Abstract
Background
The incidence of myocardial infarction (MI) and sudden cardiac death (SCD) is significantly higher in individuals with Type 2 Diabetes Mellitus (T2DM) than in the general population. Strategies for the prevention of fatal arrhythmias are often insufficient, highlighting the need for additional non-invasive diagnostic tools. The T-wave heterogeneity (TWH) index measures variations in ventricular repolarization and has emerged as a promising predictor for severe ventricular arrhythmias. Although the EMPA-REG trial reported reduced cardiovascular mortality with empagliflozin, the underlying mechanisms remain unclear. This study investigates the potential of empagliflozin in mitigating cardiac electrical instability in patients with T2DM and coronary heart disease (CHD) by examining changes in TWH.
Methods
Participants were adult outpatients with T2DM and CHD who exhibited TWH > 80 µV at baseline. They received a 25 mg daily dose of empagliflozin and were evaluated clinically including electrocardiogram (ECG) measurements at baseline and after 4 weeks. TWH was computed from leads V4, V5, and V6 using a validated technique. The primary study outcome was a significant (p < 0.05) change in TWH following empagliflozin administration.
Results
An initial review of 6,000 medical records pinpointed 800 patients for TWH evaluation. Of these, 412 exhibited TWH above 80 µV, with 97 completing clinical assessments and 90 meeting the criteria for high cardiovascular risk enrollment. Empagliflozin adherence exceeded 80%, resulting in notable reductions in blood pressure without affecting heart rate. Side effects were generally mild, with 13.3% experiencing Level 1 hypoglycemia, alongside infrequent urinary and genital infections. The treatment consistently reduced mean TWH from 116 to 103 µV (p = 0.01).
Conclusions
The EMPATHY-HEART trial preliminarily suggests that empagliflozin decreases heterogeneity in ventricular repolarization among patients with T2DM and CHD. This reduction in TWH may provide insight into the mechanism behind the decreased cardiovascular mortality observed in previous trials, potentially offering a therapeutic pathway to mitigate the risk of severe arrhythmias in this population.
Trial registration
NCT: 04117763.
Similar content being viewed by others
Background
The global incidence of Type 2 Diabetes Mellitus (T2DM) is reaching critical levels, projected to affect approximately 600 million individuals by 2035 [1]. This condition markedly increases the prevalence of cardiovascular diseases (CVD) and generates a concern highlighted by the higher rates of post-myocardial infarction (MI) mortality and a greater frequency of sudden cardiac death (SCD) among those with diabetes, in comparison to non-diabetic individuals [2, 3].
Often, SCD is the first clinical indication of an underlying, undetected cardiac disorder. Traditional risk factors fall short in accurately predicting these fatal arrhythmias, underscoring the need for more precise predictive tools [4]. SCD is generally preceded by an electrical disturbance, prompting recent shifts towards non-invasive methods for risk assessment using electrocardiographic indicators [5]. One such marker is the T-wave heterogeneity index (TWH) that measures the variance of waveforms around the T-wave’s average waveform, effectively capturing the spatial disparity in heart repolarization [4]. TWH has proven valuable in accurately stratifying the risk of sudden cardiac death, overall cardiac mortality, and arrhythmic episodes in a diverse range of cardiovascular conditions [6,7,8,9].
