Abstract
Background
Circulating atherogenic index of plasma (AIP) levels has been proposed as a novel biomarker for dyslipidemia and as a predictor of insulin resistance (IR) risk. However, the association between AIP and the incidence of new-onset stroke, particularly in individuals with varying glucose metabolism status, remains ambiguous.
Methods
A total of 8727 participants aged 45 years or older without a history of stroke from the China Health and Retirement Longitudinal Study (CHARLS) were included in this study. The AIP was calculated using the formula log [Triglyceride (mg/dL) / High-density lipoprotein cholesterol (mg/dL)]. Participants were divided into four groups based on their baseline AIP levels: Q1 (AIP ≤ 0.122), Q2 (0.122 < AIP ≤ 0.329), Q3 (0.329 < AIP ≤ 0.562), and Q4 (AIP > 0.562). The primary endpoint was the occurrence of new-onset stroke events. The Kaplan–Meier curves, multivariate Cox proportional hazard models, and Restricted cubic spline analysis were applied to explore the association between baseline AIP levels and the risk of developing a stroke among individuals with varying glycemic metabolic states.
Results
During an average follow-up of 8.72 years, 734 participants (8.4%) had a first stroke event. The risk for stroke increased with each increasing quartile of baseline AIP levels. Kaplan–Meier curve analysis revealed a significant difference in stroke occurrence among the AIP groups in all participants, as well as in those with prediabetes mellitus (Pre-DM) and diabetes mellitus (DM) (all P values < 0.05). After adjusting for potential confounders, the risk of stroke was significantly higher in the Q2, Q3, and Q4 groups than in the Q1 group in all participants. The respective hazard ratios (95% confidence interval) for stroke in the Q2, Q3, and Q4 groups were 1.34 (1.05–1.71), 1.52 (1.19–1.93), and 1.84 (1.45–2.34). Furthermore, high levels of AIP were found to be linked to an increased risk of stroke in both pre-diabetic and diabetic participants across all three Cox models. However, this association was not observed in participants with normal glucose regulation (NGR) (p > 0.05). Restricted cubic spline analysis also demonstrated that higher baseline AIP levels were associated with higher hazard ratios for stroke in all participants and those with glucose metabolism disorders.
Conclusions
An increase in baseline AIP levels was significantly associated with the risk of stroke in middle-aged and elderly individuals, and exhibited distinct characteristics depending on the individual’s glucose metabolism status.
Similar content being viewed by others
Introduction
Stroke is a major global public health burden with high morbidity and mortality. Despite diligent primary prevention efforts, the prevalence and incidence of stroke in China continue to exhibit an alarming increase. Consequently, it is imperative to develop low-cost and reproducible indicators that can enhance early identification of high-risk individuals with stroke [1]. DM and arterial hypertension are widely recognized as the most common risk factors for stroke [2]. Many studies have elucidated that metabolic disorders, including dyslipidemia and hyperglycemia, are significant risk factors for stroke. Several metabolic indicators, such as remnant cholesterol and triglyceride-glucose index, have been utilized to assess the risk and prognosis of stroke [3,4,5], but the predictive accuracy is still limited. The AIP, a logarithmically transformed ratio of fasting triglyceride to fasting high-density lipoprotein cholesterol, is a sensitive marker of lipoprotein profiles primarily reflecting plasma lipid levels. Recent researches suggest that rising AIP can indicate the severity of IR and is closely related to the development of IR and type 2 diabetes [6,7,8]. As a robust biomarker of dyslipidemia, AIP has been considered to be a powerful independent predictor of adverse cardiovascular and cerebrovascular events. AIP potentially serves as a significant predictor of intracranial arterial stenosis, the risk of ischemic stroke, and poor stroke outcomes.
[9,10,11,12]. On the other hand, AIP serves as a reliable indicator of IR, which indicates that it may differentiate stroke risk in individuals with abnormal glucose metabolism. However, the relationship between AIP and stroke based on an individual’s glucose metabolism status has been poorly investigated. Therefore, prospective cohort studies with a large sample size are warranted to clarify the relationship.
