Abstract
The aim of this hypothesis is to propose a new approach in targeted therapy of cancer: The simultaneous, dual targeting of two single molecules, Par-4 and G6PD, rather than inhibition of full-length signaling pathways. Rationale: Targeted inhibition of especially two survival signaling pathways (PI3K/AKT/mTOR and MAPK/ERK) is frequently tried, however, a major breakthrough has not yet been reported. Inhibition of complete pathways naturally goes along with a variety of dose-limiting side effects thus contributing to poor efficacy of the administered drugs. This essay offers a synopsis of relevant studies to support the above mentioned idea—targeting of two single molecules which either are crucial for tumor growth and cancer-cell-survival: on one side, Par-4-activation selectively triggers apoptosis of tumor cells thus reversing their characteristic feature—immortality. On the other side inhibition of G6PD breaks the energy supply of tumor cells, weakens their defence against oxidative stress and thereby enhances the sensitivity of tumor cells to oxidative agents (e.g. chemotherapy). Advantage of the proposed dual Par-4/G6PD-therapy is good tolerability and—especially when administered along with conventional therapy—less frequent emergence of resistance.
Similar content being viewed by others
Background
Targeted inhibition of especially two survival signaling pathways (PI3K/AKT/mTOR and MAPK/ERK) is frequently tried, however, a major breakthrough has not yet been reported. Inhibition of complete pathways naturally goes along with a variety of dose-limiting side effects thus contributing to poor efficacy of the administered drugs. There is a good case to believe that modulation of single molecules which are crucial for the survival of tumor cells might be more successful.
Hypothesis
This manuscript deals with the assumption that two well-known molecules—glucose-6-phosphate dehydrogenase (G6PD) and prostate apoptosis response-4 (Par-4)—are some kind of physiological antagonists: G6PD is vital for cell survival whereas Par-4, on the contrary, is required for programmed cell death, apoptosis.
The idea arose that inhibition of the one (G6PD) and strenghtening of the other (Par-4) could be helpful in cancer therapy.
Supportive evidence
G6PD strengthens the oxidative defence of tumor cells
Dramatically increased activity of G6PD in cancer cells when compared with the nontransformed type was reported as early as in the middle of the past century [1–7]. This fact has repeatedly been confirmed in more recent studies [8–14] indicating that G6PD plays an important role in the metabolism of cancer cells.
G6PD—the rate limiting step of the pentose phosphate pathway (PPP)—is one of the endpoints of the mTOR-pathway [8, 15, 16] and is therefore regulated by the PI3K/Akt/mTOR-signaling. The activity of G6PD ensures steady supply of pentoses required for the synthesis of nucleic acids and, even more important, for stabilization of the NADP/NADPH-equilibrium which is crucial for antioxidative defence [17]. Both supply with NADPH and with pentoses is an essential prerequisite for the uncontrolled growth and proliferation of cells in general and particularly of tumor cells [8, 15, 18].
Prostate apoptosis response-4 (Par-4)
Likewise, another molecule plays a central role in tumor development and growth: the tumor suppressor Par-4. Evidence is given that Par-4, which was identified in 1994 in prostate cancer cells [19], plays a key function in apoptosis (for review see [20]).
One of the characteristic features of cancer cells—immortality—is based on deactivation of the Par-4-function to enable the tumor cells to escape apoptosis. Therefore, downregulation of Par-4-expression seems to be a decisive step in tumorigenesis which is vital for the viability of tumor cells [21, 22].
Over the years vast quantities of results dealing with the relevance of the two molecules—G6PD and Par-4—in tumor growth were published. This hypothesis is based on the results gained from search in relevant scientific literature.
Beginning in the late 1970-ies data regarding glucose-6-phosphate dehydrogenase (G6PD)—especially those relating to cell proliferation, oxidative defence and tumor growth—were recorded and analyzed. Research was initially carried out in university- and other scientific libraries. Since online access exists search was continued in large scientific databases like PubMed.
After discovering of prostate apoptosis response-4 (Par-4) by the end of 1990 data regarding this molecule were recorded and analyzed, too, and interpreted in the context of knowledge about the role of G6PD in normal cells as well as in tumor ones.