Empagliflozin, a sodium-glucose cotransporter-2 inhibitor (SGLT2i), has emerged as a key player in cardiovascular healthcare, a status underscored by EMPA-REG trial [10]. This study highlighted a 14% reduction in relative risk for a combined outcome of cardiovascular death, non-fatal MI, and non-fatal stroke, with a notable 38% decrease in cardiovascular mortality, including sudden death cases. These benefits were significant even in patients already under comprehensive cardiovascular risk management and were observable as soon as 27 days post-treatment initiation [11]. The rapid and significant effects of empagliflozin on cardiovascular outcomes in patients with high-risk T2DM are acknowledged, yet the mechanisms behind these effects remain partly unclear. Dapagliflozin, another SGLT2i, has been shown to reduce ventricular arrhythmias, cardiac arrests, and SCD in conjunction with standard heart failure (HF) treatments [12]. Additionally, preclinical studies bolster the hypothesis that empagliflozin may diminish the risk of fatal arrhythmias [13]. These findings are crucial for managing patients with high-risk cardiovascular diabetes and underscore the need for focused clinical trials to investigate further the potential effect of SGLT2i in the prevention of cardiac arrhythmias. While there’s evidence suggesting a potential reduction in fatal arrhythmias with empagliflozin [14], its specific anti-arrhythmic actions and influence on SCD susceptibility in T2DM and CHD patients still require further elucidation. Our study aimed to explore whether empagliflozin can attenuate ventricular arrhythmogenesis and lower the risk of SCD by assessing TWH in diabetic patients with coronary artery disease, contributing to the evolving field of cardiovascular care in patients with diabetes.
Methods
Study design
The EMPATHY-HEART Trial employed an exploratory pilot study design to investigate the influence of empagliflozin on ventricular repolarization heterogeneity in patients diagnosed with both T2DM and CHD. The choice of an exploratory design emerged from the imperative to elucidate the potential impacts of empagliflozin on ventricular electrical instability, an aspect with limited prior exploration. Given the limited number of clinical trials utilizing interlead T-wave heterogeneity, the study aimed to establish the basis for future research and hypothesis development.
Participants
Outpatients at Heart Institute of the Faculty of Medicine of the University of Sao Paulo (InCor), Brazil, were invited to participate. The study included individuals 18 years and older, diagnosed with T2DM, and showing evidence of CHD, indicated by a history of myocardial infarction, significant coronary stenosis, or a positive test for myocardial ischemia. Additionally, participants required a baseline TWH on ECG of 80 µV or higher, following the criteria established by Tan et al. [7]. Exclusion criteria comprised chronic kidney disease (glomerular filtration rate below 45 ml/min/1.73 m²); advanced hepatic disease (Child-Pugh B or C); age over 85 years and an uninterpretable 12-lead baseline ECG, due to conditions like pacemaker rhythms or signal distortions. In this context, “baseline” ECG refers to the electrocardiogram conducted at the first study visit.
Intervention
Under the guidance of a sole researcher (CL), all participants underwent comprehensive clinical assessments. They received a daily prescription of empagliflozin 25 mg, aligning with current clinical guidelines emphasizing its cardiovascular benefits in high-risk diabetic populations. This dosage selection aimed specifically to investigate empagliflozin’s effects on the prevention of cardiac arrhythmias. Tailored adjustments to hypoglycemic regimens were made as necessary to minimize the risk of hypoglycemia, ensuring a standardized yet personalized approach for participant safety and treatment efficacy.
Patients performed a 12-lead ECG at their initial visit and again after 4 weeks. During the follow-up, a clinical reassessment and treatment adherence analysis were conducted. This 4-week period aligns with findings from a post-hoc analysis indicating a notable decrease in cardiovascular death or HF hospitalization starting from day 27 post-randomization [11]. Treatment adherence was evaluated through direct questioning of each participant [15].
Data collection
Data Management and Statistical Analysis Data acquisition and management were conducted using the REDCap (Research Electronic Data Capture) platform [16].
Resting electrocardiograms were conducted using Mortara Eli 250 C (2013) or GE Healthcare MAC 2000 (2019) equipment, capturing 12 leads simultaneously in a 4 × 3 format, with a paper speed of 25 mm/s and a 10-mV gain. Electrode placement followed standard technical guidelines [17].
TWH was calculated specifically in leads V4, V5, and V6, chosen for their lower variability due to electrode positioning and patient’s body constitution [18], and their proven effectiveness in detecting electrical instability and arrhythmia risk [6, 7].
Two independent researchers, CL and GLA, blinded to the patients’ treatment status, performed the TWH calculations for each ECG.
