Abstract
Background
Coronary inflammation plays crucial role in type 2 diabetes mellitus (T2DM) induced cardiovascular complications. Both glucose-lowering drug interventions (GLDIS) and glycemic control (GC) status potentially correlate coronary inflammation, as indicated by changes in pericoronary adipose tissue (PCAT) attenuation, and thus influence cardiovascular risk. This study evaluated the impact of GLDIS and GC status on PCAT attenuation in T2DM patients.
Methods
This retrospective study collected clinical data and coronary computed tomography angiography (CCTA) images of 1,342 patients, including 547 T2DM patients and 795 non-T2DM patients in two tertiary hospitals. T2DM patients were subgroup based on two criteria: (1) GC status: well: HbA1c < 7%, moderate: 7 ≤ HbA1c ≤ 9%, and poor: HbA1c > 9%; (2) GLDIS and non-GLDIS. PCAT attenuations of the left anterior descending artery (LAD-PCAT), left circumflex artery (LCX-PCAT), and right coronary artery (RCA-PCAT) were measured. Propensity matching (PSM) was used to cross compare PCAT attenuation of non-T2DM and all subgroups of T2DM patients. Linear regressions were conducted to evaluate the impact of GC status and GLDIS on PCAT attenuation in T2DM patients.
Results
Significant differences were observed in RCA-PCAT and LCX-PCAT between poor GC-T2DM and non-T2DM patients (LCX: − 68.75 ± 7.59 HU vs. – 71.93 ± 7.25 HU, p = 0.008; RCA: − 74.37 ± 8.44 HU vs. − 77.2 ± 7.42 HU, p = 0.026). Higher PCAT attenuation was observed in LAD-PCAT, LCX-PCAT, and RCA-PCAT in non-GLDIS T2DM patients compared with GLDIS T2DM patients (LAD: − 78.11 ± 8.01 HU vs. − 75.04 ± 8.26 HU, p = 0.022; LCX: − 71.10 ± 8.13 HU vs. − 68.31 ± 7.90 HU, p = 0.037; RCA: − 78.17 ± 8.64 HU vs. − 73.35 ± 9.32 HU, p = 0.001). In the linear regression, other than sex and duration of diabetes, both metformin and acarbose were found to be significantly associated with lower LAD-PCAT (metformin: β coefficient = − 2.476, p=0.021; acarbose: β coefficient = − 1.841, p = 0.031).
Conclusion
Inadequate diabetes management, including poor GC and lack of GLDIS, may be associated with increased coronary artery inflammation in T2DM patients, as indicated by PCAT attenuation on CCTA, leading to increased cardiovascular risk. This finding could help healthcare providers identify T2DM patients with increased cardiovascular risk, develop improved cardiovascular management programs, and reduce subsequent cardiovascular related mortality.
Similar content being viewed by others
Background
Diabetes mellitus is a metabolic chronic inflammatory disease associated with increased cardiovascular risk [1,2,3]. Against the backdrop of a sharp rise in the number of diabetes patients globally, type 2 diabetes mellitus (T2DM) patients, accounting for 90% of global diabetes cases, have become the primary group for cardiovascular disease-related deaths [4,5,6]. T2DM patients face a higher risk of cardiovascular complications due to exacerbated vascular inflammation, which leads to the remodeling of blood vessel structure and function, as well as reduced vascular elasticity and efficiency [7,8,9].
Pericoronary adipose tissue (PCAT) engages in a bi-directional signaling interaction with the arterial wall [10], not only secreting inflammatory cytokines towards the vessel wall but also responding to signals from it [11, 12]. This dynamic crosstalk is crucial for regulating vascular homeostasis under normal physiological conditions [13]. PCAT adipocytes constrict due to inflammatory factor secretion [14, 15]. This ‘cachexia’ effect on adipocytes near the inflamed arterial wall leads to lipid-poor adipocytes having increased water content in the proximal-to-distal direction. CCTA accordingly exhibits a CT value gradient - as PCAT nears the inflamed coronary wall, CT values rise [16]. The complex interactions between PCAT and the arterial walls play a critical role in maintaining vascular health. CCTA attenuation, as an imaging biomarker reflecting coronary inflammation, can assist in evaluating the status of vascular inflammation and cardiovascular disease risk [17].
