Abstract
Background
Insulin resistance and chronic kidney disease are both associated with increased coronary artery disease risk. Many formulae estimating glucose disposal rate in type 1 diabetes infer insulin sensitivity from clinical data. We compare associations and performance relative to traditional risk factors and kidney disease severity between three formulae estimating the glucose disposal rate and coronary artery disease in people with type 1 diabetes.
Methods
The baseline glucose disposal rate was estimated by three (Williams, Duca, and Januszewski) formulae in FinnDiane Study participants and related to subsequent incidence of coronary artery disease, by baseline kidney status.
Results
In 3517 adults with type 1 diabetes, during median (IQR) 19.3 (14.6, 21.4) years, 539 (15.3%) experienced a coronary artery disease event, with higher rates with worsening baseline kidney status. Correlations between the three formulae estimating the glucose disposal rate were weak, but the lowest quartile of each formula was associated with higher incidence of coronary artery disease. Importantly, only the glucose disposal rate estimation by Williams showed a linear association with coronary artery disease risk in all analyses. Of the three formulae, Williams was the strongest predictor of coronary artery disease. Only age and diabetes duration were stronger predictors. The strength of associations between estimated glucose disposal rate and CAD incidence varied by formula and kidney status.
Conclusions
In type 1 diabetes, estimated glucose disposal rates are associated with subsequent coronary artery disease, modulated by kidney disease severity. Future research is merited regarding the clinical usefulness of estimating the glucose disposal rate as a coronary artery disease risk factor and potential therapeutic target.
Similar content being viewed by others
Background
Insulin resistance, commonly observed in type 2 diabetes, can also occur in people with type 1 diabetes [1]. This is sometimes referred to as ‘double diabetes’, and is characterised by the presence of components of the metabolic syndrome, such as adiposity, hyperglycaemia, hypertension, and dyslipidaemia [2], and is associated with a higher risk of micro- and macrovascular complications and death [3,4,5,6]. A definition of the metabolic syndrome has been agreed upon by an international consensus group, but notably, this definition is not specific for people with type 1 diabetes [7]. Whilst the gold standard method of quantifying insulin sensitivity, the euglycaemic hyperinsulinaemic clamp [8] is not feasible in large studies or in clinical practice. Many formulae using clinically available variables have been developed to estimate insulin sensitivity, such as the estimated glucose disposal rate (eGDR) [9,10,11,12,13]. These equations have been derived from clamp studies in several dozens of people with type 1 diabetes, usually adults, without or with only a few with chronic complications, potentially limiting generalisability. Furthermore, validation of eGDR calculated from these formulae and GDR measured in independent clamp studies showed rather weak correlations, with r < 0.3 [10, 11, 14]. Nevertheless, eGDR calculated from some formulae have been shown to be associated with subsequent diabetic kidney disease [4, 15, 16], diabetic retinopathy [4], cardiovascular disease [15, 17], and mortality [18] in people with type 1 diabetes. We are not aware of any publications that have evaluated more than one eGDR formulae in relation to subsequent chronic diabetes complications, nor evaluated eGDR performance according to diabetic kidney disease status.
We aim, thus, to compare three eGDR formulae [10,11,12] that are based on the three available clamp studies performed in adult Caucasians with type 1 diabetes, and assess their relationship with subsequent incidence of coronary artery disease (CAD) over a long follow-up in a large cohort of adults with type 1 diabetes, including evaluating eGDR formulae performance according to baseline kidney disease severity.
Methods
Study participants
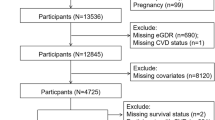
All participants are from the FinnDiane Study, an ongoing, multicentre, observational, Finnish study founded in 1997, including 77 study centres (Additional file 1: Table S1), which aims to discover clinical, genetic, and environmental risk factors for micro- and macro-vascular complications of type 1 diabetes [2]. We included 3517 participants with type 1 diabetes, defined as age of diabetes onset < 40 years and insulin treatment from within 1-year of diagnosis who had their baseline visit before the end of 2017 (median 2000, IQR 1998, 2002). We excluded individuals with pre-existing (pre-baseline) cardiovascular events, including myocardial infarction, coronary revascularisation, stroke, amputations, or peripheral artery revascularisation, as well as those with kidney replacement therapy or an estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73 m2. We further excluded individuals with missing data required for eGDR calculation by the three formulae of interest.