Participant ECGs were extracted from InCor’s electronic records as PDFs, anonymized, and converted to TIFF format. ECGScan software (AMPS-LLC, New York, NY) translated the waveforms [19], which were then turned into text files using ISHNE and CalECG softwares (AMPS-LLC, New York, NY) [20]. These files were analyzed in Microsoft Excel (365 version). Data from leads V4, V5, and V6 were overlaid, aligned by the PR segment, and synchronized at the QRS complex onset. The second central moment algorithm was applied to the J-T wave interval of each cardiac cycle to determine the point of highest morphology variance. The square root of this variance provided the TWH value in microvolts per beat. The TWH index, representing the average TWH of all recorded beats, indicates repolarization nonuniformity; higher values suggest a greater risk of life-threatening ventricular arrhythmias [7]. Detailed TWH calculation methods from resting 12-lead ECGs are described elsewhere [4, 5, 9].
Statistical analysis
The primary goal of our study was to investigate how TWH levels were altered by empagliflozin administration. To ensure reliability and agreement of ECG data analyzed by the independent researchers we utilized the Intraclass Correlation Coefficient (ICC). To enhance result precision, we excluded extreme outliers, namely, subjects whose TWH levels were more than 3 standard deviations above the population mean, to reduce data skewness. The Shapiro-Wilk test was employed for normality evaluation. Variables were analyzed using Student’s t-test for normal distributions and the Wilcoxon rank-sum test for non-parametric data, including TWH, to determine empagliflozin’s effects. Multivariate analysis utilized MANOVA, with all tests adhering to a significance threshold of p < 0.05, reflecting a 5% significance level. Statistical analyses were conducted using R, a software environment for statistical computing and graphics [21]. Data are presented as means ± standard deviation (S.D.)
Ethical considerations
Recruitment for this study occurred between October 2019 and October 2023. Each participant gave written informed consent, having been fully briefed on aims, methods, and potential risks. Adhering to the Declaration of Helsinki’s principles, this clinical trial was approved by the Institutional Review Board of Clinical Hospital of the Medical School of the University of Sao Paulo, under registration number SDC: 4732/18/083. The research is registered on ClinicalTrials.gov with the identifier NCT04117763.
Results
Patient characteristics
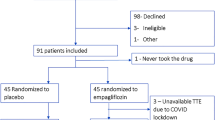
Among the potential candidates screened, 412 had TWH equal to or greater than 80 µV. Of these, 90 participants were included in the study. Figure 1 illustrates the flowchart of participant inclusion. The demographic and clinical profiles of the participants, as outlined in Table 1, indicated a cohort with a notably high cardiovascular risk. This included the majority having experienced myocardial infarctions, over half displaying a tri-arterial pattern in invasive stratification, and nearly a quarter having undergone coronary artery bypass grafting. Only one patient was included based on the criterion of a positive non-invasive test for myocardial ischemia. As expected in a specialized heart hospital setting, participants were optimized for cardiovascular risk reduction. The majority were on statins (90%) and beta-blockers (84%). Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were used by 77% of the patients. Additionally, 11% were on furosemide, 21% on spironolactone, and none on sacubitril/valsartan. No modifications were made to the HF treatment regimen of the patients, except for the addition of empagliflozin. For diabetes management, 24% were on basal insulin therapy, and 11% adhered to a basal-bolus insulin therapy regimen. Only two subjects were not on antiplatelet agents.
Clinical parameters
Adherence to treatment exceeded 80% across all participants. Consistent with prior evidence [10], our study found that empagliflozin significantly reduced blood pressure without significantly affecting heart rate (Table 2).
Hypoglycemia was noted in 13.3% of participants, with a predominant occurrence at Level 1 [15] (91.7%), and no reports of serious episodes. Urinary tract infections were observed in 4.4%, while genital fungal infections occurred in 11.1% of the patients.
T-Wave heterogeneity
TWH values for each ECG, assessed by independent researchers, showed high reliability, as reflected by ICC values of 0.9 for both baseline and follow-up phases, with p-values < 0.001. This robust concordance validated aggregating data from both researchers for subsequent data analysis and presentation.
