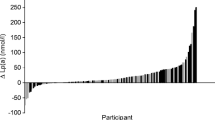
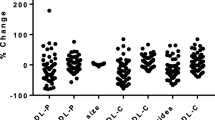
Abstract
Background
Cardiovascular disease represents a significant risk factor for mortality in individuals with type 2 diabetes mellitus (T2DM). High-density lipoprotein (HDL) is believed to play a crucial role in maintaining cardiovascular health through its multifaceted atheroprotective effects and its capacity to enhance glycemic control. The impact of dietary interventions and intermittent fasting (IF) on HDL functionality remains uncertain. The objective of this study was to assess the effects of dietary interventions and IF as a strategy to safely improve glycemic control and reduce body weight on functional parameters of HDL in individuals with T2DM.
Methods
Before the 12-week intervention, all participants (n = 41) of the INTERFAST-2 study were standardized to a uniform basal insulin regimen and randomized to an IF or non-IF group. Additionally, all participants were advised to adhere to dietary recommendations that promoted healthy eating patterns. The IF group (n = 19) followed an alternate-day fasting routine, reducing their calorie intake by 75% on fasting days. The participants’ glucose levels were continuously monitored. Other parameters were measured following the intervention: Lipoprotein composition and subclass distribution were measured by nuclear magnetic resonance spectroscopy. HDL cholesterol efflux capacity, paraoxonase 1 (PON1) activity, lecithin cholesterol acyltransferase (LCAT) activity, and cholesterol ester transfer protein (CETP) activity were assessed using cell-based assays and commercially available kits. Apolipoprotein M (apoM) levels were determined by ELISA.
Results
Following the 12-week intervention, the IF regimen significantly elevated serum apoM levels (p = 0.0144), whereas no increase was observed in the non-IF group (p = 0.9801). ApoM levels correlated with weight loss and fasting glucose levels in the IF group. Both groups exhibited a robust enhancement in HDL cholesterol efflux capacity (p < 0.0001, p = 0.0006) after 12 weeks. Notably, only the non-IF group exhibited significantly elevated activity of PON1 (p = 0.0455) and LCAT (p = 0.0117) following the 12-week intervention. In contrast, the changes observed in the IF group did not reach statistical significance.
Conclusions
A balanced diet combined with meticulous insulin management improves multiple metrics of HDL function. While additional IF increases apoM levels, it does not further enhance other aspects of HDL functionality.
Trial registration
The study was registered at the German Clinical Trial Register (DRKS) on 3 September 2019 under the number DRKS00018070.
Graphical Abstract

Similar content being viewed by others
Background
Diet and fasting are the cornerstones of lifestyle modifications and essential factors in promoting cardiovascular health. The Mediterranean diet is associated with lower cardiovascular risk and diabetes incidence [1]. Various fasting strategies, from intermittent fasting to fasting-mimicking diets, may also offer advantages for preventing and treating chronic metabolic diseases like type 2 diabetes (T2DM) [2,3,4]. Significant weight loss from short-term calorie restriction can lower blood sugar and HbA1c levels, potentially leading to remission of T2DM [5]. Previous findings of the INTERFAST-2 study demonstrated that alternate day fasting (IF) over 12 weeks in insulin-treated people with T2DM is safe, and reduces HbA1c, body weight, and total daily insulin dose, while the resting metabolic rate and the physical activity levels remained unaltered [6].
T2DM and the cluster of pathologies including glucose intolerance/insulin resistance, obesity, and high plasma triglycerides that comprise the metabolic syndrome are associated with low and dysfunctional HDL. In addition to its established positive effects on cardiovascular health, HDL is emerging as a significant factor in enhancing glycemic control. HDL lowers plasma glucose by increasing plasma insulin and activating the adenosine monophosphate-activated protein kinase (AMPK) pathway in skeletal muscle [7]. HDL binds to cell surface receptors on skeletal muscle, including ATP-binding cassette transporter A1, leading to the mobilization of intracellular calcium ions and activation of calcium/calmodulin-dependent protein kinase kinase. This cascade promotes the phosphorylation and activation of AMPK, resulting in downstream effects such as glucose uptake. The most extensively studied and significant function of HDL is its cholesterol efflux capacity, which quantifies the capacity to remove cholesterol from cells. Recent large-scale clinical studies have demonstrated a correlation between in vitro HDL cholesterol efflux capacity and the prevalence and incidence of cardiovascular disease, which appears to be independent of HDL-C concentration [8]. Paraoxonase 1 (PON1), an HDL-associated enzyme, enhances insulin sensitivity by promoting GLUT4 translocation in myocytes [9]. ApoM is a lipocalin, primarily associated with HDL particles, that has a distinct hydrophobic binding pocket that allows it to bind functional lipids such as sphingosine-1-phosphate (S1P). This interaction plays a critical role in preventing the degradation of S1P, enhancing the formation of atheroprotective preβ-HDL, and promoting insulin release [10, 11]. Lecithin–cholesterol acyltransferase (LCAT) as well as cholesterol ester transfer protein (CETP) play a crucial role in HDL maturation [12, 13]. These mechanisms suggest a potential link between low circulating HDL levels and metabolic dysfunction [7].