In the present study, we used data from the CHARLS to explore the association between baseline AIP levels and stroke under different glucose metabolic states.
Methods
Study participants
This study employed a prospective study design andall participants were derived from the CHARLS, a national cohort commenced in 2011. The cohort specifically focuses on individuals aged 45 years and older in China. The participants are traced once every 2–3 years to identify their health status. To date, five-wave periods of follow-up surveys have been completed, with data collected in 2011, 2013, 2015, 2018, and 2020. The detailed research methods have been described previously [13]. We initially enrolled 17,708 participants in CHARLS wave 1. Among these people, 8981 were excluded for meeting the following exclusion criteria: (1) missing available data on AIP, fasting blood glucose (FPG), glycosylated hemoglobin (HbA1c) (n = 6132), (2) age < 45 years old or missing data on age (n = 423), (3) personal cancer history (n = 102), (4) history of stroke (n = 345), (5) lack of data on stroke or lost to follow-up (n = 1979). Finally, 8727 participants were divided into four groups according to the baseline AIP quartiles and were followed up until 2020 (Fig. 1). The CHARLS study received ethical approval from the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015), and all participants provided their written informed consent.
Data collection
Trained interviewers collected the demographic information (such as age, sex, and marital status), health status and functioning (such as smoking, drinking, hypertension, and diabetes) of the participants with standard questionnaires. After a 15-minute rest, participants, except those with an arm injury, were instructed to undergo three left arm blood pressure measurements at a 45-second interval, and the average of three values was reported. Participants were asked to take off shoes, heavy clothes before weighed and measured for height. Body weight and height were measured using standardized scales to the nearest 0.1 kg and 0.1 cm, respectively. Venous blood samples were collected from each participant by medically trained staff following a standard phlebotomy protocol and assayed for biochemical measurements. Regrettably, nearly 8% of the participants who gave blood reported that they fasted less than 8 h before blood collection. The enzymatic colorimetric method was applied to measure FPG levels and serum lipid parameters, while HbA1c was measured using boronate affinity high performance liquid chromatography [14].
Definitions
Hypertension diagnosis was based on a self-reported physician-diagnosed, and/or any antihypertensive medication use, and/or an average systolic/diastolic blood pressure (SBP/DBP) ≥ 140/90 mmHg [15]. DM was defined as FPG ≥ 126 mg/dl or HbA1c ≥ 6.5%, and/or a self-reported physician-diagnosed, and/or taking hypoglycemic agents. Pre-DM was characterized by an FPG of 100 to 125 mg/dL or an HbA1c of 5.7–6.4%. Individuals who were without DM or Pre-DM were classified as having NGR [16]. Dyslipidemia was diagnosed by a self-reported physician-diagnosed, and/or current use of lipid-lowering drugs, and/or total cholesterol (TC) ≥ 240 mg/dl, triglyceride (TG) ≥ 150 mg/dl, high-density lipoprotein cholesterol (HDL-C) < 40 mg/dl, low-density lipoprotein cholesterol (LDL-C) ≥ 160 mg/dl [17]. Cancer and heart disease were determined by participants’ self-reported. Body mass index (BMI) was calculated as the following formula: weight/height2 (kg/m2). The AIP was calculated as log (TG/HDL-C) [18].
Follow up of endpoint events
The primary focus of the study was on the occurrence of the first stroke, which included both cerebral infarction and cerebral hemorrhage. Self-reported stroke was assessed with the following questions: “Have you been diagnosed with stroke by a doctor”; “Have you been diagnosed with stroke by a doctor since the last follow-up visit?”; “Compared to when we interviewed you last time, is your stroke condition better, about the same as it was then, or worse?”. The time of stroke events was determined by participants’ responses to questions: “When was the stroke first diagnosed or known by yourself?”; “When was your most recent stroke?”. All participants were followed up through five-wave interviews conducted from 2011 to either the occurrence of a stroke or 2020, whichever came first.