A recent study found that diabetic patients exhibited significantly higher PCAT attenuation around the right coronary artery (RCA) compared with non-diabetic patients, irrespective of stenotic severity and plaque vulnerability [18]. potentially due to inflammation around blood vessels triggered by high blood sugar levels. Case studies have documented a consistent reduction in PCAT attenuation among T2DM following treatment with the antidiabetic drug somatostatin, hinting at GLDIS’s potential in mitigating inflammation of the coronary arteries [19]. Elevated PCAT attenuation is linked to a heightened risk of cardiovascular incidents [20, 21]. Considering this association, this study aimed to evaluate the effects of diabetes management strategies, especially GLDIS and the state of GC, on PCAT attenuation, aiming to contribute to the cardiovascular risk management for T2DM patients.
Methods
Study population
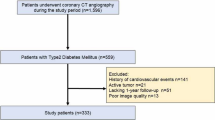
In this cross-sectional study, we retrospectively recruited consecutive T2DM patients and non-T2DM patients who underwent CCTA in two tertiary hospitals from January 2019 to January 2020. We gathered data on the clinical features, coronary CTA findings, and patient outcomes. The study protocol was approved by the Ethics Review Committee of Wuhan Central Hospital and the Ethics Review Committee of Liyuan Hospital (Wuhan Central Hospital: WHZXKYL 2023 − 168; Liyuan Hospital: [2023] IEC RYJ010), respectively. Finally, we included 1342 patients from Wuhan Central Hospital and Liyuan hospital. (Fig. 1 ).
Data collection
From January 2019 to January 2020, patients who underwent CCTA and met the following inclusion criteria were enrolled: From January 2019 to January 2020, patients who underwent CCTA in two tertiary hospitals and did not meet the following exclusion criteria were included in the study : Exclusion criteria: (1) history of diagnosed cardiovascular disease (CVD), including myocardial infarction, ischemic heart disease surgery (coronary artery bypass grafting, percutaneous transluminal coronary angioplasty), or stroke. (2) suspected infectious diseases; and (3) poor image quality. The definition of T2DM includes the following criteria: (1) previous history of diabetes; (2) fasting blood glucose ≥ 7.0 mmol/L; (3) glycated blood glucose protein ≥ 6.5%; (4) receiving hypoglycemic therapy [22]. Based on HbA1c levels, T2DM patients are divided into three groups: well GC group: HbA1c < 7%, moderate GC group: 7 ≤ HbA1c ≤ 9%, and poor GC group: HbA1c > 9% [23]. GLDIS is defined as the use of glucose-lowering drug therapy, which includes the administration of one or more oral or injectable glucose-lowering drugs and maintaining this treatment for a period of 3 months or longer. Table 1 showed the results of how the groups were categorized and matched based on propensity scores.
A total of three radiologists jointly collected the baseline features of the patients from clinical inpatient records, such as age, sex, body mass index (BMI), laboratory test data, previous medication use, and CAD risk factors. Hypertension was defined as systolic blood pressure > 140 mmHg and/or diastolic blood pressure > 90 mmHg and/or use of antihypertensive medication [24]. According to the guidelines, dyslipidemia was defined as one or more of the following: total cholesterol > 6.2 mmol/L, low-density lipoprotein (LDL) cholesterol > 4.1 mmol/L, high-density lipoprotein (HDL) cholesterol < 1.0 mmol/L, serum triglycerides > 2.3 mmol/L, or diagnosis/treatment of dyslipidemia [25]. Smoking status is defined as current smoking or non-smoking. Data System (CAD-RADS) grade 3 or above were considered to have significant stenosis.
CCTA acquisition
All patients underwent CCTA using a dual-source CT scanner (SOMATOM Definition Flash, Siemens Medical Solutions, Erlangen, Germany) or a 128-slice wide detector CT scanner (Revolution HD, GE Healthcare, USA). Patients’ heart rates were controlled to maintain approximately 70 beats per minute, and oral metoprolol was routinely recommended. CTA image acquisition was performed using prospective ECG-triggered Tube voltage (100–140 kV) and tube current was automatically adjusted based on the patient’s body size using the automatic exposure control system on the scanner.