Baseline clinical characteristics were as previously described [2]. Anthropometrics: weight in light clothing to the closest 0.1 kg, height to the closest one cm, waist circumference midway between lowest ribs and iliac crest and hip circumference at widest part of gluteal region, both to closest 0.5 cm; blood pressure (BP): the mean of two measures of systolic and diastolic BP taken seated after ten minutes rest; medical history: including history of diabetes and its complications, current medications, insulin pump use, and lifestyle by validated questionnaires; clinical chemistry: venous blood for creatinine, HbA1c, lipids, and lipoproteins; kidney status: eGFR calculated by chronic kidney disease epidemiology collaboration equation [19], albuminuria status based on albumin excretion rate (AER) in two out of three urine collections classified as: normal AER (< 20 µg/min or < 30 mg/24 h), moderately increased albuminuria (20–200 µg/min or 30–300 mg/24 h), or severely increased albuminuria (> 200 µg/min or > 300 mg/24 h). Diabetic kidney disease severity as per Kidney Disease: Improving Global Outcomes (KDIGO) risk categories: low risk (normal AER and eGFR ≥ 60 ml/min/1.73 m2, N = 2513), moderately increased risk (normal AER and eGFR 45–59 ml/min/1.73 m2, or moderately increased albuminuria and eGFR ≥ 60 ml/min/1.73 m2, N = 516), as well as high (N = 251) and very high risk (N = 237) (normal AER and eGFR < 45 ml/min/1.73 m2, moderately increased albuminuria and eGFR < 60 ml/min/1.73 m2, or severely increased albuminuria irrespective of eGFR) [20]. Due to relatively low numbers, the high and very high KDIGO risk groups were combined.
eGDR and the metabolic syndrome
eGDR was estimated by three formulae for adult Caucasians:
-
1.
Modified Williams formula: The original formula [12], modified for use of HbA1c vs. HbA1 [2]. eGDR = 24.4−(12.97*WHR)-(3.39*AHT)−(0.60*HbA1c [%]), where WHR = waist-to-hip ratio and AHT = antihypertensive treatment and/or BP ≥ 140/90 mmHg (yes = 1; no = 0).
-
2.
Duca formula: The best fit formula without adiponectin by Duca et al. [10]: eGDR = exp[4.1075-(0.01299*waist circumference [cm])-(1.05819*daily insulin dose per body weight [IU/kg]−(0.31327*triglycerides [mmol/L])−(0.00802*diastolic BP [mmHg]).
-
3.
Januszewski formula [11]: eGDR = 6.6743 + (6.1818*sex [Woman = 0; Man = 1]) + (0.0708*age [years]) + (7.4104*HDL cholesterol [mmol/L])−(0.1692*pulse pressure [mmHg])−(0.0894*serum creatinine [µmol/L]).
Insulin resistance was defined as the lowest quartile of eGDR by each formula in this study [21]. In addition, suggested eGDR cut-offs from the literature of four, six, and eight mg/kg/min [18], with numbers lower than these values being regarded as insulin resistant, have also been evaluated in relationship to incident CAD. The metabolic syndrome, as a categorical variable was as defined according to the Joint Statement criteria [7].
