation, survival, migration, and metabolism [79]. Zhao et al. reported that sestrin 2 acted as an oncogene in SCC and melanoma. Sestrin 2 knockdown facilitated apoptosis induced by vemurafenib and 5-FU in human melanoma cells (A375 and MEL624 lines) and the SCC cell line A431 [38], respectively, and suppressed the growth of A431 and A375 cells xenografted in nude mice [38]. Sestrin 2 activated AKT by decreasing PTEN membrane recruitment [38]. Therefore, the positive effect of sestrin 2 on AKT activity may be related to survival of SCC and melanoma. Using the NK-92 cell line, Wang et al. described the role of sestrin 2 in natural killer cell activity against ovarian cancer. Sestrin 2 promoted ovarian cancer cell survival by suppressing the anti-tumor effects of NK-92 cells through activation of AMPK and inhibition of the mTORC1 pathway [80]. Sorafenib is an effective drug that inhibits tumor growth and angiogenesis of HCC [47, 81]. Sorafenib treatment increased the expression of sestrin 2 in human HCC lines Bel-7404 and SNU-368, and knockdown of sestrin 2 enhanced growth inhibition and apoptosis induced by sorafenib. Therefore, sestrin 2 may have potential tumorigenic effects in HCC [31]. Sestrin 2 also has a potentially oncogenic function in lung cancer. Knockdown of sestrin 2 with shRNA inhibited proliferation, migration, and sphere formation in A549 cells [44]. In pancreatic cancer, overexpression of sestrin 2 promoted PANC-1 cells proliferation and increased glycolysis, and mTOR inhibitors suppressed these effects in vitro. Additionally, the knockdown of sestrin 2 inhibited the growth of pancreatic cancer in vivo. This latter study indicated that the mTOR signaling pathway participated in sestrin 2-mediated promotion and development of pancreatic cancer [82].
Sestrin 2 promotes anoikis and drug resistance
Metastasis is an important feature of malignant tumors and indicates a poor prognosis. Anoikis resistance is one of the pivotal mechanisms for cancer metastasis [83]. Sestrin 2 facilitates metastasis and cancer anoikis resistance. It was shown that the interaction between sestrin 2 and miR-141 affected the anoikis resistance of human endometrial cancer cell lines KLE, RL-95-2, Ishikawa, and AN3CA; inhibiting miR-141 increased sestrin 2 protein expression, resulting in enhanced anoikis resistance [32]. Another study demonstrated that sestrin 2 promoted anoikis resistance in melanoma cells and contributed to melanoma metastasis in vivo [39]. The detachment of metastatic melanoma cells from the extracellular matrix can induce the up-regulation of sestrin 2 expression because of suspension stress. The specific molecular mechanisms by which sestrin 2 inhibits anoikis include the reduction of intracellular ROS levels and regulation of endogenous apoptosis-related proteins, including B-cell lymphoma 2 and B-cell lymphoma 2 associated X-protein [39].
Drug resistance is an important reason for therapeutic failure in cancer chemotherapy [84, 85]. Exploring the reasons behind drug resistance is of great significance to fundamentally solve this problem and improve the survival rate of patients. Sestrin 2 is a crucial target molecule for chemotherapeutic drug resistance because it activates the AKT and/or AMPK pathways [31, 38]. In human SCC and melanoma cells, sestrin 2 induced chemotherapeutic drug resistance by activating the AKT pathway and regulating PTEN activity [38]. In HCC, sestrin 2 was involved in primary resistance to sorafenib by activating the AKT and AMPK pathways [31]. In A549 lung cancer cells, sestrin 2 knockdown decreased the mRNA expression of ATP-binding cassette transporter ABCG and ABCA2 (drug resistance marker genes) and increased sensitivity to doxorubicin [44]. Taken together, targeting the sestrin 2 gene may be a valuable method to overcome primary resistance to chemotherapy drugs. Figure 4 shows the pathways of sestrin 2-mediated tumorigenesis that promote tumor growth, anoikis resistance, and drug resistance.