PCAT inflammation analysis
Using professional FAI analysis software (coronary artery FAI analysis, version 1.0.4, Shukun technology [26]), the three main vessels of the coronary artery—LAD, LCX, and RCA—were tracked. To avoid the influence of aortic wall, the opening of right coronary artery 10–50 mm and the proximal end of left anterior descending branch and left circumflex branch 40 mm were measured and measured. PCAT attenuation was obtained by automatically calculating and recording the weighted average CT attenuation of adipose tissue (attenuation coefficient between − 190 and − 30 HU) within the radial distance of the outer wall of the blood vessel equidistant from the average diameter of the blood vessel [17, 27]. Figure 2 provides an example of these parameters.
Representative case of LAD-PCAT attenuation measured by CCTA. A Three-dimensional reconstruction of the heart ; B PCAT attenuation between − 190 and – 30 HU in the cross-sectional view ; C The segment of the proximal coronary artery in a straightened view ; D Around the proximal 40 mm of the left anterior descending artery. LAD left anterior descending artery, CCTA coronary computed tomography angiography, PCAT Pericoronary adipose tissue
Analysis of sample size
As there is no publicly available data on PCAT attenuation in GLDIS T2DM patients and non-GLDIS T2DM patients, the sample size was determined based on our own preliminary data. Prior to this study, we retrospectively analyzed the PCAT attenuation of 102 T2DM patients, who were not part of this study. The GLDIS T2DM patients (n = 87): RCA − 74.79 ± 7.34, non-GLDIS T2DM patients (n = 25): RCA − 78.74 ± 8.17. According to the findings, a sample size of 161 GLDIS T2DM patients and 47 non-GLDIS T2DM patients had to achieve a 90% efficacy level and observe significant differences at a unilateral significance level of 0.05 between GLDIS T2DM patients and non-GLDIS T2DM patients. Ultimately, the study included 439 GLDIS T2DM patients and 108 non-GLDIS T2DM patients, ensuring > 90% efficacy level. The sample size calculation was performed using the statistical software (PASS 2021 version 21.0.3,)
Statistical analysis
Quantitative variables with a normal distribution were expressed as means ± standard deviations (SD), while median and interquartile range (IQR) were used for variables that were not normally distributed. Categorical variables were reported as counts (%) and compared using either the Chi-square test or Fisher exact test, depending on the size of the data cell. Student’s t-test was used for normally distributed data, and the Mann-Whitney U test was used for data that was not normally distributed. The assumption of a normal distribution was checked using the one-sample Kolmogorov-Smirnov test. One-way ANOVA with Bonferroni post-hoc test was used to compare continuous variables among multiple groups; the chi-squared test was used for comparison of categorical variables. To minimize the impact of baseline characteristic differences between the two groups, propensity score matching (PSM) was employed. A one-to-one nearest neighbor matching algorithm was used to assess the propensity score of each patient. A logistic regression model was used with T2DM as the dependent variable and age, gender, BMI, cardiovascular risk factors (dyslipidemia, hypertension, smoking), tube voltage and statin use as independent variables. Considering previous studies indicating the effect of statins on coronary inflammation, statins were also included in the matching factors [28].
Multivariate stepwise linear regression was conducted to examine the association between cardiovascular risk factors (age, gender, body mass index, dyslipidemia, hypertension, and smoking), glucose-lowering drugs, antihypertensive drugs, statin use, duration of diabetes, and tube voltage as independent variables, and LAD-PCAT, LCX-PCAT, and RCA-PCAT as dependent variables. Statistical significance was assessed using a two-tailed p-value of less than 0.05. All statistical analyses were conducted using SPSS Statistical Software version 25.0 and GraphPad Prism 7.0.
Results
Baseline characteristics of study groups
In Table 2, detailed demographic data are presented. A total of 1342 patients were included (mean age: 62.43 ± 10.58 years, including 662 males). At baseline, T2DM patients (n = 547) had a higher BMI (p = 0.019), a higher prevalence of hypertension (p < 0.001), dyslipidemia (p = 0.003), statin use (p < 0.001), and significant differences in all lipid profile measures. There was no significant difference in PCAT attenuation between T2DM and non-T2DM patients in the three coronary arteries (LAD: p = 0.238, LCX: p = 0.854, RCA: p = 0.671).