Coronary artery disease (CAD)
CAD events (N = 539) were identified for all participants until the end of 2020 from the Finnish Care Register for Health Care, Finnish Institute for Health and Welfare, and from the death registry, Statistics Finland. CAD was defined as first coronary artery disease event of acute myocardial infarction (ICD (international classification of diseases)-8/9: 410 and 412; ICD-10: I21–23), coronary revascularisation (NOMESCO (Nordic Medico-Statistical Committee) Classification of Surgical Procedures: codes FNA (Connection to coronary artery from internal mammary artery), FNB (Connection to coronary artery from gastroepiploic artery), FNC (Aorto-coronary venous bypass), FND (Aorto-coronary bypass using prosthetic graft), FNE (Coronary bypass using free arterial graft), FNF (Coronary thromboendarterectomy), FNG (Expansion and recanalisation of coronary artery), TFN40 (Catherisation of heart with balloon widening of coronary vessels), FN1AT (Endovascular dilatation of coronary arteries), FN1BT (Extensive endovascular dilatation of coronary arteries), FN1YT (Percutaneous insertion of coronary artery stent), and FN2 (Other procedures on coronary arteries)), or CAD as immediate or underlying cause of death (ICD-9: 410–414; ICD-10: 120–I25). Individuals were followed up for at least half a year until their first CAD event, death, or until the end of 2020 for a median of 19.3 years (IQR 14.6, 21.4) years, in total 59,501 person-years of follow-up.
P-values for normally distributed continuous variables were calculated using a t-test, for non-normally distributed continuous variables a Mann–Whitney test, and for categorical variables a χ2 test. P-values to compare more than two groups were calculated using a Kruskal–Wallis test. Correlations between eGDR scores were estimated by Spearman correlation coefficients. Kaplan–Meier survival curves were constructed by quartiles of all eGDR scores and for the presence of the metabolic syndrome. Time-to-event analyses with CAD as outcome were performed using Cox proportional hazard regression models [22]. The proportional hazard assumption was tested based on Schoenfeld residuals and if violated we restricted the follow-up to 15 years as the hazards were proportional up to that time. As the different formulae use different variables to calculate eGDR, we did not adjust the analyses with further covariates to ensure a fair comparison. As the cohort was selected in a way to avoid any missing information, there was no need to address missing information. When eGDR scores were modelled as a continuous variable, we tested for linearity using the Wald test and if indicated modelled the relationship with cubic splines. Martingale residuals were assessed, and variables were log transformed when necessary. The performance of the three formulae and all their components on predicting CAD were compared using the Harrell C-Index. The analyses were repeated on three subsets of individuals with varying KDIGO status: low, moderate and those with a high or a very high status combined in one set. All analyses were performed in R (R Core Team version 4.2.2, Vienna, Austria).
Results
Baseline characteristics
Demographics are provided in Table 1, including a subdivision by subsequent CAD status. From 3517 FinnDiane Study participants, 539 experienced a CAD event during the 19-year follow-up. At baseline those who subsequently developed CAD vs. those who did not, were older, had longer diabetes duration, higher BP, higher BMI and waist height ratio, higher triglycerides, total and LDL cholesterol concentrations, lower HDL cholesterol concentrations, and were more likely to be in the KDIGO category high or very high. Additionally, they were more likely to have the metabolic syndrome, lower eGDR scores, to be on antihypertensive and lipid-lowering drugs, and more likely to have a history of smoking. Insulin pumps were used by 3.5 vs. 6.3% of those who did vs. did not develop CAD respectively (p = 0.02).
Comparison of insulin resistance by eGDR formulae’s lowest quartile and metabolic syndrome
There was little overlap in the number of insulin-resistant individuals when defined as being in the lowest quartile in each of the eGDR formulae (Additional file 1: Fig. S1). The frequency of the metabolic syndrome in participants considered insulin-resistant, i.e., the lowest quartile of eGDR by the Williams, Duca, and Januszewski eGDR formulae, was 64, 71, and 64%, respectively. In comparison, for those in the highest quartile of eGDR, the corresponding frequencies were 17, 16, and 17%.