Understanding the factors influencing HDL function in T2DM could pave the way for novel biomarkers to track disease progression and develop personalized treatment plans. While specific dietary components have demonstrated improvements in HDL function [14], the influence of fasting on HDL structure, function, and metabolism remains unclear. In this study, we examined the effects of IF on various functional parameters of HDL in patients with T2DM, including (i) HDL subclass distribution, (ii) serum apoM levels, (iii) HDL cholesterol efflux capacity, (iv) PON1 activity and (v) serum LCAT and CETP activities.
Materials and methods
This is an analysis of the INTERFAST-2 study, a single-center, randomized, controlled trial, investigating the effect of intermittent fasting in people with T2DM already injecting insulin (INTERFAST-2). This study was conducted at the University Hospital Graz, Austria, and approved by the ethics committee of the Medical University of Graz, Austria (EK 30–350 ex 17/18). This research adhered to the tenets of the Declaration of Helsinki, and GCP-ICH guidelines, and complied with the protocol and requirements of the relevant regulatory authorities. The study population consisted of individuals with T2DM, aged 18 to 75 years, with glycated hemoglobin A1c ≥ 7.0% (≥ 53 mmol/mol). The primary inclusion criteria were as follows: a total daily insulin dose of ≥ 0.3 units per kilogram of body weight and stable body weight over the previous three months (weight change < ± 3 kg). Participants had to be willing to comply with the study procedures, attend the study site, participate in the required protocols, and adhere to the fasting protocols.
Major exclusion criteria included active known malignancy within the past year (excluding prostate, gastrointestinal, and basal cell carcinoma intraepithelial neoplasia), pregnancy or intent to become pregnant, lactation, and any chronic disease that might interfere with the interpretation of study results. In addition, participants were excluded if they had started a new hormonal supplement or changed their hormonal contraceptive in the previous two months. Participants with type 1 diabetes mellitus or other forms of diabetes mellitus, acute or chronic inflammatory diseases, or who consumed more than 15 standard alcoholic drinks per week were also excluded. In addition, individuals who worked night shifts or used illicit substances were not included.
Four weeks before the start of the dietary intervention, participants were switched to the same basal insulin regimen. A trained physician made dose adjustments for participants in both groups during the intervention period. Further information can be found in the published study protocol [15].
A registered dietitian provided an educational intervention focused on individualized dietary strategies to promote optimal health outcomes. The session emphasized on creating a balanced and varied plate with a rainbow of vegetables. Participants were encouraged to reduce their added sugars and salt intake while adding more whole grains and legumes to their meals. All patients had the same number of interactions with the dietitian during both on-site and telephone visits. Adherence to the diet was continuously monitored voluntarily.
Blood samples were collected from the participants at the outset of the study, which occurred after the insulin switch phase but before the commencement of the intervention. The second blood draw was conducted after 12 weeks of intervention. Blood samples were drawn after a minimum of 8 h of overnight fasting.
ApoB-Depletion of serum
Apolipoprotein B (apoB) was depleted from serum using a polyethylene glycol (PEG) precipitation method. A 20% (w/v) stock solution of PEG (P1458, Sigma-Aldrich, Darmstadt, Germany) was prepared by dissolving it in 200 mmol/L glycine buffer. Forty microliters (µL) of this PEG solution were then added to 100 µL of serum. The mixture was gently mixed and incubated at room temperature for 20 min. Following incubation, the samples were centrifuged at 10,100 × g for 30 min at 4 °C. The resulting HDL-containing apoB-depleted serum was collected and stored at − 70 °C for further analysis.
Lecithin–cholesteryl acyltransferase (LCAT) activity
LCAT activity was assessed using a commercially available kit (MAK107, Merck, Darmstadt, Germany) following the manufacturer’s guidelines. The serum samples were incubated with the LCAT substrate for four hours at 37 °C. The fluorescent substrate emits at 470 nm, and upon LCAT-mediated hydrolysis, a monomer with fluorescence at 390 nm is released. LCAT activity was quantified by monitoring the change in the ratio of emission intensities at λ = 470 nm and λ = 390 nm over time.