Comparison of clinical characteristics and CT parameters between non-T2DM and T2DM patients with different GC status
After adjusting for age, gender, BMI, cardiovascular risk factors (dyslipidemia, hypertension, smoking), tube voltage, CAD-RADS grade, and statin use, we observed a significant difference in RCA-PCAT between poor GC-T2DM patients and non-T2DM patients (− 74.37 ± 8.44 vs. − 77.2 ± 7.42, p = 0.026) (Table 3). Additionally, PCAT attenuation differences between well GC-T2DM patients or moderate GC-T2DM patients and non-T2DM patients were not significant. (Table 3) The pairwise comparisons of baseline PCAT attenuation around LAD, LCX, and RCA among T2DM patients with different GC statuses were shown in Fig. 3.
The baseline data for T2DM patients divided into three groups based on GC statuses is shown in Additional file 1: Table S1. The results showed that there were significant differences in LAD-PCAT among the three groups from the baseline data (p = 0.008).
Comparison of clinical characteristics and CT parameters in GLDIS T2DM patients and non-GLDIS T2DM patients
For T2DM patients, the presence or absence of GLDIS made a significant difference in RCA-PCAT, LAD-PCAT, and LCX-PCAT (GLDIS T2DM patients vs. non-GLDIS T2DM patients, LAD: − 75.29 ± 8.33 vs. − 78.29 ± 8.17, p = 0.002 ;LCX: − 68.74 ± 7.97 vs. − 72.09 ± 7.93, p = 0.001;RCA: − 73.66 ± 9.10 vs. − 77.67 ± 8.53, p < 0.001) (Additional file 1: Table S2 and Fig. S1). This significant difference remained after conducting PSM to adjust for cardiovascular risk factors (age, sex, body mass index, dyslipidemia, hypertension and smoking) and statin use rates ( p<0.05 for all, Table 4; Fig. 4 ).
Subgroup analysis of PCAT attenuation in GLDIS T2DM patients and non-T2DM patients, as well as non-GLDIS T2DM patients and non-T2DM patients, is presented in Additional file 1: Table S3-S4. LCX-PCAT and RCA-PCAT were significantly higher in non-GLDIS T2DM patients compared with non-T2DM patients, while this difference was not observed between GLDIS T2DM patients and non-T2DM patients. The result remains the same even after PSM.
Results from stepwise regression analysis
Multivariate stepwise linear regression analysis selected five significant factors associated with the attenuation of LAD-PCAT, LCX-PCAT, and RCA-PCAT. Metformin, acarbose and duration of diabetes were found to have a significant impact on LAD-PCAT (metformin : β coefficient = − 2.476, p=0.021; acarbose : β coefficient = − 1.841, p = 0.031; Duration of diabetes: β coefficient = 0.23, p<0.001). Additionally, Tube voltage had a significant impact on all three dependent variables (LAD-PCAT, LCX-PCAT, RCA-PCAT). The independent variables that significantly affected LCX-PCAT and RCA-PCAT are presented in Table 5.
Discussion
To our knowledge, this is a multi-center study with a large sample size, the first to explore more comprehensively the relationship between coronary inflammation based on PCAT attenuation and diabetes management (including GC and GLDIS) in T2DM patients. Firstly, our study revealed that, after adjusting for confounding factors, the RCA-PCAT in poor GC-T2DM patients was significantly higher compared with non-T2DM patients. Secondly, RCA-PCAT, LAD-PCAT, and LCX-PCAT were significantly higher in non-GLDIS T2DM patients than GLDIS T2DM patients. Thirdly, in the multivariate stepwise linear regression, metformin and acarbose were both significantly associated with lower LAD-PCAT. The results indicated inadequate diabetes management, including poor GC and lack of GLDIS, may aggravate coronary inflammation.
This research further substantiates earlier findings of the relationship between T2DM and coronary artery inflammation. A prior study by Yu et al. [18] showed diabetic patients had elevated levels of coronary inflammation compared to non-diabetic populations. Furthermore, a matched case-control study utilizing 18 F-FDG-PET/CT demonstrated type 1 diabetes individuals had increased vascular wall inflammation, correlating with inflammatory blood proteins [29]. Consistently, our results indicated that poor GC-T2DM patients exhibited significantly higher RCA-PCAT versus non-T2DM patients after PSM.
Additionally, this study revealed the potential importance of GLDIS for improving coronary inflammation in T2DM patients. As a new non-invasive technique based on CCTA imaging to directly detect coronary artery inflammation, PCAT attenuation was markedly higher in non-GLDIS T2DM patients compared with GLDIS T2DM patients. This result indicates that non-GLDIS T2DM patients have higher coronary inflammation. