Abstract
Background
Most bacteria are grown in a binary fission way meaning a bacterial cell is equally divided into two. Polyhydroxyalkanoates (PHA) can be accumulated as inclusion bodies by bacteria. The cell division way and morphology have been shown to play an important role in regulating the bacterial growth and PHA storages.
Results
The common growth pattern of Escherichia coli was changed to multiple fission patterns by deleting fission related genes minC and minD together, allowing the formation of multiple fission rings (Z-rings) in several positions of an elongated cell, thus a bacterial cell was observed to be divided into more than two daughter cells at same time. To further improve cell growth and PHA production, some genes related with division process including ftsQ, ftsL, ftsW, ftsN and ftsZ, together with the cell shape control gene mreB, were all overexpressed in E. coli JM109 ∆minCD. The changing pattern of E. coli cell growth and morphology resulted in more cell dry weights (CDW) and more than 80 % polyhydroxybutyrate (PHB) accumulation increases compared to its binary fission control grown under the same conditions.
Conclusions
This study clearly demonstrated that combined over-expression genes ftsQ, ftsW, ftsN, ftsL and ftsZ together with shape control gene mreB in multiple division bacterial E. coli JM109 ∆minCD benefited PHA accumulation. Our study provides useful information on increasing the yield of PHA by changing the cell division pattern and cell morphology of E. coli.
Similar content being viewed by others
Background
In Escherichia coli, cell division is regulated strictly to ensure that daughter cells contain proper cellular components [1]. Most bacteria are divided via binary fission, allowing a parent cell to split in two [2]. During this process, several genes and their products are important, such as the tubulin-like protein FtsZ [3], and MinC, MinD and MinE that regulate the formations of division sites [4, 5]. The MinC and MinD are inhibitors able to block the formation of the FtsZ ring at all sites, while MinE relieves the division block at the mid-cell site, resulting in binary fission [4, 6]. In the absence of MinC and MinD, the divisions appear to form at all division sites since FtsZ rings can be generated at both polar and medial positions [7, 8]. On the other hand, deletion of the ‘min’ system results in the production of some mini-cells, products of divisions near the E. coli cell poles [9]. The cell division pattern could be changed by disrupting the ‘min’ system, allowing the common binary fission be changed to multiple fissions [10].
To change the replication process of a bacterial cell, the formation of FtsZ ring and proper septation should be manipulated [6]. There are at least ten genes that have been shown to be essential for formation of the FtsZ ring and regulation the division process [11, 12]. Among the essential genes, FtsQ and FtsL are two membrane proteins localizing to the cell septum during division process [13, 14], and the location of FtsW is dependent on the prior localization of FtsQ and FtsL [15, 16]. As the last protein acted in cell division, FtsN causes the disassembly of other elements from the division ring [17, 18]. FtsZ interacts with FtsQ, FtsL, FtsW and FtsN in the progression and completion of cytokinesis [12]. FtsZ also plays an important role in the bacterial cell division process as a tubulin-like protein [12, 19, 20].
Polyhydroxyalkanoates (PHA), a family of biodegradable and biocompatible thermal polyesters or bioplastics, are accumulated as inclusion bodies by bacteria under unbalanced growth conditions [21–23]. Polyhydroxybutyrate (PHB) is the model PHA used for many demonstration studies, and it has been developed as environmentally friendly bioplastics with promising applications [24, 25]. High cost of PHA production has been a key limiting factor on its commercial application [26]. Efforts on process optimization, use of cheap carbon sources and pathway engineering were made to cut cost [27–31]. Although the cost of PHA production can be reduced under these efforts, it is still significantly higher compared with the petrochemical plastics such as polyethylene (PE) [24]. Therefore, other methods are needed to reduce the cost of PHA [32, 33].
Since PHA are produced by bacteria as inclusion bodies, cell shapes of the host strain can affect the amount of PHA granules and the quantity of PHA that can be stored [34, 35]. The change of cell division process could produce more daughter cells in various shapes at the same time, possibly leading to more PHA, as was indicated by previous studies that PHA synthesis is also limited by the small cell size, a large cell size with more space can allow more PHA granules to be accumulated. Bacterial peptidoglycan cell wall and the actin-like protein MreB cytoskeleton are major determinants of cell shape in rod-shaped bacteria such as E. coli [36–38].
In this study, we aimed to change the cell division pattern and thus cell morphology, and to use the multiple fission cells for possible enhanced PHB accumulation.
Results
Changing E. coli growth pattern: from binary division to multiple fission
In this study, genes minC and minD regulating fission ring locations were deleted in E. coli JM109 using homologous recombination method. E. coli JM109 ∆minCD became several folds longer than the wild type when cultivated in LB medium (Fig. 1), sizes extended from 1–3 μm for the wild type (Fig. 1a) to around 5 μm for the E. coli JM109 ∆minCD (Fig. 1d), accompanied by some mini-cells attached around the elongated cells. Interestingly, the individual mutant of minC and minD in E. coli JM109, respectively, namely, E. coli JM109 ∆minC (Fig. 1b) and E. coli JM109 ∆minD (Fig. 1c), displayed a similar morphology to E. coli JM109 ∆minCD (Fig. 1d). The reason may be attributed to joint efforts of minC and minD to decide FtsZ ring formation [7]. Both are essential for the function of ‘min’ system.