Arylesterase activity of Paraoxonase
The Ca2+-dependent arylesterase activity of PON1 was determined using a photometric assay involving phenylacetate substrate, following a previously described protocol [16]. Briefly, 1.5 µL of 1:10 phosphate-buffered saline diluted apoB-depleted serum was added to a 200 µL buffer solution containing 100 mM Tris, 2 mM CaCl2 (pH 8.0), and 1 mM phenylacetate. The hydrolysis of phenylacetate was monitored at a wavelength of 270 nm. The enzymatic activity was determined using the Beer-Lambert law, with a molar extinction coefficient of 1,310 L mol-1 cm-1.
Cholesterol Ester Transfer Protein (CETP) activity
Serum CETP activity was determined using a commercially available kit (MAK106, Merck, Darmstadt, Germany) following the manufacturer’s instructions. The CETP Activity Assay Kit uses a proprietary substrate to measure CETP-mediated neutral lipid transfer. 3 µl of serum samples diluted 1:10 in phosphate-buffered saline are incubated with the donor and acceptor molecules at 37 °C for three hours. The reaction produces a fluorescent signal (λEx = 465 nm/λEm = 535 nm) proportional to CETP activity.
Serum levels of apolipoprotein M
Serum levels of apoM were quantified using a sandwich enzyme-linked immunosorbent assay method described in a prior study [17]. For apoM measurement, capture antibody (clone 1G9) (Abnova, Taipei City, Taiwan) detection antibody (clone EPR2904) (Abcam, Cambridge, UK), and HRP-conjugated anti-rabbit IgG antibody (cat. No. PO448) (DAKO, Glostrup, Denmark) were used. Briefly, a high-binding ELISA plate (Corning, Arizona, US) was coated with a capture antibody overnight and blocked with 2% bovine serum albumin. Serum samples (10 µl) were treated with 1,4-dithiothreitol (Sigma-Aldrich) and iodoacetamide (Sigma-Aldrich) to cleave disulfide bonds in apoM. The 1:50 diluted samples (in tris-buffered saline + 1% bovine serum albumin) were incubated overnight in the ELISA plate. After washing and the addition of detection and secondary antibodies, the absorbance of the colorimetric reaction was measured at 492 nm to determine the apoM concentration.
Cholesterol efflux capacity
The cholesterol efflux capacity of apoB-depleted serum was determined following established protocols [18, 19]. In brief, J774.2 cells (Sigma Aldrich, Darmstadt, Germany) were labeled with 0.5 µCi/mL radiolabeled [3 H]-cholesterol (Hartmann Analytic, Braunschweig, Germany) in DMEM media containing 2% FBS, 1% penicillin/streptomycin, and 8(4-chlorophenylthio)-cyclic adenosine monophosphate (0.3 mM) (Sigma-Aldrich, Darmstadt, Germany) overnight. After two washes, the cells were equilibrated for 2 h in serum-free DMEM supplemented with 2% bovine serum albumin from Sigma-Aldrich (Darmstadt, Germany). The cells were then rinsed and incubated with 2.8% apoB-depleted serum samples for 3 h. Cholesterol efflux capacity was expressed as the ratio of radioactivity in the media to the total radioactivity in the media and lysed cells.
NMR analysis
HDL subclasses and composition were assessed using a Bruker 600 MHz Avance Neo NMR spectrometer (Bruker, Rheinstetten, Germany) according to the Bruker IVDr Lipoprotein Subclass Analysis Protocol. Lipoprotein quantification was performed by analyzing the data using the Bruker IVDr Lipoprotein Subclass Analysis (B.I.LISATM) method as described previously [20]. The Bruker IVDr Lipoprotein Subclass Analysis identifies four HDL subclasses, labeled HDL-1 through HDL-4, based on increasing density and decreasing size. The defined density ranges for these subclasses are HDL-1 (1.063 to 1.100 kg/L), HDL-2 (1.100 to 1.112 kg/L), HDL-3 (1.112 to 1.125 kg/L), and HDL-4 (1.125 to 1.210 kg/L). For simplicity, these subclasses are designated as L-HDL (HDL-1), M-HDL (HDL-2), S-HDL (HDL-3), and XS-HDL (HDL-4).
Statistical analysis
All statistical analyses were performed with SPSS (version 29.0.0.0) (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 8.0. A p-value of < 0.05 was used to determine statistical significance. Participant characteristics are reported as means ± standard deviation, median with interquartile range or counts, and proportions. Statistical differences between the groups were calculated using paired t-test or Wilcoxon signed rank test, depending on the normality of the data. Fisher’s exact test was used to identify differences in comorbidities and used medication. The Spearman correlation coefficient was used to assess correlations between HDL functions and clinical parameters. The results are presented as a scatterplot. Differences before and after intervention within each group were calculated using the paired t-test or Wilcoxon test, depending on the normality of the data.