Sestrin 2 is required for cancer cell survival under stress conditions
Sestrin 2 is a stress-induced protein family member activated by diverse stressors, such as glucose starvation, nutritional deficiency, ER stress, oxidative stress, hypoxia, and DNA damage [14, 18]. Activated sestrin 2 has been found to be beneficial for tumor cell survival under diverse stress conditions [86,87,88,89].
Glucose is one of the primary sources of energy for cancer cell growth. Ben-Sahra et al. demonstrated that sestrin 2 was a key molecular for cancer cell survival when glycolysis was blocked [86]. The expression of sestrin 2 was upregulated by an AKT-dependent but p53-independent mechanism, and sestrin 2 was essential for mTOR suppression under a state of energy stress caused by glycolysis inhibition [86]. Currently, sestrin 2 is regarded as a novel energy stress sensor [86]. Recently, a study by Kumar et al. showed that under glucose starvation conditions (with adequate glutamine), sestrin 2 promoted HepG2 cell survival by activating PPAR-γ coactivator-1 alpha (a stress sensor in cancer cells) through the modulation of glutamine metabolism [89]. This study demonstrated the crucial role of sestrin 2 in regulating cancer cell survival under glucose starvation conditions.
Glutamine is an essential nutrient for tumor cell proliferation. However, how tumor cells survive during glutamine deficiency remains unknown. Byun et al. revealed that after glutamine deficiency, sestrin 2 expression was upregulated through a ROS-p38 MAPK-CCAAT/enhancer-binding protein β-dependent pathway. The depletion of glutamine resulted in the binding of sestrin 2 to mTORC2 to increase the stability of the sestrin 2 protein and reduce the activity of mTORC1, thus, preventing lung cancer cell death [88]. The differential regulation of mTORC1 and mTORC2 by sestrin 2 can prevent ATP depletion and maintain redox balance [88]. The positive feedback loop between sestrin 2 and mTORC2 is vital for lung cancer cell survival during glutamine deficiency [88].
Kim et al. explored the response of cancer cells to heme iron-induced stress. Hemin (Fe3+ heme) induced the expression of sestrin 2 by activating ROS and nuclear factor (erythroid-derived 2)-like 2, and, together with hemin, sestrin 2 overexpression protected colon cancer cells from death including HCT116 and RKO cells and promoted MC38 tumor growth both in vitro and in vivo. This study suggested that sestrin 2 has a promotive effect on colon cancer during high iron conditions [28].
ER stress is involved in various biological processes of cancer and is closely related to biological functions, such as proliferation, apoptosis, drug resistance, and autophagy [90,91,92]. Sestrin 2 expression is upregulated under ER stress in cancer cells through the regulation of the activating transcription factor 4 [93], inositol-requiring enzyme 1/X-box binding protein 1, and protein kinase RNA-like endoplasmic reticulum kinase signaling pathways [87, 94]. Sestrin 2 knockdown reduced ER stress-induced autophagy and promoted ER stress-induced cell death by activating the mTORC1 pathway in breast cancer cells lines HCC1806 and MCF7 [87]. This study emphasizes that sestrin 2 induction mediated by ER stress contributes to tumor cell survival and that targeting sestrin 2 may be a mechanism for enhancing ER stress-induced cell death in breast cancer cells [87].
Together, these studies revealed that sestrin 2 is a vital regulator of cancer cell survival under the conditions of glycolysis inhibition, glutamine deficiency, glucose starvation, high iron, and ER stress. These pathways are summarized in Fig. 5.