In the absences of minC and/or minD, the FtsZ ring fails to locate to the middle of a cell (Fig. 2a; Additional file 1: Video S1). Under this circumstance, E. coli JM109 ∆minCD changes not only the cell morphology (Fig. 1) but also the way of cell division (Fig. 2a). As multiple FtsZ rings were randomly and simultaneously formed in various positions of an elongated cell of E. coli JM109 ∆minCD, one elongated bacterial cell was broken into more than two daughter cells (Additional file 1: Video S1). For example, a cell of an elongated E. coli JM109 ∆minCD was divided into three daughter cells when two FtsZ rings were formed and located in two different positions of the elongated cell. The size of the daughter cell is dependent on the position where the FtsZ ring is formed. Some mini-cells were observed during the multiple fission process when the FtsZ ring was formed at a polar site where no nucleic acid was available for encapsulating into the cellular space (Fig. 2a). E. coli JM109 ∆minCD changes not only its cell size but also its way of growth.
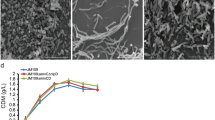
Cell division pattern, morphology and PHA production of E. coli JM109∆minCD. a The division pattern of E. coli JM109 ∆minCD and E. coli JM109. Arrows the division position of cell. E. coli JM109 ∆minCD had more than two division rings. Scale bar 2 μm. The OD600 (b) and cell dry weight (c) of recombinant E. coli JM109 ∆minCD. Cells were cultivated in LB medium under 37 °C for 24 h, respectively. Errors are s.d. (n = 3). d The CDW and PHA production by recombinants harboring pBHR68 cultivated in LB medium by addition of 20 g/L glucose at 37 °C for 48 h. Error bars are s.d. (n = 3)
Growth rate from the binary division to multiple fission was investigated in terms of the OD600 and cell dry weight (CDW) of the wild type and mutant grown in LB medium for 24 h, respectively. Even though OD600 of E. coli JM109 ∆minCD showed a little bit higher than that of the wild type (Fig. 2b), the cell dry weights indicated only little significant difference between the mutant and wild type (Fig. 2c). OD values were not only related to cell density but also to the cell morphology. Thus, the mutant strain could achieve higher OD value since the cell length was changed. However, the cell dry weight was not enhanced, the reason could be attributed to the mini-cells that could not make a contribution to the final CDW.
When E. coli JM109∆minCD was used to express PHB synthesis operon phbCAB encoded by plasmid pBHR68, it was found that the mutant accumulated slightly more PHB compared with its wild type under the same conditions. When grown in a mineral medium supplemented with yeast extract, the amount of PHB produced by E. coli JM109 ∆minCD had a little increase compared with the wild type E. coli JM109. However, the different amounts of yeast extract added into the MM medium (mineral or minimal growth medium) had little effect on the enhancements on cell growth and PHB accumulation (Table 1). When E. coli JM109∆minCD was cultured in MM medium containing 1 g/L yeast extract, the cell dry weight was 5.12 g/L and PHB contents was 43.08 %, which was higher than the control one and other culture conditions in MM medium. On the other hand, when the mutant was cultivated in LB medium for 48 h, the cell dry weight could reach 8.35 g/L containing 60.23 % PHB, much higher than the control E. coli JM109 having only 53.22 % PHB (Fig. 2d). In all cases, LB medium supported better cell growth and PHB synthesis compared with the mineral medium added with yeast extracts (Table 1; Fig. 2d). Since the LB medium was more suitable for E. coli JM109∆minCD, subsequent study was employed only the LB medium. It was concluded that multiple fission promoted the PHB accumulation compared to the common binary fission way of growth when cultivations were conducted in LB medium.
FtsZ, overexpression in E. coli JM109∆minCD led to multiple dividing cells accumulating more PHB
As an essential cell division protein, FtsZ forms a contractile ring structure (FtsZ ring) at the cell division site, regulation of FtsZ ring assembly controls the timing and the location of cell division [39, 40]. FtsZ ring formed by FtsZ assembly, is a very dynamic process.
Since location of FtsZ ring was random in E. coli JM109∆minCD, the elongated cell needed more than two FtsZ rings in order to realize the multiple division. As the correct FtsZ concentration is required for the formation of FtsZ ring, plasmid (p15a-ftsZ) was constructed to regulate the expression level of ftsZ under the control of arabinose (Fig. 3). E. coli JM109ΔminCD harboring plasmid p15a-ftsZ was added with 0.2 % arabinose, E. coli JM109ΔminCD and E. coli JM109 harboring plasmid p15a-blank were used as control. After 2 h inoculation, E. coli JM109∆minCD (p15a-ftsZ) showed longer cell shape compared with the control groups (Figs. 4a, b, c). The length of E.coli JM109∆minCD was improved further by overexpression ftsZ, and the reason may be that the high expression level of FtsZ disrupted the normal division.