Statistical analysis
Normally distributed continuous data were presented as mean ± standard deviation and analyzed for statistical significance using a one-way ANOVA. Non-normally distributed continuous data were expressed as median and interquartile range and analyzed by the Kruskal–Wallis test. Categorical data were described with counts and percentages and assessed using the chi-square test. Missing data of the participants included in this study were presented in Table S1 (see Additional file), and the multiple imputations method was applied to impute the missing data, assuming that the data was randomly missing.
Participants were divided into four groups according to the quartile level of the AIP: Quartile 1 (Q1), AIP ≤ 0.122; Quartile 2 (Q2), 0.122 < AIP ≤ 0.329; Quartile 3 (Q3), 0.329 < AIP ≤ 0.562; Quartile 4 (Q4), AIP > 0.562. In addition to its use as a categorical variable, AIP was also analyzed as a continuous variable to enhance the reliability of the results. Based on AIP grouping, the Kaplan–Meier method was used to estimate the cumulative incidence of stroke, and differences were compared using the log-rank test. Cox proportional hazard regression analyses were conducted to investigate the relationship between baseline AIP and the occurrence of stroke and to calculate the hazard ratio (HR) and 95% confidence interval (CI). Before analyzing, the assumption of proportional hazards was visually assessed by calculating the schoenfeld residuals. Three models were estimated: Model 1 applied an unadjusted model to estimate crude HR; Model 2 included adjustments for age, gender, marital status, drinking, smoking, residence, SBP, DBP, and BMI; Model 3 included adjustments for the variables in Model 2 as well as history of hypertension and heart disease, TC, FPG, HbA1c. The quartile 1 group was set as the reference in all models. In addition, restricted cubic splines analysis based on multivariable-adjusted Cox regression was conducted to visualize the linear or nonlinear relationship between baseline AIP levels and the risk of stroke. Moreover, to determine the prognostic value of AIP for stroke in different glucose metabolic states, we analyzed participants with NGR, Pre-DM, and DM, respectively. Subgroup analyses were stratified by baseline age (< 60 and ≥ 60 years), gender, BMI (< 24 and ≥ 24 kg/m2), residence (rural and urban), hypertension, and glucose metabolic states (NGR, Pre-DM, and DM) to assess the consistency of the adverse effect of AIP on new-onset stroke. To ensure the reliability of our findings, we performed two additional sensitivity analyses. Firstly, participants who had fasted for less than 8 h before blood collection were excluded. Secondly, we excluded participants with missing data for SBP, DBP, HR and BMI.
All statistical analyses were performed using IBM SPSS Statistics (Version 26), R (Version 4.3.2) and Rstudio (Version 1.3.1093). A two-sided P-value < 0.05 was considered to indicate statistical significance in the present study.
Results
General characteristics of participants
The baseline clinical and demographic characteristics of participants grouped by AIP quartile were presented in Table 1. The average age of participants at baseline was 58.04 ± 8.75 years, with 4742 (54.3%) being female. Participants in higher AIP quartiles tended to be younger, female, married, and better-educated compared to those in the lowest quartile. Additionally, they had lower proportions of rural residents, current smokers, and current alcohol consumers. The prevalence of hypertension, diabetes, dyslipidemia, and heart disease was higher among those in higher AIP quartiles. Moreover, SBP, DBP, heart rate, BMI, FPG, HbA1c, TC, TG, and LDL levels were elevated, while HDL-C levels were lower in these groups. The general characteristics were compared between the included and excluded participants in Table S2 (see Additional file). No significant differences were observed in hypertension, FPG, and TG between the included and excluded subjects.