Research, analyse and interpretation of the findings was done by the author itself over an about 40-year period, beginning with elaboration of PhD thesis in 1976 (“Role of G6PD and its isozymes in human organism”) and continued by personal interest and curiosity until today.
Significant reduction of Par-4-activity was documented in almost all examined tumor-types, among others in kidney- [23], different neurological [24, 25], endometrial [26], breast- [27], prostate- [28], colon- [29] as well as in cholangiocarcinoma-cells [30] thus confirming that reduced Par-4-activity is an important feature of tumor cells.
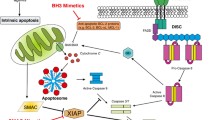
Evidence is given that this feature is—one could say—programmed straight from the first step of carcinogenesis. The vast majority of tumors develop because of oncogenic mutations in the PI3K, Akt, PTEN, [31–35], ras [36–39], and other key genes [21, 31, 40, 41]. These genes are—among others—directly involved in initiating of PI3K/Akt/mTOR and/or MAPK/ERK signaling pathways which are vital for fast growing cells and cell proliferation [35]. Mutations of these genes frequently go along with accidental activation of either survival pathways. Both PI3K/Akt/mTOR and MAPK/ERK pathways act in activated state as Par-4-suppressors (see Fig. 1).
Molecular links between the two major survival pathways MAPK/ERK and PI3K/AKT/mTOR. The vast majority of tumors develop because of oncogenic mutations in the PI3K, Akt, PTEN, ras and related key genes, which are directly involved in initiating of PI3K/Akt/mTOR and/or MAPK/ERK signaling pathways. These pathways are vital for fast growing cells and cell proliferation. In tumor cells, MAPK/ERK and PI3K/AKT/mTOR pathways cooperate in downregulating of the pro-apoptotic tumor suppressor Par-4. Activation of MEK via ras (MAPK/ERK-pathway) induces upregulation of DNA-methylases (Dnmt-1 and Dnmt-3) which methylate specific sites in the promoter of the Par-4 gene hereby silencing the Par-4-gene. On the other hand, activated Akt, a key member of PI3K/Akt/mTOR-signaling, phosphorylates the Par-4 molecule at serine residue 249 hereby triggering downregulation of the Par-4 activity. Deactivation of the Par-4-function enables tumor cells to escape apoptosis and thus ensures their longevity. At the same time, activated PI3K/Akt/mTOR-signaling regulates the activity of G6PD via SREBP1-c. Activated G6PD supplies tumor cells with pentoses for the synthesis of nucleic acids and, even more important, ensures NADP/NADPH-equilibrium, which is vital for antioxidative defense. Withaferin A and 3,3′-diindolylmethane are known Par-4-activators. Aspirin, nicotinamide, steroids are inhibitors of G6PD
Downregulation of Par-4 via MAPK/ERK
Activation of MEK via ras (MAPK/ERK-pathway) induces upregulation of DNA-methylases (Dnmt-1 and Dnmt-3) which for their part methylate specific sites in the promoter of the Par-4 gene hereby inactivating Par-4. The causative relation between ras, MAPK/ERK and Par-4 was confirmed: Inhibition of MEK causes downregulation of both the DNA-methylases whereon the function of the Par-4 promoter is restored and the Par-4-activity raises again [42, 43].
Downregulation of Par-4 via PI3K/Akt/mTOR
Likewise, PI3K/Akt/mTOR-signaling is directly involved in downregulation of Par-4. Activated Akt phosphorylates the Par-4 molecule at serine residue 249 in this manner triggering downregulation of the Par-4 activity [44].
As already mentioned several therapeutic approaches based on inhibition of the PI3K/Akt/mTOR and MAPK/ERK pathways were developed [32], i.e. mTOR-Inhibitor Everolimus [45] and BRAF-Inhibitors Vemurafenib and Dobrafenib [46, 47]. These therapies didn’t fulfill the hopes because of dose-limiting side effects [46, 48, 49] and/or development of resistance to the inhibitor [50–53].
Restoration of Par-4-acitivity causes tumor reduction
In many cases, tumor reduction after restoring of Par-4-activity was reported. So Chakraborty and colleagues observed that injection of Par-4 via adenovirus into prostate tumor induced apoptosis in cancer cells followed by dramatic reduction in tumor volume [54]. Similar findings were reported after Par-4 transfection in melanomas [55]. Vetterkind and colleagues reported that ectopic expression of Par-4 was sufficient to induce apoptosis in tumor cells of the central nervous system [25]. In animal experiments intravenous injection of recombinant Par-4 was sufficient to inhibit the formation of metastases [56]. Yang et colleagues noticed that in vitro activation of Par-4-expression by small activating RNA (saRNA) induced growth inhibition and apoptosis in tumor cells [42].