Conclusion
In this review, we summarized the dual roles and mechanisms of sestrin 2 in various cancers. There are currently many unanswered questions regarding the role of sestrin 2 in tumors. First, it will be necessary to understand why the expression of sestrin 2 is inconsistent in different tumors. The evaluation of a greater number of clinical samples will be required in subsequent studies. Second, the functions of sestrin 2 in different tumors requires further exploration. Some studies have shown that sestrin 2 plays a role as a tumor suppressor gene in bladder and prostate cancer. However, sestrin 2 acts as an oncogene in SCC, pancreatic cancer, and ovarian cancer. It remains controversial whether sestrin 2 serves as a tumor-suppressing gene or oncogene in lung, liver, colorectal, breast, melanoma, and endometrial cancers. Furthermore, it is unclear whether sestrin 2 acts as a tumor suppressor or promoter in thyroid cancer, head and neck cancer, neuroblastoma, and nasopharyngeal carcinoma, and verification requires more data. Third, the mechanisms by which sestrin 2 functions should be clarified. According to this literature review, the mechanisms used by sestrin 2 to inhibit tumor growth are mainly associated with mTOR, iNOS, XIAP, and HIF-1α signaling pathways. However, the mechanisms by which sestrin 2 promotes tumor growth are relatively complex, especially under stress conditions. When tumors are under stress, such as during hypoxia or nutritional deficiency, they can cope with the crisis through a series of protective mechanisms, including reducing metabolic energy consumption, delaying cell growth, and inhibiting apoptosis. Sestrin 2 can be regarded as a protective factor for cell survival during stressful conditions. We discussed the possible mechanisms of sestrin 2 function and considered that differences in metabolic status and tumor types may be the reasons for the contradictory roles of sestrin 2. However, to a certain extent, this conclusion is not satisfactory because of the lack of more favorable experimental evidence and the role of sestrin 2 in cancer requires further validation.
Sestrin 2 plays an essential role in various tumors and is a promising diagnostic and therapeutic target. For diagnosis, sestrin 2 protein may be useful as an auxiliary characteristic to determine tumor classification and prognosis. For treatment, sestrin 2 can be used as an effective target for the development of anticancer drugs. However, the function of sestrin 2 in regulating cancer is bidirectional, and the underlying mechanisms need further exploration and verification.
Availability of data and materials
Not applicable.
Abbreviations
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin complex 1
- AMPK:
-
5ʹ-Adenosine monophosphate-activated protein kinase
- TNM:
-
Tumor, node, and metastasis
- ER:
-
Endoplasmic reticulum
- CRC:
-
Colorectal cancer
- SCC:
-
Squamous cell carcinoma
- NSCLC:
-
Non-small cell lung cancer
- HCC:
-
Hepatocellular carcinoma
- PI3K:
-
Phosphoinositide 3-kinase
- ERK:
-
Extracellular signal-regulated kinase
- MAPK:
-
Mitogen-activated protein kinase
- p70S6K:
-
P70 ribosomal protein S6 kinase
- shRNA:
-
Short hairpin RNA
- 5-FU:
-
5-Fluorouracil
- IMCA:
-
2-Imino-6-methoxy-2H-chromene-3-carbothioamide
- CAP:
-
Cold atmospheric plasma
- iNOS:
-
Nitric oxide synthase
- ROS:
-
Reactive oxygen species
- XIAP:
-
X-linked inhibitor of apoptosis protein
- HIF-1α:
-
Hypoxia inducible factor-1α
- ISO:
-
Isorhapontigenin
- miR:
-
MicroRNA
- PTEN:
-
Tensin homolog deleted on chromosome ten
- CRC:
-
Colorectal cancer
- EC:
-
Endometrial cancer
- MTC:
-
Medullary thyroid cancer
- EC:
-
Endometrial cancer
- FOXO1:
-
Forkhead box 1 transcription factor
- C/EBPβ:
-
CCAAT/enhancer binding protein β
- PERK:
-
Phosphorylated-protein kinase RNA-like endoplasmic reticulum kinase
- IRE1:
-
Inositol-requiring enzyme 1
- XBP1:
-
X-box binding protein 1
- ATF4:
-
Activating transcription factor 4
- Up:
-
Upregulation of Sestrin2
- Down:
-
Downregulation of Sestrin2
- SGC:
-
Sebaceous gland carcinoma
- NB:
-
Neuroblastomas
- SCC:
-
Skin squamous cell carcinoma
- LSD1:
-
Lysine-specific demethylase
- TCGA:
-
The Cancer Genome Atlas
- UCEC:
-
TCGA-Uterine Corpus Endometrial Carcinoma
- GEO:
-
Gene Expression Omnibus
- GSE:
-
GEO Series
- HBV:
-
Hepatitis B viral
- HCV:
-
Hepatitis C viral
- DDIT3:
-
DNA damage inducible transcript 3
- N/A:
-
not available
- Nrf2:
-
Nuclear factor E2-related factor 2
- AP-1:
-
Activating protein-1
- Sp-1:
-
Specificity protein 1
References
Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–8.
