Abstract
Importance
Diabetes mellitus (DM) is thought to be closely related to arterial stenotic or occlusive disease caused by atherosclerosis. However, there is still no definitive clinical evidence to confirm that patients with diabetes have a higher risk of restenosis.
Objective
This meta-analysis was conducted to determine the effect of DM on restenosis among patients undergoing endovascular treatment, such as percutaneous transluminal angioplasty (PTA) or stenting.
Data sources and study selection
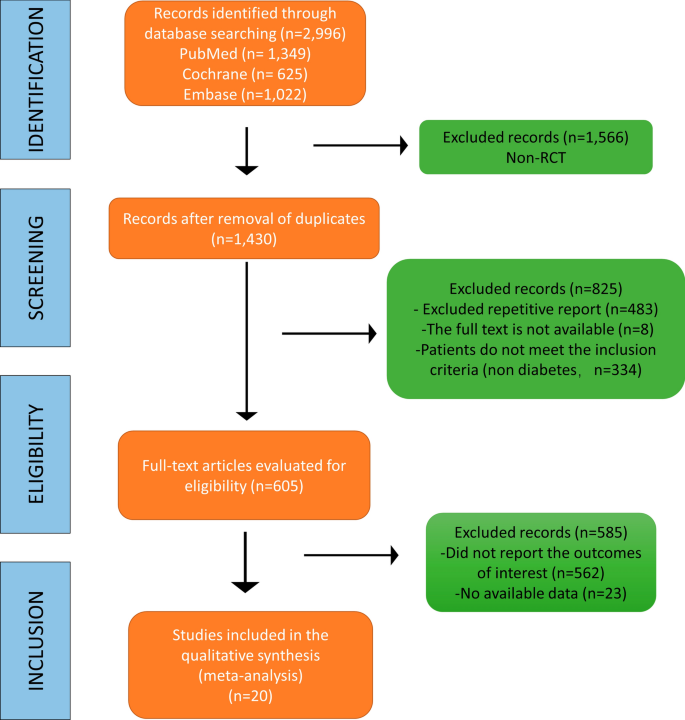
The PubMed/Medline, EMBASE and Cochrane Library electronic databases were searched from 01/1990 to 12/2022, without language restrictions. Trials were included if they satisfied the following eligibility criteria: (1) RCTs of patients with or without DM; (2) lesions confined to the coronary arteries or femoral popliteal artery; (3) endovascular treatment via PTA or stenting; and (4) an outcome of restenosis at the target lesion site. The exclusion criteria included the following: (1) greater than 20% of patients lost to follow-up and (2) a secondary restenosis operation.
Data extraction and synthesis
Two researchers independently screened the titles and abstracts for relevance, obtained full texts of potentially eligible studies, and assessed suitability based on inclusion and exclusion criteria.. Disagreements were resolved through consultation with a third researcher. Treatment effects were measured by relative ratios (RRs) with 95% confidence intervals (CIs) using random effects models. The quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.
Main outcomes and measures
The main observation endpoint was restenosis, including > 50% stenosis at angiography, or TLR of the primary operation lesion during the follow-up period.
Results
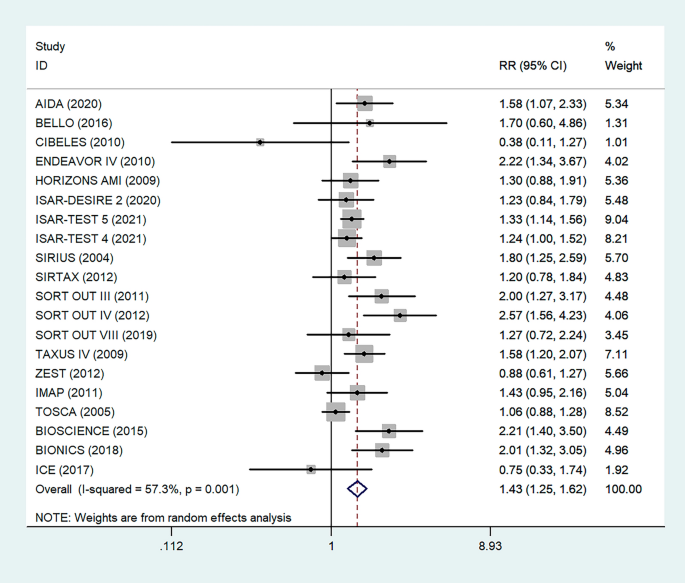
A total of 31,066 patients from 20 RCTs were included. Patients with DM had a higher risk of primary restenosis after endovascular treatment (RR = 1.43, 95% CI: 1.25–1.62; p = 0.001).
Conclusions and relevance
This meta-analysis of all currently available RCTs showed that patients with DM are more prone to primary restenosis after endovascular treatment.
Similar content being viewed by others
Cardiovascular disease (CVD) has the highest mortality rate worldwide [1], and vascular stenotic and occlusive lesions are the main pathological cause of death and disability for CVD patients [2]. At present, the development of intravascular therapy technology provides a reliable method for the treatment of vascular occlusive diseases, and quite a few of the difficulties in treatment have been resolved. However, restenosis after endovascular repair is a major problem that confuses clinicians and affects their choice of treatment [3]. Studies have shown that 30% to 50% of patients with coronary ischemic disease experience restenosis after endovascular therapy within five years; although the application of drug-coated balloons or drug-eluting stents has clearly reduced the occurrence of restenosis, restenosis still occurs in 10% to 20% of patients within one year [4, 5]. Restenosis after endovascular treatment causes a large number of patients to stop working or even die, which causes considerable damage to people's health and social and economic development. Thus, this is an urgent unsolved clinical problem. Identifying potential exposure and protective factors could make health care more effective in controlling restenosis after endovascular treatment.
Diabetes mellitus (DM) and its complications constitute one of the greatest human health problems worldwide. The prevalence of DM will increase globally from 371 million individuals in 2013 to 552 million individuals in 2030 [6]. DM is an important risk factor for the development of atherosclerotic diseases such as coronary heart disease (CHD), cerebrovascular disease, and peripheral artery disease (PAD). Cardiovascular complications are the leading cause of mortality among individuals with DM, and > 50% of patients die from a cardiovascular event, especially coronary artery disease but also stroke and peripheral vascular disease [7]. Insulin resistance and hyperglycemia in diabetes patients increase the risk of adverse cardiovascular events [8]. Studies have shown that hyperglycemia, insulin resistance, and an increase in advanced glycation end products are important conditions for a 2–fourfold increased risk of coronary artery disease (CAD) and PAD among people with diabetes [9,10,11,12,13,14]. However, at present, there is insufficient clinical evidence to confirm that diabetes increases the risk of restenosis after endovascular treatment. Therefore, to determine the effect of DM on restenosis among patients following endovascular interventional therapy, we designed this meta-analysis based on current clinical randomized controlled trial (RCT) data.
Methods
Study design and search strategy
We have designed the research and register for the study on the INPLASY website, and the registration number is INPLASY202370034 (DOI: 10.37766 / inplasy2023.7.0034). Ethical approval was acquired from the Ethic Committee of Southwest Medical University (No. 20220217–013). Two researchers independently scanned the titles and abstracts of the retrieved studies for the topic, and then obtained the full texts of potentially eligible studies and examined these independently for their suitability according to the inclusion criteria. In the case of disagreement between the two researchers, a third researcher was consulted to reach a consensus on whether to include the report or not. They documented the selection process with a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA [15]) flow chart. Trials were included if they satisfied the following eligibility criteria: (1) The studies included had to be randomized controlled studies (RCTs) including patients with or without DM; (2) had to involve lesions confined to the coronary arteries or femoral popliteal artery; (3) had to involve endovascular treatment via percutaneous transluminal angioplasty (PTA) or stenting; and (4) had to include an outcome of restenosis at the target lesion site. The exclusion criteria were as follows: (1) the proportion of patients lost to follow-up was higher than 20%; (2) a secondary restenosis operation; and (3) a sub-analysis or post-hoc analysis of RCTs. We developed and adhered to a standard protocol for study identification, inclusion, and data abstraction for all steps of our systematic review. Our major endpoint was restenosis, defined as a stenosis diameter > 50% in the in-segment area assessed by angiography, including the stent area as well as 5-mm margins proximal and distal to the stent. Meanwhile, clinically driven target lesion revascularization (CD-TLR) was also included, defined as any procedure performed to restore luminal patency after there has been late luminal loss of the target lesion (confirmed by angiography).