Following empagliflozin treatment, we noted a significant reduction in TWH values, indicating a decrease in ventricular repolarization heterogeneity. Specifically, mean baseline TWH values fell from 127 µV to 114 µV at follow-up, with a median reduction from 116 µV to 103 µV. This change, statistically significant with a p-value of 0.01, underscores a meaningful difference attributed to empagliflozin treatment.
Visual analysis of the data presented in the Boxplot in Fig. 2 indicates increased variability in TWH values post-treatment, as shown by the rise in standard deviation from 43.9 to 55.7 µV and in the interquartile range from 46.2 to 67.8 µV. The presence of outliers above the third quartile, both before and after treatment, highlights a persistent dispersion in TWH values, irrespective of therapy.
Representative digitized ECG tracings for a patient during the study are provided in Fig. 3. The baseline ECG, depicted in the upper panel, shows pronounced T-wave heterogeneity, with significant variability in T-wave morphology across different leads. Following a 4-week treatment with empagliflozin, the lower panel presents ECG tracings that demonstrate a noticeable reduction in TWH, evidenced by a decrease in variability among T-wave forms.
Figure 4 reveals a trend toward lower TWH levels after treatment across the study population. About one-third of the participants achieved a TWH reduction to 80 µV or below post-treatment. Predominantly, the optimal responders – those whose TWH diminished to 80 uV or less – initially had TWH values below 100 uV.
A multivariate analytical method (MANOVA) was employed to explore the connections among clinical variables, including HbA1c levels, time since diabetes diagnosis, incidence of angina, previous MI, MI localization, classification in invasive stratification, and Left Ventricular Ejection Fraction (LVEF), with the goal of identifying a significant association with TWH reduction. In this analysis, LVEF was categorized into two groups: patients with an LVEF greater than or equal to 50%, representing preserved ejection fraction, and those with an LVEF below 50%, representing reduced ejection fraction. HbA1c levels were similarly categorized with a cutoff at 8%. Single-vessel disease and diabetes duration emerged as significant factors, leading to their inclusion in a refined linear multivariate analysis model, which revealed that only single-vessel disease had a significant effect on reducing TWH (p = 0.027). Inspecting this group of patients with single-vessel disease, it was observed that the majority had a prior myocardial infarction, with the anterior wall being the most affected. The model, with a multiple R2 of 0.321 and an adjusted R2 of 0.2791, alongside a p-value of 6.183e-06, reveals moderate explanatory power and significant statistical validity.
Discussion
Participants characterized by high cardiovascular risk and treated at a specialized cardiology hospital, had increased baseline TWH, indicating a heightened susceptibility to arrhythmias or sudden cardiac death. Therapeutically, these patients were following optimal regimens, marked by widespread use of beta-blockers. Consequently, the positive effect of empagliflozin observed in this cohort point to a possible additional therapeutic benefit in addition to beta-blockers. This finding raises the intriguing possibility that beta-blockers might have slightly concealed an even greater potential of empagliflozin to diminish ventricular electrical instability. We employed the TWH level of 80 µV established by previous research to demonstrate that the risk of severe arrhythmias and SCD increases significantly when TWH levels exceed this level [7, 8]. Electrocardiograms were performed in an ambulatory setting without provocative testing for ischemia or exertion. Within a relatively brief period of four weeks of empagliflozin treatment, there was a statistically significant reduction in the TWH median.
This proof-of-concept trial showed that empagliflozin significantly reduces ventricular electrical vulnerability in patients with T2DM and CHD, evidenced by reduced T-wave heterogeneity. The findings complement recent evidence, offering new insights into these phenomena and their implications in a clinical setting for the first time.
Research into the role of SGLT2i in arrhythmia prevention is rapidly evolving. Recent studies highlight SGLT2i’s potential to reduce arrhythmia risk and SCD in diabetic and non-diabetic individuals. Key findings include the EMBODY trial’s demonstration of empagliflozin’s improvement in heart rate variability [22], and studies from Taiwan [23] and the SGLT2-I AMI PROTECT [24] indicating a 17% reduction in new arrhythmias and fewer severe arrhythmic events among SGLT2i users, respectively.