Hoekstra HJ, Wobbes T, Heineman E, Haryono S, Aryandono T, Balch CM. Fighting global disparities in cancer care: a surgical oncology view. Ann Surg Oncol. 2016;23(7):2131–6.
Xu H, Liu L, Li W, Zou D, Yu J, Wang L, Wong CC. Transcription factors in colorectal cancer: molecular mechanism and therapeutic implications. Oncogene. 2021;40(9):1555–69.
Ding L, Lan Z, Xiong X, Ao H, Feng Y, Gu H, Yu M, Cui Q. The dual role of microRNAs in colorectal cancer progression. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19092791.
Oura K, Morishita A, Masaki T. Molecular and functional roles of microRNAs in the progression of hepatocellular carcinoma—a review. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21218362.
Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, Liao QX, Zhang H, Zeng LS, Cui SZ. LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/beta-catenin pathway. Mol Cancer. 2018;17(1):126.
Jie M, Wu Y, Gao M, Li X, Liu C, Ouyang Q, Tang Q, Shan C, Lv Y, Zhang K, et al. CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol Cancer. 2020;19(1):56.
Seoane J, Gomis RR. TGF-beta family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol. 2017. https://doi.org/10.1101/cshperspect.a022277.
Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10(6):415–24.
Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75.
Ershaid N, Sharon Y, Doron H, Raz Y, Shani O, Cohen N, Monteran L, Leider-Trejo L, Ben-Shmuel A, Yassin M, et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun. 2019;10(1):4375.
Gambardella V, Castillo J, Tarazona N, Gimeno-Valiente F, Martinez-Ciarpaglini C, Cabeza-Segura M, Rosello S, Roda D, Huerta M, Cervantes A, et al. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. 2020;86: 102015.
Liu YC, Yeh CT, Lin KH. Molecular functions of thyroid hormone signaling in regulation of cancer progression and anti-apoptosis. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20204986.
Pasha M, Eid AH, Eid AA, Gorin Y, Munusamy S. Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid Med Cell Longev. 2017;2017:3296294.
Shen M, Xie S, Rowicki M, Michel S, Wei Y, Hang X, Wan L, Lu X, Yuan M, Jin JF, et al. Therapeutic targeting of metadherin suppresses colorectal and lung cancer progression and metastasis. Cancer Res. 2021;81(4):1014–25.
Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 2018;834:188–96.
Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–60.
Wang LX, Zhu XM, Yao YM. Sestrin 2: its potential role and regulatory mechanism in host immune response in diseases. Front Immunol. 2019;10:2797.
Hu HJ, Shi ZY, Lin XL, Chen SM, Wang QY, Tang SY. Upregulation of Sestrin2 expression protects against macrophage apoptosis induced by oxidized low-density lipoprotein. DNA Cell Biol. 2015;34(4):296–302.
Xiao T, Zhang L, Huang Y, Shi Y, Wang J, Ji Q, Ye J, Lin Y, Liu H. Sestrin2 increases in aortas and plasma from aortic dissection patients and alleviates angiotensin II-induced smooth muscle cell apoptosis via the Nrf2 pathway. Life Sci. 2019;218:132–8.