Results
Baseline characteristics of the study cohort
Baseline characteristics of the study cohort are presented in Table 1. The INTERFAST-2 trial included 41 participants, with 19 (45%) assigned to the IF group and 22 (55%) to the non-IF group. The mean age of the study cohort was 63 ± 7 years, and body mass index (BMI) was 34 ± 5 kg/m². While lipid profiles were generally within normal limits, glucose metabolism was impaired, indicated by elevated fasting glucose (184 ± 42), insulin (18.3 ± 9.4), and glycated hemoglobin (HbA1c) levels (68 ± 12 mmol/mol). Hypertension was common, with elevated systolic blood pressure (141 ± 22 mmHg). Additionally, elevated levels of inflammatory markers, CRP and IL-6, suggested low-grade inflammation, highlighting the metabolic and inflammatory burden associated with the study population. A significant proportion of the study cohort also had other comorbidities such as heart failure, coronary artery disease, and myocardial infarction. Participants were taking a variety of medications for diabetes management, including metformin, DPP-4 inhibitors, SGLT2 inhibitors, GLP-1 receptor agonists, and basal insulin. While the groups were generally well-matched, some differences have to be noted in age (61 ± 7; 66 ± 6), HDL-C (53 ± 11; 45 ± 10), and triglyceride levels (212 [93, 528]; 161 [52, 321]). No significant differences between other baseline characteristics were observed.
Effects of IF on HDL composition
Lipoprotein profile analysis by NMR revealed changes in HDL composition after 12 weeks. When comparing the IF group to the non-IF group, we observed a non-significant upward trend in several components of large HDL (L-HDL) after the intervention in the IF group. This includes apoA-I, apoA-II, cholesterol, and phospholipids (p = 0.083, 0.126, 0.198, and 0.061, respectively) (Fig. 1A). Plasma apoM levels increased significantly after 12 weeks of IF (Fig. 1B). In agreement with our results, previous studies have shown an association between apoM and BMI [21, 22]. Consistent with this observation, our data showed a negative correlation between changes in BMI due to weight loss and changes in apoM levels (Fig. 1C). In addition, we observed a negative association between the change in apoM (delta apoM) and the change in fasting glucose (delta fasting glucose) (Fig. 1D).
Effects of IF on HDL composition. (A) HDL compositional parameters measured by NMR spectroscopy. (B) Fold change of apoM levels after 12 weeks of intervention. (C) Correlation analysis of delta apoM levels with delta BMI of the IF group; difference from before to after 12 weeks intervention measurements. (D) Correlation analysis of delta apoM with delta fasting glucose parameters of the IF group. HDL, high-density lipoprotein; HDL-A1, HDL-associated apolipoprotein A1; HDL-A2, HDL-associated apolipoprotein A2; HDL-C, HDL cholesterol; HDL-FC, HDL free cholesterol; HDL-TG, HDL triglycerides; HDL-PL, HDL phospholipids; LDL-ApoB, LDL associated apolipoprotein B; LDL-C, LDL cholesterol
Effects of IF on metrics of HDL function and metabolism
Next, we determined whether IF affects the functional metrics of HDL. Interestingly, the ability of HDL to remove cholesterol from macrophages was elevated in both the IF and non-IF groups after 12 weeks of intervention (Fig. 2A). To gain insight into the changes in serum antioxidant and anti-inflammatory activities upon fasting, we examined the activity of the HDL-associated enzyme PON1. After 12 weeks, there was a significant increase in PON1 activity among the non-IF group, while the increase in the IF group did not reach significance (Fig. 2B). LCAT is an enzyme that plays a crucial role in the maturation of HDL particles [23]. Interestingly, we observed a significant increase in LCAT activity in the non-IF group, whereas again the increase in the IF group did not reach significance (Fig. 2C). CETP facilitates the redistribution of cholesteryl esters, triglycerides, and phospholipids between plasma lipoproteins. Notably, no change in CETP activity was observed in either of the groups following the intervention period (Fig. 2D).
Effects of IF on HDL functionality and metabolism. Line plots of cholesterol efflux capacity, enzymes involved in HDL functionality, and metabolism before and after the intervention period. (A) Cholesterol efflux capacity (% efflux); (B) PON1 activity (mM/min/ml); (C) LCAT activity (% substrate turnover); (D) CETP activity (pg transfer/h). Differences between baseline and after intervention (12 weeks) were analyzed by paired t-test or Wilcoxon test depending on the normality of the data. CETP, cholesterol ester transfer protein; LCAT, lecithin cholesterol acyltransferase; PON1, paraoxonase 1
Discussion
While fasting is practiced by billions worldwide for health or religious reasons, the larger picture of human adaptation to prolonged food deprivation remains elusive. Furthermore, the long-term health effects, both positive and negative, are areas of ongoing research. In patients with T2DM, previous studies suggested that IF may offer several benefits such as weight loss, which may lead to a lower daily insulin requirement [6, 24]. In addition, integrating a balanced diet and proper insulin management with IF may positively impact HDL metabolism and function. As previously shown, a 12-week IF regimen in insulin-treated people with T2DM is safe, and reduces HbA1c, body weight, and total daily insulin dose. IF may be a promising approach for some T2DM patients, potentially improving blood sugar control [6].
This study explored the effects of dietary intervention and fasting on HDL metabolism and function in individuals with type 2 diabetes. Our findings underscore the potential of nutritional modifications to enhance various HDL-related functional parameters after a 12-week intervention. These results are particularly noteworthy because improved HDL functionality is closely linked to reduced cardiovascular risk and better glycemic control. Metrics of HDL function, such as cholesterol efflux capacity, have been demonstrated to strongly correlate with a decreased risk of cardiovascular disease [8]. It is interesting to note that HDL cholesterol efflux capacity is inversely related to T2DM in the EPIC Norfolk study [25]. HDL-associated PON1 plays a crucial role in preventing LDL oxidation and atherosclerosis by hydrolyzing lipid peroxides [26]. Oxidized LDL is associated with insulin resistance. The prevention of LDL oxidation by PON1 helps to maintain insulin sensitivity [9]. Moreover, PON1 reduces insulin resistance in mice fed a high-fat diet, and promotes GLUT4 overexpression in myocytes, via the IRS-1/Akt pathway [27]. Beyond its primary role in cholesterol esterification, LCAT exhibits additional antioxidant and anti-inflammatory properties. LCAT can neutralize the platelet-activating factor and oxidized phospholipids [12], and its activity has been independently associated with all-cause mortality in patients with chronic kidney disease [28]. Moreover, HDL-associated apoM inhibits the degradation of S1P, stimulates the formation of atheroprotective small preβ-HDL particles, and facilitates insulin release [10, 11]. These findings suggest a potential correlation between low or dysfunctional circulating HDL levels and metabolic dysfunction. Notably, the administration of reconstituted HDL to patients with T2DM has been shown to decrease plasma glucose by increasing plasma insulin and activating skeletal muscle AMP-activated protein kinase [29], which is in line with the aforementioned assumption. These results indicate that therapies aimed at raising functional HDL levels may have broader clinical applications beyond atherosclerosis in the management of T2DM.
Notably, whereas HDL cholesterol efflux capacity increased in both the IF and non-IF groups, the activities of PON1 and LCAT were significantly enhanced only in the non-IF group. IF increased apoM levels after 12 weeks. The activity of CETP, a protein that facilitates the redistribution of cholesterol esters and triglycerides between lipoproteins [30], was not altered in both groups.
All participants were given recommendations for a diet similar to the Mediterranean diet. This included eating at least three servings of vegetables and two servings of fruit per day, which was not possible for the IF group on fasting days. This could explain why some parameters such as PON1 and LCAT activity only increased significantly in the non-IF group. Additionally, participants were encouraged to reduce sugar and salt intake, choose whole grains and legumes over refined options, and limit red meat, dairy products, and saturated fats. Simmering was recommended as a healthier cooking method compared to frying [31]. Previous research demonstrated, that the Mediterranean diet markedly improves HDL functional parameters [14]. In the PREDIMED trial, the cholesterol efflux capacity of HDL was increased after 1 year of intervention compared to baseline levels [32]. The Mediterranean diet, especially when supplemented with extra virgin olive oil rich in phenolic compounds, has been shown to markedly improve metrics of HDL functionality. Particularly, the phenolic compounds of extra virgin olive oil seem to exert significant positive effects on HDL function [14]. Moreover, supplementation of anthocyanins as well as antioxidants such as lycopene or the omega-3 fatty acid eicosapentaenoic acid improve parameters of HDL function. Especially the consumption of nuts, legumes, and fish was previously reported to be associated with elevated PON1 antioxidant activity [33]. It is documented that the consumption of tomatoes is associated with an increase in LCAT activity, which is believed to be linked to the high lycopene content of tomatoes [34].
While the recommended Mediterranean-style diet rich in fruits, vegetables, and whole grains might be the primary driver of the improved cholesterol efflux observed in both groups, the lack of a significant PON1 and LCAT activity increase in the IF group remains unclear and further research is needed to draw firm conclusions.
It is important to note that alternate-day fasting appears to have the opposite effect in mice lacking LDL receptors. Two studies reported that alternate-day fasting unexpectedly increased the development of atherosclerosis in mice lacking LDL receptors [35, 36].