Structures of plasmids used in this study. Plasmid p15a-ftsZ, under an arabinose promoter, was used to control the expression of ftsZ; Plasmid ptk-mreB with a mreB promoter was constructed; Plasmid p15a-Pbad-ftsQLWN, under the arabinose promoter, was used to control the expression of ftsQLWN; Plasmid p15a-Pglta-ftsQLWN, under the glta promoter, regulated the expression of ftsQLWN
Electron microscopy studies on cell morphology and PHA production of E. coli JM109 ∆minCD overexpressing ftsZ. SEM morphology studies on: a E. coli JM109 (p15a-blank). b E. coli JM109 ∆minCD (p15a-blank). c E. coli JM109 ∆minCD (p15a-ftsZ). Cells were cultivated in LB medium. Scale bars, 10 μm. d The cell dry weight and PHA accumulation by E. coli JM109 ∆minCD overexpressing ftsZ with pBHR68. Cells were cultivated in LB medium at 37 °C for 48 h, 0.2 % arabinose was added into the medium after 2 h of inoculation, and 20 g/L glucose was added 6 h later. Error bars are s.d. (n = 3)
Then, we investigated the influence of ftsZ on PHB production. When over-expressing ftsZ and PHB synthesis operon phbCAB encoded in plasmid pBHR68, E. coli JM109∆minCD (p15a-ftsZ, pBHR68) grew to 10.15 g/L containing 73.31 % PHB in the cell dry weights, significantly higher than the wild type JM109 (p15a-blank, pBHR68) grew to 7.49 g/L containing 55.62 % PHB (Fig. 4d). Compared with the other control groups, JM109 (p15a-ftsZ, pBHR68) grew to 8.13 g/L with 65.63 % PHB and JM109∆minCD (p15a-blank, pBHR68) contained 62.15 % PHB, the ability to produce PHB was improved by overexpressing ftsZ in the multiple fission E. coli JM109∆minCD.
Over-expressing genes ftsQ, ftsL, ftsW and ftsN in E. coli JM109∆minCD improved the PHB contents
To enhance the growth of E. coli JM109∆minCD, the formation of FtsZ ring should be accelerated to reduce time spent for the cell division (or cell fission) process. Among many essential genes related to the division process, some have showed important functions, including ftsQ, ftsL, ftsW and ftsN. FtsZ interacts with these genes encoded proteins to complete cytokinesis [12, 41].
To investigate the influence on growth rate and ability of PHB production by genes ftsQ, ftsL, ftsW and ftsN, two types of plasmids were constructed, one was expressed under the control of a constitutive promoter glta (p15a-Pglta-ftsQLWN), another plasmid under an arabinose promoter inducible using arabinose (p15a-Pbad-ftsQLWN) (Fig. 3).
Cell morphology and growth rate of the strains were investigated in LB medium. The length of E. coli JM109∆minCD (p15a-Pbad-ftsQLWN) was longer than the control (Fig. 5a, b, c). The growth rate of E. coli JM109∆minCD (p15a-Pbad-ftsQLWN) was a little higher than E. coli JM109 (p15a-blank) when cultivated in LB medium adding 0.2 % arabinose after 2 h (Fig. 5d). The growth rate between E. coli JM109∆minCD (p15a-Pglta-ftsQLWN) and E. coli JM109 (p15a-blank) was similar (Additional file 2: Figure S1), indicating the arabinose promoter is better under this situation.
Effect of ftsQLWN over-expression on cell morphology and PHA accumulation in E. coli JM109 ∆minCD. SEM morphology studies on: a E. coli JM109 (p15a-blank). b E. coli JM109 ∆minCD (p15a-blank). c E. coli JM109 ∆minCD (p15a-Pbad-ftsQLWN). Scale bars, 10 μm. d The cell growth rate of E. coli JM109 ∆minCD harboring plasmid p15a-Pbad-ftsQLWN.Strains were grown in the LB medium, and 0.2 % arabinose was added into the medium after 2 h of inoculation. Error bars are s.d. (n = 3). e The cell dry weight and PHA accumulation by E. coli JM109 ∆minCD (p15a-Pglta-ftsQLWN) with pBHR68, Cells were cultivated in LB mediums referred to containing 20 g/L glucose at 37 °C for 48 h. Error bars are s.d. (n = 3). f The cell growth and PHA production by E. coli JM109 ∆minCD (p15a-Pbad-ftsQLWN) with pBHR68. The strains were grown in the LB medium at 37 °C to an OD600 = 0.4–0.6 (2 h after injection), followed by induction with 0.2 % arabinose for 6 h, then addition of 20 g/l glucose for another 40 h cultivation. Error bars are s.d. (n = 3)
Escherichia coli JM109ΔminCD harboring plasmid p15a-Pbad-ftsQLWN was added with 0.2 % arabinose after 2 h inoculation. And E. coli JM109ΔminCD with plasmid p15a-Pglta-ftsQLWN was added with 20 g/L glucose without arabinose. After cultured in LB medium for 48 h, E. coli JM109ΔminCD (p15a-Pglta-ftsQLWN) could reach 9.77 g/L cell dry weights containing 66.83 % PHB which was also higher than the control accumulating less than 60 % PHB (Fig. 5e). On the other hand, E. coli JM109ΔminCD (p15a-Pbad-ftsQLWN, pBHR68) grew to 11.39 g/L containing 70.06 % PHB, significantly higher than the control group (Fig. 5f). It was therefore concluded that the cell dry weights and PHB contents were improved by overexpressing the division proteins in the multiple fission E. coli JM109∆minCD, the fast assembly of divisome complex could help the minCD mutant to accumulate more PHB granules. Furthermore, the cell dry weights and PHB contents were both higher in the mutant harboring plasmid p15a-Pbad-ftsQLWN than in the plasmid p15a-Pglta-ftsQLWN, indicating that an inducible promoter was better for PHB production.