Chen M, Xi Y, Chen K, Xiao P, Li S, Sun X, Han Z. Upregulation Sestrin2 protects against hydrogen peroxide-induced oxidative damage bovine mammary epithelial cells via a Keap1-Nrf2/ARE pathway. J Cell Physiol. 2021;236(1):392–404.
Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21(39):6017–31.
Kim H, An S, Ro SH, Teixeira F, Park GJ, Kim C, Cho CS, Kim JS, Jakob U, Lee JH, et al. Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat Commun. 2015;6:10025.
Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43–8.
Ren D, He Z, Fedorova J, Zhang J, Wood E, Zhang X, Kang DE, Li J. Sestrin2 maintains OXPHOS integrity to modulate cardiac substrate metabolism during ischemia and reperfusion. Redox Biol. 2021;38: 101824.
Sun X, Han F, Lu Q, Li X, Ren D, Zhang J, Han Y, Xiang YK, Li J. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating Sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat diet-induced obese mice. Diabetes. 2020;69(6):1292–305.
Han X, Ding C, Zhang G, Pan R, Liu Y, Huang N, Hou N, Han F, Xu W, Sun X. Liraglutide ameliorates obesity-related nonalcoholic fatty liver disease by regulating Sestrin2-mediated Nrf2/HO-1 pathway. Biochem Biophys Res Commun. 2020;525(4):895–901.
Kim H, Yin K, Falcon DM, Xue X. The interaction of Hemin and Sestrin2 modulates oxidative stress and colon tumor growth. Toxicol Appl Pharmacol. 2019;374:77–85.
Zhang XY, Wu XQ, Deng R, Sun T, Feng GK, Zhu XF. Upregulation of sestrin 2 expression via JNK pathway activation contributes to autophagy induction in cancer cells. Cell Signal. 2013;25(1):150–8.
Wei JL, Fu ZX, Fang M, Guo JB, Zhao QN, Lu WD, Zhou QY. Decreased expression of sestrin 2 predicts unfavorable outcome in colorectal cancer. Oncol Rep. 2015;33(3):1349–57.
Dai J, Huang Q, Niu K, Wang B, Li Y, Dai C, Chen Z, Tao K, Dai J. Sestrin 2 confers primary resistance to sorafenib by simultaneously activating AKT and AMPK in hepatocellular carcinoma. Cancer Med. 2018;7(11):5691–703.
Kozak J, Wdowiak P, Maciejewski R, Torres A. Interactions between microRNA-200 family and Sestrin proteins in endometrial cancer cell lines and their significance to anoikis. Mol Cell Biochem. 2019;459(1–2):21–34.
Ro SH, Xue X, Ramakrishnan SK, Cho CS, Namkoong S, Jang I, Semple IA, Ho A, Park HW, Shah YM, et al. Tumor suppressive role of sestrin2 during colitis and colon carcinogenesis. Elife. 2016;5: e12204.
Jayaraj P, Sen S, Rangarajan S, Ray N, Vasu K, Singh VK, Phartyal R, Yadav S, Verma A. Immunohistochemical evaluation of stress-responsive protein sestrin2 and its correlation with p53 mutational status in eyelid sebaceous gland carcinoma. Br J Ophthalmol. 2018;102(6):848–54.
Ambrosio S, Sacca CD, Amente S, Paladino S, Lania L, Majello B. Lysine-specific demethylase LSD1 regulates autophagy in neuroblastoma through SESN2-dependent pathway. Oncogene. 2017;36(48):6701–11.
Liang Y, Zhu J, Huang H, Xiang D, Li Y, Zhang D, Li J, Wang Y, Jin H, Jiang G, et al. SESN2/sestrin 2 induction-mediated autophagy and inhibitory effect of isorhapontigenin (ISO) on human bladder cancers. Autophagy. 2016;12(8):1229–39.