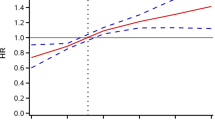
Association of AIP and the risk of stroke using a multivariable-adjusted restricted cubic spines model. Restricted cubic spline analysis has four knots at the 5th, 35th, 65th, and 95th percentiles of AIP. A total participants; B participants with NGR; C participants with Pre-DM. D participants with DM
Predictive value of baseline AIP for the first stroke
During an average follow-up period of 8.72 years, 734 (8.4%) participants experienced their first stroke. According to the AIP quartiles, the incidences of stroke, from Q1 to Q4, were 5.65, 7.99, 10.18, 13.55 per 1000 person-years, respectively. Analysis of the Kaplan–Meier cumulative incidence curve revealed a gradual increase in stroke events from the Q1 to Q4 groups, with statistically significant difference observed (Fig. 2A log-rank test P < 0.0001). The Cox proportional hazard models confirmed a significant relationship between baseline AIP levels and new-onset stroke. Baseline AIP was analyzed as both a continuous variable and a categorical variable (quartiles). Following adjustment for potential confounding factors, per 1-unit increase in baseline AIP was associated with a 90% higher risk of stroke in Model 3 (HR 1.90, 95% CI 1.52–2.36). Furthermore, the risk of stroke showed an increasing trend across quartiles of AIP in Model 3 (HR 1.34, 95% CI 1.05–1.71 for Q2; HR 1.52, 95% CI 1.19–1.93 for Q3; HR 1.84, 95% CI 1.45–2.34 for Q4; p-trend 0.001, Table 2). Multivariable-adjusted restricted cubic splines analysis also demonstrated a significant dose-response relationship between the AIP as a continuous variable and the risk of stroke (P for overall trend < 0.001; P for nonlinear = 0.2551)(Fig. 3A).
Associations between AIP and stroke regulated by individual glucose metabolic states
During the follow-up period, 219 (6.3%) participants with NGR, 352 (8.7%) participants with Pre-DM and 163 (13.4%) participants with DM were detected with the first stroke. The Kaplan–Meier curves (Fig. 2B–D) showed a significant difference in the cumulative incidence of stroke among Pre-DM and DM across the four AIP groups (P < 0.0001), while no significant difference was observed for NGR (P = 0.57). The results presented in Table 3 indicated that, in comparison to Q1, other AIP groups showed a significant association with an increased risk of stroke in individuals with Pre-DM and DM in Model 3. Specifically, for individuals with Pre-DM, HR were 1.49 (95% CI 1.03–2.16) for Q2, 1.80 (95% CI 1.26–2.57) for Q3, and 2.27 (95% CI 1.60–3.23) for Q4, with a p-trend of 0.001. In individuals with DM, HR were 3.08 (95% CI 1.16–8.20) for Q2, 3.95 (95% CI 1.54–10.12) for Q3, and 4.58 (95% CI 1.83–11.47) for Q4, with a p-trend of 0.001. However, no significant difference was found among AIP groups in individuals with NGR in the three Cox models (all p-values > 0.05). The restricted cubic splines analysis showed a notable increase in the risk of stroke in individuals with Pre-DM and DM as baseline AIP rises, demonstrating a linear relationship (Pre-DM: P for nonlinear = 0.1193; DM: P for nonlinear = 0.3121) (Fig. 3C–D). Conversely, the analysis did not reveal a significant dose-response correlation between AIP and the risk of stroke in individuals with NGR (Fig. 3B).
Subgroup analysis
To further explore the association between baseline AIP and the first stroke event, we performed a subgroup analysis stratified by potential risk factors. As shown in Table 4, elevated AIP levels were associated with a higher incidence of stroke, which was consistent across different subgroups including age, gender, BMI, residence, and hypertension. Among individuals with Pre-DM and DM, increased AIP levels were linked to a higher stroke risk, whereas this association was not observed in the NGR groups. Significant interactions were noted between AIP and BMI (P value for interaction = 0.011) as well as between AIP and glucose metabolic status (P value for interaction = 0.031). However, no significant interactions were detected between AIP and other variables (all P values for interaction > 0.05).
Sensitivity analyses
Several sensitivity analyses were carried out to assess the robustness of the results. After excluding 707 participants who fasted for less than 8 h prior to blood collection, Cox regression analyses generated consistent results with the primary analysis (see Additional file, Table S3, S4). Furthermore, there was no substantial change in the association between baseline AIP and stroke risk even after excluding participants with missing data for SBP, DBP, BMI and heart rate (see Additional file, Table S5, S6).
Discussion
In the large national longitudinal survey cohort of middle-aged and elderly individuals, a significant correlation was elaborated between higher baseline AIP levels and an increased risk of a new-onset stroke. This association was particularly prominent in those with abnormal glucose metabolism, including Pre-DM and DM. The study suggested that baseline AIP might be a dependable biomarker for stratifying stroke risk. Maintaining a low AIP level might be beneficial for the primary prevention of stroke in individuals with abnormal glucose metabolism.
Dyslipidaemia and IR are features of the metabolic syndrome, both of which contribute to the risk of stroke [19,20,21]. The AIP is widely recognized as a reliable marker of dyslipidemia and atherosclerosis. Several studies have demonstrated that both baseline and cumulative AIP exposure are linked to cardiovascular diseases, particularly coronary artery disease [18, 22,23,24]. Notably, the impact of AIP on cardiovascular diseases may differ depending on an individual’s glucose metabolic state. Min et al. have found that individuals with abnormal glucose metabolism and higher AIP levels may have a greater risk of developing cardiovascular diseases [25]. In addition, higher levels of AIP have been found to be positively correlated with the risk of hypertension and non-alcoholic fatty liver disease, which is potentially influenced by the glucose metabolic states [26,27,28]. Interestingly, a cross-sectional study has demonstrated a strong association among elevated AIP levels, an increased risk of IR, and the onset of type 2 diabetes [6]. Elevation of TG or low HDL-C levels in the fasting or post-prandial state is observed in approximately half of individuals with type 2 diabetes, and is also frequently found in individuals with IR or impaired glucose tolerance [29, 30]. Research suggests that atherogenic dyslipidaemia is a significant risk factor for cardiovascular disease in individuals with abnormal glucose metabolism [31]. Further investigation is warranted to determine if AIP levels can serve as a marker for Pre-DM and DM. Recent research has explored the association between AIP and cerebrovascular disease, revealing that higher AIP levels are correlated with a higher incidence of atherosclerotic stenosis in the carotid and intracranial arteries [10, 32]. In the general population, increased baseline and cumulative AIP levels are associated with a greater risk of ischemic stroke [9, 11]. To the best of our knowledge, this study is the first to demonstrate the significant predictive value of baseline AIP level for stroke in individuals with glycemic dysregulation.
This study found that high baseline AIP levels were associated with new-onset stroke in individuals with Pre-DM and DM, which was consistent with previous reports that high AIP was associated with the risk and prognosis of stroke. Therefore, assessing AIP levels in middle-aged and elderly individuals with glycemic dysregulation would have clinical significance. Further studies are warranted to investigate the baseline levels of AIP, which is able to predict and identify stroke risk. Clinical studies have shown a significant correlation between stroke prognosis and the topography of stroke [33]. Consequently, it is valuable to investigate the potential impact of baseline AIP on the location of cerebrovascular topography in stroke patients. Lowering LDL-cholesterol has been traditionally believed to be beneficial in preventing overall stroke, with statins being commonly used for this purpose [34]. However, a multicenter clinical trial shows that patients with high triglycerides levels are accompanied with a high risk of ischemic stroke, despite statin therapy [35]. Recent data also advocated for lowering triglycerides as a strategy to prevent stroke [36]. Therefore, it is plausible to consider that lowering triglycerides, which is equivalent to lowering AIP levels, contributes to stroke prevention.
Although the mechanisms underlying the relationship between AIP and stroke remain unclear, several possible explanations have been proposed. Firstly, triglycerides appear to be related to vascular inflammation and subclinical atherosclerosis. Raised serum triglycerides can potentiate inflammatory responses in vascular endothelial cells and vascular smooth muscle cells, especially in diabetic patients [37, 38]. Emerging evidence indicates that triglycerides-rich lipoproteins like chylomicrons and very low-density lipoproteins may play a role in atherosclerotic lesion formation [39, 40]. Besides, HDL particles exhibit various vasoprotective properties, such as reducing cellular death, dampening inflammatory response, and shielding against pathological oxidation [41]. Therefore, it can be inferred that as AIP increase, higher levels of triglycerides lead to more significant damage to vascular structure and function, while lower levels of HDL offer less protection to the vasculature. Secondly, the level of AIP has been investigated to be closely associated with traditional risk factors for stroke, including BMI, hypertension, diabetes, dyslipidemia, and heart disease. The results of the present study are consistent with these studies. Elevated AIP levels may interact with other cerebrovascular risk factors and potentially exacerbate the progression of stroke. However, further research is necessary to fully understand the underlying mechanisms.
Strengths and limitations
This is the largest population-based investigation into the association between AIP and stroke among middle-aged and elderly individuals with glycemic dysregulation. The data was obtained from a high-quality, nationally representative longitudinal survey of the middle-aged and elderly population across various regions of China, including urban and rural areas. In order to obtain robust results, we included potential confounders to exclude interference in the results. With nearly a decade of follow-up, our analysis indicated that baseline AIP was a reliable predictor of stroke in middle-aged and elderly individuals with dysglycaemia. Moreover, since standard assay for TG and HDL-C are widely used in clinical practice and it is straightforward to calculate AIP from TG and HDL-C, it is sensible to recommend AIP as an efficient and convenient indicator for assessing the risk of stroke.
However, there are several limitations of the study that require consideration. Firstly, baseline medications such as antihypertensive, lipid-lowering, and glucose-lowering medications were not taken into account in the Cox proportional hazards model, potentially impacting the results. Secondly, while the diagnostic criteria for glucose metabolism status have been clearly defined, it remains a slight chance of misclassification for participants with borderline glycaemic status. Thirdly, some participants from the CHARLS cohort were excluded from this research due to strict exclusion criteria, resulting in a limited number of participants included. This may have introduced attrition bias. Fourthly, the study was based on middle-aged and elderly Chinese, and further validation in other ethnic and age groups is needed. Fifthly, this study focused on the impact of baseline AIP level and did not examine the longitudinal changes in AIP during the follow-up period. Sixthly, due to a lack of data on stroke subtypes in CHARLS, the study was unable to assess the effect of AIP on ischemic or hemorrhagic stroke, respectively. Finally, despite efforts to control for confounding variables, there is a possibility that some confounders were not accounted for. Further investigations are needed to verify our findings in other large cohort studies
Conclusions
In the pursuit of primary prevention strategies to reduce stroke incidence, this longitudinal prospective study found that baseline high AIP levels in individuals with glycemic dysregulation might indicate subgroups at a higher risk of developing stroke, particularly among individuals under 60 years old with a BMI ≥ 24 kg/m2 residing in rural areas. However, the levels of AIP in middle-aged and older adults without dysglycaemia did not affect the occurrence of stroke.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the China Health and Retirement Longitudinal Study repository, which can be accessed online at http://charls.pku.edu.cn. You can register as a CHARLS user to access all published data by following the necessary procedure.
Abbreviations
- AIP:
-
Atherogenic index of plasma
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- BMI:
-
Body mass index
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Hemoglobin A1c
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- GMS:
-
glucose metabolic states
- NGR:
-
Normal glucose regulation
- Pre-DM:
-
Prediabetes mellitus
- DM:
-
Diabetes mellitus
References
Owolabi MO, Thrift AG, Mahal A, Ishida M, Martins S, Johnson WD, et al. Primary stroke prevention worldwide: translating evidence into action. Lancet Public Health. 2022;7(1):e74–85.
Arboix A, Massons J, García-Eroles L, Targa C, Comes E, Parra O, et al. Nineteen-year trends in risk factors, clinical characteristics and prognosis in lacunar infarcts. Neuroepidemiology. 2010;35(3):231–6.
Wadström BN, Wulff AB, Pedersen KM, Jensen GB, Nordestgaard BG. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort-based study. Eur Heart J. 2022;43(34):3258–69.
Cai W, Xu J, Wu X, Chen Z, Zeng L, Song X, et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22(1):138.
Ago T, Matsuo R, Hata J, Wakisaka Y, Kuroda J, Kitazono T, et al. Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology. 2018;90(17):e1470–7.