As a continuous score calculated with the three assessed formulae, the eGDRs were significantly (p < 0.001), albeit weakly, correlated: The Spearman correlation coefficient was 0.42 for Williams vs. Duca scores; 0.10 for Williams vs. Januszewski; and 0.10 for Duca vs. Januszewski.
eGDR, the metabolic syndrome, and KDIGO risk categories
Baseline characteristics by KDIGO categories are provided in Additional file 1: Table S2. Worsening kidney disease was associated with higher rates of CAD, male sex, longer diabetes duration, younger age of diabetes onset, higher HbA1c concentrations, and worse traditional risk factors, such as adiposity, BP, and lipids. For all three formulae, the eGDR decreased with worsening KDIGO risk category, and, in addition, the percentage of individuals with metabolic syndrome increased with higher KDIGO category.
Associations between baseline eGDR quartiles and subsequent CAD
Kaplan–Meier curves for incidence of CAD (Additional file 1: Fig. S2) by eGDR quartiles showed increasing separation of curves over follow-up time, with different patterns of spread between formulae, reaching statistical significance for all eGDR formulae: eGDR by Williams and Januszewski, both p < 0.001, and eGDR by Duca, p = 0.015. Similarly, metabolic syndrome status curves separated significantly (p < 0.001) over time, with higher CAD rates in those with vs. without the metabolic syndrome at baseline (Additional file 1: Fig. S3).
Risk for CAD based on baseline eGDR score and by kidney disease severity
At all the proposed cut-offs for eGDR calculated by the Williams and Januszewski formulae, we found an association with the CAD incidence (Table 2). The strengths of the associations varied depending on the cut-off, but was stronger for scores based on the Williams formula at all cut-offs. For the Duca formula, only the lowest quartile of its eGDR was associated with incidence of CAD. When restricting the follow-up time to maximum of 15 years, the hazard ratio (HR) for CAD incidence decreased linearly with an increasing eGDR score for all three formulae (Fig. 1A–C), indicating that any increase in eGDR (improvement in insulin sensitivity) was cardioprotective. In Fig. 1D, the HRs are defined per score percentile and therefore allow for a direct comparison of the strength of the association for all three formulae. When using the C-index, the Williams-derived eGDR discriminated individuals with regards to CAD incidence either better or at least equally well compared to the other formulae. Only in the lowest score percentiles, the HRs based on Januszewski were higher, but their 95% confidence intervals (CI) overlapped with the Williams formula, e.g., in the 0.28 percentile Januszewski-based HR was 9.29 [6.46, 13.36], whereas the Williams-based HR was 7.69 [5.78, 10.24].
Additional file 1: Figures S4, S5, and S6 show the HR for the 15 years incidence of CAD, separately for each KDIGO category. In all three categories, when using the eGDR score by Williams, the HR for CAD decreased linearly with increasing insulin sensitivity.
Comparison of baseline eGDR scores with other risk factors for subsequent CAD
As shown in Fig. 2 and Additional file 1: Tables S3–S6, age and diabetes duration showed the highest C-index for the association with incident CAD for the whole cohort and for each KDIGO risk category. In the full cohort, the eGDR score by Williams had a higher C-index compared to the Januszewski score (0.69, 95% CI [0.67, 0.72] vs. 0.62 [0.60, 0.65]) and the Duca score (0.53 [0.51, 0.56]). This was observed in the low KDIGO category (Additional file 1: Table S4), however in the moderate (Additional file 1: Table S5) as well as the pooled high and very high KDIGO categories (Additional file 1: Table S6), there were no significant differences between the eGDR scores among the three formulae as the 95% CIs overlapped. However, in the KDIGO categories high and very high, the C-index for Januszewski was nominally higher than the C-index for Williams, but the CIs overlapped (0.58, [0.54, 0.63] vs. 0.57 [0.52, 0.61]).