The results of our study suggest that a balanced Mediterranean-style diet enhances HDL function. However, the addition of fasting might negate some of these advantages, with the notable exception of increasing apoM levels. Our findings align with previous research showing that apoM levels are lower in individuals with obesity and metabolic syndrome, conditions often characterized by insulin resistance, and that caloric restriction increases the production of apoM [37]. ApoM expression is controlled by transcription factors associated with hepatic glucose and lipid metabolism [38]. ApoM, a carrier for S1P has been shown to enhance insulin secretion through S1P signaling, potentially impacting glycemic control [39]. The observed association between apoM levels and blood glucose in the IF group is consistent with this concept. Furthermore, the observed rise in apoM levels in the IF group could potentially lead to improved blood sugar regulation, given that apoM has been shown to enhance insulin secretion through S1P [10].
In addition to diet and fasting, regular aerobic exercise training is tightly linked with improved glycemic control and can significantly improve lipid profiles in healthy and obese individuals [40,41,42,43]. In individuals with T2DM, supervised and structured aerobic exercise training was associated with increased HDL cholesterol levels and reductions in plasma triglycerides and LDL cholesterol [44]. Combining a Mediterranean diet, intermittent fasting, and regular physical activity could further improve the health of T2DM patients.
Some limitations of our study have to be noted. Primarily, the relatively small sample size limits the generalizability of our findings. Secondly, the open-label design may have introduced bias. Participant’s awareness of their fasting status may have altered their behavior, impacting the results. Those fasting may have indulged in unhealthy food choices post-fasting, potentially negating some benefits. Conversely, participants in the non-IF group may have consciously adhered to a healthier diet. Moreover, the non-IF group had higher baseline HDL cholesterol and triglyceride levels, which might have influenced the results. If baseline HDL cholesterol levels are already high, there might be a limited capacity for further improvement through dietary and fasting interventions. However, both groups showed significant improvements in HDL cholesterol efflux capacity, suggesting that baseline differences likely did not substantially affect the results. The slight age difference of approximately five years between the groups is unlikely to have impacted the findings, as previous research suggests that cholesterol efflux capacity and LCAT activity are generally independent of age [45]. While PON1 activity declines in individuals over 65 in comparison to individuals of about 26 years of age [45], the relatively small age difference in our study is unlikely to have had a significant impact.
Conclusions
Our findings underscore the continued importance of a healthy lifestyle, particularly the Mediterranean diet, in addition to well-managed insulin therapy for individuals with T2DM. While we observed improvements in HDL function, as indicated by increased cholesterol efflux capacity, in both the non-IF and IF groups, the exclusive increase in apoM levels in the IF group suggests an additional mechanism that may contribute to the metabolic benefits. The established role of apoM in promoting insulin secretion is consistent with the observed improvements in glycemic control. While the recommended Mediterranean-style diet rich in fruits, vegetables, and whole grains might be the primary driver of the improved cholesterol efflux observed in both groups, the lack of a significant PON1 and LCAT activity increase in the IF group remains unclear and further research is needed to draw firm conclusions.
In conclusion, our study highlights the importance of a comprehensive approach to managing T2DM, which includes a healthy diet, well-regulated insulin therapy, and potentially intermittent fasting, as strategies to reduce cardiovascular risk and enhance overall metabolic health. Additionally, increasing functional HDL levels may offer broader clinical benefits in T2DM management, extending beyond just the prevention of atherosclerosis.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- Apo:
-
Apolipoprotein
- CETP:
-
Cholesterol ester transfer protein
- HDL:
-
High-density lipoprotein
- IF:
-
Intermittent fasting
- LCAT:
-
Lecithin-cholesterol acyltransferase
- PON1:
-
Paraoxonase 1
- S1P:
-
Sphingosine-1-phosphate
- T2DM:
-
Type 2 diabetes mellitus
References
Martín-Peláez S, Fito M, Castaner O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. Rev Nutrients. 2020;12:2236.
Lewgood J, Oliveira B, Korzepa M, Forbes SC, Little JP, Breen L et al. Efficacy of Dietary and Supplementation interventions for individuals with type 2 diabetes. Nutrients. 2021;13.
Cienfuegos S, McStay M, Gabel K, Varady KA. Time restricted eating for the prevention of type 2 diabetes. J Physiol. 2022;600:1253–64.
van den Burg EL, Schoonakker MP, van Peet PG, van den Akker-van Marle EM, Lamb HJ, Longo VD et al. Integration of a fasting-mimicking diet programme in primary care for type 2 diabetes reduces the need for medication and improves glycaemic control: a 12-month randomised controlled trial. Diabetologia. 2024.
Magkos F, Hjorth MF, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16:545–55.
Obermayer A, Tripolt NJ, Pferschy PN, Kojzar H, Aziz F, Müller A, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—A Randomized Controlled Trial. Diabetes Care. 2022;46:463–8.