mreB in E. coli JM109∆minCD allowed more PHB accumulation in multiple dividing cells
Since the mini-cells formed during the division process waste time and energy reducing space for PHB accumulation, it would be much better if the sizes of the mini-cells can become larger to be a viable cell. It has been known that enzyme complexes responsible for synthesizing cell elongation specific peptidoglycan are organized by the actin homolog MreB [35, 42].
Escherichia coli changes to spherical shape from rod shape when the expression level of mreB changed. By overexpressing mreB, sizes of E. coli JM109∆minCD became larger than that of the control and wild types (Fig. 6a, b, c). When over-expressing mreB and PHB synthesis operon phbCAB encoded in plasmid pBHR68, E. coli JM109∆minCD (ptk-mreB, pBHR68) grew to 10.55 g/L containing 70.51 % PHB in the cell dry weights, significantly higher than the wild type grown to 9.11 g/L containing 48.52 % PHB (Fig. 6d). E. coli JM109∆minCD (ptk-mreB,pBHR68) showed longer and larger sizes (accompanied by many larger mini-cells) compared with the wild type, showing more PHB granule accumulation. Sizes of mini-cells were also improved significantly, permitting more storage of PHB granules.
SEM morphology studies and PHA production of E. coli JM109 ∆minCD overexpressing mreB. SEM morphology studies on: a E. coli JM109 (ptk-blank). b E. coli JM109 ∆minCD (ptk-blank). c E. coli JM109 ∆minCD (ptk-mreB). Scale bars,10 μm. d E. coli JM109 ∆minCD overexpressing mreB and harboring pBHR68 were cultivated in LB medium, containing 20 g/L glucose at 30 °C for 48 h. Error bars are s.d. (n = 3)
Over-expressing genes ftsQLWN and ftsZ together with scaffold gene mreB in E. coli JM109∆minCD increased cell growth and PHB production
The overexpression of FtsZ could speed up the formation of FtsZ ring, while FtsZ ring related genes ftsQ, ftsL, ftsW and ftsN (ftsQLWN) could accelerate the division process, additional manipulation of mreB could enlarge the cell size for more PHB granules accumulation. It was expected that the growth rate of E. coli JM109∆minCD could be enhanced if all the above genes were functionally expressed.
Genes ftsQ, ftsL,ftsW and ftsN (ftsQLWN) were constructed into vector pBBR1-MCS1 to form plasmid pBBR-Pbad-ftsQLWN, which could stably co-exist together with ptk-mreB-ftsZ and pBHR68 in one cell of E. coli JM109∆minCD (Fig. 7a).
The six genes co-expression system and the influence on cell morphology, PHA production. a The co-expression of six genes in E. coli JM109 ∆minCD. The pathway presented in a was the order of functional proteins in the division process. FtsA and ZipA bind directly to FtsZ polymers at the division site, followed by the sequential addition of FtsK, FtsQ, FtsL, FtsW, FtsI and FtsN. b The cell growth CDW by E. coli JM109 ∆minCD containing plasmid pBBR-Pbad-ftsQLWN and ptk-mreB-ftsZ, the arabinose was added to the culture after 2 h of inoculation. The control was cultivated under the same condition. Error bars are s.d. (n = 3). c The cell dry weight and PHA contents by the recombinant strain. The strains were grown in the LB medium at 30 °C to an OD600 = 0.4–0.6, followed by induction with 0.2 % arabinose for 6 h, then addition of 20 g/L glucose. Error bars are s.d. (n = 3). The SEM results of strains E. coli JM109 (pBBR1-MCS1, ptk-blank,pBHR68) (d) and E. coli JM109 ∆minCD(pBBR-Pbad-ftsQLWN,ptk-mreB,pBHR68) (e), Scale bars,10 μm. The TEM of PHB accumulation in E. coli JM109 (pBBR1-MCS1, ptk-blank,pBHR68) (f) and E. coli JM109 ∆minCD (pBBR-Pbad-ftsQLWN,ptk-mreB,pBHR68) (g), Scale bars, 2 μm. (d–g) All the strains were grown in the LB medium at 30 °C to an OD600 = 0.4–0.6, followed by induction with 0.2 % arabinose for 6 h, then addition of 20 g/L glucose for another 40 h of cultivation
Escherichia coli JM109∆minCD (pBBR-Pbad-ftsQLWN, ptk-mreB-ftsZ) was then cultivated in LB medium without extra glucose. 20 mL culture broth was sampled regularly during the growth process for CDW. Arabinose was added to the cultures 2 and 4 h after inoculation, respectively. When Z-ring related genes ftsQLWN and ftsZ were over-expressed at the early stage of growth, the recombinant grew faster to reach 2.8 g/L, higher than 2.2 g/L of the control (Fig. 7b). However, cell growth was not enhanced more when arabinose induction was conducted 4 h after inoculation (Additional file 2: Figure S2). It seemed that gene overexpression in the early stage of cell growth had better effect on cell growth. Thus, arabinose induction was initiated after 2 h of inoculation in subsequent studies.
When over-expressing ftsQLWN, ftsZ and mreB together in the recombinant strain, E. coli JM109∆minCD (pBBR-Pbad-ftsQLWN, ptk-mreB-ftsZ, pBHR68) produced 82.13 % PHB in 11.58 g/L CDW, this means a one fold increase in PHB production over the control group (Fig. 7c). It was concluded that overexpression of these six genes together could increase the cell growth rate together with a significant increase on PHB accumulation.
Cell morphologies of recombinants E. coli JM109∆minCD overexpressing the six genes and PHB synthesis operon were studied under both SEM and TEM (Fig. 7d, e, f, g). It was observed that JM109∆minCD (pBBR-Pbad-ftsQLWN, ptk-mreB-ftsZ, pBHR68) was much larger than the control E. coli JM109 (pBBR1-MCS1, ptk-blank, pBHR68) (Fig. 7d, e). It was clearly observed that over expression on FtsZ ring and mreB genes resulted not only in large cell sizes but also in cells of various morphologies, which could be attributed to the unequal multiple fissions of the cells. The recombinant strain showed more PHB granule accumulation compared with the control group (Fig. 7f, g).
Discussion
In E. coli, the FtsZ ring at mid-cell ensures the binary fission, and the genes encoding proteins MinC and MinD inhibit formation of the FtsZ ring. As a result, overexpression of MinC and MinD in E. coli has been known to generate filament cells without FtsZ rings [19]. In contrast, the absence of MinC and/or MinD allows Z rings be formed in any position of a cell either at polar or medial positions [5]. The division pattern of E. coli JM109∆minCD changed from binary division to multiple fission, and uneven divisions resulted in some larger cells and some normal or smaller size cells. The influence on cell growth rate was limited by E. coli JM109∆minCD, however, the ability to produce PHB was enhanced, which could be attributed to the long cells generated from the multiple fission possessing more spaces for PHB granule storage, while wild type cells usually have equal small cell sizes.
Since FtsZ ring is important in cell division process, the concentration of FtsZ affects the cell morphology and cell division pattern as reported previously [39]. The length of E. coli JM109∆minCD was enhanced further by overexpressing ftsZ. PHB accumulation in JM109∆minCD (p15a-ftsZ) was elevated and the reason may be attributed to the long shape cell morphology. The high expression level of FtsZ could make cell longer which helped bacterial to store more inclusion bodies, but had little effect on cell growth rate.
In order to enhance the growth rate of E. coli JM109∆minCD, the division speed should be accelerated. After the formation of FtsZ ring, many genes showed important functions in assembly of the septal ring components, including ftsQ, ftsL, ftsW and ftsN [12, 41]. We investigated the function of these four genes together. The cell growth rate had improved a little when overexpress ftsQ, ftsL, ftsW and ftsN genes together in E. coli JM109∆minCD, and the shake flask results demonstrated that the PHB production ability of E. coli JM109ΔminCD (p15a-Pbad-ftsQLWN, pBHR68) was enhanced significantly. The reason may be attributed to the longer cells with more Z rings of the overexpressing mutant, which could be divided into many daughter cells immediately as soon as the multiple FtsZ rings were formed. Due to the formations of many mini-cells during this multiple fission process, which did not contribute to the cell growth and PHB accumulation as mini-cells had no nucleic acid, the reduction on formations of mini-cells, by overexpression division protein, may be able to improve both cell growth and PHB accumulations.
Since the mini-cells formed during the division process waste time and energy reducing space for PHB accumulation, it would be much better if the sizes of the mini-cells can become larger to be a viable cell. It has been known that enzyme complexes responsible for synthesizing cell elongation specific peptidoglycan are organized by the actin homolog MreB [35]. When overexpressing mreB in E. coli JM109∆minCD, the width of the cell was enhanced and size was bigger compared with wild type, especially within PHB granules. As a result, the ability of PHB production was enhanced by E. coli JM109ΔminCD overexpressing mreB.
As the ftsZ and ftsQLWN genes could enhance the cell length, and mreB gene could enhance the cell width, we constructed two plasmids expressing the six genes together in E. coli JM109∆minCD. The cell growth rate was accelerated under the function of the high expression level of the six genes. Furthermore, the size of the cell was bigger than the wild type, and the big volume could contain more PHB. The PHB production ability of JM109∆minCD (pBBR-Pbad-ftsQLWN, ptk-mreB-ftsZ) was elevated significantly. In conclusion, multiple division and larger cell morphology could enhance the storage space in the cells, and we provide new insights into the PHB production mechanism.