In this meta-analysis, we identified related published studies through a computerized literature search of the PubMed/Medline, Cochrane Library, and EMBASE (from 01/1990 to 12/2022) electronic databases. Two independent researchers checked citations for inclusion in the systematic review by using a hierarchical approach. The researchers assessed the title, abstract, and full text of these manuscripts. In addition, another reviewer manually searched the bibliographies of journal articles and relevant reviews to locate additional studies.
Reviewers extracted data using a data extraction form designed and piloted by the authors. If studies were reported in multiple publications, data were extracted from the different publications and then combined into a single data extraction form so that no data were omitted. The following characteristics of the included studies were registered in the data extraction form: methods and study design, participants, interventions and outcomes, including the outcome of restenosis. For all unclear restenosis analysis results, emails were sent to the corresponding authors to request the raw data; unfortunately, up to the time that the manuscript was written, no reply had been received.
Data synthesis and statistical analysis
For dichotomous data, we calculated Mantel‒Haenszel relative ratios (RRs) and 95% confidence intervals (CIs). Heterogeneity across studies was assessed by Cochran’s Q statistic with a P value set at 0.1. The I2 statistic was also taken into account regardless of the P value. An I2 of ≥ 50% was prespecified as the threshold considered too high to provide consistent analysis. A random effects model was used for the analysis. Tests were two-tailed, and a P value of < 0.05 was considered statistically significant. Funnel plots were used to assess publication bias. STATA 12.0 (StataCorp, USA) was used to analyze the data.
Assessment of the risk of bias in the included studies
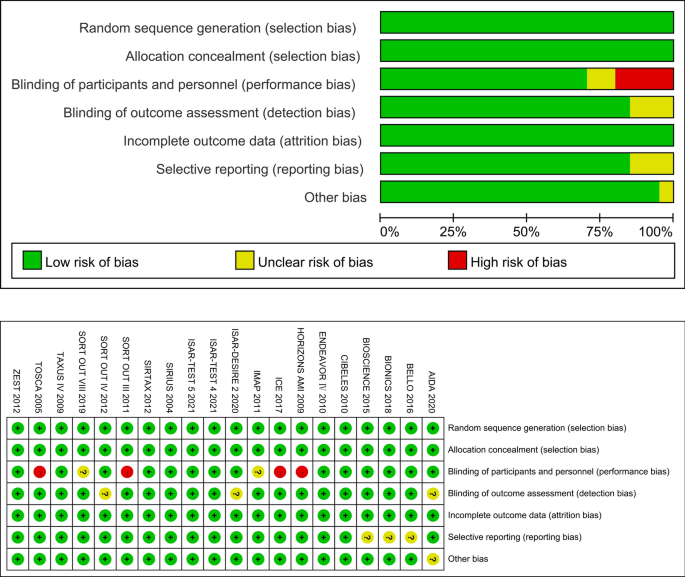
Two reviewers assessed the risk of bias independently for the included studies using the Cochrane risk of bias assessment tool [16]. We evaluated all included studies for the following: adequacy of sequence generation and allocation concealment; adequacy of blinding of couples, providers and outcome assessors; completeness of outcome data; risk of selective outcome reporting; and risk of other potential sources of bias. The results of the risk of bias assessment are presented in Fig. 2.
Results
The results of the literature search
In this analysis, a total of 2,996 RCTs were retrieved from the database. After eliminating duplicate studies, 1,430 studies remained. After browsing titles and abstracts, 605 full-text articles were assessed for eligibility, of which 585 were excluded because of the absence of relevant endpoint data (no surveillance undertaken, restenosis rates not reported separately). Finally, 20 studies were included in the meta-analysis for qualitative and quantitative analyses (Fig. 1). As a result, 31,066 patients were enrolled in this study. Table 1 details the case numbers and baseline characteristics, inclusion/exclusion criteria, number and type of stent/PTA procedure, strategies for follow-up, criteria for diagnosing restenosis, and the number of cases of restenosis with DM for each RCT. All authors of the studies included in this meta-analysis were requested to supply missing data and details of their studies. Unfortunately, no author supplied the requested information.
Description of the prospective randomized pooled trials and quality assessment of the included studies
As the aim of this study was to analyze the PTA- or stenting-related outcomes among patients who suffered from cardiovascular stenotic or occlusive disease with or without DM, the efficacy endpoint was the rate of restenosis (> 50% stenosis at angiography or TLR during the follow-up period). As a result, 31,066 patients were enrolled in this study. Table 1 summarizes the characteristics of the 20 included RCTs. All trials met the inclusion criteria and had a low risk of bias according to the Cochrane tool [16] for assessing risk of bias in RCTs (Fig. 2). Four studies (TOSCA, SORT OUT III, ICE, HORIZONS AMI) were designed to be open-labeled, and the BASKET-SMALL 2 study did not describe its randomization method (Table 2).
Meta-analysis results
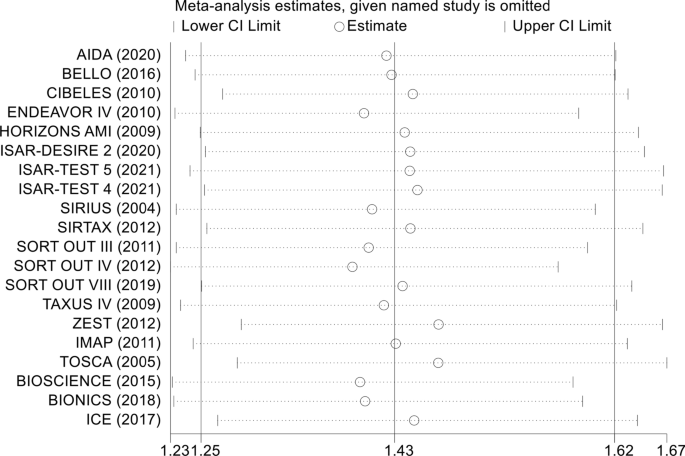
There were 20 RCTs that included a total of 31,066 patients that reported data on DM and restenosis. Since there was high heterogeneity (I2 = 57.3%, P = 0.001), a meta-analysis was conducted through a random effects model. Overall, the pooled results showed that DM was significantly associated with a higher risk of a major endpoint (RR = 1.43, 95% CI: 1.25–1.62; Fig. 3). The heterogeneity test showed significant differences among individual studies (P < 0.01, I2 = 57.3%). The sensitivity analysis showed that heterogeneity mainly came from BIONICS (2018), but the outcome did not change with removal of this study (RR 1.40,95% CI: 1.23–1.59) (Fig. 9).
Forest plot of the association between DM and restenosis. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 3 (95% CI, 1.25–1.62; I2= 57.53%, P = 0.001). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Subgroup analysis
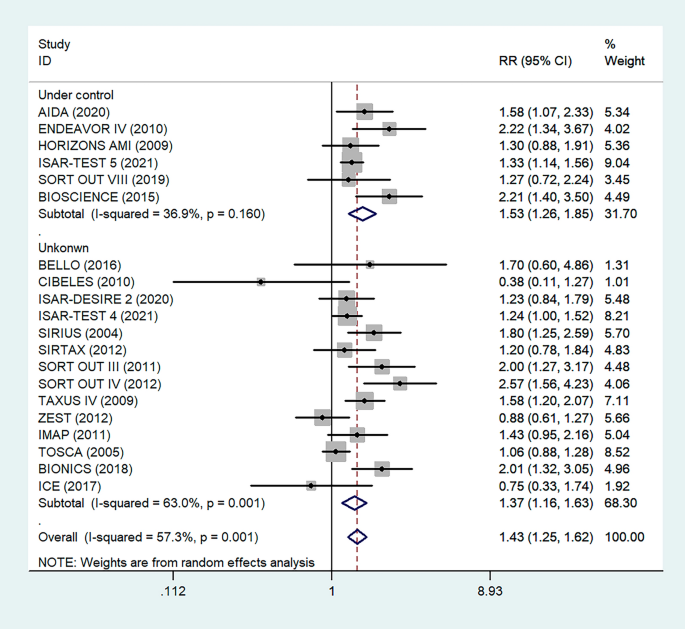
To determine the relationship between glycemic control levels and the incidence of postoperative restenosis, we performed a subgroup analysis of the included studies. Since detailed blood glucose levels of patients could not be obtained, we divided the included studies into the "under control" group and the "unknown" group according to whether the proportion of overall medicine glycemic control (oral hypoglycemic agents or insulin) of diabetic patients in each study was more than 80% (Fig. 4). As a result, there was no significant difference in restenosis rates between the under-control group (RR = 1.53, 95% CI 1.26–1.84, P < 0.05) and the unknown group (RR = 1.37, 95% CI 1.16–1.63, P = 0.001).
Subgroup analysis of the association between restenosis and glycemic control levels. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 4 (‘Under Control’ subgroup, RR = 1.53, 95% CI, 1.25–1.62; I2 = 36.9%, P < 0.05, ‘Unknown’ subgroup, RR = 1.37, 95% CI, 1.16–1.63; I2 = 63%, P = 0.001.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
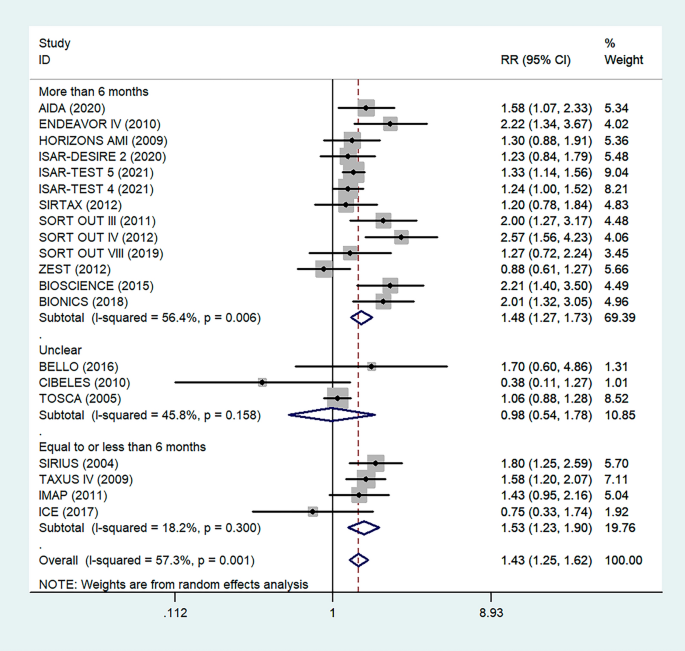
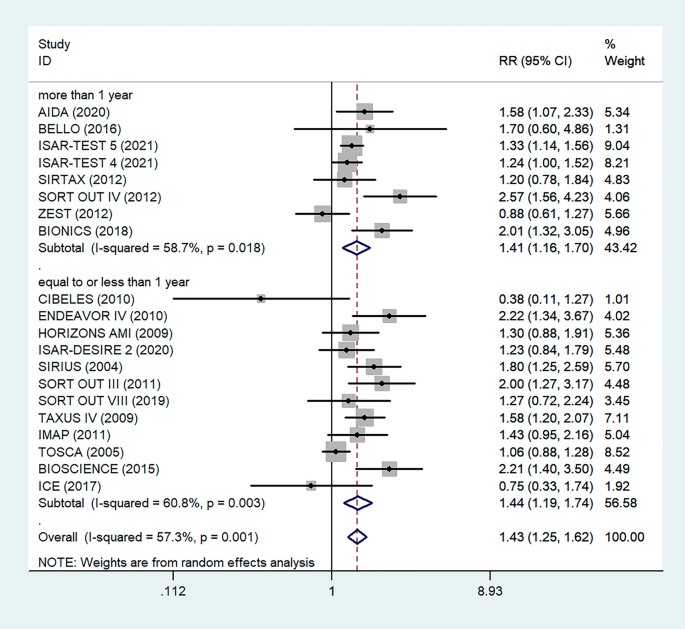
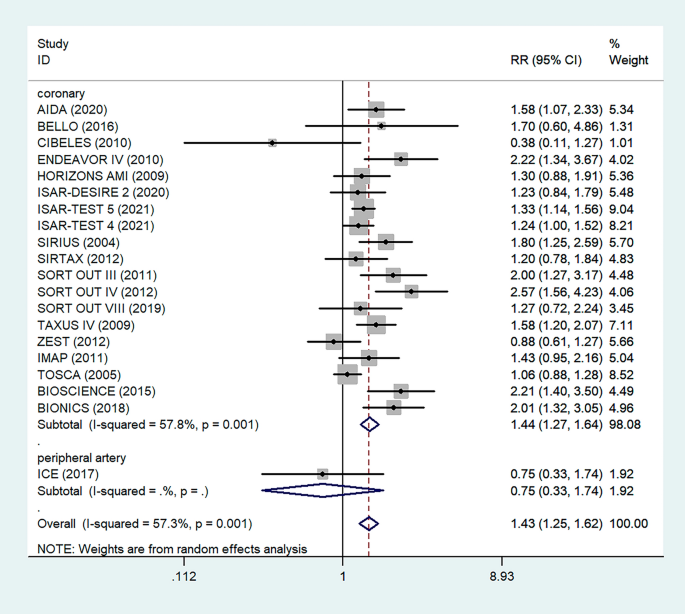
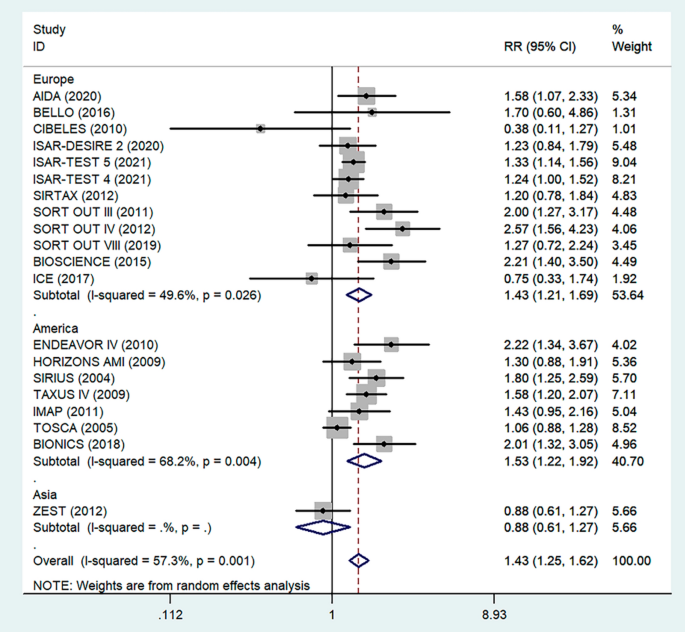
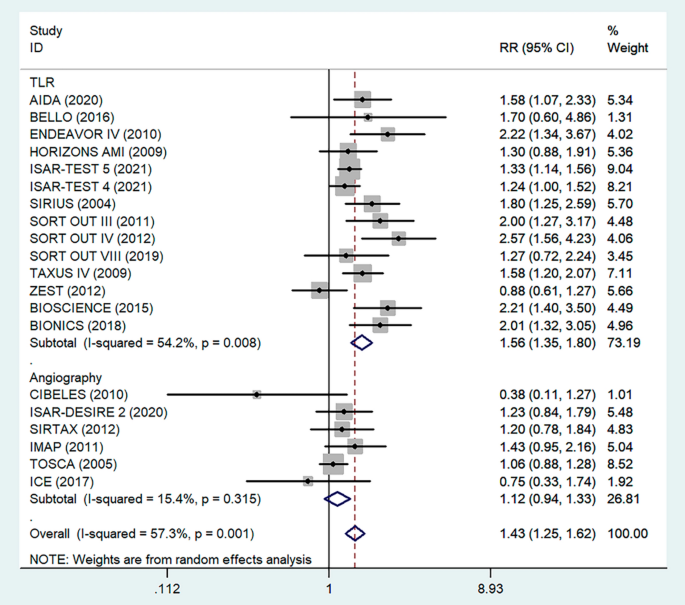
Antiplatelet therapy and anticoagulant therapy after vascular intervention are closely related to long-term efficacy [17]. Studies have demonstrated that an adequate treatment course of antiplatelet therapy after coronary stenting or angioplasty of lower extremity peripheral arteries is beneficial to improve long-term patency rates [18, 19]. We also analyzed the relationship between the postoperative dual antiplatelet therapy (DAPT) duration and restenosis in the studies. The results showed that regardless of the duration of DAPT, either less than (RR = 1.53, 95% CI 1.23–1.90, P < 0.05) or more than 6 months (RR = 1.48, 95% CI 1.27–1.73, P = 0.006) had no effect on restenosis outcome (Fig. 5). In addition, subgroup analysis was performed based on a 1-year follow-up. The results showed that there was no significant difference in the incidence of restenosis based on follow-up duration in the more than 1 year group (RR = 1.41, 95% CI 1.16–1.70, P = 0.018) and the less than or equal to 1 year group (RR = 1.44, 95% CI 1.19–1.74, P = 0.003) (Fig. 6). Furthermore, in these 20 studies, the major target vessel was the coronary artery, except for the ICE study, which evaluated peripheral arteries. Then, we performed a subgroup analysis by different target lesions. As shown in the forest plot, the restenosis rate after endovascular therapy was related to the primary site of the vascular lesion (coronary artery (RR = 1.44, 95% CI 1.27–1.64) or lower limb artery (RR = 0.75, 95% CI 0.33–1.74)) (Fig. 7). Moreover, subgroup analysis was also performed to account for continental differences in the included populations. Since there was only one study conducted among Asians, our subgroup included European and American participants (without distinguishing between South and North America), and it showed no significant differences in the endpoints between Americans (RR = 1.53, 95% CI 1.22–1.92, P = 0.004) and Europeans (RR = 1.43, 95% CI 1.21–1.69, P < 0.05) (Fig. 8). According to the diagnosis of restenosis, including angiography and TLR, we conducted a subgroup analysis of the two modalities, and the results showed that patients with diabetes had a higher risk of TLR (RR = 1.53, 95% CI 1.34–1.76, P = 0.010) (Fig. 9).
Subgroup analysis of the association between restenosis and the duration of DAPT. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 5 (‘More than 6 months’ subgroup, RR = 1.48, 95% CI, 1.27–1.73; I2 = 56.4%, P = 0.006, ‘Equal to or less than 6 months’ subgroup, RR = 1.53, 95% CI, 1.23–1.90; I2 = 18.2%, P < 0.05.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Subgroup analysis of the association between restenosis and different follow-up times. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 6 (‘More than 1 year’ subgroup, RR = 1.41, 95% CI, 1.16–1.70; I2 = 58.7%, P = 0.018, ‘Equal to or less than 1 year’ subgroup, RR = 1.44, 95% CI, 1.19–1.74; I2 = 60.8%, P = 0.003.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Subgroup analysis of the association between restenosis and types of lesion vessels. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 7 (‘Coronary’ subgroup, RR = 1.44, 95% CI, 1.27–1.64; I2 = 57.8%, P = 0.001, ‘Peripheral’ subgroup, RR = 0.75, 95% CI, 0.33–1.74.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Subgroup analysis of the association between restenosis and different continents. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 8 (‘Europe’ subgroup, RR = 1.43, 95% CI, 1.21–1.69; I2 = 49.6%, P < 0.05. ‘America’ subgroup, RR = 1.53, 95% CI, 1.22–1.92; I2 = 68.2%, P = 0.004.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Subgroup analysis of the association between restenosis and diagnostic methods. The vertical dashed lines indicate the pooled summary estimate (95% CI) for all studies in Fig. 9 (‘TLR’ subgroup, RR = 1.56, 95% CI, 1.35–1.80; I2 = 49.6%, P = 0.008, ‘Angiography’ subgroup, RR = 1.12, 95% CI, 0.94–1.33; I2 = 15.4%, P = 0.315.). The area of each square is proportional to the inverse variance of the estimate. The horizontal lines indicate the 95% confidence intervals of the estimate
Quantified covariable analysis-meta-regression analyses
With the aim of performing a comprehensive literature review on restenosis after interventional endovascular treatments of PTA or stenting in patients with diabetes (1) across different “interventions” (we performed a regression analysis according to different interventional modalities, i.e., aspirin, PTA or stent placement) (2) among patients with different health conditions (the proportion of patients with smoking exposure, hypertension, or hyperlipemia, was distinguished and regression analysis was performed) (3) and in different periods of these interventional endovascular techniques (presented as the publication times), meta-regression was employed. RRs, using variable rates as the dependent variable, and the different interventions, the different health conditions, and the publication times as the independent variables, were determined. There was no evidence that the different interventions, the different health conditions and the publication times were confounding factors in this subgroup analysis. Data from the analyses of moderator variables are presented in Table 3.
Sensitivity analysis
In the sensitivity analysis, each included study was removed one by one, and a summary analysis of the remaining studies was performed to assess whether a single included study had an excessive impact on the results of the entire meta-analysis (Fig. 10). None of the studies had an excessive impact on the results of the meta-analysis, indicating that the results of the meta-analysis were stable and reliable.
Publication bias
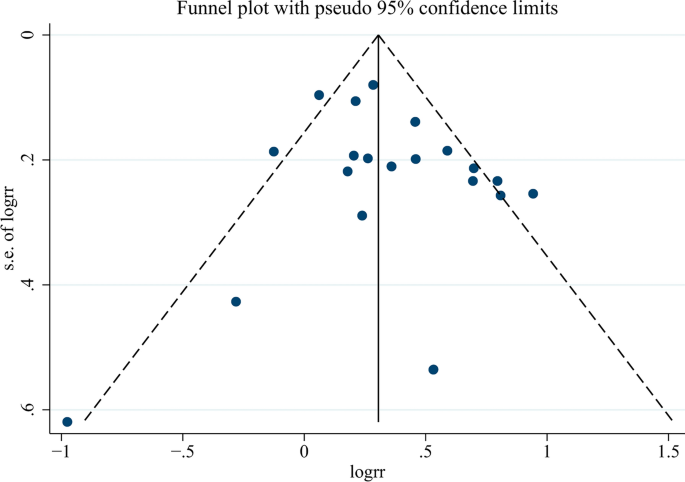
The probability of publication bias in the spread of the meta-analysis by funnel diagram and Begg’s test at a significance level of 0.05 indicated no bias of spread in the present study (p = 0.344) (Fig. 11). According to the results of the diagram, the publication offset of the included studies was small, and the results of the meta-analysis had high uniformity.
Meta-analysis GRADE assessment
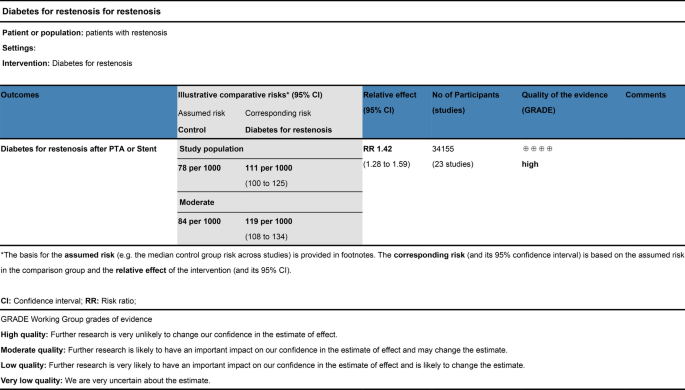
The evidence was assessed according to the GRADE process for the purposes of making clinical practice recommendations. We used GRADE to evaluate the quality of evidence, as shown in Fig. 12. Judgments about evidence quality (high, moderate, low or very low) were made by two review authors who worked independently and resolved disagreements by discussion. Conclusions were justified, documented, and incorporated into the reporting of results for each outcome. A ‘high’ level of evidence score was obtained according to the GRADE scoring rule after assessing the risks of inconsistency, indirectness, imprecision and publication bias.
Meta-analysis GRADE assessment. Search strategy of PubMed/Medline. PubMed platform. Searched from 1990 to December 12, 2022. #1 (((diabetes mellitus) AND percutaneous transluminal angioplasty)) OR ((diabetes mellitus) AND stent) (798). #2 ((Percutaneous Transluminal Angioplasty) OR (Transluminal Angioplasty) OR (Endoluminal Repair) OR (Angioplasty) OR (Stent) OR (Endovascular Stent Grafting) OR (Stent Grafting) OR (Stents)) AND ((Diabetes mellitus) OR (Diabetes) OR (Diabetic)) AND ((Restenosis) OR (Graft Restenosis) OR (Restenoses)) AND ((random) OR (randomized) OR (randomised)) (235). #3 ((((diabetes mellitus) OR (diabetes)) OR (melituria)) OR (diabetic)) AND (restenosis) (316)
Discussion
At present, PTA or stent implantation is the main treatment for cardiovascular stenosis or occlusion; however, restenosis after endovascular treatment is still a challenge [20]. Indeed, as the number of stent placements has risen to an estimate of over 3 million annually worldwide, revascularization procedures have become much more common [21]. However, restenosis after endovascular therapy is a major problem, and studies have shown that 30% to 50% of patients with coronary ischemic disease experience restenosis after endovascular therapy. To date, DM has been recognized as a high-risk factor for cardiovascular events [7, 22]. Epidemiological investigations have shown that patients with concomitant DM and PAD are at high risk for major complications, such as amputation [23]. Technical progress, such as the application of drug-coated balloons or drug-eluting stents [20, 24], has been found to potentially increase patency after endovascular treatment and thus reduce restenosis. The studies we enrolled included a large number of RCTs that involved drug-coated balloons and drug-eluting stents. Although the incidence of restenosis was significantly lower than that of traditional balloons or bare-metal stents, restenosis remained at ahigh rate and was difficult to resolve.
A study published in 1999 found that the long-term need for TLR increased with higher classes of in-stent restenosis (ISR) (hazard ratio (HR) = 1.7; P = 0.0380) and with the presence of diabetes (HR = 2.8; P = 0.0003) [25]. Taken together with other evidence, DM is suggested to be a strong determinant of restenosis (neointimal hyperplasia) [26, 27]. Michael Jonas et al. [28] used the insulin resistance model of the Zucker fatty rat and found that insulin-resistant Zucker fatty rats developed a thicker neointima and a narrower lumen area 2 weeks after implantation of an abdominal aortic stent compared with normal Zucker lean rats. Additionally, Manikandan Panchatcharam et al. [29] established a femoral artery guide wire injury model in hyperglycemic mice and confirmed that hyperglycemia had an obvious accelerating effect on intimal regeneration after vascular injury by promoting smooth muscle cell proliferation and migration. These animal studies demonstrated the role of hyperglycemia and ISR in regulating the function of vascular smooth muscle cells (VSMCs) and promoting neointimal hyperplasia. Moreover, advanced glycation end products (AGEs) also play an important role in promoting the progression of diabetic vascular disease. Zhongmin Zhou et al. [30] demonstrated a prominently increased accumulation of AGEs and immunoreactivities of receptor for advanced glycation end products (RAGEs) in response to balloon injury in diabetic compared with nondiabetic rats. Additionally, blockade of RAGE/ligand interaction significantly decreased VSMC proliferation in vitro and bromodeoxyuridine (BrdU)-labeled proliferating VSMCs in vivo, suppressed neointimal formation and increased luminal area in both diabetic and nondiabetic rats. These animal studies demonstrated that the pathophysiological features of diabetes, including ISR, metabolic syndrome, hyperglycemia, and increased AGEs, are involved in neointima after balloon dilation or stent implantation.
The current mainstream view is that DM increases the risk of restenosis [31]. Studies have shown that patients with insulin-dependent DM are at particularly high risk for adverse events after percutaneous coronary intervention (PCI) [27]. The universally accepted hypothesis for this phenomenon is that hyperglycemia induces endothelial dysfunction and a proinflammatory state that promote the production of growth factors and cytokines, leading to extensive neointimal formation and thus contributing to the progression of restenosis [32, 33]. Besides the endothelial cell inflammation hypothesis, researches indicated that endothelial progenitor cells played a significant role in restenosis [34, 35]. Balestrieri ML et al [36] revealed that high glucose concentration decreased the quantity of endothelial progenitor cells via SIRT1 signaling pathway. In addition, the prethrombotic environment of patients with diabetes ultimately increases the risk of restenosis [37]. However, until now, there has been no conclusive, large-scale clinical evidence to support this view. Our meta-analysis involved 20 RCTs from multiple countries and time spans, with up to 31,066 patients. The results of the meta-analysis confirmed for the first time that DM is a high-risk factor for restenosis after endovascular treatment in a human cohort.
Studies [38] have shown that the type of glucose-controlling drug can affect postoperative restenosis in diabetic patients after coronary stent or balloon dilation. Their data, especially regarding metformin and thiazolidinediones, indicate beneficial results compared to insulin and sulfonylurea for restenosis. However, no large trials have been undertaken in which the effect of glucose-lowering agents on restenosis is associated with improved outcomes. Indeed, experts believe that maintaining proper glycemic control is crucial for diabetic patients who have undergone revascularization procedures [21]. In several recent prospective studies, high glycemic levels [39, 40] and insulin resistance [41] have increased restenosis rates after coronary stenting or balloon angioplasty, but these results were based on statistical analysis of clinical phenomena, lacking evidence from high-quality controlled studies. Marfella R et al [42] conducted a RCT involving 165 patients with high blood glycemic and ST-segment elevation myocardial infarction (STEMI) undergoing PCI treatment, randomly assigning these patients to an interventional-glycemic-control group and an intensive-glycemic-control group. The results showed that the restenosis rate of patients in the intensive-glycemic-control group after PCI reduced by half (48% and 24%) at 6 months. The study confirmed the positive correlation between blood glucose levels and restenosis. However, it solely concentrated on blood glucose levels without addressing whether the patients had diabetes or specific subtypes. Our research indicates that restenosis of the diabetic patients after interventional treatment is not directly related to blood glucose level. This implies that diabetes may pose other risks aside from high blood glucose, such as AGEs and insulin resistance, which could potentially contribute to restenosis. Although Mone P et al [43] demonstrated the effect of high glucose on the risk of restenosis in STEMI patients without DM, the restenosis of high glucose-DM group (18.5%) is still higher than high glucose non-DM group (14.0%) at one-year follow-up, which indicates that diabetic patients may be influenced by additional pathogenic factors beyond elevated blood glucose levels. In animal studies, it has been demonstrated that hyperglycemia and insulin resistance lead to intimal hyperplasia after vascular injury in rats [28,29,30, 44, 45], which seems to be of crucial importance in determining exaggerated neointimal hyperplasia after balloon angioplasty in diabetic animals. However, our meta-analysis showed that blood glucose levels in diabetic patients did not affect the incidence of restenosis after endovascular therapy (Fig. 5). In animal experiments, hyperglycemia can contribute to neointimal hyperplasia, which was inconsistent with the results of our meta-analysis, suggesting that there may be other important risk factors involved in restenosis in diabetic patients. A prospective observational study involving 377 participants discovered that postoperative restenosis rates differed among patients with type 2 diabetes and acute myocardial infarction (AMI) based on whether they were prescribed oral sodium/glucose cotransporter 2 (SGLT2) inhibitors [46]. The study confirmed that the administration of SGLT2 inhibitors to type 2 diabetes patients was associated with a reduced frequency of ISR-related events, independent of glycemic control. This research highlights the importance of non-glycemic factors in the reduction of postoperative restenosis among diabetes patients. AGEs and their receptor isoforms seem to have an important contribution to both the pathogenesis and clinical outcome of restenosis. AGEs have been shown to promote carotid intimal regeneration in rats after balloon injury [30], suggesting that AGEs may act as a stimulus for restenosis. Cristiano Spadaccio et al. found that soluble RAGE (sRAGE) levels and total circulating AGEs were positively correlated with an increased risk of stent restenosis [47, 48]. This suggests that AGEs may play a more important role in restenosis than hyperglycemia. Currently, there is a lack of clinical studies on the relationship between restenosis and AGE levels, and further RCT studies may be of great significance.
DAPT has become essential in daily clinical practice. In fact, current practice guidelines recommend aspirin and clopidogrel DAPT for patients suffering from CAD or monotherapy for patients with symptomatic PAD, regardless of clinical background [49]. However, there is controversy over the duration of antiplatelet therapy in view of the different lesion sites and stent types [50]. Cristian A Dámazo-Escobedo et al [51]. confirmed that long-term antiplatelet therapy after coronary stenting would be justified by the high incidence of thrombosis-restenosis through a prospective observational study. However, based on the current European guidelines for management after coronary stent placement, there is no consensus on the ideal duration of DAPT to prevent stent thrombosis-restenosis without a significant increase in bleeding risk. In our meta-analysis, based on the circumstances of antiplatelet therapy included in the study after revascularization, we took 6 months of DAPT as the line of demarcation and found that different durations of antiplatelet therapy had no significant effect on the incidence of restenosis after endovascular therapy. Moreover, anticoagulation therapy after revascularization in PAD is particularly important compared to that after coronary revascularization. The COMPASS and VOYAGER PAD RCTs showed that a low-dose oral anticoagulant combined with aspirin (Rivaroxaban 2.5 mg twice a day; Aspirin 100 mg once a day) improved the long-term patency rate of lower extremity artery disease after endovascular therapy, reduced the incidence of major limb adverse events and cardiovascular events, and did not increase the risk of fatal major bleeding [52,53,54]. Regrettably, due to the lack of available anticoagulant therapy regimens in the included studies, we did not perform a subgroup meta-analysis of anticoagulant therapy and restenosis in this study. Anticoagulation combined with antiplatelet therapy may be beneficial in future RCTs.
It is worth mentioning that according to the subgroup analysis, patients with diabetes mellitus had a higher TLR rate, while patients with restenosis detected by angiographic follow-up showed no significant difference. This implies that patients with diabetes may be more prone to symptomatic restenosis and require surgical reintervention, suggesting to clinicians that patients with diabetes may have increased reoperation rates. Therefore, diabetic patients should take this characteristic into full consideration when choosing the type of balloon or stent, such as the choice of a drug-eluting balloon to reduce restenosis. Since this study did not involve comparisons of balloon types or stent types, research in this direction may be of great significance for diabetic patients.
The findings of the meta-analysis involving 31,066 individuals affirm that patients with diabetes mellitus (DM) have a higher risk of restenosis following intravascular treatment. Nevertheless, there are still limitations to this meta-analysis. More detailed baseline characteristics of patients were not obtained from some of the included studies, even after contact these corresponding authors, such as the medical history time of DM, detailed blood glucose levels and hypoglycemic methods (such as diet, exercise or drug therapy), which may be important factors and could affect the analysis of restenosis. Due to the high rank of the GRADE results, we also suggest a cautious interpretation for this meta-analysis, and further high-quality RCTs are needed to improve the current conclusion.
Conclusions
In summary, the findings of this systematic review and meta-analysis provided convincing evidence that patients with DM had an increased risk of primary restenosis after PTA or stenting, suggesting that DM is a high-risk factor for restenosis after endovascular treatment, irrespective of blood glucose level, antiplatelet therapy duration, targeted lesion vessel and continent. In conclusion, our meta-analysis provides a reliable suggestion for the health management of diabetic patients with vascular occlusive disease after endovascular therapy.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ABR:
-
Angiographic binary restenosis
- AGEs:
-
Advanced glycation end products
- BrdU:
-
Bromodeoxyuridine
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- DAPT:
-
Dual antiplatelet therapy
- DM:
-
Diabetes mellitus
- FU-T:
-
Follow-up time
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- HR:
-
Hazard ratio
- ISR:
-
In-stent restenosis
- MACE(s):
-
Major adverse cardiac event(s)
- PAD:
-
Peripheral artery disease
- PCI:
-
Percutaneous coronary intervention
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PTA:
-
Percutaneous transluminal angioplasty
- QA-R:
-
Quantitative angiography-confirmed restenosis
- RAGEs:
-
Receptor for advanced glycation end products
- RCTs:
-
Randomized controlled trials
- RR:
-
Relative ratio
- SGLT2:
-
Sodium/glucose cotransporter 2
- sRAGE:
-
Soluble RAGE
- STEMI:
-
ST-segment elevation myocardial infarction
- TLR:
-
Targeted lesion revascularization
- VSMCs:
-
Vascular smooth muscle cells
References
Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–40. https://doi.org/10.1038/nrcardio.2017.154.
Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010.
Gori T. Restenosis after coronary stent implantation: cellular mechanisms and potential of endothelial progenitor cells (A Short Guide for the Interventional Cardiologist). Cells. 2022. https://doi.org/10.3390/cells11132094.
Kereiakes DJ, Kandzari DE, Zidar JP. Drug-coated balloons for in-stent restenosis. J Am Coll Cardiol. 2020;76:1391–2. https://doi.org/10.1016/j.jacc.2020.06.082.
Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–725. https://doi.org/10.1161/CIR.0000000000000470.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. https://doi.org/10.1038/nrendo.2014.29.
Newman JD, Schwartzbard AZ, Weintraub HS, Goldberg IJ, Berger JS. Primary prevention of cardiovascular disease in diabetes mellitus. J Am Coll Cardiol. 2017;70:883–93. https://doi.org/10.1016/j.jacc.2017.07.001.
Eckel RH, Bornfeldt KE, Goldberg IJ. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 2021;33:1519–45. https://doi.org/10.1016/j.cmet.2021.07.001.
Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018;24:59. https://doi.org/10.1186/s10020-018-0060-3.
Henry H. Ruiz1, Ravichandran Ramasamy1, Ann Marie Schmidt1. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrine Society 2019. doi: https://doi.org/10.1210/endocr/bqz006/5602541
Jankauskas SS, Kansakar U, Varzideh F, et al. Heart failure in diabetes. Metabolism. 2021;125: 154910. https://doi.org/10.1016/j.metabol.2021.154910.
Shu J, Matarese A, Santulli G. Diabetes, body fat, skeletal muscle, and hypertension: the ominous chiasmus? J Clin Hypertens (Greenwich). 2019;21:239–42. https://doi.org/10.1111/jch.13453.
Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14:453. https://doi.org/10.1007/s11892-013-0453-1.
Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis. 2018;275:379–81. https://doi.org/10.1016/j.atherosclerosis.2018.05.033.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. https://doi.org/10.1136/bmj.b2700.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. https://doi.org/10.1136/bmj.d5928.
Miyazaki Y, Suwannasom P, Sotomi Y, et al. Single or dual antiplatelet therapy after PCI. Nat Rev Cardiol. 2017;14:294–303. https://doi.org/10.1038/nrcardio.2017.12.
Mohan J, Yelamanchili VS, Zacharias SK. Acute Coronary Syndrome Catheter Interventions. In. StatPearls. Treasure Island (FL); 2022.
De Luca L, Bonaca MP, Magnani G. Antithrombotic strategies for patients with coronary and lower extremity peripheral artery diseases: a narrative review. Expert Rev Cardiovasc Ther. 2020;18:881–9. https://doi.org/10.1080/14779072.2020.1833719.
Wang J, Jin X, Huang Y, et al. Endovascular stent-induced alterations in host artery mechanical environments and their roles in stent restenosis and late thrombosis. Regen Biomater. 2018;5:177–87. https://doi.org/10.1093/rb/rby006.
Wilson S, Mone P, Kansakar U, et al. Diabetes and restenosis. Cardiovasc Diabetol. 2022;21:23. https://doi.org/10.1186/s12933-022-01460-5.
Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600–9. https://doi.org/10.1016/j.phrs.2016.09.040.
Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40:1808–17. https://doi.org/10.1161/ATVBAHA.120.314595.
Nestelberger T, Kaiser C, Jeger R. Drug-coated balloons in cardiovascular disease: benefits, challenges, and clinical applications. Expert Opin Drug Deliv. 2020;17:201–11. https://doi.org/10.1080/17425247.2020.1714590.
Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–8. https://doi.org/10.1161/01.cir.100.18.1872.
Liu MW, Roubin GS, King SB. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–87. https://doi.org/10.1161/01.cir.79.6.1374.
Abizaid A, Kornowski R, Mintz GS, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998;32:584–9. https://doi.org/10.1016/s0735-1097(98)00286-1.
Jonas M, Edelman ER, Groothuis A, et al. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–33. https://doi.org/10.1161/01.RES.0000183730.52908.C6.
Panchatcharam M, Miriyala S, Yang F, et al. Enhanced proliferation and migration of vascular smooth muscle cells in response to vascular injury under hyperglycemic conditions is controlled by beta3 integrin signaling. Int J Biochem Cell Biol. 2010;42:965–74. https://doi.org/10.1016/j.biocel.2010.02.009.
Zhou Z, Wang K, Penn MS, et al. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003;107:2238–43. https://doi.org/10.1161/01.CIR.0000063577.32819.23.
Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9:53–62. https://doi.org/10.1038/nrcardio.2011.132.
Lee MS, David EM, Makkar RR, Wilentz JR. Molecular and cellular basis of restenosis after percutaneous coronary intervention: the intertwining roles of platelets, leukocytes, and the coagulation-fibrinolysis system. J Pathol. 2004;203:861–70. https://doi.org/10.1002/path.1598.
Aronson D, Edelman ER. Revascularization for coronary artery disease in diabetes mellitus: angioplasty, stents and coronary artery bypass grafting. Rev Endocr Metab Disord. 2010;11:75–86. https://doi.org/10.1007/s11154-010-9135-3.
Hibbert B, O’Brien ER. Circulating endothelial progenitor cells and in-stent restenosis: friend, foe, or none of the above? Can J Cardiol. 2014;30:6–7. https://doi.org/10.1016/j.cjca.2013.11.010.
Park KS, Kang SN, Kim DH, et al. Late endothelial progenitor cell-capture stents with CD146 antibody and nanostructure reduce in-stent restenosis and thrombosis. Acta Biomater. 2020;111:91–101. https://doi.org/10.1016/j.actbio.2020.05.011.
Balestrieri ML, Servillo L, Esposito A, et al. Poor glycaemic control in type 2 diabetes patients reduces endothelial progenitor cell number by influencing SIRT1 signalling via platelet-activating factor receptor activation. Diabetologia. 2013;56:162–72. https://doi.org/10.1007/s00125-012-2749-0.
Elezi S, Kastrati A, Pache J, et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998;32:1866–73. https://doi.org/10.1016/s0735-1097(98)00467-7.
Lexis CP, Rahel BM, Meeder JG, Zijlstra F, van der Horst IC. The role of glucose lowering agents on restenosis after percutaneous coronary intervention in patients with diabetes mellitus. Cardiovasc Diabetol. 2009;8:41. https://doi.org/10.1186/1475-2840-8-41.
Liu C, Zhao Q, Zhao Z, et al. Correlation between estimated glucose disposal rate and in-stent restenosis following percutaneous coronary intervention in individuals with non-ST-segment elevation acute coronary syndrome. Front Endocrinol (Lausanne). 2022;13:1033354. https://doi.org/10.3389/fendo.2022.1033354.
Zhu Y, Liu K, Chen M, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20:137. https://doi.org/10.1186/s12933-021-01332-4.
Sasso FC, Pafundi PC, Marfella R, et al. Adiponectin and insulin resistance are related to restenosis and overall new PCI in subjects with normal glucose tolerance: the prospective AIRE Study. Cardiovasc Diabetol. 2019;18:24. https://doi.org/10.1186/s12933-019-0826-0.
Marfella R, Sasso FC, Siniscalchi M, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction. J Clin Endocrinol Metab. 2012;97:2862–71. https://doi.org/10.1210/jc.2012-1364.
Mone P, Gambardella J, Minicucci F, et al. Hyperglycemia Drives Stent Restenosis in STEMI Patients. Diabetes Care. 2021;44:e192–3. https://doi.org/10.2337/dc21-0939.
Desouza CV, Gerety M, Hamel FG. Neointimal hyperplasia and vascular endothelial growth factor expression are increased in normoglycemic, insulin resistant, obese fatty rats. Atherosclerosis. 2006;184:283–9. https://doi.org/10.1016/j.atherosclerosis.2005.04.015.
Indolfi C, Torella D, Cavuto L, et al. Effects of balloon injury on neointimal hyperplasia in streptozotocin-induced diabetes and in hyperinsulinemic nondiabetic pancreatic islet-transplanted rats. Circulation. 2001;103:2980–6. https://doi.org/10.1161/01.cir.103.24.2980.
Marfella R, Sardu C, D’Onofrio N, et al. SGLT-2 inhibitors and in-stent restenosis-related events after acute myocardial infarction: an observational study in patients with type 2 diabetes. BMC Med. 2023;21:71. https://doi.org/10.1186/s12916-023-02781-2.
Spadaccio C, Patti G, De Marco F, et al. Usefulness of preprocedural levels of advanced glycation end products to predict restenosis in patients with controlled diabetes mellitus undergoing drug-eluting stent implantation for stable angina pectoris (from the Prospective ARMYDA-AGEs Study). Am J Cardiol. 2013;112:21–6. https://doi.org/10.1016/j.amjcard.2013.02.046.
Basta G, Berti S, Cocci F, et al. Plasma N-epsilon-(carboxymethyl)lysine levels are associated with the extent of vessel injury after coronary arterial stenting. Coron Artery Dis. 2008;19:299–305. https://doi.org/10.1097/MCA.0b013e3282fec058.
Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–79. https://doi.org/10.1161/CIR.0000000000000471.
Zhang Y, Zhang X, Dong Q, et al. Duration of dual antiplatelet therapy after implantation of drug-coated balloon. Front Cardiovasc Med. 2021;8: 762391. https://doi.org/10.3389/fcvm.2021.762391.
Damazo-Escobedo CA, Vieyra-Herrera G, Martinez-Rios MA, Garcia-Navarrete MG, Silva-Ruz C. Coronary stent and prolonged dual antiplatelet therapy. A longitudinal study. Gac Med Mex. 2022;158:216–21. https://doi.org/10.24875/GMM.M22000677.
Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–29. https://doi.org/10.1016/S0140-6736(17)32409-1.
Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N Engl J Med. 2020;382:1994–2004. https://doi.org/10.1056/NEJMoa2000052.
Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71:2306–15. https://doi.org/10.1016/j.jacc.2018.03.008.
Kerkmeijer LSM, Tijssen RYG, Hofma SH, et al. Three-year clinical outcomes of the absorb bioresorbable vascular scaffold compared to Xience everolimus-eluting stent in routine PCI in patients with diabetes mellitus-AIDA sub-study. Catheter Cardiovasc Interv. 2021;98:713–20. https://doi.org/10.1002/ccd.29329.
Giannini F, Latib A, Jabbour RJ, et al. Comparison of paclitaxel drug-eluting balloon and paclitaxel-eluting stent in small coronary vessels in diabetic and nondiabetic patients—results from the BELLO (balloon elution and late loss optimization) trial. Cardiovasc Revasc Med. 2017;18:4–9. https://doi.org/10.1016/j.carrev.2016.12.008.
Konigstein M, Ben-Yehuda O, Smits PC, et al. Outcomes among diabetic patients undergoing percutaneous coronary intervention with contemporary drug-eluting stents: analysis from the BIONICS randomized trial. JACC Cardiovasc Interv. 2018;11:2467–76. https://doi.org/10.1016/j.jcin.2018.09.033.
Franzone A, Pilgrim T, Heg D, et al. Clinical outcomes according to diabetic status in patients treated with biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents: prespecified subgroup analysis of the BIOSCIENCE trial. Circ Cardiovasc Interv. 2015. https://doi.org/10.1161/CIRCINTERVENTIONS.114.002319.
Ruiz-Garcia J, Teles R, Rumoroso JR, et al. Comparison between diabetic and non-diabetic patients after successful percutaneous coronary intervention for chronic total occlusions in the drug-eluting stent era. Rev Port Cardiol. 2015;34:263–70. https://doi.org/10.1016/j.repc.2014.10.009.
Kirtane AJ, Patel R, O’Shaughnessy C, et al. Clinical and angiographic outcomes in diabetics from the ENDEAVOR IV trial: randomized comparison of zotarolimus- and paclitaxel-eluting stents in patients with coronary artery disease. JACC Cardiovasc Interv. 2009;2:967–76. https://doi.org/10.1016/j.jcin.2009.08.008.
Brener SJ, Mehran R, Dressler O, Cristea E, Stone GW. Diabetes mellitus, myocardial reperfusion, and outcome in patients with acute ST-elevation myocardial infarction treated with primary angioplasty (from HORIZONS AMI). Am J Cardiol. 2012;109:1111–6. https://doi.org/10.1016/j.amjcard.2011.11.046.
Krankenberg H, Zeller T, Ingwersen M, et al. Self-expanding versus balloon-expandable stents for iliac artery occlusive disease: the randomized ICE trial. JACC Cardiovasc Interv. 2017;10:1694–704. https://doi.org/10.1016/j.jcin.2017.05.015.
Abreu Filho LM, Forte AA, Sumita MK, Favarato D, Meireles GC. Influence of metal alloy and the profile of coronary stents in patients with multivessel coronary disease. Clinics (Sao Paulo). 2011;66:985–9. https://doi.org/10.1590/s1807-59322011000600011.
Kufner S, Byrne RA, de Waha A, et al. Sirolimus-eluting versus paclitaxel-eluting stents in diabetic and non-diabetic patients within sirolimus-eluting stent restenosis: results from the ISAR-DESIRE 2 trial. Cardiovasc Revasc Med. 2014;15:69–75. https://doi.org/10.1016/j.carrev.2014.02.001.
Lenz T, Koch T, Joner M, et al. Ten-year clinical outcomes of biodegradable versus durable polymer new-generation drug-eluting stent in patients with coronary artery disease with and without diabetes mellitus. J Am Heart Assoc. 2021;10: e020165. https://doi.org/10.1161/JAHA.120.020165.
Koch T, Lenz T, Joner M, et al. Ten-year clinical outcomes of polymer-free versus durable polymer new-generation drug-eluting stent in patients with coronary artery disease with and without diabetes mellitus : results of the intracoronary stenting and angiographic results: test Efficacy of Sirolimus- and Probucol- and Zotarolimus-Eluting Stents (ISAR-TEST 5) trial. Clin Res Cardiol. 2021;110:1586–98. https://doi.org/10.1007/s00392-021-01854-7.
Moussa I, Leon MB, Baim DS, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109:2273–8. https://doi.org/10.1161/01.CIR.0000129767.45513.71.
Billinger M, Beutler J, Taghetchian KR, et al. Two-year clinical outcome after implantation of sirolimus-eluting and paclitaxel-eluting stents in diabetic patients. Eur Heart J. 2008;29:718–25. https://doi.org/10.1093/eurheartj/ehn021.
Maeng M, Jensen LO, Tilsted HH, et al. Outcome of sirolimus-eluting versus zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a SORT OUT III Substudy). Am J Cardiol. 2011;108:1232–7. https://doi.org/10.1016/j.amjcard.2011.06.037.
Jensen LO, Thayssen P, Junker A, et al. Comparison of outcomes in patients with versus without diabetes mellitus after revascularization with everolimus- and sirolimus-eluting stents (from the SORT OUT IV trial). Am J Cardiol. 2012;110:1585–91. https://doi.org/10.1016/j.amjcard.2012.07.022.
Gyldenkerne C, Olesen KKW, Jensen LO, et al. Everolimus-eluting versus biolimus-eluting coronary stent implantation in patients with and without diabetes mellitus. Am J Cardiol. 2019;124:671–7. https://doi.org/10.1016/j.amjcard.2019.05.060.
Hermiller JB, Raizner A, Cannon L, et al. Outcomes with the polymer-based paclitaxel-eluting TAXUS stent in patients with diabetes mellitus: the TAXUS-IV trial. J Am Coll Cardiol. 2005;45:1172–9. https://doi.org/10.1016/j.jacc.2004.10.075.
Yee KM, Buller CE, Catellier D, et al. Effect of bare metal stenting on angiographic and clinical outcomes in diabetic and nondiabetic patients undergoing percutaneous coronary intervention of nonacute occluded coronary arteries: a report from the Total Occlusion Study of Canada (TOSCA). Catheter Cardiovasc Interv. 2005;66:178–84. https://doi.org/10.1002/ccd.20410.
Jang S-J, Park D-W, Kim W-J, et al. Differential long-term outcomes of zotarolimus-eluting stents compared with sirolimus-eluting and paclitaxel-eluting stents in diabetic and nondiabetic patients: two-year subgroup analysis of the ZEST randomized trial. Catheter Cardiovasc Interv. 2013;81:1106–14. https://doi.org/10.1002/ccd.24603.
Acknowledgements
None.
Funding
This study was supported by the International Science and Technology Innovation Cooperation Project of Sichuan Province (22GJHZ0278), the Sichuan Science and Technology Program (2022YFS0614; 2022YFS0617), the Medical Research Project of Sichuan Province (S21020), the Science and Technology Strategic Cooperation Project of Luzhou Municipal People's Government and Southwest Medical University (2021LZXNYD-D10; 23YKDCXY0003), and the Doctoral Research Initiation Program of the Affiliated Hospital of Southwest Medical University (19041).
Author information
Authors and Affiliations
Contributions
X.S. conceptualized and designed this study. J.W., X.Z., and X.S. revised and edited the manuscript. Y.M. and C.Z. collected and analyzed the data. C.Z. and X.S. prepared the draft of the manuscript. J.W. and Y.H. reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, X., Zhang, C., Ma, Y. et al. Association between diabetes mellitus and primary restenosis following endovascular treatment: a comprehensive meta-analysis of randomized controlled trials. Cardiovasc Diabetol 23, 132 (2024). https://doi.org/10.1186/s12933-024-02201-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02201-6