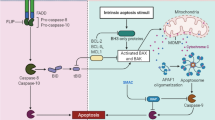
Abstract
Aim and background
Cancer represents a major health problem with an exceedingly high toll on the patients, their families, and the economy. Cancers are also associated with high mortality rates. Existing therapies for cancer are generally ineffective with many side effects.
Method
A search was conducted on Pubmed, Google Scholar, Scopus, and web of science databases, and articles related to anticancer effects of resveratrol were collected.
Results
Resveratrol is a natural compound that can activate the Nrf2 transcription factor. Nfr2 translocates to the nucleus and induces antioxidant gene expression. In different cell lines, resveratrol can increase apoptosis and inhibit the proliferation of cancer cells.
Conclusion
We found that resveratrol shows efficacy for the treatment of cancer, but due to high controversy on the Nrf2 signaling pathway and mechanisms of resveratrol action, additional studies should be conducted to better characterize its mode-of-action in cancer.
Graphical Abstract

Similar content being viewed by others
Introduction
Resveratrol belongs to the flavonoids group and can be found in various fruits (e.g. berries, grapes, red wine, and peanuts). In addition to its anticancer effects, it has anti-diabetic, antioxidant, anti-inflammatory effects. Resveratrol has beneficial effects against drug resistance in cancer and can increase the sensitivity of cells to chemotherapeutic drugs. Resveratrol also has protective properties on the liver, heart, and brain [1]. Cancer and cardiovascular disease are two major health problems with high mortality rates [2]. Cancer is responsible for many deaths that occur in a year. In 2020, it is estimated that about 10 million cancer death has occurred and 19.3 million new cases were diagnosed with cancer [3]. Existing therapies for cancer are generally not effective. Current therapies for cancer including chemotherapy and radiotherapy have multiple side effects and resistance to them may develop over time. Chemotherapeutic drugs simultaneously affect both normal and cancer cells. Cancer patients treated with chemotherapy lose their hair and their bone marrow is damaged which may lead to aplastic anemia [4]. Patients who are treated with radiotherapy display many side effects including lymphopenia, thrombocytopenia, and neutropenia [5,6,7]. Radiotherapy damage to stem cells of bone marrow may be teratogenic for the fetus [8, 9]. It also affects the skin and causes radiodermatitis and increases the risk of secondary cancer following therapy [10, 11]. In addition, radiotherapy damages the DNA and causes apoptosis and cell death [12]. Thus, discovering new cancer therapeutic approaches is necessary and timely. Resveratrol is a natural product and has anticancer effects. It can activate the Nrf2 signaling pathway and reduce oxidative stress. In this review, we summarized the anticancer effects of resveratrol which are mediated via the activation of the Nrf2 signaling pathway.
Oxidative stress and cancer pathogenesis
Oxidative stress is a trigger and occurs in many diseases, such as diabetes, cancers, and neurological disorders. Various metabolic pathways lead to the production of reactive oxygen species, referred to as ROS (e.g. O2–, H2O2, OH–, O3). For instance, UV radiation, enzymes such as NADPH oxidase, and chemical substances, such as alcohol can produce oxidative stress in cells. Cells also possess antioxidant enzymes (e.g. catalase and superoxide dismutase) that can reduce ROS and decrease restore their redox status. HO-1 (heme oxygenase-1) is an antioxidant enzyme and its levels increase upon resveratrol treatment [13,14,15]. In normal cells, low levels of ROS have been implicated in signal transduction, phagocytosis, inflammation, and activation of enzymes [4, 15]. In turn, ROS production in tumor cells is elevated cells as a consequence of increased metabolic rate, gene mutation, and relative hypoxia, and excess ROS are quenched by increased antioxidant enzymatic and non-enzymatic pathways in the same cells. Furthermore, ROS activates signaling pathways related to the metastasis of tumors. ROS can induce apoptosis by activation of caspase enzymes and several antioxidant substances prevent cells from undergoing this process [15]. In addition, oxidative conditions in cancer cells increase VEGF levels for angiogenesis. In cancer therapy, there are anti-angiogenesis antibodies that can block the VEGF receptor [16] (Fig. 1).
Nrf2 signaling pathway
Cap’n’collar (Cnc) transcription factors family has several members including Nrf2, Nrf3, Nrf1. Nrf2 (nuclear erythroid-2 related factor 2) is a transcription factor that can stimulate the antioxidant enzymes. It can regulate the oxidative stress of cells by activating the genes which are related to cellular stress [1, 17]. Nrf2 has tumor suppressor effects and also can increase the proliferation in cancer cells. It has been shown that in many cancers the expression level of Nrf2 is elevated [18]. In addition, in cancer cells, the overexpression of Nrf2 leads to resistance to chemotherapy and radiotherapy. Nrf2 has seven domains (Neh1 to Neh7) and two binding sites (ETGE and DLG). The most important domain for Nrf2 is Neh2 that has seven lysin amino acids [18]. The activation of the Nrf2 transcription factor due to its antioxidant properties may be effective in cancer therapy. But there is a controversy on whether activation of Nrf2 is of clinical benefit in cancer therapy or is a carcinogen? Nrf2 has been referred to as a double-edged sword. In addition to its cytoprotective and chemoprevention effects, the activation of Nrf2 results in inhibition of apoptosis, induction of proliferation, and also enhancement of cell survival [19]. During chemotherapy, antioxidant levels of β-carotene, vitamin C, and E are decreased. In addition, chemotherapeutic and antineoplastic drugs (e.g. daunorubicin, and epirubicin) can increase ROS levels and induce oxidative stress and attenuate cancer cells death. It has also been suggested that reduction in oxidative conditions in cancer cells may enhance the anticancer effects of antineoplastic drugs [4]. In a study by DeNicola et al., it was shown that Oncogenes including Kras, Myc, and Braf genes suppressed ROS production and increased the transcription of Nrf2 in cells [20] (Table 1).
The keap1-Nrf2 pathway
The Keap1-Nrf2 signaling pathway is essential for the regulation of oxidative stress [19, 21]. In the basal condition, Nrf2 and keap1 are connected and whenever cells are placed in oxidative condition, Nrf2 is separated from Keap1, transfer to the nucleus, and activate the antioxidant genes [19].
Keap1 has three domains that can bind to ETGE and DLF motifs from the Nrf2 protein. Keap1 and Nrf2 form a complex with Cullin3 and E3 ubiquitin ligase. The oxidation of cysteine sulfhydryl groups in the oxidative stress condition causes the separation of Nrf2 from Keap-1. Then Next, Nrf2 translocates to the nucleus forming a heterodimer complex with Maf (musculoaponeurotic fibrosarcoma) and binds to an ARE (antioxidant response element) enhancer [18, 22].
Nrf2 binds to the NF-E2 site of the β-globin gene. This molecule has cytoprotective and chemoprevention activity [23]. Several substances induce Nrf2 activation (e.g. Hydrogen sulfide, nitrogen oxide, physical activity, lipid peroxidation, and curcumin). Keap1 (formerly known as an Nrf2 inducer) is a protein that the stress molecule can bind to the cysteine amino acid. Indeed, Keap1 protein is a negative controller of the Nrf2. Keap1 has oxidative sensors and can detect oxidative stress such as ROS in the cells [23, 24]. The Keap1-Nrf2 pathway regulates the anabolic pathways in the cells that are necessary for the reduction of oxidant (for example, NADPH that is produced in the pentose phosphate pathway) [24, 25].
PI3K/AKT pathway
The class IA of the PI3K family has been shown to be responsible for cancer progression. It (class IA) has two subunits (p85 and p110 subunits). The PIP2 (phosphatidylinositol-4,5-bisphosphate) is the substrate of PI3K. when growth factors bind to their receptors on the surface of cells, the inhibitory effect of the p85 subunit dissociates from the p110 subunit. In addition, the p110 subunit can be activated by ROS. The phosphorylation of PIP2 by p110 results in PIP3 (phosphatidylinositol-3,4,5-trisphosphate) production. Next, PIP3 binds to PDK1 and AKT proteins, leading to phosphorylation of AKT protein by PDK1 and activation of numerous enzymes. AKT can phosphorylate transcription factors and proteins involved in cell survival [26]. In a leukemia cell line activation of the PI3K/AKT pathway has been shown to increase Nrf2 expression [27].
Effects of resveratrol on various types of cancers
Estrogen is a steroid hormone that increases the risk of breast cancer. Due to the reduction of estrogen in menopausal women, the risk of osteoporosis and cardiovascular disease is increased in this group. As a treatment for this condition, estrogen as a hormone therapy has been administrated to menopausal women. Yet, estrogen is a carcinogen, and can significantly increase the risk of breast cancer [28, 29]. Thus, the regulation of estrogen levels is important in the prevention of breast cancer.
Catechol estrogen is a carcinogen for breast cancer. UGT1A8 is an enzyme that can metabolize the catechol estrogen. Resveratrol can increase the expression of UGT1A8 through activation of the Nrf2 gene expression and degrade the catechol estrogen. Indeed, Nrf2 affects the promoter of the UGT1A8 gene and induces UGT1A8 gene activation [30]. Anwesha et al. synthesized two analogs of resveratrol (HPIMBD and TIMBD) and compared their antioxidant and cytotoxicity effects in the presence or absence of resveratrol. They reported that these analogs do not have antiproliferative or cytotoxicity effects against the MCF-10A cell line. But, compared to resveratrol can more efficaciously induce Nrf2 expression. HPIMBD and TIMBD also increased SOD3 enzyme expression which is responsible for the detoxification of ROS, significantly attenuating ROS generation in this cell line [31].
As noted above, oxidative stress may trigger carcinogenesis and increase cell proliferation [32]. By activating the Nrf2 signaling pathway resveratrol protects cells from oxidative stress-induced damage. Zhang et al. found that resveratrol can increase the Nrf2 and HO-1 expression and in contrast, it reduces the ROS production and Keap1 expression. When treated with resveratrol, cell proliferation was inhibited and apoptosis was induced secondary to suppression of the Bcl-2 protein and increased expression of Bax protein [32].
Cheng et al. showed that resveratrol can induce apoptosis and inhibit cell proliferation. Resveratrol activated the Nrf2 through ROS production [13]. Lee et al. used the combination of resveratrol and clofarabine on the MSTO-211H cell line. When combined, their inhibitory effects against cell growth were promoted. Reduction in Nrf2 protein expression levels and increased cell viability were reported in cells are treated with the combination of resveratrol and clofarabine [33].
Soeur et al. used keratinocytes to investigate the antioxidant properties of resveratrol in skin cells, showing the latter can increase the antioxidant enzymes, such as glutathione peroxidase-2 by activating the Nrf2-Keap1 pathway [34]. Reduction in Nrf2 expression by resveratrol was also reported. HEO et al. showed the antiproliferative effects of resveratrol against malignant melanoma cells, reporting that resveratrol induced apoptosis by increasing Bcl-2 expression levels, but decreased Nrf2 expression level in melanoma cells [35]. In a leukemia cell line, aberrant activation of the PI3K/AKT/Nrf2 pathway inhibited apoptosis and increased cell proliferation [27].
The effects of resveratrol in pancreatic and renal cell carcinoma also have been investigated in vitro. Shanel et al. reported the ameliorative activity of resveratrol against toxicity induced by ochratoxin in human embryonic kidney cells (HEK293 cell). Some fungi such as Penicillium and Aspergillus can produce it. Ochratoxin can induce oxidative stress. It has nephrotoxin activity and causes renal dysfunction. Results showed that after 48 h resveratrol elevate the expression of Nrf2 cells. in conclusion, resveratrol can be regarded as a good choice for Ochratoxin-induced toxicity and has chemo-preventive properties [36].
Resveratrol increases the pancreatic cancer cells' sensitivity to gemcitabine by its effect on NAF-1 (nutrient-deprivation autophagy factor-1) and Nrf2 signaling. Liang et al. showed that Resveratrol can activate the Nrf2 signaling and reduce the expression of NAF-1 that has anti-apoptotic activity. in addition to induction of apoptosis, resveratrol showed the antiproliferative activity against pancreatic cancer cells. in conclusion, new drugs for reducing the transcription or activity of NAF-1 (e.g. resveratrol) may be effective in the treatment of pancreatic cancer [13].