Our research corroborates a retrospective analysis of 46 T2DM patients, showing that SGLT2i decreases QTc dispersion on 12-lead ECGs without affecting heart rate, QTc interval, or Tpeak–Tend interval, particularly in those with elevated baseline QTc dispersion [25]. While not associated with HbA1c changes, a relationship was observed with systolic blood pressure variations, and post-treatment serum electrolyte levels were stable. The findings suggest that SGLT2i improves disparity in ventricular recovery times, irrespective of its effects on blood sugar levels [26]. This effect underscores the direct cardioprotective actions of SGLT2i, supported by evidence that glycemic control alone does not significantly affect QT and Tpeak-Tend dispersion [27].
While the exact mechanisms by which SGLT2i reduces arrhythmias are under investigation, it is believed that they involve multiple processes underlying cardiac arrhythmogenesis. Interest areas include their impact on cardiovascular autonomic function. Studies have shown SGLT2i decreases sympathetic activity and increases parasympathetic activity, improving autonomic balance [22]. Their influence on specific ionic currents in cardiomyocytes, reducing late-INa and spontaneous calcium transients similarly to ranolazine and lidocaine [28], has also been explored. It is relevant that blockade of sympathetic activity [29], increasing cardiac vagal tone [30], and blockade of late-INa current [31] have been shown to reduce T-wave heterogeneity. Experimental studies suggest empagliflozin directly inhibits the NHE1 exchanger and SGLT1 in cardiomyocytes [32], reducing sodium content, improving mitochondrial function, and decreasing oxidative stress, which are additional mechanisms proposed for arrhythmia reduction. Regarding ischemia-reperfusion-related arrhythmias, experimental studies on non-diabetic rats showed empagliflozin significantly reduced ventricular arrhythmias, including ventricular tachycardia and fibrillation, and eliminated SCD vulnerability [13]. Control groups had a 69.2% mortality rate, whereas empagliflozin-treated groups recorded no sudden cardiac death, indicating empagliflozin’s cardioprotective capacity through ERK1/2 phosphorylation pathway activation. Similarly, in rabbit models, empagliflozin reduced ventricular arrhythmias by improving calcium cycling and mitochondrial function [33]. Pre-ischemic use of dapagliflozin significantly reduced infarct size and cardiomyocyte apoptosis, underscoring its cardioprotective ability and potential value in minimizing cardiac damage and enhancing post-injury cardiac function [34].
In our hypothesis-generating study, we employed the TWH index as a surrogate marker for arrhythmia and SCD, given the well-established efficacy of this marker [6, 7, 9]. The significance of TWH rests on three fundamental pillars: its capacity to stratify risk by identifying individuals with high susceptibility to adverse cardiac events; its effectiveness in predicting responses to therapeutic interventions, exemplified by cardiac resynchronization therapy; and its capability in monitoring the progression of cardiac electrical instability, aiding in the evaluation of treatment efficacy [9].
Following treatment, slightly over one-third of participants experienced a reduction in TWH to below the safety threshold of 80 µV, primarily among those with initially TWH levels around 100 µV. This finding could suggest that individuals with lower baseline TWH are more likely to experience significant treatment benefits, aligning with safer TWH levels post-treatment.
However, patients’ responses to the treatment varied, as indicated by the wide distribution and high standard deviation of observed changes. This variation could be clinically significant, suggesting that while there is a general trend towards TWH reduction, individual reactions to the treatment can vary significantly. Surprisingly, a few patients experienced an increase in TWH after the intervention. This finding, emerging from this pilot study, signals a complexity that exceeds the initial scope of the research but warrants future investigation.