Siebel AL, Heywood SE, Kingwell BA. HDL and glucose metabolism: current evidence and therapeutic potential. Front Pharmacol. 2015;6.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and Incident Cardiovascular events. N Engl J Med. 2014;371:2383–93.
van Diepen JA, Berbée JFP, Havekes LM, Rensen PCN. Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis. 2013;228:306–15.
Kurano M, Hara M, Tsuneyama K, Sakoda H, Shimizu T, Tsukamoto K, et al. Induction of insulin secretion by apolipoprotein M, a carrier for sphingosine 1-phosphate. Biochimica et Biophysica Acta (BBA) -. Mol Cell Biology Lipids. 2014;1841:1217–26.
Wolfrum C, Poy MN, Stoffel M. Apolipoprotein M is required for preβ-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat Med. 2005;11:418–22.
Subramanian VS, Goyal J, Miwa M, Sugatami J, Akiyama M, Liu M, et al. Role of lecithin-cholesterol acyltransferase in the metabolism of oxidized phospholipids in plasma: studies with platelet-activating factor-acetyl hydrolase-deficient plasma. Biochim et Biophys Acta (BBA) - Mol Cell Biology Lipids. 1999;1439:95–109.
Fielding CJ, Havel RJ. Cholesteryl Ester transfer protein: friend or foe? J Clin Invest. 1996;97:2687–8.
Stadler JT, Marsche G. Dietary Strategies to Improve Cardiovascular Health: Focus on Increasing High-Density Lipoprotein Functionality. Front Nutr. 2021;8.
Obermayer A, Tripolt NJ, Pferschy PN, Kojzar H, Jacan A, Schauer M, et al. INTERmittent FASTing in people with insulin-treated type 2 diabetes mellitus– the INTERFAST-2 study protocol. Diabet Med. 2022;39:e14813.
Holzer M, Zangger K, El-Gamal D, Binder V, Curcic S, Konya V, et al. Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: novel pathways generating dysfunctional high-density lipoprotein. Antioxid Redox Signal. 2012;17:1043–52.
Bosteen MH, Dahlbäck B, Nielsen LB, Christoffersen C. Protein unfolding allows use of commercial antibodies in an apolipoprotein M sandwich ELISA[S]. J Lipid Res. 2015;56:754–9.
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35.
Marsche G, Zelzer S, Meinitzer A, Kern S, Meissl S, Pregartner G, et al. Adiponectin predicts high-density lipoprotein cholesterol efflux capacity in adults irrespective of body mass index and fat distribution. J Clin Endocrinol Metabolism. 2017;102:4117–23.
Stadler JT, Habisch H, Prüller F, Mangge H, Bärnthaler T, Kargl J, et al. HDL-Related parameters and COVID-19 mortality: the importance of HDL function. Antioxidants. 2023;12:2009.
Li T, Yang L, Zhao S, Zhang S. Correlation between apolipoprotein M and inflammatory factors in obese patients. Med Sci Monit. 2018;24:5698–703.
Ooi EMM, Watts GF, Chan DC, Nielsen LB, Plomgaard P, Dahlbäck B, et al. Association of apolipoprotein M with high-density lipoprotein kinetics in overweight-obese men. Atherosclerosis. 2010;210:326–30.
Ong K-L, Cochran BJ, Manandhar B, Thomas S, Rye K-A. HDL maturation and remodelling. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of lipids. 2022;1867:159119.
Houmard JA, Tanner CJ, Yu C, Cunningham PG, Pories WJ, MacDonald KG, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty Acyl-CoAs in morbidly obese subjects. Diabetes. 2002;51:2959–63.
Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–13 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4648056/.
Durrington PN, Bashir B, Soran H. Paraoxonase 1 and atherosclerosis. Front Cardiovasc Med. 2023;10.
Koren-Gluzer M, Aviram M, Hayek T. Paraoxonase1 (PON1) reduces insulin resistance in mice fed a high-fat diet, and promotes GLUT4 overexpression in myocytes, via the IRS-1/Akt pathway. Atherosclerosis. 2013;229:71–8 https://www.sciencedirect.com/science/article/pii/S0021915013002049.
Stadler JT, Bärnthaler T, Borenich A, Emrich IE, Habisch H, Rani A et al. Low LCAT activity is linked to Acute Decompensated Heart failure and mortality in CKD patients. J Lipid Res. 2024;100624.
Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, et al. High-Density Lipoprotein Modulates Glucose Metabolism in Patients With Type 2 Diabetes Mellitus. Circulation. 2009;. https://doi.org/10.1161/CIRCULATIONAHA.108.843219https://www.ahajournals.org/doi/.
Hatakeyama K. CETP activity: a link between lipid metabolism and Coagulation System. J Atheroscler Thromb. 2016;23:1144.
Guasch-Ferré M, Willett WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. 2021;290:549–66.