Treatment of therapy resistant glioma stem cells with a combination of ectopic Par-4 and Tamoxifen caused inhibition of the Akt- and the ERK-pathways followed by subsequent apoptosis in the tumor stem cells. This success was seen only in combination of the two substances while monotherapy either with Tamoxifen or with Par-4 alone failed [57].
Thus, the proapoptotic protein Par-4 seems to be crucial for apoptosis induction in tumor cells; many (if not the vast majority of) cancers are able to develop only because of inactivity (or reduced activity) of this tumor suppressor.
Inhibition of G6PD arrests tumor growth
Likewise, a direct connection between tumor growth and G6PD was confirmed. Several researchers consider a high G6PD activity in tumors as an independent negative prognostic marker in cancer [8, 56]. Overexpression of G6PD is considered as a predictor of high risk of recurrent metastasis in breast cancer patients [58].
First evidence that reduction of G6PD activity may be capable to reduce tumor growth was given as early as in the 1970-ies [59]; the same was recently approved by more modern methods [9]. It has been shown that reduction of the G6PD activity in tumor cells up to 80% is sufficient for significant reduction of cell proliferation, migration and invasiveness as well as for significant decrease of colony forming efficiency; coincidentally the rate of tumor cell apoptosis increased [9]. Furthermore, it has been shown that inhibition of G6PD activity triggers the sensitivity of tumor cells against oxidative stress and consequently leads to an increased susceptibility of these cells to apoptosis [9, 60].
Adult organisms are only marginally dependent on G6PD-activity
In resting cells the G6PD-activity is often barely detectable; only regenerating [61] and embryonic cells [62] and, as above mentioned, tumor cells, are dependent on sufficient G6PD-activity. As early as in 1965 Beaconsfield realized that cancer mortality seemed to be lower in populations where G6PD-deficiency is common due to endemic occurrence of deficient G6PD-variants [63]. The well-known finding that regular use of medications with G6PD-inhibiting properties (e.g. aspirin) goes along with a lower cancer risk when compared with the general population underpins the crucial role of active G6PD in tumor formation and growth [64, 65].
Discussion and conclusions
Targeted inhibition of the prosurvival, antiapoptotic G6PD-activity combined with simultaneous or consecutive targeted activation of the pro-apoptotic tumor suppressor Par-4 might be a promising approach in cancer therapy. Of particular advantage seems to be the fact that combination of the proposed dual targeted therapy with conventional cancer therapies promises to be more effective than either monotherapy [57] notably because of the additional increase of the sensitivity of tumor cells against chemotherapy because of impairment of antioxidative defence mechanisms [66].
Targeting of G6PD and Par-4 is well tolerated
Controlled G6PD-inhibition is generally well tolerated: serious adverse effects from G6PD-inhibition—at least for a defined period—are in general scarce to be expected (except in case of pregnancy or regenerating processes). In G6PD-deficient individuals hemolytic anemia possibly can occur which has to be clinically managed. The most suitable Inhibitors of G6PD-activity are aspirin [67, 68] and, yet better tolerated, Niacin (6-Aminonicotinamide) [69].
Likewise, Par-4-activation is well tolerated. The proapoptotic effect of Par-4 is selectively restricted to tumor cells. Even activated Par-4 is not capable to induce apoptosis in normal cells; instead of that cells with active Par-4 are senisitized towards apoptotic signals [54, 70, 71].
Evidence is given from vitro- and in vivo-animal studies that several steroids of plant origin (Withaferin A; 3,3′-diindolylmethane) are known as Par-4-activators and are capable to induce apoptosis and growth arrests in prostate [72], cholangio-[73] and other carcinoma cells [73–78]. These natural occuring compounds are generally well tolerated (e.g. Withaferin A is well known from Ayurveda therapy).
