Abstract
Background
The Triglyceride Glucose-Body Mass Index (TyG-BMI) has been established as a robust indicator of insulin resistance (IR), reflecting metabolic health across various populations. In general, lower TyG-BMI values are often associated with better metabolic health outcomes and a reduced risk of adverse health events in non-critically ill populations. Previous studies have highlighted a significant negative association between TyG-BMI and all-cause mortality (ACM) among critically ill atrial fibrillation patients. Given the high prevalence and severe outcomes associated with stroke, understanding how TyG-BMI at the time of ICU admission correlates with ACM in critically ill stroke patients becomes imperative. This study aims to assess the correlation between TyG-BMI and ACM in this specific patient cohort, exploring how traditional associations between TyG-BMI and metabolic health may differ in the context of acute, life-threatening illness.
Methods
Patient data were retrieved by accessing the Medical Information Mart for Intensive Care IV (MIMIC-IV 2.2) database, categorizing patients into three groups on the basis of TyG-BMI tertiles. The study evaluated both primary and secondary outcomes: the primary outcomes included the 90-day, 180-day, and 1-year ACM, while secondary outcomes encompassed ICU, in-hospital, and 30-day ACM. Our study employed the Kaplan–Meier (K–M) curve method for outcome comparison across the groups while utilizing multivariate Cox proportional-hazards regression models and restricted cubic splines (RCS) to explore TyG-BMI association with these outcomes. Additionally, interaction and subgroup analyses were performed, focusing on different mortality time points.
Results
Among a cohort of 1707 individuals diagnosed with stroke, the average age was 68 years (interquartile range [IQR]: 58–78 years), with 946 (55.42%) of the participants being male. The analysis of K-M curves suggested that patients having a lower TyG-BMI level faced a heightened risk of long-term ACM, whereas the short-term ACM exhibited no statistically significant differences across the three TyG-BMI groups. Furthermore, Cox proportional-hazards regression analysis validated a statistically significant increased risk of long-term ACM among patients belonging to the lowest TyG-BMI tertile. Additionally, RCS analysis results demonstrated L-shaped correlations between the TyG-BMI index and both short- and long-term ACM. These findings underscore the TyG-BMI predictive value for long-term mortality in stroke patients, highlighting a nuanced relationship that varies over different time frames. The results revealed no interactions between TyG-BMI and the stratified variables, with the exception of age.
Conclusion
In our study, lower TyG-BMI levels in critically ill stroke patients are significantly related to a higher risk of long-term ACM within the context of the United States. This finding suggests the potential of TyG-BMI as a marker for stratifying long-term risk in this patient population. However, it's crucial to note that this association was not observed for short-term ACM, indicating that the utility of TyG-BMI may be more pronounced in long-term outcome prediction. Additionally, our conclusion that TyG-BMI could serve as a reliable indicator for managing and stratifying stroke patients over the long term is preliminary. To confirm our findings and assess the universal applicability of TyG-BMI as a prognostic tool, it is crucial to conduct rigorously designed research across various populations.
Similar content being viewed by others
Introduction
Epidemiological studies have indicated that cerebrovascular disease (CVD) rendered it the primary contributor to fatality and disability in American adults except for cardiovascular diseases [1, 2]. As the CVD predominant component, the incidence of stroke is on the rise, marking it as a significant global health issue responsible for substantial mortality and long-term disability [3]. Strokes are primarily categorized into ischemic and hemorrhagic types. Ischemic strokes, which constitute around 85% of all stroke cases, occur due to obstructions in the arteries that supply blood to the brain, often owing to thrombosis or embolism [4]. Hemorrhagic stroke, conversely, involves bleeding into or around the brain, usually because of weakened blood vessel rupture and includes subtypes such as subarachnoid hemorrhage (SAH) and non-traumatic intracerebral hemorrhage (ICH). ICH experiences a yearly rise of over 3.41 million instances [5], whereas SAH represents 5% of all strokes [6]. Both ischemic and hemorrhagic strokes lead to high mortality rates and extensive disabilities [7,8,9]. The increasing worldwide population aging causes a substantial stroke burden, especially in intensive care units (ICU). Thus, identifying prognostic indicators that can foresee adverse outcomes in stroke patients is of paramount importance. These indicators should be straightforward, user-friendly, cost-efficient, and readily applicable in clinical settings.
Stroke severity, a critical determinant of patient outcomes, is traditionally assessed using scales such as the NIH Stroke Scale and the Canadian Neurological Scale. These tools, while invaluable, have limitations, including complexity, time consumption, and the need for specialized training. In the search for more accessible and efficient prognostic tools, insulin resistance (IR) has been related to stroke [10] and is characterized by the body's compensatory excessive insulin production to counteract diminished insulin efficacy in glucose uptake and utilization, leading to hyperinsulinemia to maintain normoglycemia. IR represents a characteristic feature of diabetes Mellitus (DM) type 2 and metabolic syndrome [11]. The hyperinsulinemic-euglycemic clamp (HEC) represents the gold standard for IR assessment across various populations [12]. However, the homeostasis model assessment-estimated IR (HOMA-IR) index, a reliable IR measure, necessitates specialized equipment, trained personnel, substantial expenses, an extended period, and several blood samples, besides being less patient-friendly. Due to these limitations, HOMA-IR is mainly confined to research settings and not widely adopted in clinical practice due to its operational complexities and bio-efficacy concerns.