Yin B, Wu Z, Xia Y, Xiao S, Chen L, Li Y. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2023;22(1):157.
Shi Y, Wen M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011–2018 population. Cardiovasc Diabetol. 2023;22(1):19.
Tan MH, Johns D, Glazer NB. Pioglitazone reduces atherogenic index of plasma in patients with type 2 diabetes. Clin Chem. 2004;50(7):1184–8.
Zhang Y, Chen S, Tian X, Xu Q, Xia X, Zhang X, et al. Elevated atherogenic index of plasma associated with stroke risk in general Chinese. Endocrine. 2024. https://doi.org/10.1007/s12020-023-03677-0.
Yu S, Yan L, Yan J, Sun X, Fan M, Liu H, et al. The predictive value of nontraditional lipid parameters for intracranial and extracranial atherosclerotic stenosis: a hospital-based observational study in China. Lipids Health Dis. 2023;22(1):16.
Zheng H, Wu K, Wu W, Chen G, Chen Z, Cai Z, et al. Relationship between the cumulative exposure to atherogenic index of plasma and ischemic stroke: a retrospective cohort study. Cardiovasc Diabetol. 2023;22(1):313.
Liu H, Liu K, Pei L, Li S, Zhao J, Zhang K, et al. Atherogenic index of plasma predicts outcomes in Acute ischemic stroke. Front Neurol. 2021;12:741754.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8.
Chen X, Crimmins E, Hu PP, Kim JK, Meng Q, Strauss J, et al. Venous blood-based biomarkers in the China Health and Retirement Longitudinal Study: Rationale, Design, and results from the 2015 Wave. Am J Epidemiol. 2019;188(11):1871–7.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–40.
Zhou Z, Ong KL, Whelton SP, Allison MA, Curtis AJ, Blaha MJ, et al. Impact of blood lipids on 10-year cardiovascular risk in individuals without dyslipidemia and with low risk factor burden. Mayo Clin Proc. 2022;97(10):1883–93.
Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic index of plasma: Novel Predictive Biomarker for Cardiovascular illnesses. Arch Med Res. 2019;50(5):285–94.
DeBoer MD, Filipp SL, Sims M, Musani SK, Gurka MJ. Risk of ischemic stroke increases over the spectrum of metabolic syndrome severity. Stroke. 2020;51(8):2548–52.
He Q, Wang W, Li H, Xiong Y, Tao C, Ma L, et al. Genetic insights into the risk of metabolic syndrome and its components on stroke and its subtypes: bidirectional mendelian randomization. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2023;43(2suppl):126–37.
Zhang F, Liu L, Zhang C, Ji S, Mei Z, Li T. Association of metabolic syndrome and its components with risk of Stroke recurrence and mortality: a Meta-analysis. Neurology. 2021;97(7):e695–705.
Kim SH, Cho YK, Kim YJ, Jung CH, Lee WJ, Park JY, et al. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovasc Diabetol. 2022;21(1):81.
Alifu J, Xiang L, Zhang W, Qi P, Chen H, Liu L, et al. Association between the atherogenic index of plasma and adverse long-term prognosis in patients diagnosed with chronic coronary syndrome. Cardiovasc Diabetol. 2023;22(1):255.
Wang Y, Wang S, Sun S, Li F, Zhao W, Yang H, et al. The predictive value of atherogenic index of plasma for cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention with LDL-C below 1.8mmol/L. Cardiovasc Diabetol. 2023;22(1):150.
Min Q, Wu Z, Yao J, Wang S, Duan L, Liu S, et al. Association between atherogenic index of plasma control level and incident cardiovascular disease in middle-aged and elderly Chinese individuals with abnormal glucose metabolism. Cardiovasc Diabetol. 2024;23(1):54.
Li K, Li J, Cheng X, Wang J, Li J. Association between the atherogenic index of plasma and new-onset non-alcoholic fatty liver disease in non-obese participants. Front Endocrinol. 2022;13:969783.
Samimi S, Rajabzadeh S, Rabizadeh S, Nakhjavani M, Nakhaei P, Avanaki FA, et al. Atherogenic index of plasma is an independent predictor of metabolic-associated fatty liver disease in patients with type 2 diabetes. Eur J Med Res. 2022;27(1):112.