Fu H, Song W, Wang Y, Deng W, Tang T, Fan W, Qu S. Radiosensitizing effects of Sestrin2 in PC3 prostate cancer cells. Iran J Basic Med Sci. 2018;21(6):621–4.
Zhao B, Shah P, Budanov AV, Qiang L, Ming M, Aplin A, Sims DM, He YY. Sestrin2 protein positively regulates AKT enzyme signaling and survival in human squamous cell carcinoma and melanoma cells. J Biol Chem. 2014;289(52):35806–14.
Zhu G, Xu P, Guo S, Yi X, Wang H, Yang Y, Liu L, Shi Q, Gao T, Li C. Metastatic melanoma cells rely on Sestrin2 to acquire anoikis resistance via detoxifying intracellular ros. J Invest Dermatol. 2020;140(3):666-675 e662.
Shin J, Bae J, Park S, Kang HG, Shin SM, Won G, Kim JS, Cho SG, Choi Y, Oh SM, et al. mTOR-dependent role of Sestrin2 in regulating tumor progression of human endometrial cancer. Cancers. 2020. https://doi.org/10.3390/cancers12092515.
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85.
Chen KB, Xuan Y, Shi WJ, Chi F, Xing R, Zeng YC. Sestrin2 expression is a favorable prognostic factor in patients with non-small cell lung cancer. Am J Transl Res. 2016;8(4):1903–9.
Xu H, Sun H, Zhang H, Liu J, Fan F, Li Y, Ning X, Sun Y, Dai S, Liu B, et al. An ShRNA based genetic screen identified Sesn2 as a potential tumor suppressor in lung cancer via suppression of Akt-mTOR-p70S6K signaling. PLoS ONE. 2015;10(5):e0124033.
Chae HS, Gil M, Saha SK, Kwak HJ, Park HW, Vellingiri B, Cho SG. Sestrin2 expression has regulatory properties and prognostic value in lung cancer. J Pers Med. 2020. https://doi.org/10.3390/jpm10030109.
Lin LT, Liu SY, Leu JD, Chang CY, Chiou SH, Lee TC, Lee YJ. Arsenic trioxide-mediated suppression of miR-182-5p is associated with potent anti-oxidant effects through up-regulation of SESN2. Oncotarget. 2018;9(22):16028–42.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Mody K, Abou-Alfa GK. Systemic therapy for advanced hepatocellular carcinoma in an evolving landscape. Curr Treat Options Oncol. 2019;20(2):3.
Chen S, Yan W, Lang W, Yu J, Xu L, Xu X, Liu Y, Bao H. SESN2 correlates with advantageous prognosis in hepatocellular carcinoma. Diagn Pathol. 2017;12(1):13.
Qi W, Li Z, Yang C, Jiangshan Dai J, Zhang Q, Wang D, Wu C, Xia L, Xu S. Inhibitory mechanism of muscone in liver cancer involves the induction of apoptosis and autophagy. Oncol Rep. 2020;43(3):839–50.
Mossmann D, Park S, Hall MN. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18(12):744–57.
Murugan AK. mTOR: role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019;59:92–111.
Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10(3):457–68.
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–302.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–76.
Populo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–918.
Wei JL, Fang M, Fu ZX, Zhang SR, Guo JB, Wang R, Lv ZB, Xiong YF. Sestrin 2 suppresses cells proliferation through AMPK/mTORC1 pathway activation in colorectal cancer. Oncotarget. 2017;8(30):49318–28.
Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20133177.
Kim GT, Lee SH, Kim YM. Quercetin regulates Sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ros in HCT116 colon cancer cells. J Cancer Prev. 2013;18(3):264–70.
Seo K, Ki SH, Park EY, Shin SM. 5-Fluorouracil inhibits cell migration by induction of Sestrin2 in colon cancer cells. Arch Pharm Res. 2017;40(2):231–9.
Won DH, Chung SH, Shin JA, Hong KO, Yang IH, Yun JW, Cho SD. Induction of sestrin 2 is associated with fisetin-mediated apoptosis in human head and neck cancer cell lines. J Clin Biochem Nutr. 2019;64(2):97–105.