Recent clinical trials have highlighted the efficacy of empagliflozin in reducing the risk of hospitalization and mortality due to HF. This effect has been observed in patients with both reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF), and extends to individuals with or without diabetes.The EMPEROR-Reduced study [35] found that empagliflozin significantly decreased the risk of the primary composite outcome of cardiovascular death or hospitalization for worsening HF in patients with HFrEF by 25%. The EMPEROR-Preserved study [36] identified a 21% reduction in the risk of cardiovascular death or hospitalization for HF in patients with HFpEF, primarily due to a 29% reduction in HF hospitalizations. Both EMPEROR studies focused on evaluating the effects of empagliflozin in HF patients, regardless of the specific cause of their HF. In addition, it’s important to note that EMPEROR trials addressed HF as syndrome and no mechanistic insights were provided. In our study population, the prevalence of HF was notable, with 74 of 90 patients (82%) diagnosed with HF. In addition, it’s important to note that EMPEROR study addressed HF as syndrome and no mechanistic insights were provided. The etiology of HF in our patients was ischemic cardiomyopathy. For 7 patients, there was no information on left ventricular ejection fraction (LVEF); 50 patients had an LVEF above 50%, 13 had an LVEF below 40%, and 14 had an LVEF between 40% and 49%. The main contribution of our study is to provide a possible mechanism to explain the benefits of empagliflozin. Indeed, we hypothesized that the significant changes observed in TWH after empagliflozin treatment, the main finding of our study, are either exclusively or partially related to HF. However, this hypothesis requires further investigation in future studies.
Our study faces certain limitations, including a small sample size, limited duration, and a non-randomized design, which may affect the generalizability of our results. Additionally, the particular demographics of our patient cohort call for careful consideration when applying these findings to wider populations. To substantiate and broaden our conclusions, future research should involve larger, more varied groups and longer observation times.
The exact molecular mechanisms by which empagliflozin attenuates ventricular arrhythmogenesis remain to be fully elucidated. This gap highlights an opportunity for future research, particularly at the molecular and cellular levels, to unravel the intricate pathways involved in the antiarrhythmic effects of SGLT2 inhibitors.
Our findings add a piece to the complex puzzle of cardiovascular management in diabetes mellitus, underscoring empagliflozin’s potential in mitigating arrhythmic risks. While enhancing our understanding of empagliflozin’s cardiovascular benefits, our study paves the way for novel research directions and clinical applications, promising significant advancements in patient care.
Conclusions
This pilot study demonstrated that empagliflozin reduces ventricular repolarization heterogeneity in patients with T2D and CAD, suggesting that a reduction in severe arrhythmias may be among the mechanisms contributing to the observed decrease in cardiovascular mortality with this treatment.
Exploring TWH in diabetic patients with coronary artery disease opens new avenues for standardizing treatment and simplifying methodologies across this patient population. By delving into this area, we can lay the groundwork for developing products that directly calculate TWH. This approach not only has the potential to enhance clinical outcomes but also offers a basis for innovation in patient management and treatment optimization. Such advancements could significantly contribute to individualized medicine, ensuring that interventions are more accurately tailored to individual patient profiles, thereby improving efficacy and patient care in the field of coronary and diabetic health.
Data availability
Data is available upon request.
Abbreviations
- CHD:
-
Coronary Heart Disease
- ECG:
-
Electrocardiogram
- HF:
-
Heart Failure
- ICC:
-
Intraclass Correlation Coefficient
- InCor:
-
Heart Institute of the Faculty of Medicine of the University of Sao Paulo
- Late-INa:
-
Late Sodium Current
- LVEF:
-
Left Ventricular Ejection Fraction
- MANOVA:
-
Multivariate Analysis of Variance
- MI:
-
Myocardial Infarction
- PDF:
-
Portable Document Format
- REDCap:
-
Research Electronic Data Capture
- SCD:
-
Sudden Cardiac Death
- SD:
-
Standard Deviation
- SGLT2i:
-
Sodium-glucose Cotransporter-2 Inhibitor
- T2DM:
-
Type 2 Diabetes Mellitus
- TIFF:
-
Tagged Image File Format
- TWH:
-
T-Wave Heterogeneity
References
Forouhi NG, Wareham NJ. Epidemiology of diabetes. Med (Abingdon England: UK). 2014;42:698–702.
Sarwar N, Gao P, Seshasai SRK, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (London England). 2010;375:2215–22.
Mukamal KJ, Nesto RW, Cohen MC, Muller JE, Maclure M, Sherwood JB, et al. Impact of diabetes on long-term survival after acute myocardial infarction. Diabetes Care. 2001;24:1422–7.
Verrier RL, Huikuri H. Tracking interlead heterogeneity of R- and T-wave morphology to disclose latent risk for sudden cardiac death. Heart Rhythm. 2017;14:1466–75.
Nearing BD, Verrier RL. Tracking cardiac electrical instability by computing interlead heterogeneity of T-wave morphology. J Appl Physiol. 2003;95:2265–72.
Kenttä TV, Nearing BD, Porthan K, Tikkanen JT, Viitasalo M, Nieminen MS, et al. Prediction of sudden cardiac death with automated high-throughput analysis of heterogeneity in standard resting 12-lead electrocardiograms. Heart Rhythm. 2016;13:713–20.
Tan AY, Nearing BD, Rosenberg M, Nezafat R, Josephson ME, Verrier RL. Interlead heterogeneity of R- and T‐wave morphology in standard 12‐lead ECGs predicts sustained ventricular tachycardia/fibrillation and arrhythmic death in patients with cardiomyopathy. J Cardiovasc Electrophysiol. 2017;28:1324–33.
Hekkanen JJ, Kenttä TV, Haukilahti MAE, Rahola JT, Holmström L, Vähätalo J, et al. Increased beat-to-beat variability of T-wave heterogeneity measured from standard 12-lead electrocardiogram is associated with sudden cardiac death: a case-control study. Front Physiol. 2020;11:1045.
Verrier RL, Nearing BD, D’Avila A. Spectrum of clinical applications of interlead ECG heterogeneity assessment: from myocardial ischemia detection to sudden cardiac death risk stratification. Ann Noninvasive Electrocardiol. 2021;26:e12894.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Verma S, Leiter LA, Sharma A, Zinman B, Mattheus M, Fitchett DH, et al. How early after treatment initiation are the CV benefits of empagliflozin apparent? A post Hoc analysis of EMPA-REG OUTCOME. Diabetes. 2020;69:28–OR.
Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42:3727–38.
Hu Z, Ju F, Du L, Abbott GW. Empagliflozin protects the heart against ischemia/reperfusion-induced sudden cardiac death. Cardiovasc Diabetol. 2021;20:199.
Fernandes GC, Fernandes A, Cardoso R, Penalver J, Knijnik L, Mitrani RD, et al. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: a meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021;18:1098–105.
Agiostratidou G, Anhalt H, Ball D, Blonde L, Gourgari E, Harriman KN, et al. Standardizing clinically meaningful outcome measures beyond HbA(1c) for type 1 diabetes: a consensus report of the American association of clinical endocrinologists, the American association of diabetes educators, the American diabetes association, the endocrine society, JDRF international, the Leona M. and Harry B. Helmsley charitable trust, the pediatric endocrine society, and the T1D exchange. Diabetes Care. 2017;40:1622–30.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81.
Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, et al. Recommendations for the standardization and interpretation of the Electrocardiogram. Circulation. 2007;115:1306–24.
Kania M, Rix H, Fereniec M, Zavala-Fernandez H, Janusek D, Mroczka T, et al. The effect of precordial lead displacement on ECG morphology. Med Biol Eng Comput. 2014;52:109–19.
Badilini F, Erdem T, Zareba W, Moss AJ. ECGScan: a method for conversion of paper electrocardiographic printouts to digital electrocardiographic files. J Electrocardiol. 2005;38:310–8.
Badilini F. The ISHNE holter standard output file format. Ann Noninvasive Electrocardiol. 1998;3:263–6.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2023.
Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19:148.
Chen HY, Huang JY, Siao WZ, Jong GP. The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19:73.