Tan M, Zhang Y, Jin L, Wang Y, Cui W, Nasifu L, et al. Association between atherogenic index of plasma and prehypertension or hypertension among normoglycemia subjects in a Japan population: a cross-sectional study. Lipids Health Dis. 2023;22(1):87.
Scott R, O’Brien R, Fulcher G, Pardy C, D’Emden M, Tse D, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate intervention and event lowering in diabetes (FIELD) study. Diabetes Care. 2009;32(3):493–8.
Annuzzi G, Giacco R, Patti L, Di Marino L, De Natale C, Costabile G, et al. Postprandial chylomicrons and adipose tissue lipoprotein lipase are altered in type 2 diabetes independently of obesity and whole-body insulin resistance. Nutrition, metabolism, and cardiovascular diseases. NMCD. 2008;18(8):531–8.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Huang Q, Liu Z, Wei M, Huang Q, Feng J, Liu Z, et al. The atherogenic index of plasma and carotid atherosclerosis in a community population: a population-based cohort study in China. Cardiovasc Diabetol. 2023;22(1):125.
Arboix A, García-Eroles L, Sellarés N, Raga A, Oliveres M, Massons J. Infarction in the territory of the anterior cerebral artery: clinical study of 51 patients. BMC Neurol. 2009;9:30.
Sun L, Clarke R, Bennett D, Guo Y, Walters RG, Hill M, et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med. 2019;25(4):569–74.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular Risk reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22.
Aradine E, Hou Y, Cronin CA, Chaturvedi S. Current status of Dyslipidemia Treatment for Stroke Prevention. Curr Neurol Neurosci Rep. 2020;20(8):31.
Wang YI, Schulze J, Raymond N, Tomita T, Tam K, Simon SI, et al. Endothelial inflammation correlates with subject triglycerides and waist size after a high-fat meal. Am J Physiol Heart Circ Physiol. 2011;300(3):H784–91.
Gordillo-Moscoso A, Ruiz E, Carnero M, Reguillo F, Rodriguez E, Tejerina T, et al. Relationship between serum levels of triglycerides and vascular inflammation, measured as COX-2, in arteries from diabetic patients: a translational study. Lipids Health Dis. 2013;12:62.
Öörni K, Lehti S, Sjövall P, Kovanen PT. Triglyceride-Rich Lipoproteins as a source of Proinflammatory Lipids in the arterial wall. Curr Med Chem. 2019;26(9):1701–10.
Raposeiras-Roubin S, Rosselló X, Oliva B, Fernández-Friera L, Mendiguren JM, Andrés V, et al. Triglycerides and residual atherosclerotic risk. J Am Coll Cardiol. 2021;77(24):3031–41.
Kontush A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovasc Res. 2014;103(3):341–9.
Acknowledgements
The authors would like to thank all participants of the CHARLS.
Funding
This research was supported by the Natural Science Foundation of Jiangsu Province (BK20211005 to J.J., BK20231120 to X.Z.), the Key Research and Development Program of Jiangsu Province of China (BE2020620 to Y.X.), the STI2030-Major Projects (2022ZD0211800 to Y.X.), the Jiangsu Province Key Medical Discipline (ZDXK202216 to Y.X.) and Nanjing Medical Science and Technology Development Foundation (ZKX22025 to X.Z.).
Author information
Authors and Affiliations
Contributions
JJ, XZ and LQ were involved in the design of this study; LQ and SF contributed to manuscript writing; SX and YP were reponsible for data collection and data management; LQ, JJ, and ZL contributed to the statistical analysis; JJ, XZ and YX participated in data review and manuscript revision. All authors have reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015). The participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qu, L., Fang, S., Lan, Z. et al. Association between atherogenic index of plasma and new-onset stroke in individuals with different glucose metabolism status: insights from CHARLS. Cardiovasc Diabetol 23, 215 (2024). https://doi.org/10.1186/s12933-024-02314-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02314-y