Discussion
In the present study, we compared the performance of three different eGDR formulae with respect to their ability to predict subsequent incident CAD at different stages of kidney disease severity in people with type 1 diabetes. We found that correlations between the three assessed formulae were weak keeping with findings from a cross-sectional clamp study series [11]. Importantly, eGDR formulae are derived from different individuals, may have had variations in clamp methodology, and include different factors with different weightings. It is also recognised that eGDR likely reflects other processes, which modulate the insulin sensitivity. However, despite the weak correlations between the eGDR formulae, the lowest eGDR quartiles of all formulae, which represents the individuals with insulin resistance, were associated with higher risk of CAD. This was true also for the metabolic syndrome, in line with previous studies [23]. Regarding the group with the lowest quartile of eGDR, the overlapping number of subjects for two or three formulae was low, indicating that the different formulae identify different individuals with lower insulin sensitivity, which also makes comparison of the formulae’s risk prediction performance relevant.
Whilst both measured and estimated insulin sensitivity may vary with kidney disease severity [2, 24], clamp studies usually exclude people with moderate or severe kidney disease, and eGDR formulae specific for type 1 diabetic kidney disease have not been developed, nor have existent formulae been systematically assessed at different stages of kidney disease, although kidney disease is a major predictor of CAD. Therefore, it is an important and novel finding that the performance of the three eGDR formulae for subsequent CAD events varied by eGDR formulae and KDIGO risk category.
Nonetheless, the Williams eGDR score was the strongest (based on C-index) predictor of CAD overall. Only the non-modifiable risk factors age and diabetes duration were stronger predictors than eGDR by Williams. Some differences in the Williams, Duca, and Januszewski clamp cohorts could potentially explain the different abilities of their respective eGDR formulae to predict CAD (Additional file 1: Table S7). The individuals in the Duca cohort were somewhat older compared to the Williams cohort (mean age 45.6 vs. 35.9 years), but the Williams cohort had a higher HbA1c (9.5%) compared both to the Duca cohort (7.6%) as well as the Januszewski cohort (7.7%). As both age and glycaemic control are independent and important risk factors for CAD, the cohorts are at different risks of developing CAD to begin with, which might also influence the models’ different abilities to predict CAD. It is, however, worth noting that although the characteristics differ between the FinnDiane participants and the Williams cohort, the eGDR by Williams was a strong predictor of CAD.
Particularly with the increasing global incidence and prevalence rates of type 1 diabetes [25] and also of obesity in people with type 1 diabetes, eGDR formulae may be useful as a surrogate endpoint in clinical research and clinical practice. In the full cohort, the Williams eGDR score ranked higher than its components, but systolic BP and pulse pressure predicted CAD equally well. In the moderate KDIGO category, the components of the Williams formula performed equally well, but waist-height ratio performed nominally better than the Williams score. In the KDIGO low category, pulse pressure, a component of the Januszewski formula, outperformed the score, and in all other settings, pulse pressure performed at least equally well. In addition, the Januszewski formula also includes serum creatinine, which possibly explains its performance in the KDIGO high and very high cohort. In the overall cohort, the continuous Williams eGDR score was a better predictor of CAD than the dichotomous metabolic syndrome score. Of note, the waist-height ratio which is a marker of central fatness and is linked to insulin sensitivity, performed similar to the presence of the metabolic syndrome for the prediction of CAD in the entire cohort and in all KDIGO categories. This finding highlights the relevance of central obesity in the metabolic syndrome and is also aligned with a previous publication showing that the waist-height ratio is associated with visceral fat mass independent of sex and kidney disease status in adults with type 1 diabetes [26].
Importantly, only the eGDR by Williams showed a linear association with CAD risk in all (sub-) analyses. Therefore, eGDR based on Williams would lend itself well to evaluating the effects of interventions, which may improve insulin sensitivity and might be a better choice than a categorical variable such as the metabolic syndrome. Potential interventions are weight-loss, exercise and muscle gain, insulin sensitiser drugs (e.g., metformin, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and incretin-based drugs), and the use of insulin pumps. There are past and ongoing trials of adjunct therapy in people with type 1 diabetes and large real-world databases, which could be used to test the facility of eGDR scores in clinical trials and in clinical practice.
Study strengths include the large, observational ‘real-world’ FinnDiane cohort with detailed characterisation of participants, long and ongoing follow-up, moderately high rates of CAD, and wide range of kidney status. Limitations are that not all existent insulin sensitivity estimating formulae have been evaluated [27]. However, importantly, the eGDR formulae are derived from a small number of clamp studies. We included one formula each from the clamp studies from Williams, Duca, and Januszewski, and excluded the clamp studies from youth and non-Caucasian populations. Furthermore, this small number of clamp studies included only few people with kidney disease and were performed in different populations, limiting the generalisability of eGDR formulae for other ages, ethnicities, and body habitus. The original formula by Duca (and also another formula by Januszewski) included serum adiponectin, which associates strongly with insulin sensitivity. Due to limited data on adiponectin, for this study we selected the best fit formula without adiponectin, which might have impacted on the weaker results observed for the Duca eGDR formula. There is uncertainty as to the effects of different types of lifestyles, drugs, and insulin delivery modality on eGDR. Furthermore, CAD may be silent in people with diabetes, which if anything would dilute our data.
Conclusions
Scoring adults with type 1 diabetes based on three formulae to estimate insulin sensitivity matters, as the lowest quartile of each score was associated with CAD. While some individual components of the eGDR formulae performed better than the eGDR score in predicting incident CAD, an eGDR score provides insight to insulin sensitivity, beyond CAD risk estimation, and offers a broader risk score that could succinctly evaluate treatment or lifestyle interventions. Notably, the strength of association varies by formula and kidney disease status. As a continuous measure to assess CAD risk, the Williams eGDR score appears particularly promising due to its linear association that is independent of kidney disease subclass.
Availability of data and materials
The datasets are not publicly available due to the consent provided by the participant at the time of data collection. The data access, which is subject to local regulations, can be obtained upon reasonable request by contacting: Maaria Puupponen (email: maaria.puupponen@helsinki.fi), Research Program Coordinator, Clinical and Molecular Metabolism (CAMM), University of Helsinki. Upon approval, analysis needs to be performed on a user-specific local server (with protected access) and requires the applicant to sign non-disclosure and secrecy agreements.
Abbreviations
- AER:
-
Albumin excretion rate
- AHT:
-
Antihypertensive treatment
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- C-index:
-
Concordance index
- eGFR:
-
Estimated glomerular filtration rate
- eGDR:
-
Estimated glucose disposal rate
- FN1AT:
-
Endovascular dilatation of coronary arteries
- FN1BT:
-
Extensive endovascular dilatation of coronary arteries
- FN1YT:
-
Percutaneous insertion of coronary artery stent
- FN2:
-
Other procedures on coronary arteries
- FNA:
-
Connection to coronary artery from internal mammary artery
- FNB:
-
Connection to coronary artery from gastroepiploic artery
- FNC:
-
Aorto-coronary venous bypass
- FND:
-
Aorto-coronary bypass using prosthetic graft
- FNE:
-
Coronary bypass using free arterial graft
- FNF:
-
Coronary thromboendarterectomy
- FNG:
-
Expansion and recanalization of coronary artery
- HbA1c :
-
Glycated haemoglobin
- HDL:
-
High density lipoprotein
- HR:
-
Hazard ratio
- ICD:
-
International classification of diseases
- IQR:
-
Interquartile range
- LDL:
-
Low density lipoprotein
- NOMESCO:
-
Nordic Medico-Statistical Committee
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- SGLT2:
-
Sodium-glucose cotransporter-2
- TFN40:
-
Catherisation of heart with balloon widening of coronary vessels
- WHR:
-
Waist-to-hip ratio
References
Donga E, Dekkers OM, Corssmit EP, Romijn JA. Insulin resistance in patients with type 1 diabetes assessed by glucose clamp studies: systematic review and meta-analysis. Eur J Endocrinol. 2015;173:101–9.
Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, Waden J, Ronnback M, Rosengard-Barlund M, Bjorkesten CG, Taskinen MR, Groop PH, FinnDiane Study G. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care. 2005;28:2019–24.