Hernáez Á, Castañer O, Elosua R, Pintó X, Estruch R, Salas-Salvadó J, et al. Mediterranean Diet improves high-density lipoprotein function in High-Cardiovascular-Risk individuals. Circulation. 2017;135:633–43.
Hernáez Á, Sanllorente A, Castañer O, Martínez-González MÁ, Ros E, Pintó X, et al. Increased Consumption of Virgin Olive Oil, nuts, legumes, whole grains, and Fish promotes HDL functions in humans. Mol Nutr Food Res. 2019;63:1800847.
McEneny J, Wade L, Young IS, Masson L, Duthie G, McGinty A, et al. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J Nutr Biochem. 2013;24:163–8.
Dorighello GG, Rovani JC, Luhman CJF, Paim BA, Raposo HF, Vercesi AE, et al. Food restriction by intermittent fasting induces diabetes and obesity and aggravates spontaneous atherosclerosis development in hypercholesterolaemic mice. Br J Nutr. 2014;111:979–86.
Deng Y, Yang X, Ye X, Yuan Y, Zhang Y, Teng F et al. Alternate day fasting aggravates atherosclerosis through the suppression of hepatic ATF3 in apoe–/– mice. Life Metabolism. 2024;loae009.
Sramkova V, Berend S, Siklova M, Caspar-Bauguil S, Carayol J, Bonnel S, et al. Apolipoprotein M: a novel adipokine decreasing with obesity and upregulated by calorie restriction. Am J Clin Nutr. 2019;109:1499–510.
Nielsen LB, Christoffersen C, Ahnström J, Dahlbäck B. ApoM: gene regulation and effects on HDL metabolism. Trends Endocrinol Metabolism. 2009;20:66–71.
Truman J-P, García-Barros M, Obeid LM, Hannun YA. Evolving concepts in cancer therapy through targeting sphingolipid metabolism. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of lipids. 2014;1841:1174–88.
Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29:901.
Hardman AE. Physical activity, obesity and blood lipids. Int J Obes. 1999;23:S64–71.
Despres JP, Pouliot MC, Moorjani S, Nadeau A, Tremblay A, Lupien PJ, et al. Loss of abdominal fat and metabolic response to exercise training in obese women. Am J Physiology-Endocrinology Metabolism. 1991;261:E159–67.
Couillard C, Després J-P, Lamarche B, Bergeron J, Gagnon J, Leon AS, et al. Effects of endurance Exercise Training on plasma HDL cholesterol levels depend on levels of triglycerides. Arterioscler Thromb Vasc Biol. 2001;21:1226–32.
Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc. 2001;33:S502.
Holzer M, Trieb M, Konya V, Wadsack C, Heinemann A, Marsche G. Aging affects high-density lipoprotein composition and function. Biochim et Biophys Acta (BBA) - Mol Cell Biology Lipids. 2013;1831:1442–8.
Acknowledgements
We thank Sabine Meissl for the valuable technical support.
Funding
The research of G.M was funded by the Austrian Science Fund (FWF) [Grant DOI https://doi.org/10.55776/DOC129] (doc. funds RESPImmun) and ApoA-I Mimetic Peptide Lipid Assemblies (Grant-DOI: https://doi.org/10.55776/I5703), (KLI 851-B) and (KLI-1076) to H.S. The research of T.M. was supported by the Austrian Science Fund (FWF) (Grant DOI https://doi.org/10.55776/P28854, https://doi.org/10.55776/I3792, https://doi.org/10.55776/DOC130, and https://doi.org/10.55776/W1226), the Austrian Research Promotion Agency (FFG) grants 864690 and 870454; the Integrative Metabolism Research Center Graz; the Austrian Infrastructure Program 2016/2017; the Styrian Government (Zukunftsfonds, doc. fund program); the City of Graz; and BioTechMed-Graz (flagship project).
For open access purposes, the author has applied a CC BY public copyright license to any author-accepted manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
Authors’ contributions: Conceptualization, G.M., H.S.; methodology, A.P., H.H., T.M., formal analysis, A.P.; investigation, A.P., G.M.; writing—original draft preparation: A.P., G.M. writing—review and editing, A.P., G.M., J.T.S., A.O., P.P., N.J.T., H.H., T.M., H.S.; visualization A.P.; supervision G.M; project administration, G.M., A.O.,P.P., H.S.; funding acquisition, G.M., T.M., H.S. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the Ethics Committee of the Medical University of Graz (EK 30–350 ex 17/18).
Consent for publication
All participants gave written consent before any study-related procedure.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pammer, A., Obermayer, A., Stadler, J.T. et al. Effects of dietary interventions and intermittent fasting on HDL function in obese individuals with T2DM: a randomized controlled trial. Cardiovasc Diabetol 23, 339 (2024). https://doi.org/10.1186/s12933-024-02426-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02426-5