The triglyceride-glucose index (TyG-i) has recently been a straightforward surrogate IR marker. Prior research has validated its predictive capacity for cardiovascular disease outcomes that included acute myocardial infarction (AMI), coronary heart disease (CHD), heart failure (HF), stroke, and hypertension (HTN) [11, 13,14,15,16]. Notably, integrating the TyG-i along with obesity measures, including body mass index (BMI), waist circumference (WC), and waist-to-height ratio (WtHR), has significantly enhanced IR assessment accuracy [16]. The combined TyG-BMI, particularly, has shown promising alignment with HOMA-IR in evaluating IR [17] and is closely linked to HTN prevalence, non-alcoholic fatty liver disorder, and cardiovascular outcomes in coronary artery disease patients [18,19,20]. Based on the study of TyG-BMI and mortality in critically ill atrial fibrillation patients [21], We hypothesis that a significant negative association between TyG-BMI and ACM among critically ill stroke patients still existed. While the TyG-BMI's positive association with stroke risk in the Chinese general population has been documented [22,23,24], its impact on outcomes for stroke patients in the ICU remains underexplored. Given the high prevalence and severe outcomes associated with critical ill stroke patients, understanding how TyG-BMI at the time of ICU admission correlates with all-cause mortality (ACM) in critically ill stroke patients becomes imperative. This study aims to assess the correlation between TyG-BMI and ACM in this specific patient cohort, exploring how traditional associations between TyG-BMI and metabolic health may differ in the context of acute, life-threatening illness. The findings could offer new avenues for the early identification and prognostic improvement of critically ill stroke patients, addressing a significant gap in current clinical practice and research.
Methods
Source of data
This retrospective study used data sourced from the publicly accessible Medical Information Mart for Intensive Care IV database (MIMIC-IV, version 2.2), which is an enhancement of its predecessor, MIMIC-III, that features updated data and some structural modifications to tables. The dataset includes clinical information from over 190,000 unique patient admissions at Beth Israel Deaconess Medical Center (BIDMC; Boston, MA, USA) from 2008 to 2019. The database provides comprehensive details on patient demographics, vital statistics, medications, laboratory tests, surgical procedures, diagnoses, treatment regimens, and survival outcomes. To access this data, we completed the National Institutes of Health (NIH) training course on the protection of human research participants and passed the Collaborative Institutional Training Initiative's tests. An informed consent waiver was obtained because the database does not contain any protected health information, and the patients remain anonymous.
Study design and population
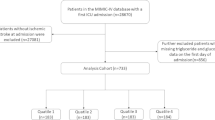
The study focused on patients who experienced a stroke and were subsequently hospitalized and admitted to the ICU for the first time. We identified a cohort of 17,860 stroke patients using the search terms “non-traumatic ICH,” “non-traumatic SAH,” and “cerebral infarction” within the International Classification of Diseases, Ninth (ICD-9) and Tenth Revision (ICD-10) diagnostic codes. The exclusion criteria were established to ensure data accuracy and relevance: (1) absence of data on triglycerides (TG), fasting blood glucose (FBG), height, or weight on first-day ICU admission; (2) patients younger than 18 years; (3) ICU stays shorter than 24 h; and (4) patients experienced multiple ICU admissions for stroke, with only the first admission’s data being considered. Following these criteria, 1,707 patients were selected and categorized into three groups depending on TyG-BMI tertiles for further analysis (Fig. 1).
Data extraction
Data extraction was performed using Navicat Premium (Version 16.1.15) employing structured query language (SQL). Comprehensive data regarding each patient at admission time were collected, such as demographic variables: gender, age, height (m), weigh (kg), and ethnicity; clinical severity scores: baseline Glasgow Coma score, sequential organ failure assessment (SOFA) score, simplified acute physiology score (SAPS)-II, acute physiology (APS) score-III, oxford acute severity of illness (OASIS) score, systemic inflammatory response syndrome (SIRS) score; baseline vital signs: the mean blood pressure (mmHg), mean heart rate (beats/min), systolic blood pressure (SBP, mmHg), respiratory rate (times/min), diastolic blood pressure (mmHg), and saturation of pulse oxygen (SPO2, %); Laboratory variables: red blood cell (RBC, 109/L), hemoglobin (Hb, g/L), platelet (109/L), white blood cell (WBC, 109/L), sodium (mmol/L); blood urea nitrogen (BUN, mg/dL); creatinine (mg/24 h); triglyceride (TG, mg/dL); fasting blood glucose (FBG, mg/dL); potassium (mmol/L), anion gap (mmol/L), prothrombin time (s), activated partial thromboplastin time (s); international normalized ratio; treatment methods: mechanical ventilation (MT), vasopressors, oxygen, thrombolysis, thrombectomy; comorbidities: HTN, DM, HF, cardiac arrhythmias, AMI, peripheral vascular disease, chronic obstructive pulmonary disease, respiratory failure, ventilator associated pneumonia (VAP), chronic kidney disease, hyperlipidemia, malignancy, renal failure, sepsis, liver disease, Charlson comorbidity index; outcomes: Length of stay (LOS) in ICU, LOS in hospital; ICU, in-hospital, 30-day; 90-day, 180-day, 1-year ACM. All blood indicators were recorded at the first measurement post-ICU admission. The dataset had no variables exceeding 20% missing values, and any missing data were imputed using multiple interpolation techniques.
Outcomes
The study focused on assessing the ACM outcomes for stroke patients within the MIMIC-IV database at various intervals. The primary endpoints were designated as long-term ACM, which included assessments at 90-day, 180-day, and 1-year post-admission. The secondary outcomes encompassed short-term ACM metrics, such as mortality within the ICU, in-hospital, and 30-day periods following admission.
TyG-BMI calculation
The TyG-i was calculated as follows: Ln[FBG (mg/dL) × TG (mg/dL)/2] while calculating BMI by dividing the body weight (kg) by the square of height (m) (kg/m2). The TyG-BMI, a composite metric, was then derived by multiplying the TyG-i by the BMI, according to the formula: TyG × BMI. This combined measure was used for the correlation assessment between metabolic status and outcomes in stroke patients.
Statistical analysis
Initial testing was performed to assess the normality of continuous variables. Student t-tests and one-way ANOVAs were utilized for normally distributed data, expressing the results as mean ± standard deviation (SD). For non-normally distributed data, the Wilcoxon rank-sum test was applied, with findings reported as medians alongside interquartile ranges (IQR). Moreover, we analyzed categorical variables through Chi-square or Fisher’s exact tests, reporting data as absolute numbers and percentages.
To evaluate the primary and secondary outcome incidence rates based on TyG-BMI stratification, we employed Kaplan–Meier (K-M) curves. Univariable Cox regression analysis was deployed to explore the relationships between TyG-BMI and both short- and long-term ACM. The multivariate Cox proportional-hazard regression model was then utilized, incorporating variables of clinical relevance or those demonstrating a univariate association with outcomes. The final model variables were selected considering the availability of event data. Model 1 was unjusted while Model 2 adjusted for age, gender, and ethnicity and Model 3 further adjusted for HTN, DM, HF, thrombolysis, thrombectomy, WBC, RBC, SBP, and SOFA score, using the lowest TyG-BMI tertile as the reference. Furthermore, TyG-BMI was examined as a continuous variable through restricted cubic splines (RCS) to elucidate dose–effect relationships with primary and secondary outcome risk. In cases of nonlinear correlations, a recursive algorithm determined the inflection points between TyG-BMI and short- and long-term ACM. This study conducted stratified analyses on the basis of age (< 60 or ≥ 60 years old), sex, race/ethnicity, HTN, and DM. Statistical analyses were performed through SPSS (version 22.0, IBM Corporation, United States) and R software (version 4.3.2, R Foundation for Statistical Computing, Austria), with P < 0.05 considering statistical significance.
Results
Baseline characteristics of study individuals
Herein, out of 17,860 stroke patients listed in the MIMIC-IV database, 1,707 met the inclusion criteria and were subsequently analyzed. They had an average age of 68 years, with an IQR of 58–78 years, and 55.4% of them were male. Our study categorized the participants into tertiles relying on their TyG-BMI upon admission: Tertile 1 (< 224.6), Tertile 2 (224.6–276.1), and Tertile 3 (> 276.1). Baseline characteristics (Table 1) revealed varying TyG-BMI levels across the groups: 196.6 for Tertile 1 (IQR: 175.8–210.3), 248.1 for Tertile 2 (IQR: 236.4–261.3), and 323.1 for Tertile 3 (IQR: 296.4–369.7).
The analysis indicated that the lowest TyG-BMI group (Tertile 1) participants were older and had lower weight, BMI, and prevalence rates of respiratory failure, hyperlipidemia, malignancy, sepsis, and VAP. They also showed lower utilization rates of MT, vasopressors, and oxygen therapy. Additionally, lower levels of RBC, Hb, WBC, BUN, creatinine, TG, and FBG, as well as shorter LOS in both the ICU and hospital, were observed in the Tertile 1 group. Conversely, this group exhibited higher levels of SpO2 than those with higher TyG-BMI levels. Regarding outcomes, the Tertile 1 group exhibited higher long-term ACM compared to the other two groups, while the short-term ACM did not significantly differ among the groups. For validity of the results, we also reported the baseline characteristics and outcomes of the excluded and included patients. More detailed results are presented in Additional file 1: Table S1.
Primary outcomes
The K-M analysis revealed varying ACM at 90-day, 180-day, and 1-year intervals across TyG-BMI tertiles (Fig. 2). Patients within the lowest TyG-BMI tertile exhibited significantly lower long-term survival rates than those with higher TyG-BMI levels, with log-rank P-values of 0.00016, 0.0016, and 0.00012, respectively. In contrast, differences in short-term ACM, including ICU, in-hospital, and 30-day mortality, among the three groups did not reach statistical significance, with log-rank P-values of 0.52, 0.68, and 0.067, respectively (Additional file 1: Fig. S1).
The clinical TyG-BMI utility for mortality prediction was determined through receiver operating characteristic (ROC) curves. However, the area under the curve (AUC) values indicated limited predictive power (ICU death AUC: 0.52; in-hospital death AUC: 0.50; 30-day death AUC: 0.53; 90-day death AUC: 0.55; 180-day death AUC: 0.56; 1-year death AUC: 0.55;) (Additional file 1: Fig. S2).
Correlation between TyG-BMI and clinical outcomes of patients having a stroke
To explore the independent TyG-BMI impact on ACM, three Cox proportional hazard regression models were employed (Table 2), adjusting Model 1 for no adjustment, Model 2 for age, gender, and ethnicity, and Model 3 for Model 2 variables, HTN, DM, HF, thrombolysis, thrombectomy, WBC, RBC, SBP, and SOFA score. In Model 3, using the lowest TyG-BMI tertile (Q1) as the reference, the hazard ratios (HRs) and 95% confidence intervals (CIs) for Q2/Q3 were 0.74 (0.59–0.92) and 0.74 (0.59–0.94) for 90-day ACM, 0.74 (0.60–0.91) and 0.69 (0.55–0.86) for 180-day ACM, and 0.75 (0.61–0.91) and 0.67 (0.54–0.83) for 1-year ACM, respectively. These findings indicate that patients with lower TyG-BMI levels faced a higher long-term ACM risk than those with a TyG-BMI index ≥ 224.6. Nonetheless, the TyG-BMI and short-term ACM relationship exhibited no significant differences (Additional file 1: Table S1).
The detection of nonlinear relationships
The RCS analyses revealed L-shaped correlations between TyG-BMI and long-term and short-term ACM, indicating a threshold effect beyond which the risk of mortality does not significantly change. Specifically, the RCS curves for long-term ACM showed significant non-linearity (Pnonlinearity = 0.16 for 90-day, Pnonlinearity = 0.09 for 180-day, Pnonlinearity = 0.09 for 1-year, depicted in Fig. 3A–C). Similarly, the short-term ACM analyses also demonstrated significant nonlinear relationships (Pnonlinearity = 0.82 for ICU, Pnonlinearity = 0.93 for in-hospital, Pnonlinearity = 0.34 for 30-day, illustrated in Additional file 1: Fig. S3A–C).
These findings suggest that beyond a certain level of TyG-BMI, the risk of both short- and long-term ACM plateaus, highlighting the significance of identifying and managing patients at risk based on their TyG-BMI levels. The exact points of non-linearity are critical for understanding the thresholds at which TyG-BMI begins to have a diminished impact on mortality risk, guiding clinical decisions regarding the monitoring and intervention for patients with stroke in both acute and long-term care settings.
Subgroup analysis
The TyG-BMI's risk stratification value for primary endpoints was further examined in multiple subgroups of the participants, including sex, age, ethnicity, HTN, and DM (Table 3, Fig. 4A–C). The TyG-BMI was significantly correlated with a lower mortality risk in stroke patients subgroups of those aged over 60 years for 90-day [HR (95% CI) 0.972 (0.956–0.988)], 180-day [HR (95% CI) 0.965 (0.949–0.980)], and 1-year [HR (95% CI) 0.966 (0.952–0.981)]. Additionally, TyG-BMI was related to a higher mortality risk in stroke patients subgroups of those aged under 60 years for 90-day [HR (95% CI) 1.032 (1.008–1.057)] and 180-day [HR (95% CI) 1.027 (1.005–1.051)]. Regarding sex, TyG-BMI was significantly correlated with a lower mortality risk in stroke patients subgroups of females for 180-day [HR (95% CI) 0.983 (0.968–0.999)], and 1-year [HR (95% CI) 0.980 (0.965–0.996)]. Regarding race/ethnicity, the TyG-BMI was significantly linked to a lower mortality risk in stroke patients subgroups of White for 90-day [HR (95% CI) 0.976 (0.958–0.995)], 180-day [HR (95% CI) 0.971 (0.953–0.989)], and 1-year [HR (95% CI) 0.970 (0.953–0.987)]. Concerning HTN, TyG-BMI had a significant relation to a lower mortality risk in stroke patients subgroups of non-HTN for 180-day [HR (95% CI) 0.978 (0.960–0.996)], both non-HTN and HTN for 1-year [HR (95% CI) 0.978 (0.962–0.995); 0.984 (0.969–0.999)]. Concerning DM, TyG-i showed a significant relation to a lower mortality risk in stroke patients subgroups of DM for 90-day [HR (95% CI) 0.978 (0.956–0.999)], 180-day [HR (95% CI) 0.970 (0.949–0.991)], and 1-year [HR (95% CI) 0.970 (0.951–0.989)]. Forest plots in Additional file 1: Fig. S4A–C depict the stratified analyses of TyG-BMI and short-term ACM.