Resveratrol effect on tumor microenvironment
Resveratrol can regulate the tumor microenvironment via modulating oxidative stress, angiogenesis, fibrosis, and the immune system [37]. In the tumor cell microenvironment, ROS levels increase and lead to apoptosis by activating p53. It was found that resveratrol has two contradictory impacts on oxidative stress. In its therapeutic effect, it elevates oxidative stress to prevent cancer cell progression [38]. In its chemopreventive effect, it can act as a ROS scavenger to sustain cells from mutations. Resveratrol can affect different innate immune cells which are involved in the regulation of tumor microenvironment. It was found that resveratrol inhibited the activation of M2 macrophage and also induced repolarization of tumor-associated macrophages (TAM) from M2 to M1, resulted in tumor suppression and metastasis. M1 is the active form of macrophage in normal cells that produces several cytokines. In the tumor microenvironment condition, reprogramming M2 toward the M1 phenotype is associated with the overproduction of inflammatory cytokines leading to cell destruction [39]. Resveratrol can also decrease immune tolerance in tumor cells by inhibiting the enzyme indoleamine 2,3-dioxygenase (IDO) expression and activity in dendritic cells resulted in regulation of cytotoxic T cell polarization to increase its antitumor effect. Treatments with anti-angiogenesis agents have been focused on as a suitable strategy among patients with solid tumors to prevent tumor progression. Resveratrol was effective on angiogenesis through an inhibitory direct effect on vascular endothelial growth factor (VEGF) generation and also inhibiting the hypoxia-inducible factor (HIF)-1generation and leads to preventing VEGF secretion [40]. Fibroblasts are involved in the tumor’s progression by producing platelet-derived growth factor (PDGF), stromal cell-derived factor 1 (SDF1), VEGF, and basic fibroblast growth factor (bFGF). Resveratrol can inhibit the tumor cell viability by decreasing several fibrogenic mediators including a-SMA, type I collagen, and fibronectin [40].
Clinical trial studies related to anti-tumor effects of resveratrol
There are few clinical studies related to the anti-cancer activity of Res. A clinical trial conducted on the protective impact of plant-based Res on colon cancer patients showed that this agent could not inhibit the expression of Wnt, myc, and cyclin D1genes in a sample of patients [41]. Patel et al. reported that resveratrol and its metabolites (resveratrol-3-O-glucuronide, resveratrol-4′-O-glucuronide, resveratrol-3-O-sulfate, resveratrol-4′-O-sulfate, resveratrol sulfate glucuronide and resveratrol disulfate) were present in the operated colorectal tissue [42]. Howells et al. reported higher levels of resveratrol in plasma and hepatic tissues after SRT501administration in patients with colorectal cancer and hepatic metastasis who were scheduled to undergo hepatectomy [43]. No significant alteration was observed in AKT1, GSK-3, survivin, and PARP biomarkers [43]. Zhu et al. evaluated the resveratrol impact on the methylation of certain proteins in women with breast cancer. Sample biopsy demonstrated invasive breast cancer with atypical hyperplasia [44]. It was found that 5 or 50 mg/2 per day of trans-resveratrol for 12 weeks reduced methylation of RASSF-1a, leading to a decrease in prostaglandin E2 (PGE2) expression in breast cancer [45]. Brown et al. [46] reported that administration of 4000 mg/patient of Res was safe among patients with recurring prostate cancer [47]. Another trial indicated use of two doses of resveratrol for 4 months decreased blood androstenedione, dehydroepiandrosterone, and dehydroepiandrosterone-sulfate concentrations without change in the size of the prostate among patients with benign prostate hyperplasia [48]. However, there are some reports related to the side effects of Res in cancer patients. It was indicated that administration of Res (5 mg/day for 6 days) increased protein carbonyl levels in patients with colorectal cancer [49]. SRT501 supplements daily caused kidney toxicity in patients with multiple myeloma at the second phase of the clinical trial, led to a patient’s death [50]).
Conclusion and future perspectives
The evidence from experimental studies suggests that resveratrol has a protective effect against several cancers by inhibiting the expression and levels of Nrf2 in cancerous samples. In addition, it can induce apoptosis and inhibit cell proliferation. Resveratrol may be effective in combination with other chemotherapeutics agents. Although, most of the studies indicated the safety of Resveratrol; however, there are some reports related to its toxicity due to dosing regimen. Current data related to trials on the effectiveness of resveratrol in patients with a different type of cancer treatment are still very few. In addition, the studies have a low sample size. The molecular mechanisms involved in the protective effects of Res against cancer were not evaluated in human samples. Therefore, more clinical trials are needed to find the exact doses and duration for cancer treatment and prevention and also determine molecular targets triggered by Res. In addition, a novel formulation of Res with nano delivery systems should be designed and evaluated their pharmacokinetic and pharmacodynamics in cancer patients.
Availability of data and materials
All data are available in the manuscript.
References
Farkhondeh T, Folgado SL, Pourbagher-Shahri AM, Ashrafizadeh M, Samarghandian S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed Pharmacother. 2020;127:110234.
Samarghandian S, Azimi-Nezhad M, Farkhondeh T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. J Cell Physiol. 2019;234(7):10421–31. https://doi.org/10.1002/jcp.27710.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3(4):294–300.
Abravan A, Faivre-Finn C, Kennedy J, McWilliam A, van Herk M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J Thorac Oncol. 2020;15(10):1624–35.
Hendrix A, Yeo A-E, Lejeune S, Seront E. Rare case of life-threatening thrombocytopenia occurring after radiotherapy in a patient treated with immune checkpoint inhibitor. BMJ Case Reports CP. 2020;13(6):e235249.
Yao J-J, Yu X-L, Zhang F, Zhang W-J, Zhou G-Q, Tang L-L, et al. Radiotherapy with neoadjuvant chemotherapy versus concurrent chemoradiotherapy for ascending-type nasopharyngeal carcinoma: a retrospective comparison of toxicity and prognosis. Chin J Cancer. 2017;36(1):1–8.
Arnon J, Meirow D, Lewis-Roness H, Ornoy A. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update. 2001;7(4):394–403.
Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol *Biol* Phys. 1995;31(5):1319–39.
Schnur JB, Love B, Scheckner BL, Green S, Wernicke AG, Montgomery GH. A systematic review of patient-rated measures of radiodermatitis in breast cancer radiotherapy. Am J Clin Oncol. 2011;34(5):529.
Lee B, Lee S, Sung J, Yoon M. Radiotherapy-induced secondary cancer risk for breast cancer: 3D conformal therapy versus IMRT versus VMAT. J Radiol Prot. 2014;34(2):325.
Carvalho HdA, Villar RC. Radiotherapy and immune response: the systemic effects of a local treatment. Clinics. 2018;73.
Cheng L, Yan B, Chen K, Jiang Z, Zhou C, Cao J, et al. Resveratrol-induced downregulation of NAF-1 enhances the sensitivity of pancreatic cancer cells to gemcitabine via the ROS/Nrf2 signaling pathways. Oxid Med Cell Longev. 2018;2018:9482018.
Samarghandian S, Samini F, Azimi-Nezhad M, Farkhondeh T. Anti-oxidative effects of safranal on immobilization-induced oxidative damage in rat brain. Neurosci Lett. 2017;17(659):26–32. https://doi.org/10.1016/j.neulet.2017.08.065.
Wu M, Ma L, Xue L, Ye W, Lu Z, Li X, et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging (Albany NY). 2019;11(3):1030.
Farkhondeh T, Samarghandian S, Pourbagher-Shahri AM, Sedaghat M. The impact of curcumin and its modified formulations on Alzheimer's disease. J Cell Physiol. 2019;234(10):16953–65.
Liu Y, Tao S, Liao L, Li Y, Li H, Li Z, et al. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat Commun. 2020;11(1):348.
Jaramillo MC, Zhang DD. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev. 2013;27(20):2179–91.
Wu S, Lu H, Bai Y. Nrf2 in cancers: a double-edged sword. Cancer Med. 2019;8(5):2252–67.
DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–9.
Kim JH, Park EY, Ha HK, Jo CM, Lee WJ, Lee SS, et al. Resveratrol-loaded nanoparticles induce antioxidant activity against oxidative stress. Asian-Australas J Anim Sci. 2016;29(2):288–98.
Li R, Jia Z, Zhu H. Regulation of Nrf2 signaling. Reactive Oxygen Species (Apex, NC). 2019;8(24):312.
Baird L, Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 2020;40(13).
Wu WL, Papagiannakopoulos T. The pleiotropic role of the KEAP1/NRF2 pathway in cancer. Annu Rev Cancer Biol. 2020;4:413–35.
Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, et al. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE. 2019;14(5):e0216711.
Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28.
Li Y, Guo Y, Feng Z, Bergan R, Li B, Qin Y, et al. Involvement of the PI3K/Akt/Nrf2 signaling pathway in resveratrol-mediated reversal of drug resistance in HL-60/ADR cells. Nutr Cancer. 2019;71(6):1007–18.