Ekstrand AV, Groop PH, Gronhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1998;13:3079–83.
Linn W, Persson M, Rathsman B, Ludvigsson J, Lind M, Andersson Franko M, Nystrom T. Estimated glucose disposal rate is associated with retinopathy and kidney disease in young people with type 1 diabetes: a nationwide observational study. Cardiovasc Diabetol. 2023;22:61.
Martin FI, Hopper JL. The relationship of acute insulin sensitivity to the progression of vascular disease in long-term type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987;30:149–53.
Pambianco G, Costacou T, Orchard TJ. The prediction of major outcomes of type 1 diabetes: a 12-year prospective evaluation of three separate definitions of the metabolic syndrome and their components and estimated glucose disposal rate: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes Care. 2007;30:1248–54.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr., International Diabetes Federation Task Force on E, Prevention, Hational Heart L, Blood I, American Heart A, World Heart F, International Atherosclerosis S, International Association for the Study of O. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214-223.
Dabelea D, D’Agostino RB Jr, Mason CC, West N, Hamman RF, Mayer-Davis EJ, Maahs D, Klingensmith G, Knowler WC, Nadeau K. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2011;54:78–86.
Duca LM, Maahs DM, Schauer IE, Bergman BC, Nadeau KJ, Bjornstad P, Rewers M, Snell-Bergeon JK. Development and validation of a method to estimate insulin sensitivity in patients with and without type 1 diabetes. J Clin Endocrinol Metab. 2016;101:686–95.
Januszewski AS, Sachithanandan N, Ward G, Karschimkus CS, O’Neal DN, Jenkins AJ. Estimated insulin sensitivity in Type 1 diabetes adults using clinical and research biomarkers. Diabetes Res Clin Pract. 2020;167: 108359.
Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–32.
Zheng X, Huang B, Luo S, Yang D, Bao W, Li J, Yao B, Weng J, Yan J. A new model to estimate insulin resistance via clinical parameters in adults with type 1 diabetes. Diabetes Metab Res Rev. 2017;33: e2880.
Uruska A, Zozulinska-Ziolkiewicz D, Niedzwiecki P, Pietrzak M, Wierusz-Wysocka B. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J Clin Lipidol. 2018;12:734–40.
Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care. 2007;30:707–12.
Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002;62:963–70.
Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–9.
Nystrom T, Holzmann MJ, Eliasson B, Svensson AM, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20:556–63.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53.
Therneau TM, Grambsch PM. Modeling survival data: extending the cox model. New York: Springer; 2000.
Thorn LM, Forsblom C, Waden J, Saraheimo M, Tolonen N, Hietala K, Groop PH, Finnish Diabetic Nephropathy Study G. Metabolic syndrome as a risk factor for cardiovascular disease, mortality, and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2009;32:950–2.
Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–7.
Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, Donaghue KC, International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest G, Magliano DJ, Maniam J, Orchard TJ, Rai P, Ogle GD. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10:741–60.
Parente EB, Mutter S, Harjutsalo V, Ahola AJ, Forsblom C, Groop PH. Waist-height ratio and waist are the best estimators of visceral fat in type 1 diabetes. Sci Rep. 2020;10:18575.
Januszewski AS, Jenkins AJ. Assessing insulin sensitivity in people with type 1 diabetes without euglycemic-hyperinsulinemic clamps. In: Patel VB, Preedy VR, editors. Biomarkers in diabetes biomarkers in disease methods discoveries and applications. Cham: Springer; 2022.
Acknowledgements
The authors are indebted to the late Carol Forsblom (1964–2022), the international coordinator of the FinnDiane Study Group, for his considerable contribution throughout the years and for this specific study. He took part in designing the study, prepared the dataset, and performed some of the preliminary analyses. We also acknowledge A. Sandelin, and K. Uljala (Folkhälsan Research Center, Helsinki, Finland) for their technical assistance, as well as all the physicians and nurses at each center participating in the collection of the study population (Additional file 1: Table S1).