Compared with other efforts to improve PHB production, such as process optimization and PHB pathway engineering et al., the multiple division and larger cell morphology could enhance the production further and provide a new vision on improved PHB production. In order to satisfy the industrialization purpose, there are still many further engineering solutions needed in the new system, such as the improvement on stability of recombinant strain, the reduction of time spending on bacterial division. The work on division pattern and cell morphology of E. coli can help improve cell factories more efficiently.
Conclusions
Bacterial cells are grown in a binary fission way meaning a bacterial cell is equally divided into two (1–2 division). This common growth pattern of E. coli can be changed to multiple fission patterns (1 to n, n > 2) by deleting fission related genes minC and minD together or individually, allowing the formation of multiple fission rings (FtsZ-rings) in several positions of an elongated E. coli cell. However, even though a bacterial cell was able to divide into more than two daughter cells, we could not observe significant faster cell growth. To further improve cell growth, some genes related to the divison including ftsQ, ftsW, ftsN, ftsL and ftsZ, together with the cell shape control gene mreB, were all overexpressed in E. coli JM109∆minCD. Different degrees of cell growth changes were visible. The changing pattern of E. coli cell growth resulted in more cell dry weights (CDW) and more than 80 % PHB accumulation increases compared to its binary fission control grown under the same conditions. This study clearly demonstrated that the disrupted min system can change the cell division pattern from binary to multiple fission. Combined over-expression of Z ring synthesis genes ftsQ, ftsW, ftsN, ftsL and ftsZ together with shape control gene mreB led to enhanced PHB accumulation.
Methods
Bacterial strains, plasmids and culture conditions
All the microorganisms and plasmids used in this study are listed in Table 2. E. coli strains were cultivated in Luria–Bertani (LB) medium or mineral medium (MM). LB medium contains (g/L) 10 tryptone, 5 yeast extract and 10 NaCl. MM medium consists of (g/L): (NH4)2SO4 2.0, MgSO4 0.2, Na2HPO4·12H2O 9.65, KH2PO4 1.5, trace element solution I 10 ml/L and trace element solution II 1 ml/L. The trace element solution I consists of (mg/L): Fe(III)–NH4–citrate 5, CaCl2 2 and HCl 1 M. The trace element solution II contains of (mg/L): ZnSO4·7H2O 100, MnCl2·4H2O 30, H3BO3 300, CoCl2·6H2O 200, CuSO4·5H2O 10, NiCl2·6H2O 20, NaMoO4·2 H2O 30 and HCl 0.5 M. When antibiotic selection pressure was required, the medium was supplemented with ampicillin (100 μg/mL), kanamycin (50 μg/mL) or chloramphenicol (30 μg/mL). In order to produce PHA by E. coli, 20 g/L glucose was added into the culture medium as a carbon source.
Construction of plasmids and recombinant strains
Molecular cloning standard procedures including DNA amplification, restriction enzyme digestion and other DNA manipulations were employed for plasmids construction [43]. The kits of DNA purification, plasmids isolation and ligation were purchased from Biomed (Beijing, China) and Thermo (Beijing, China). Primers used in this study were all synthesized by Invitrogen Company (Shanghai, China). In order to construct larger plasmid, the Gibson assembly kit was used in this work (NEB, China) [44].
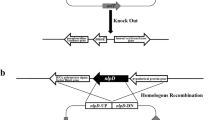
Genes minC and minD knockout in E. coli
Escherichia coli JM109ΔminCD mutant was constructed by one-step disruption on the chromosome [45]. 500 bp homologous upstream and downstream of target gene DNA, and the KanR gene flanked by FLP recognition target (FRT) sites from plasmid pKD13, were used to form the deletion fragment by PCR amplification. The primers used in this study are listed in Additional file 3: Table S1.
After induction by 0.2 % l-arabinose, E. coli JM109 with help plasmid pKD46RecA was maintained ice-cold for 30 min and 10 μl of the deletion fragment was added into the competent cells (Bio-Rad Inc., USA). After PCR verification for screening the positive colony and elimination of the KanR by plasmid pCP20, the deletions were confirmed by PCR analysis and DNA sequencing.
Finally, DNA sequencing was employed to confirm the gene knockout. At the end, chromosomal minC and minD genes deleted strain E. coli JM109 was obtained.
Scanning electron microscopy (SEM)
Cells were first fixed with 2.5 % (v/v) glutaraldehyde for more than 4 h, and then washed with 0.1 M phosphate-buffered saline (PBS) (pH 7.3) (3 times, 10 min each). After that, the fixed cells were washed by ethanol in concentration gradient (v/v) of 50, 70, 80, 90 and 100 % in a sequential way, and further dehydrated by tertiary butyl alcohol mixed with ethanol in a ratio of 1:1. The sample was treated with pure tertiary butyl alcohol and used for imaging after lyophilized.
Ultra-thin-section TEM
After 48 h fermentation, the cells were harvested and re-suspended in 0.1 M phosphate-buffered saline (PBS) (pH 7.3), and then chemically fixed in 2.5 % (v/v) glutaraldehyde. After treatments to prepare ultrathin sections, a H-7650B instrument (Hitachi Ltd, Japan) at 120 kV was used in the electron microscopy study.