Cesaro A, Gragnano F, Paolisso P, Bergamaschi L, Gallinoro E, Sardu C, et al. In-hospital arrhythmic burden reduction in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: insights from the SGLT2-I AMI PROTECT study. Front Cardiovasc Med. 2022;9:1012220.
Sato T, Miki T, Ohnishi H, Yamashita T, Takada A, Yano T, et al. Effect of sodium-glucose co‐transporter‐2 inhibitors on impaired ventricular repolarization in people with type 2 diabetes. Diabet Med. 2017;34:1367–71.
Inzucchi SE, Kosiborod M, Fitchett D, Wanner C, Hehnke U, Kaspers S, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation. 2018;138:1904–7.
Miki T, Tobisawa T, Sato T, Tanno M, Yano T, Akasaka H, et al. Does glycemic control reverse dispersion of ventricular repolarization in type 2 diabetes? Cardiovasc Diabetol. 2014;13:125.
Philippaert K, Kalyaanamoorthy S, Fatehi M, Long W, Soni S, Byrne NJ, et al. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation. 2021;143:2188–204.
Justo F, Fuller H, Nearing BD, Rajamani S, Belardinelli L, Verrier RL. Inhibition of the cardiac late sodium current with eleclazine protects against ischemia-induced vulnerability to atrial fibrillation and reduces atrial and ventricular repolarization abnormalities in the absence and presence of concurrent adrenergic stimulation. Heart Rhythm. 2016;13:1860–7.
Nearing BD, Anand IS, Libbus I, Dicarlo LA, Kenknight BH, Verrier RL. Vagus nerve stimulation provides multiyear improvements in autonomic function and cardiac electrical stability in the ANTHEM-HF study. J Card Fail. 2021;27:208–16.
Bonatti R, Silva AFG, Batatinha JAP, Sobrado LF, Machado AD, Varone BB, et al. Selective late sodium current blockade with GS-458967 markedly reduces ischemia-induced atrial and ventricular repolarization alternans and ECG heterogeneity. Heart Rhythm. 2014;11:1827–35.
Joshi SS, Singh T, Newby DE, Singh J. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart. 2021;107:1032–8.
Azam MA, Chakraborty P, Si D, Du B, Massé S, Lai PFH, et al. Anti-arrhythmic and inotropic effects of empagliflozin following myocardial ischemia. Life Sci. 2021;276:119440.
Lahnwong C, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol. 2020;19:91.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with Empagliflozin in Heart failure. N Engl J Med. 2020;383(15):1413–24. https://doi.org/10.1056/nejmoa2022190.
Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61. https://doi.org/10.1056/nejmoa2107038.
Acknowledgements
We extend our deepest gratitude to the patients for their willing participation, which has significantly enriched our study. Our thanks also go to the teams at the Interdisciplinary Medicine Unit in Cardiology, the Fulvio Pileggi Research Center at the Heart Institute, and Professor Verrier’s team at Harvard for their support and knowledge sharing.
Funding
Funding for this research was obtained from The Sao Paulo Research Foundation (FAPESP) under the record 2020/02668-2 and The National Council for Scientific and Technological Development (CNPQ) under the record 309454/2020-4.
Author information
Authors and Affiliations
Contributions
CL conducted the study as part of her PhD project. GLA and FGS assisted in screening participants and calculating TWH. AEF assisted in organizing participant visits and in editing figures. RLV developed the TWH analysis technique, reviewed the manuscript, and provided suggestions. ACCG and BC supervised the project’s execution and contributed with suggestions and guidance.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The main study protocol was approved by the Institutional Review Board of Clinical Hospital of the Medical School of the University of São Paulo, under registration number SDC: 4732/18/083. All participants gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lauretti, C., Antonio, G.L., Fernandes, A.E. et al. Empagliflozin’s role in reducing ventricular repolarization heterogeneity: insights into cardiovascular mortality decline from the EMPATHY-HEART trial. Cardiovasc Diabetol 23, 221 (2024). https://doi.org/10.1186/s12933-024-02311-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02311-1