Summary: The special advantage of a dual targeted G6PD- and Par-4-therapy rather than inhibition of complete signaling pathways (e.g. PI3K/Akt/mTOR and/or MAPK/ERK) might be good tolerability, no mentionable adverse effects, and—especially in combination with conventional oncological therapy—less frequent emergence of resistance.
Abbreviations
- Akt:
-
serin/threonin-kinase (proteinkinase B, PKB)
- BRAF:
-
proto-oncogene protein B-raf
- Dnmt:
-
DNA methyltransferases
- ERK:
-
extracellular-signal regulated kinase
- G6PD:
-
glucose-6-phosphate dehydrogenase
- MAPK:
-
mitogen-activated protein kinase
- MEK:
-
MAPK/ERK kinase
- mTOR:
-
mechanistic target of rapamycin (also known as mammalian target of Rapamycin)
- Par-4:
-
prostate apoptosis response-4, also known as PAWR
- PI3K:
-
phosphoinositide 3-kinase
- PPP:
-
pentose phosphate pathway, also known as ‘hexose monophosphate shunt’ or ‘phosphogluconate pathway’
- PTEN:
-
phosphatase and tensin homolog
- ras:
-
proto-oncogene (rat sarcoma)
- saRNA:
-
short activating RNA
References
Wenner CE, Weinhouse SJ. Metabolism in neoplastic tissues. IX. An isotope tracer study of glucose catabolism pathways in normal and neoplastic tissues. J Biol Chem. 1956;222:399–414.
Sahasrabudhe MB. A new approach to the chemotherapy of cancer. Nature. 1958;182:163–5.
Dickens F, Glock GE, McLean P. Some problems in the choice of oxidative pathways of carbohydrate metabolism. In: Wolstenholme GEW, Connor CM, editors. Ciba foundation symposium on the regulation of cell metabolism. London: J.& A.Churhill Ltd; 1959. p. 173–4.
Knox E. The enzymatic pattern of neoplastic tissue. Adv Cancer Res. 1967;10:117–61.
Gumaa KA, Greenslade KR, McLean P. Enzymes and intermediates of the pentose phosphate pathway in liver and hepatomas. Biochim Biophys Acta. 1968;158:300–2.
Singh M, Singh VN, August TJ, Horecker BL. Alterations in glucose metabolism in chick embryo cells transformed by Rous sarcoma virus. Transformation-specific changes in the activities of key enzymes of the glycolytic and hexose monophosphate shunt pathways. Arch Biochem Biophys. 1974;165:240–6.
Oertel GW, Benes P. The effects of steroids on glucose-6-phosphate dehydrogenase. J Steroid Biochem. 1972;3:493–6.
Tsouko E, Khan AS, White MA, Han JJ, Shi Y, Merchant FA, et al. Regulation of the pentose phosphate pathway by an androgen receptor—mTOR- mediated mechanism and its role in prostate cancer cell growth. Oncogenesis. 2014;3:e103. doi:10.1038/oncsis2014.18.
Li D, Zhu Y, Tang Q, Lu H, Li H, Yang Y, et al. A new G6PD knockdown tumor-cell line with reduced proliferation and increased susceptbility to oxidative stress. Cancer Biother Radiopharm. 2009;24:81–90.
Zampella EJ, Bradley EL Jr, Pretlow TG 2nd. Glucose-6-phosphate dehydrogenase: a possible clinical indicator for prostatic carcinoma. Cancer. 1982;49:384–7.
Wang J, Yuan W, Chen Z, Wu S, Chen J, Ge J, et al. Overexpression of G6PD is associated with poor clinical outcome in gastric cancer. Tumour Biol. 2012;33:95–101.
Kuo W, Lin J, Tang TK. Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. Int J Cancer. 2000;85:857–64.
Rao X, Duan X, Mao W, Li X, Li Z, Li Q, et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat Commun. 2015;6:8468.
Yang L, Hou Y, Yuan J, Tang S, Zhang H, Zhu Q, et al. Twist promotes reprogramming of glucose metabolism in breast cancer cells through PI3K/AKT and p53 signalling pathways. Oncotarget. 2015;6:25755–69.
Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR compex 1. Mol Cell. 2010;39:171–83.
Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and Liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–8.
Ho HY, Wei TT, Cheng ML, Chiu DT. Green tea polyphenol epigallocatechin-3-gallate protects cells against peroxynitrite-induced cytotoxicity: modulatory effect of cellular G6PD status. J Agric Food Chem. 2006;54:1638–45.
Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39:347–54.
Sells SF, Wood DP Jr, Joshi-Barve SS, et al. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and independent prostate cells. Cell Growth Differ. 1994;5:457–66.
Shrestha-Bhattarai T, Rangnekar VM. Review: cancer-selective apoptotic effects of extracellular and intracellular Par-4. Oncogene. 2010;29:3873–80.
Joshi J, Fernandez-Marcos PJ, Galvez A, Amanchy R, Linares JF, Duran A, et al. Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis. EMBO J. 2008;27:2181–93.
Barradas M, Monjas A, Diaz-Meco MT, Serrano M, Moscat J. The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J. 1999;18:6362–9.
Cook J, Krishnan S, Ananth S, Sells SF, Shi Y, Walther MM, et al. Decreased expression of the pro-apoptotic protein Par-4 in renal cell caarcinoma. Oncogene. 1999;18:1205–8.
Kögel D, Reimertz C, Mech P, Poppe M, Frühwald MC, Engemann H, et al. Dlk/ZIP kinase-induced apoptosis in human medulloblastoma cells: requirement of the mitochondrial apoptosis pathway. Br J Cancer. 2001;85:1801–8.
Vetterkind S, Boosen M, Scheidtmann KH, Preuss U. Ectopic expression of Par-4 leads to induction of apoptosis in CNS tumor cell lines. Int J Oncol. 2005;26:159–67.
Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, Tendero MJ, Rodriguez-Pinilla SM, Garcia-Cao I, et al. Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res. 2007;67:1927–34.
Zapata-Benavides P, Méndez-Vásquez JL, González-Rocha TR, Zamora-Avil DE, Franco-Molina MA, Garza-Garza R, et al. Expression of prostate apoptosis response (Par-4) is associated with progesterone receptor in breast cancer. Arch Med Res. 2009;40:595–9.
Fernandez-Marcos PJ, Abu-Backer S, Joshi J, Galvez A, Castilla E, Canamero M, et al. Simultaneous inactivation of Par-4 and PTEN in vivo leads to synergistic NF-kB activation and invasive prostate carcinoma. PNAS. 2009;106:12962–7.
Wang BD, Kline Chr LB, Pastor DM, Olson TL, Frank B, Luu T, et al. Prostate apoptosis response protein 4 sensitizes human colon cancer cells to chemotherapeutic 5-FU through mediation of an NFkB and microRNA network. Mol Cancer. 2010;9:98.
Franchitto A, Torrice A, Semeraro R, Napoli C, Nuzzo G, Giuliante F, et al. Prostate apoptosis response-4 is expressed in normal cholangiocytes, is down-regulated in human cholangiocaarcioma, and promotes apoptosis off neoplastic cholangiocytes when induced pharmacologically. Am J Pathol. 2010;177:1779–90.
Chalhoub N, Baker SJ. PTEN and the PI3-Kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50.
Abraham J. PI3 K/AKT/mTOR pathway inhibitors: the ideal combination partners for breast cancer therapies? Expert Rev Anticancer Ther. 2015;15:51–68.
Sun Z, Wang Z, Liu X, Wang D. New development of inhibitors targeting the PI3 K/AKT/mTOR pathway in personalized treatment o non-small-cell lung cancer. Anticancer Drugs. 2015;26:1–14.
Joshi MC, Kumar K, Kumar V. Potent phosphatidylinositol 3-kinase inhibitors and their biology. Curr Drug Discov Technol. 2014;11:113–26.
Dillon LM, Bean JR, Yang W, Shee K, Symmonds LK, Balko JM, et al. P-REX1 creates a positive feedback loop to activate growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast cancer. Oncogene. 2015;34:3968–76.
Tolcher AW, Khan K, Ong M, Banerji U, Papadimitrakopoulou V, Gandara DR. Anti-tumor activity in RAS-driven tumours by blocking AKT and MEK. Clin Cancer Res. 2015;21:739–48.
Dickson MA, Gordon MS, Edelman G, Bendell JC, Kudchadkar RR, LoRusso PM, et al. Phase I study of XL281 (BMS-908662), a potent oral RAF kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2015;33:349–56.