Zhang L, Liu W, Wang Q, Li Q, Wang H, Wang J, Teng T, Chen M, Ji A, Li Y. New drug candidate targeting the 4A1 orphan nuclear receptor for medullary thyroid cancer therapy. Molecules. 2018. https://doi.org/10.3390/molecules23030565.
Chen X, Wang Y. Adiponectin and breast cancer. Med Oncol. 2011;28(4):1288–95.
Di Zazzo E, Polito R, Bartollino S, Nigro E, Porcile C, Bianco A, Daniele A, Moncharmont B. Adiponectin as link factor between adipose tissue and cancer. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20040839.
Pham DV, Raut PK, Pandit M, Chang JH, Katila N, Choi DY, Jeong JH, Park PH. Globular adiponectin inhibits breast cancer cell growth through modulation of inflammasome activation: critical role of Sestrin2 and AMPK signaling. Cancers. 2020. https://doi.org/10.3390/cancers12030613.
Xia J, Zeng W, Xia Y, Wang B, Xu D, Liu D, Kong MG, Dong Y. Cold atmospheric plasma induces apoptosis of melanoma cells via Sestrin2-mediated nitric oxide synthase signaling. J Biophotonics. 2019;12(1): e201800046.
Dai X, Bazaka K, Thompson EW, Ostrikov KK. Cold atmospheric plasma: a promising controller of cancer cell states. Cancers. 2020. https://doi.org/10.3390/cancers12113360.
Ayachi O, Barlin M, Broxtermann PN, Kashkar H, Mauch C, Zigrino P. The X-linked inhibitor of apoptosis protein (XIAP) is involved in melanoma invasion by regulating cell migration and survival. Cell Oncol (Dordr). 2019;42(3):319–29.
Ding B, Parmigiani A, Yang C, Budanov AV. Sestrin2 facilitates death receptor-induced apoptosis in lung adenocarcinoma cells through regulation of XIAP degradation. Cell Cycle. 2015;14(20):3231–41.
Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138(5):1058–66.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32.
Seo K, Seo S, Ki SH, Shin SM. Sestrin2 inhibits hypoxia-inducible factor-1alpha accumulation via AMPK-mediated prolyl hydroxylase regulation. Free Radic Biol Med. 2016;101:511–23.
Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, Carnuccio R, Kroemer G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle. 2009;8(10):1571–6.
Jin HR, Du CH, Wang CZ, Yuan CS, Du W. Ginseng metabolite Protopanaxadiol induces Sestrin2 expression and AMPK activation through GCN2 and PERK. Cell Death Dis. 2019;10(4):311.
Shan L, Tong L, Hang L, Fan H. Fangchinoline supplementation attenuates inflammatory markers in experimental rheumatoid arthritis-induced rats. Biomed Pharmacother. 2019;111:142–50.
Zhou L, Hong G, Li S, Liu Q, Song F, Zhao J, Yuan J, Tickner J, Xu J. Fangchinoline protects against bone loss in OVX mice via inhibiting osteoclast formation, bone resorption and RANKL-induced signaling. Int J Biol Sci. 2020;16(2):309–19.
Wang N, Pan W, Zhu M, Zhang M, Hao X, Liang G, Feng Y. Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br J Pharmacol. 2011;164(2b):731–42.
Hua X, Xu J, Deng X, Xu J, Li J, Zhu DQ, Zhu J, Jin H, Tian Z, Huang H, et al. New compound ChlA-F induces autophagy-dependent anti-cancer effect via upregulating Sestrin-2 in human bladder cancer. Cancer Lett. 2018;436:38–51.
Sanli T, Linher-Melville K, Tsakiridis T, Singh G. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PLoS ONE. 2012;7(2): e32035.