Discussion
This retrospective study marks the first examining the correlation between TyG-BMI levels and ACM in critically ill stroke patients. Our findings identified significant L-shaped associations between TyG-BMI levels and long-term ACM, demonstrating that lower TyG-BMI levels relate to a heightened long-term ACM risk in this patient population within the United States context. While L-shaped correlations observed between TyG-BMI levels and short-term ACM did not achieve statistical significance. These insights could inform the development of clinical guidelines aimed at minimizing mortality among these patients. Accordingly, TyG-BMI emerges as a viable marker for stratifying and managing stroke patients during long-term follow-up care.
IR constitutes a standard feature of both metabolic syndrome and obesity. The global rise in obesity rates is directly linked to an increase in IR and related CVD occurrence and frequency [25]. The mechanisms by which IR can lead to stroke are multifaceted and include hyperinsulinemia and hyperglycemia, chronic low-grade inflammation, endothelial dysfunction, lipid abnormalities, HTN, and blood clotting abnormalities. IR, a documented key risk factor for stroke development and progression, can damage blood vessels and endothelium, reduce the ability of blood vessels to dilate, and alter the balance between clotting and anti-clotting factors in the blood, leading to atherosclerosis (hardening and narrowing of the arteries), increased blood pressure and a higher risk of clot formation or bleeding, ultimately causing stroke development and progression.
The TyG-i, introduced in 2008, has been acknowledged as a cost-effective, uncomplicated, and dependable substitute indicator for IR [26]. It has undergone validation and is strongly linked with stroke, acting as an independent stroke onset and progression predictor in hospitalized individuals [23, 27]. The BMI, commonly used to gauge obesity, shows a significant positive correlation with stroke incidence, as evidenced by multiple studies [28,29,30]. The combined TyG-BMI metric is a valuable tool for assessing IR [18, 21,22,23,24, 31], demonstrating its utility in evaluating major adverse cardiovascular event risk, particularly in patients having ST-elevation myocardial infarction (STEMI) and those with pre-HTN or HTN [32, 33]. Moreover, elevated TyG-BMI levels have been found to be related to an increased stroke incidence, though this relationship has primarily been documented within the Chinese population, highlighting the need for further research across different ethnicities to corroborate these findings [21,22,23,24].
The significance of the TyG-BMI in forecasting cardiovascular incidents and stroke risk is well-documented. Hou et al. have manifested that TyG-BMI and ACM possessed a U-shaped correlation in individuals suffering from CHD and HTN, thereby affirming the index's predictive accuracy for ACM within this demographic [34]. Further research underscores its utility, with one study indicating that TyG-BMI is a significant independent predictor of AF recurrence post-ablation, showcasing the highest predictive value among studied markers [35]. In intravenous thrombolysis-treated AIS patients, TyG-BMI has been identified as indicative of a negative prognosis [36]. Additionally, among patients receiving peritoneal dialysis, an elevated baseline TyG-BMI correlates with an increased ACM risk [37], reinforcing the index’s prognostic value across diverse medical scenarios. However, research on its connection with ACM in critically ill stroke patients remains scarce. Our investigation reveals that TyG-BMI can forecast long-term ACM in such patients, with a TyG-BMI and ACM L-shaped relationship being observed. This insight could be pivotal for identifying high-risk individuals in critical care settings, potentially aiding clinical trial designs. Subgroup analyses indicate that critically ill stroke patients, particularly those over 60 and White individuals, lower TyG-BMI levels exhibit higher ACM rates over 90-day, 180-day, and 1-year intervals and TyG-BMI is significant negative associated with ACM which is a protective factor. Conversely, ages under 60 years, the forest plots demonstrated that TyG-BMI is significant positive associated with ACM which is a risk factor. Furthermore, an elevated TyG-BMI appears to be beneficial for critically ill stroke patients who have non-HTN or DM, suggesting the index's broader applicability in managing and understanding stroke outcomes across diverse patient profiles. Our study shows that TyG-BMI is a highly effective clinical indicator for identifying critically ill stroke patients. Managing ICU-admitted critically ill patients is a significant subject and the primary focus of clinical practice. TyG-BMI, a readily available parameter upon ICU admission, might effectively assist clinicians in promptly identifying high-risk patients, hence reducing fatality rates and enhancing patient prognosis.
Given the robust evidence indicating that elevated TyG-BMI levels correlate with an increased risk of stroke [22,23,24], the discovery of an inverse relationship between TyG-BMI levels and mortality in our study may appear counterintuitive at first glance. This investigation centers on a cohort of patients who have not only experienced a stroke but are also critically ill. The health status and prognosis of this specific group diverge markedly from the general population that has not encountered a stroke, necessitating a nuanced interpretation of risk factors. Critically ill patients exhibit a spectrum of complex physiological alterations and metabolic dysregulations, potentially leading to a divergent pattern of risk factors compared to the general population. Furthermore, TyG-BMI serves as an indicator of insulin resistance and metabolic health. In the broader population, elevated TyG-BMI levels signal deteriorating metabolic health, thereby elevating the risk of cardiovascular incidents, including strokes. Conversely, among stroke survivors, diminished TyG-BMI levels may reflect severe malnutrition or metabolic exhaustion, conditions that are intimately linked with disease severity and an elevated mortality risk. Our findings corroborate the hypothesis of a significant negative correlation between TyG-BMI and ACM in critically ill stroke patients, aligning with previous studies [21, 38]. This alignment underscores the importance of TyG-BMI levels within the specific health trajectory of critically ill stroke patients, differentiating their risk profile from that of the general population.
Our study faces multiple constraints that merit attention. First, the retrospective nature and single-center design, relying on MIMIC-IV database-retrieved observational data, make the establishment of a definitive causal correlation challenging. Despite adjustments for numerous variables and conducting subgroup analyses, the potential impact of unaccounted confounders on our findings cannot be entirely ruled out such as subtypes of stroke, eliminations based on the National Institutes of Health Stroke Scale, stroke onset timing, and specific death causes being inaccessible within the utilized database. Second, the study's moderate sample size calls for validation through larger-scale cohort studies to reinforce our conclusions. Third, the initial blood glucose and lipid measurements were taken from patients upon their ICU admission, raising uncertainties about whether these readings were from fasting individuals. This ambiguity could affect the reliability of these variables in our analysis. Fourth, Despite the comprehensive analysis presented in this cohort, it is important to note that the study predominantly involved African-American and other racial groups within the United States. Consequently, the applicability of our results is primarily limited to the American context. Given this limitation, the extrapolation of our findings to predict critically ill stroke’s prognosis in other continents such as Europe, Asia, and Australia warrants further investigation to understand potential variations across different ethnic and geographical populations. Fifth, our investigation does not explore the biological mechanisms underpinning the TyG-BMI and ACM relation in critically ill stroke patients, leaving a gap in understanding the causative pathways. Additionally, the inability to determine the precise timing of stroke onset and identify the primary causes of death diminishes the clinical applicability of our findings. Addressing these limitations in upcoming studies could offer a more in-depth understanding of the TyG-BMI's role in predicting outcomes for critically ill stroke patients.
Conclusion
In our study, lower TyG-BMI levels in critically ill stroke patients are significantly related to a higher risk of long-term ACM within the context of the United States. This finding suggests the potential of TyG-BMI as a marker for stratifying long-term risk in this patient population. However, it's crucial to note that this association was not observed for short-term ACM, indicating that the utility of TyG-BMI may be more pronounced in long-term outcome prediction. Additionally, our conclusion that TyG-BMI could serve as a reliable indicator for managing and stratifying stroke patients over the long term is preliminary. To confirm our findings and assess the universal applicability of TyG-BMI as a prognostic tool, it is crucial to conduct rigorously designed research across various populations.
Data availability
Our contributions are detailed within the article and its Supplementary Material. For additional concerns regarding the research, please contact the corresponding author. The Supplementary Material is available online for reference.
References
Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8:345–54. https://doi.org/10.1016/S1474-4422(09)70023-7.
Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–12. https://doi.org/10.1038/s41569-018-0119-4.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, GBD-NHLBI-JACC global burden of cardiovascular diseases writing group, et al. . Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010.
Randolph SA. Ischemic stroke. Workplace Health Saf. 2016;64:444. https://doi.org/10.1177/2165079916665400.
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. https://doi.org/10.1016/S1474-4422(21)00252-0.
Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–96. https://doi.org/10.1056/NEJMra052732.
Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–48. https://doi.org/10.1161/CIRCRESAHA.116.308413.
O’Carroll CB, Brown BL, Freeman WD. Intracerebral hemorrhage: a common yet disproportionately deadly stroke subtype. Mayo Clin Proc. 2021;96:1639–54. https://doi.org/10.1016/j.mayocp.2020.10.034.
Wang YJ, Li ZX, Gu HQ, Zhai Y, Zhou Q, Jiang Y, China Stroke Statistics Writing Committee, et al. China Stroke Statistics: an update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. 2022;7:415–50. https://doi.org/10.1136/svn-2021-001374.
Ding PF, Zhang HS, Wang J, Gao YY, Mao JN, Hang CH, et al. Insulin resistance in ischemic stroke: mechanisms and therapeutic approaches. Front Endocrinol (Lausanne). 2022;13:1092431. https://doi.org/10.3389/fendo.2022.1092431.
Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr Metab Cardiovasc Dis. 2020;30:2351–62. https://doi.org/10.1016/j.numecd.2020.07.041.
Kolterman OG, Insel J, Saekow M, Olefsky JM. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980;65:1272–84. https://doi.org/10.1172/JCI109790.
Zhang R, Shi S, Chen W, Wang Y, Lin X, Zhao Y, et al. Independent effects of the triglyceride-glucose index on all-cause mortality in critically ill patients with coronary heart disease: analysis of the MIMIC-III database. Cardiovasc Diabetol. 2023;22:10. https://doi.org/10.1186/s12933-023-01737-3.
Li X, Chan JSK, Guan B, Peng S, Wu X, Lu X, et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc Diabetol. 2022;21:229. https://doi.org/10.1186/s12933-022-01658-7.
Cai W, Xu J, Wu X, Chen Z, Zeng L, Song X, et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22:138. https://doi.org/10.1186/s12933-023-01864-x.
Bala C, Gheorghe-Fronea O, Pop D, Pop C, Caloian B, Comsa H, et al. The association between six surrogate insulin resistance indexes and hypertension: a population-based study. Metab Syndr Relat Disord. 2019;17:328–33. https://doi.org/10.1089/met.2018.0122.
Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11: e0149731. https://doi.org/10.1371/journal.pone.0149731.
Wang M, Chang M, Shen P, Wei W, Li H, Shen G. Application value of triglyceride-glucose index and triglyceride-glucose body mass index in evaluating the degree of hepatic steatosis in non-alcoholic fatty liver disease. Lipids Health Dis. 2023;22:186. https://doi.org/10.1186/s12944-023-01954-5.
Nikbakht HR, Najafi F, Shakiba E, Darbandi M, Navabi J, Pasdar Y. Triglyceride glucose-body mass index and hypertension risk in iranian adults: a population-based study. BMC Endocr Disord. 2023;23:156. https://doi.org/10.1186/s12902-023-01411-5.
Cheng Y, Fang Z, Zhang X, Wen Y, Lu J, He S, et al. Association between triglyceride glucose-body mass index and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc Diabetol. 2023;22:75. https://doi.org/10.1186/s12933-023-01794-8.
Hu Y, Zhao Y, Zhang J, Li C. The association between triglyceride glucose-body mass index and all-cause mortality in critically ill patients with atrial fibrillation: a retrospective study from MIMIC-IV database. Cardiovasc Diabetol. 2024;23:64. https://doi.org/10.1186/s12933-024-02153-x.
Shao Y, Hu H, Li Q, Cao C, Liu D, Han Y. Link between triglyceride-glucose-body mass index and future stroke risk in middle-aged and elderly Chinese: a nationwide prospective cohort study. Cardiovasc Diabetol. 2024;23:81. https://doi.org/10.1186/s12933-024-02165-7.
Huang Q, Yin L, Liu Z, Wei M, Feng J, Huang Q, et al. Association of novel lipid indicators with the risk of stroke among participants in Central China: a population-based prospective study. Front Endocrinol (Lausanne). 2023;14:1266552. https://doi.org/10.3389/fendo.2023.1266552.
Du Z, Xing L, Lin M, Sun Y. Estimate of prevalent ischemic stroke from triglyceride glucose-body mass index in the general population. BMC Cardiovasc Disord. 2020;12(20):483. https://doi.org/10.1186/s12872-020-01768-8.
Chen T, Qian Y, Deng X. Triglyceride glucose index is a significant predictor of severe disturbance of consciousness and all-cause mortality in critical cerebrovascular disease patients. Cardiovasc Diabetol. 2023;22:156. https://doi.org/10.1186/s12933-023-01893-6.
RamdasNayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride Glucose (TyG) Index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022;72:986–8. https://doi.org/10.47391/JPMA.22-63.
Yang Y, Huang X, Wang Y, Leng L, Xu J, Feng L, et al. The impact of triglyceride-glucose index on ischemic stroke: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22:2. https://doi.org/10.1186/s12933-022-01732-0.
Wang X, Huang Y, Chen Y, Yang T, Su W, Chen X, et al. The relationship between body mass index and stroke: a systemic review and meta-analysis. J Neurol. 2022;269:6279–89. https://doi.org/10.1007/s00415-022-11318-1.