Study on cell growth and PHB production
Escherichia coli cells were harvested after 48 h cultivation and washed with distilled water. Cell dry weight (CDW) was measured after lyophilization overnight, and the PHB was analyzed by a gas chromatograph after methanolysis reaction at 100 °C for 4 h. A Spectra System P2000 (Thermo Separation, USA) was used to determine the intracellular PHB contents, while pure PHB (Sigma, American) was used as a standard sample in this study.
Abbreviations
- PHA:
-
polyhydroxyalkanoates
- PHB:
-
poly(3-hydroxybutyrate)
- OD600 :
-
the optical density at 600 nm
- CDW:
-
cell dry weight
References
Adams DW, Wu LJ, Errington J. Cell cycle regulation by the bacterial nucleoid. Curr Opin Microbiol. 2014;22:94–101.
Wang JD, Levin PA. Metabolism, cell growth and the bacterial cell cycle. Nat Rev Microbiol. 2009;7:822–7.
Pazos M, Casanova M, Palacios P, Margolin W, Natale P, Vicente M. FtsZ placement in nucleoid-free bacteria. PLoS ONE. 2014;9:e91984.
Reyes-Lamothe R, Nicolas E, Sherratt DJ. Chromosome replication and segregation in bacteria. Annu Rev Genet. 2012;46:121–43.
Zhou H, Lutkenhaus J. MinC mutants deficient in MinD- and DicB-mediated cell division inhibition due to loss of interaction with MinD, DicB, or a septal component. J Bacteriol. 2005;187:2846–57.
Anderson DE, Gueiros-Filho FJ, Erickson HP. Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J Bacteriol. 2004;186:5775–81.
Pichoff S, Lutkenhaus J. Escherichia coli division inhibitor MinCD blocks septation by preventing Z-ring formation. J Bacteriol. 2001;183:6630–5.
Howard M. A mechanism for polar protein localization in bacteria. J Mol Biol. 2004;335:655–63.
Akerlund T, Gullbrand B, Nordström K. Effects of the Min system on nucleoid segregation in Escherichia coli. Microbiology. 2001;10:3213–22.
Rowlett VW, Margolin W. The bacterial Min system. Curr Biol. 2013;23:R553–6.
Zhang Z, Morgan JJ, Lindahl PA. Mathematical model for positioning the FtsZ contractile ring in Escherichia coli. J Math Biol. 2014;68:911–30.
Margolin W. FtsZ and the division of prokaryotic cells and organelles. Nat Rev Mol Cell Biol. 2005;6:862–71.
Villanelo F, Ordenes A, Brunet J, Lagos R, Monasterio O. A model for the Escherichia coli FtsB/FtsL/FtsQ cell division complex. BMC Struct Biol. 2011;11:28.
Carson MJ, Barondess J, Beckwith J. The FtsQ protein of Escherichia coli: membrane topology, abundance, and cell division phenotypes due to overproduction and insertion mutations. J Bacteriol. 1991;173:2187–95.
Boyle DS, Khattar MM, Addinall SG, Lutkenhaus J, Donachie WD. ftsW is an essential cell-division gene in Escherichia coli. Mol Microbiol. 1997;24:1263–73.
Fraipont C, Alexeeva S, Wolf B, van der Ploeg R, Schloesser M, den Blaauwen T, Nguyen-Disteche M. The integral membrane FtsW protein and peptidoglycan synthase PBP3 form a subcomplex in Escherichia coli. Microbiology. 2011;157:251–9.
Hale CA, de Boer PA. ZipA is required for recruitment of FtsK, FtsQ, FtsL, and FtsN to the septal ring in Escherichia coli. J Bacteriol. 2002;184:2552–6.
Addinall SG, Cao C, Lutkenhaus J. FtsN, a late recruit to the septum in Escherichia coli. Mol Microbiol. 1997;25:303–9.
Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–25.
Loose M, Mitchison TJ. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat Cell Biol. 2014;16:38.
Matsumoto K, Taguchi S. Enzyme and metabolic engineering for the production of novel biopolymers: crossover of biological and chemical processes. Curr Opin Biotechnol. 2013;24:1054–60.
Chen GQ, Hajnal I, Wu H, Lv L, Ye J. Engineering biosynthesis mechanisms for diversifying polyhydroxyalkanoates. Trends Biotechnol. 2014;33:565–74.
You M, Peng G, Li J, Ma P, Wang Z, Shu W, Peng S, Chen GQ. Chondrogenic differentiation of human bone marrow mesenchymal stem cells on polyhydroxyalkanoate (PHA) scaffolds coated with PHA granule binding protein PhaP fused with RGD peptide. Biomaterials. 2010;32:2305–13.
Meng DC, Shen R, Yao H, Chen JC, Wu Q, Chen GQ. Engineering the diversity of polyesters. Curr Opin Biotechnol. 2014;29:24–33.
Gumel AM, Annuar MSM, Heidelberg T. Current application of controlled degradation processes in polymer modification and functionalization. J Appl Polym Sci. 2013;129:3079–88.
Rodriguez-Carmona E, Cano-Garrido O, Seras-Franzoso J, Villaverde A, Garcia-Fruitos E. Isolation of cell-free bacterial inclusion bodies. Microb Cell Fact. 2010;9:71.