Jiang X, Randhawa SB, Kagan R. Estrogen and estrogen analogs for prevention and treatment of osteoporosis. Marcus and Feldman’s Osteoporosis. Elsevier; 2021. p. 1711–9.
Ueda K, Adachi Y, Liu P, Fukuma N, Takimoto E. Regulatory actions of estrogen receptor signaling in the cardiovascular system. Front Endocrinol. 2020;10:909.
Zhou X, Zhao Y, Wang J, Wang X, Chen C, Yin D, et al. Resveratrol represses estrogen-induced mammary carcinogenesis through NRF2-UGT1A8-estrogen metabolic axis activation. Biochem Pharmacol. 2018;155:252–63.
Chatterjee A, Ronghe A, Padhye SB, Spade DA, Bhat NK, Bhat HK. Antioxidant activities of novel resveratrol analogs in breast cancer. J Biochem Mol Toxicol. 2018;32(1):e21925.
Zhang Y, Wang G, Wang T, Cao W, Zhang L, Chen X. Nrf2-Keap1 pathway-mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide-treated rheumatoid arthritis fibroblast-like synoviocytes. Ann N Y Acad Sci. 2019;1457:166–78.
Lee YJ, Im JH, Lee DM, Park JS, Won SY, Cho MK, et al. Synergistic inhibition of mesothelioma cell growth by the combination of clofarabine and resveratrol involves Nrf2 downregulation. BMB Rep. 2012;45(11):647–52.
Soeur J, Eilstein J, Léreaux G, Jones C, Marrot L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free Radic Biol Med. 2015;78:213–23.
Heo JR, Kim SM, Hwang KA, Kang JH, Choi KC. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int J Mol Med. 2018;42(3):1427–35.
Raghubeer S, Nagiah S, Phulukdaree A, Chuturgoon A. The phytoalexin resveratrol ameliorates ochratoxin a toxicity in human embryonic kidney (HEK293) cells. J Cell Biochem. 2015;116(12):2947–55.
Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Export Opin. 2007;7:1705–22.
Farkhondeh T, Samarghandian S, Shahri AM, Samini F. The neuroprotective effects of thymoquinone: a review. Dose-Response. 2018;16(2):1559325818761455.
Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Export Opin. 2007;7:1705–22.
Talib WH, Alsayed AR, Farhan F, Al Kury LT. Resveratrol and tumor microenvironment: mechanistic basis and therapeutic targets. Molecules. 2020;25(18):4282. https://doi.org/10.3390/molecules25184282.
Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. 2009;1:25.
Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–9. https://doi.org/10.1158/0008-5472.CAN-10-2027.
Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward W, Gescher AJ. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res. 2011;4:1419–25. https://doi.org/10.1158/1940-6207.CAPR-11-0148.
Zhu W, Qin W, Zhang K, Rottinghaus GE, Chen Y-C, Kliethermes B, Sauter ER. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012;64:393–400. https://doi.org/10.1080/01635581.2012.654926.
Soleas GJ, Grass L, Josephy PD, Goldberg DM, Diamandis EP. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin Biochem. 2006;39:492–7. https://doi.org/10.1016/S0009-9120(02)00275-8.
Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–11. https://doi.org/10.1158/0008-5472.CAN-10-2364.
Samarghandian S, Azimi-Nezhad M, Farkhondeh T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. J Cell Physiol. 2019;234(7):10421–31.
Kjaer TN, Ornstrup MJ, Poulsen MM, Jørgensen JO, Hougaard DM, Cohen AS, Neghabat S, Richelsen B, Pedersen SB. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate. 2015;75:1255–63. https://doi.org/10.1002/pros.23006.
Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A, Britton RG, Horner-Glister E, Greaves P, Jawad D. Cancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med. 2015;7:298117.
Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, Jacobson E, Gumbleton T, Oakervee H, Cavenagh J. A phase 2 study of SRT 501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160:714–7.
Funding
This research did not receive funding.
Author information
Authors and Affiliations
Contributions
SMMAD and SS involved in the conceptualization; validation of resources, and data extraction. TF and SMMAD performed writing the manuscript, SS, and MA reviewed and edited the manuscript. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There is no competing of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alavi, M., Farkhondeh, T., Aschner, M. et al. Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell Int 21, 579 (2021). https://doi.org/10.1186/s12935-021-02280-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02280-5