Funding
This research was funded by grants from Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Liv och Hälsa Society, Sigrid Juselius Foundation, Finska Läkaresällskapet (Medical Society of Finland), Diabetes Research Foundation, and State funding for university-level health research by Helsinki University Hospital (TYH2023403). Open Access funding was provided by the Helsinki University Library.
Author information
Authors and Affiliations
Consortia
Contributions
S.M., E.B.P., A.S.J., P.H.G., A.J.J., and L.M.T. were responsible for the study design. E.B.P., R.S., V.H., P.H.G, and L.M.T. were responsible for the acquisition of the clinical data. S.M. was responsible for the statistical analyses. S.M., A.S.J., A.J.J., and L.M.T were responsible preparation of the first draft of the manuscript. All authors interpreted the results and contributed to the critical revision of the manuscript. All authors reviewed the manuscript and approved the final version. P.H.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee at Helsinki and Uusimaa Hospital District. Each participant gave written informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
S.M. reports receiving lecture honoraria from Encore Medical Education. E.B.P. reports receiving lecture honorariums from Sanofi and Astra Zeneca. P.H.G. reports receiving lecture honorariums from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Elo Water, Medscape, MSD, Mundipharma, Novo Nordisk, PeerVoice, Sanofi, Sciarc, and being an advisory board member of AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Medscape, MSD, Mundipharma, Nestlé, Novo Nordisk, and Sanofi. All other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Physicians and nurses at each of the FinnDiane centres participating in patient recruitment and characterisation. Table S2. Baseline clinical characteristics at according to the Kidney Disease Improving Global Outcomes (KDIGO) risk categories. Table S3. C-indexes and 95% confidence intervals (CI) with regards to coronary artery disease (CAD) for three estimated glucose disposal rate (eGDR) formulae, the metabolic syndrome and their components for the full cohort. Table S4. C-indexes and 95% confidence intervals (CI) with regards to coronary artery disease (CAD) for three estimated glucose disposal rate (eGDR) formulae, the metabolic syndrome and their components for individuals in Kidney Disease Improving Global Outcomes (KDIGO) category low. Table S5. C-indexes and 95% confidence intervals (CI) with regards to coronary artery disease (CAD) for three estimated glucose disposal rate (eGDR) formulae, the metabolic syndrome and their components for individuals in Kidney Disease Improving Global Outcomes (KDIGO) category moderate. Table S6. C-indexes and 95% confidence intervals (CI) with regards to coronary artery disease (CAD) for three estimated glucose disposal rate (eGDR) formulae, the metabolic syndrome and their components for individuals in Kidney Disease Improving Global Outcomes (KDIGO) categories high and very high. Table S7. Comparison of the FinnDiane study participants to those in the clamp studies. Figure S1. A Venn diagram for insulin resistance defined as those individuals that were ranked in the lowest quartile of each estimated glucose disposal rate (eGDR) score. Figure S2. Kaplan–Meier curves for subsequent coronary artery disease (CAD) based on baseline estimated glucose disposal rete (eGDR) quartiles for (A) eGDR by Williams; (B) eGDR by Duca, and (C) eGDR by Januszewski. Figure S3. Kaplan–Meier curves for subsequent coronary artery disease (CAD) based on metabolic syndrome (yes vs. no). Figure S4. Hazard ratio plot for coronary artery disease according to different eGDR formulae in individuals at KDIGO category low. Figure S5. Hazard ratio plot for coronary artery disease according to different eGDR formulae in individuals at KDIGO category moderate. Figure S6. Hazard ratio plot for coronary artery disease according to different eGDR formulae in individuals at KDIGO category high & very high.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mutter, S., Parente, E.B., Januszewski, A.S. et al. Insulin sensitivity estimates and their longitudinal association with coronary artery disease in type 1 diabetes. Does it matter?. Cardiovasc Diabetol 23, 152 (2024). https://doi.org/10.1186/s12933-024-02234-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02234-x