Li ZJ, Shi ZY, Jian J, Guo YY, Wu Q, Chen GQ. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab Eng. 2010;12:352–9.
Choi J, Lee SY. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol Bioeng. 1999;62:546–53.
Koutinas M, Menelaou M, Nicolaou EN. Development of a hybrid fermentation-enzymatic bioprocess for the production of ethyl lactate from dairy waste. Bioresour Technol. 2014;165:343–9.
Kachrimanidou V, Kopsahelis N, Papanikolaou S, Kookos IK, De Bruyn M, Clark JH, Koutinas AA. Sunflower-based biorefinery: poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from crude glycerol, sunflower meal and levulinic acid. Bioresour Technol. 2014;172:121–30.
Tsakona S, Kopsahelis N, Chatzifragkou A, Papanikolaou S, Kookos IK, Koutinas AA. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J Biotechnol. 2014;189:36–45.
Wu H, Wang H, Chen J, Chen GQ. Effects of cascaded vgb promoters on poly(hydroxybutyrate) (PHB) synthesis by recombinant Escherichia coli grown micro-aerobically. Appl Microbiol Biotechnol. 2014;98:10013–21.
Jones JA, Toparlak OD, Koffas MA. Metabolic pathway balancing and its role in the production of biofuels and chemicals. Curr Opin Biotechnol. 2015;33:52–9.
Wang Y, Wu H, Jiang XR, Chen GQ. Engineering Escherichia coli for enhanced production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in larger cellular space. Metab Eng. 2014;25:183–93.
Jiang XR, Wang H, Shen R, Chen GQ. Engineering the bacterial shapes for enhanced inclusion bodies accumulation. Metab Eng. 2015;29:227–37.
Dominguez-Escobar J, Chastanet A, Crevenna AH, Fromion V, Wedlich-Soldner R, Carballido-Lopez R. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science. 2011;333:225–8.
Garner EC, Bernard R, Wang WQ, Zhuang XW, Rudner DZ, Mitchison T. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science. 2011;333:222–5.
Kocaoglu O, Calvo RA, Sham LT, Cozy LM, Lanning BR, Francis S, Winkler ME, Kearns DB, Carlson EE. Selective penicillin-binding protein imaging probes reveal substructure in bacterial cell division. ACS Chem Biol. 2012;7:1746–53.
Bi E, Lutkenhaus J. Ftsz ring structure associated with division in Escherichia coli. Nature. 1991;354:161–4.
Meier EL, Goley ED. Form and function of the bacterial cytokinetic ring. Curr Opin Cell Biol. 2014;26:19–27.
Goehring NW, Gueiros F, Beckwith J. Premature targeting of a cell division protein to midcell allows dissection of divisome assembly in Escherichia coli. Genes Dev. 2005;19:127–37.
Rueff AS, Chastanet A, Dominguez-Escobar J, Yao ZZ, Yates J, Prejean MV, Delumeau O, Noirot P, Wedlich-Soldner R, Filipe SR, Carballido-Lopez R. An early cytoplasmic step of peptidoglycan synthesis is associated to MreB in Bacillus subtilis. Mol Microbiol. 2014;91:348–62.
Chong L. Molecular cloning - A laboratory manual, 3rd edition. Science. 2001;292:446.
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Meth. 2009;6:343–5.
Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–5.
Spiekermann P, Rehm BH, Kalscheuer R, Baumeister D, Steinbűchel A. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch Microbiol. 1999;171:73–80.
Authors’ contributions
HW designed and carried out the experiments, analyzed the data and drafted the manuscript. ZF, XJ and JC contributed general advice, particularly on shake flask experiment. GQC draft the basic idea and supervised the study. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the Center of Biomedical Analysis, Tsinghua University for the SEM and TEM studies. Plasmid pBHR68 was kindly donated by Professor Alexander Steinbüchel of Münster University in Germany.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Gene sequences used in this project are from Gen-bank (http://www.ncbi.nlm.nih.gov/) and tools from NEB were used to construct plasmids (http://nebuilder.neb.com/).
Ethics, consent and permissions
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This research was financially supported by the State Basic Science Foundation 973 (Grant no. 2012CB725201) and National Natural Science Foundation of China (Grant nos. 31430003 and 31270146). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional files
12934_2016_531_MOESM2_ESM.docx
Additional file 2: Figure S1. Growth of E. coli JM109∆minCD (p15a-pglta-ftsQLWN) and E. coli JM109 (p15a-blank) in a LB medium. Error bars are s.d. (n=3). Figure S2. Growth of E. coli JM109 ∆minCD containing plasmid pBBR-Pbad-ftsQLWN and ptk-mreB-ftsZ, respectively. Arabinose was added to the culture after 4 h of inoculation. The control was cultivated under the same condition. Error bars are s.d. (n=3).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wu, H., Fan, Z., Jiang, X. et al. Enhanced production of polyhydroxybutyrate by multiple dividing E. coli . Microb Cell Fact 15, 128 (2016). https://doi.org/10.1186/s12934-016-0531-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-016-0531-6