Kordi-Tamandani DM, Saberi E, Jamali S, Ladiz MA. ERK and RAF1 genes: analysis of methylation and expression profiles in patients with oral squamous cell carcinoma. Br J Biomed Sci. 2014;71:100–3.
Luke JJ, Ott PA, Shapiro GI. The biology and clinical development of MEK inhibitors for Cancer. Drugs. 2014;74:2111–28.
Aviel-Ronen S, Blackhall FH, Shepherd FA, Tsao MS. K-ras mutations in non-small-cell lung carcinoma: a review. Clin Lung Cancer. 2006;8:30–8.
Eskander RN, Tewari KS. Exploiting the therapeutic potential of the PI3 K-AKT-mTOR pathway in enriched populations of gynecologic malignancies. Expert Rev Clin Pharmacol. 2014;7:847–58.
Yang K, Shen J, Xie YQ, Lin YW, Qin J, Mao QQ, et al. Promoter-targeted double-stranded small Ras activate PAWR gene expression in human cancer cells. Int J Biochem Cell Biol. 2013;445:1338–46.
Pruitt K, Ulkü AS, Frantz K, Rojas RJ, Muniz-Medina VM, Rangnekar VM, et al. Ras-mediated loss of the pro-apoptotic response protein Par-4 is mediated by DNA hypermethylation through Raf-independent and Raf-dependent signaling cascades in epithelial cells. J Biol Chem. 2005;280:23363–70.
Boosen M, Vetterkind S, Kubicek J, Scheidtmann KH, Illenberger S, Preuss U. Par-4 is an essential downstream target of DAP-like kinase (Dlk) in Dlk/Para-4-mediated apoptosis. Mol Biol Cell. 2009;20:4010–20.
André F, Regan R, Ozguroglu M, Xu B, Jerusalem G, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast ancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–91.
Ha SH, Park JH, Jang H, Huh W, Lim HY, Kim YG, et al. Increased risk of everolimus-associated acute kidney injury in cancer patients with impaired kidney function. BMC Cancer. 2014;14:906.
Donders F, Kuypers D, Woter P, Neven P. Everolimus in acute kidney injury in a patient with breast cancer: a case report. J Med Case Rep. 2014;8:386.
Shapiro GI, Rodon J, Bedell C, Kwak EL, Baselga J, Brana I, et al. Phase I safety, pharmacokinetic, an pharmacoddynamic study of SAR245408 (XL 147), an Oral Pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:233–44.
Klempner S, Myers AP, Cantley LC. What a tangled web we weave: emerging resistance mechanisms to inhibition of the phosphoinositide-3-kinase pathway. Cancer Discov. 2013;3:1345–54.
Toulany M, Minjgee M, Saki M, Holler M, Meier F, Eicheler W, et al. ERK2-dependent reactivation of Akt mediates the limited response of tumor cells with constitutive K-RAS activity to PI3K inhibition. Cancer Biol Ther. 2014;15:317–28.
Dent P. Crostalk between ERK, AKT, and cell survival. Cancer Biol Ther. 2014;15:245–6.
Rozengurt E, Soares HP, Sinnet-Smith J. Suppression of Feedback Loops Mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol Cancer Ther. 2014;13:2477–88.
Jeannot V, Busser B, Brambilla E, Wislez M, Robin B, Cadranel J, et al. The PI3 K/AKT pathway promotes gefitinib resistance in mutant KRAS lung adenocarcinoma by a deacetylase-dependent mechanism. Int J Cancer. 2014;134:2560–71.
Chakraborty M, Qiu SG, Vasudevan KM, Rangnekar VM. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 2001;61:7255–63.
Lucas T, Pratscher B, Krishnan S, Fink D, Gunsberg P, Wolschek M, et al. Differential expression levels of Par-4 in melanoma. Melanoma Res. 2001;11:379–83.
Zhao Y, Burikhahov R, Brandon J, Qiu S, Shelton BJ, Spear B, et al. Systemic Par-4 inhibits non-autochthonous tumor growth. Cancer Biol Ther. 2011;12:152–7.
Jagtap JC, Dawood P, Shah RD, Chandrika G, Natesh K, Shiras A, et al. Expression and regulation of prostate apoptosis response-4 (Par-4) in human glioma stem cells in drug-induced apoptosis. PLoS ONE. 2014;9:e88505. doi:10.1371/journal.pone.0088505.