Perez-Ramirez C, Canadas-Garre M, Molina MA, Faus-Dader MJ, Calleja-Hernandez MA. PTEN and PI3K/AKT in non-small-cell lung cancer. Pharmacogenomics. 2015;16(16):1843–62.
Wang X, Liu W, Zhuang D, Hong S, Chen J. Sestrin2 and sestrin3 suppress NK-92 cell-mediated cytotoxic activity on ovarian cancer cells through AMPK and mTORC1 signaling. Oncotarget. 2017;8(52):90132–43.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Guo Y, Zhu H, Weng M, Zhang H, Wang C, Sun L. CC-223, NSC781406, and BGT226 exerts a cytotoxic effect against pancreatic cancer cells via mTOR signaling. Front Pharmacol. 2020;11: 580407.
Guha D, Saha T, Bose S, Chakraborty S, Dhar S, Khan P, Adhikary A, Das T, Sa G. Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis. 2019;24(11–12):958–71.
Lippert TH, Ruoff HJ, Volm M. Intrinsic and acquired drug resistance in malignant tumors. The main reason for therapeutic failure. Arzneimittelforschung. 2008;58(6):261–4.
Chen Z, Li Y, Tan B, Zhao Q, Fan L, Li F, Zhao X. Progress and current status of molecule-targeted therapy and drug resistance in gastric cancer. Drugs Today (Barc). 2020;56(7):469–82.
Ben-Sahra I, Dirat B, Laurent K, Puissant A, Auberger P, Budanov A, Tanti JF, Bost F. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ. 2013;20(4):611–9.
Saveljeva S, Cleary P, Mnich K, Ayo A, Pakos-Zebrucka K, Patterson JB, Logue SE, Samali A. Endoplasmic reticulum stress-mediated induction of SESTRIN 2 potentiates cell survival. Oncotarget. 2016;7(11):12254–66.
Byun JK, Choi YK, Kim JH, Jeong JY, Jeon HJ, Kim MK, Hwang I, Lee SY, Lee YM, Lee IK, et al. A positive feedback loop between Sestrin2 and mTORC2 is required for the survival of glutamine-depleted lung cancer cells. Cell Rep. 2017;20(3):586–99.
Kumar A, Giri S, Shaha C. Sestrin2 facilitates glutamine-dependent transcription of PGC-1alpha and survival of liver cancer cells under glucose limitation. FEBS J. 2018;285(7):1326–45.
Sisinni L, Pietrafesa M, Lepore S, Maddalena F, Condelli V, Esposito F, Landriscina M. Endoplasmic reticulum stress and unfolded protein response in breast cancer: the balance between apoptosis and autophagy and its role in drug resistance. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20040857.
Mohamed E, Cao Y, Rodriguez PC. Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy. Cancer Immunol Immunother. 2017;66(8):1069–78.
Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168(4):692–706.
Bruning A, Rahmeh M, Friese K. Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated sestrin-2 regulation. Mol Oncol. 2013;7(6):1012–8.
Yang Y, Guo G, Zhou W, Ge Y, Fan Z, Liu Q, Gao Y. Sestrin2 protects against bavachin induced ER stress through AMPK/mTORC1 signaling pathway in HepG2 cells. J Pharmacol Sci. 2021;145(2):175–86.
Acknowledgements
We thank Susan Zunino, PhD, from Liwen Bianji (Edanz), for editing the English text of this manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2019BH036, ZR2020MH106), the Science and Technology Development Project of Weifang (2018YX058), the PhD Foundation of Weifang Medical University and the public domestic visiting program of Weifang Medical University (20217-03).
Author information
Authors and Affiliations
Contributions
RP and XS designed this review article. JQ and ML researched the literature and contributed to manuscript draft. FH, NH, JZ, RP and XS conducted the figure design and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qu, J., Luo, M., Zhang, J. et al. A paradoxical role for sestrin 2 protein in tumor suppression and tumorigenesis. Cancer Cell Int 21, 606 (2021). https://doi.org/10.1186/s12935-021-02317-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02317-9