Bardugo A, Fishman B, Libruder C, Tanne D, Ram A, Hershkovitz Y, et al. Body Mass Index in 1.9 million adolescents and stroke in young adulthood. Stroke. 2021;52:2043–52. https://doi.org/10.1161/STROKEAHA.120.033595.
Gu H, Shao S, Liu J, Fan Z, Chen Y, Ni J, et al. Age- and sex-associated impacts of body mass index on stroke type risk: a 27-year prospective cohort study in a low-income population in China. Front Neurol. 2019;1(10):456. https://doi.org/10.3389/fneur.2019.00456.
Jiang C, Yang R, Kuang M, Yu M, Zhong M, Zou Y. Triglyceride glucose-body mass index in identifying high-risk groups of pre-diabetes. Lipids Health Dis. 2021;20:161. https://doi.org/10.1186/s12944-021-01594-7.
Liu M, Pan J, Meng K, Wang Y, Sun X, Ma L, et al. Triglyceride-glucose body mass index predicts prognosis in patients with ST-elevation myocardial infarction. Sci Rep. 2024;14:976. https://doi.org/10.1038/s41598-023-51136-7.
Chen L, He L, Zheng W, Liu Q, Ren Y, Kong W, et al. High triglyceride glucose-body mass index correlates with prehypertension and hypertension in east Asian populations: a population-based retrospective study. Front Cardiovasc Med. 2023;10:1139842. https://doi.org/10.3389/fcvm.2023.1139842.
Hou XZ, Lv YF, Li YS, Wu Q, Lv QY, Yang YT, et al. Association between different insulin resistance surrogates and all-cause mortality in patients with coronary heart disease and hypertension: NHANES longitudinal cohort study. Cardiovasc Diabetol. 2024;23:86. https://doi.org/10.1186/s12933-024-02173-7.
Wang Z, He H, Xie Y, Li J, Luo F, Sun Z, Zheng S, Yang F, Li X, Chen X, Chen Y, Sun Y. Non-insulin-based insulin resistance indexes in predicting atrial fibrillation recurrence following ablation: a retrospective study. Cardiovasc Diabetol. 2024;23:87. https://doi.org/10.1186/s12933-024-02158-6.
Liu H, Liu D, Zuo P. Association between insulin resistance markers and poor prognosis in patients with acute ischemic stroke after intravenous thrombolysis. Neurologist. 2024. https://doi.org/10.1097/NRL.0000000000000550.
Zhan C, Peng Y, Ye H, Diao X, Yi C, Guo Q, et al. Triglyceride glucose-body mass index and cardiovascular mortality in patients undergoing peritoneal dialysis: a retrospective cohort study. Lipids Health Dis. 2023;22:143. https://doi.org/10.1186/s12944-023-01892-2.
Dou J, Guo C, Wang Y, Peng Z, Wu R, Li Q, et al. Association between triglyceride glucose-body mass and one-year all-cause mortality of patients with heart failure: a retrospective study utilizing the MIMIC-IV database. Cardiovasc Diabetol. 2023;22:309. https://doi.org/10.1186/s12933-023-02047-4.
Acknowledgements
Our heartfelt gratitude extends to both the dedicated researchers and staff who contributed to the MIMIC-IV study, as well as its participants. Their invaluable contributions have furnished the critical data and methodological foundation pivotal to our research.
Funding
Funded by the Project of Mianyang Central Hospital (2021YJ006).
Author information
Authors and Affiliations
Contributions
HYW, LZP, and YXS devised the study, penned the initial draft, and performed the statistical analysis. These authors were pivotal in revising the manuscript and shaping the study's design. All study contributors reviewed and endorsed the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research involving human participants received approval from the Institutional Review Boards at both the Massachusetts Institute of Technology (Cambridge, MA, USA) and the Beth Israel Deaconess Medical Center (Boston, MA, USA), ensuring adherence to local laws and institutional policies. Therefore, no informed consents were needed from participants or their legal guardians/next of kin. This exemption aligns with national legislation and institutional guidelines, which did not mandate written consent for participation.
Competing interest
No competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Kaplan–Meier survival analysis curves for ACM and cumulative incidence of ICU (A), in-hospital (B), and 30-day (C) ACM. Figure S2. ROC of TyG-BMI for predicting ACM. Figure S3. Restricted cubic spline analysis of TyG-BMI and ICU (A), in-hospital (B), and 30-day (C) ACM. Figure S4. Forest plots of stratified analyses of TyG-BMI and ICU (A), in-hospital (B), and 30-day (C) ACM. Table S1. The baseline characteristics and outcomes between excluded and included participants. Table S2. Multivariable Cox proportional hazard models for short-term ACM. Table S3. Subgroup analyses of TyG-BMI and short-term ACM.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, Y., Li, Z. & Yin, X. Long-term survival in stroke patients: insights into triglyceride-glucose body mass index from ICU data. Cardiovasc Diabetol 23, 137 (2024). https://doi.org/10.1186/s12933-024-02231-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02231-0