Pu H, Zhang Q, Zhao C, Shi L, Wang Y, Wang J, et al. Overexpression of G6PD is associated with high risks of recurrent metastasis and poor progression-free survival in primary breast carcinoma. World J Surg Oncol. 2015;13:323.
Avenarius I, Schwartz E. Modification of the growth of Ehrlich ascites tumor by dehydroepiandrosterone. Naturwissenschaften. 1975;62:44.
Polimeni M, Voena C, Kopecka J, Riganti C, Pescarmona G, Bosia A, et al. Modulation of doxorubicin resistance by the glucose-6-phosphate dehydrogenase activity. Biochem J. 2011;439:141–9.
Love NR, Ziegler M, Chen Y, Amaya E. Carbohydrate metabolism during vertebrate appendage regeneration: what is its role? How is it regulated? A postulation that regenerating vertebrate appendages facilitate glycolytic and pentose pathways to fuel macromolecule biosynthesis. BioEssays. 2014;36:27–33.
Longo L, Camacho Vanegas O, Patel M, Rosti V, Li H, Waka J, et al. Maternally transmitted severe glucose-6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO J. 2002;21:4229–39.
Beaconsfield P. Malaria, Glucose-6-phosphate dehydrogenase deficiency, and HbS. Br Med J. 1967;2:174–5.
Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–50.
Bardia A, Olson JE, Vachon CM, Lazowich D, Vierkant RA, Wang AH, et al. Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat. 2011;126:149–55.
Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, et al. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004;11:823–31.
Alfonso LF, Srivenugopal KS, Bhat GJ. Does aspirin acetylate multiple cellular proteins? (review). Mol Med Rep. 2009;2:533–7.
Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX. L. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 2014;33:1304–20.
Libri V, Yandim C, Athanasopoulos S, Loyse N, Natisvili T, Law PP, et al. Epigenetic and neurological effects and safety of high-dose nicotinammide in patients with Friedrich´s ataxia: an expoloratory, open-label, dose-escalation study. Lancet. 2014;384:504–13.
Nalca A, Qiu SG, El-Guendy N, Krishnan S, Rangnekar VM. Oncogenic Ras sensitizes cells to apoptosis by Par-4. J Biol Chem. 1999;274:29976–83.
El-Guendy N, Rangnekar VM. Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp Cell Res. 2003;283:51–66.
Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chenil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–53.
Sarkar S, Jain S, Rai V, Sahoo DK, Raha S, Suklabaidya S, et al. Plant-derived SAC domain of PAR-4 (prostate apoptosis response 4) exhibits growth inhibitory effects in prostate cancer cells. Front Plant Sci. 2015;6:822.
Rah B, Amin H, Yousuf K, Khan S, Jamwal G, Mukherjee D, et al. A novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PLoS ONE. 2012;7:2012. doi:10.1371/journal.pone.0044039.
Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone. AAPS J. 2014;16:1–10.
Roy RV, Suman S, Das T, Luevano JE, Damodaran C. Withaferin A, a steroidal lactone from Withania somnifera, induces mitotic catastrophe and growth arrest in prostate cancer cells. J Nat Prod. 2013;76:1909–15.
Rah B, ur Rasool R, Nayak D, Yousuf SK, Mukherjee D, Kumar LD, et al. PAWR-mediated suppression of BCL2 promotes switching of 3-azido withaferin A (3-AWA)-induced autophagy to apoptosis in prostate cancer cells. Autophagy. 2015;11:314–31.
Azmi AS, Ahmad A, Banerjee S, Rangnekar VM, Mohammad RM, Sarkar FH. Chemoprevention of pancreatic cancer: characterization of Par-4 and its modulation by 3,3′-diindolylmethane (DIM). Pharm Res. 2008;25:2117–24.
Acknowledgements
Not applicable.
Competing interests
The author declare that he has no competing interests.
Funding
There was no funding. The research was carried out and the paper was authored in spare-time.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cernaj, I.E. Simultaneous dual targeting of Par-4 and G6PD: a promising new approach in cancer therapy? Quintessence of a literature review on survival requirements of tumor cells. Cancer Cell Int 16, 87 (2016). https://doi.org/10.1186/s12935-016-0363-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-016-0363-9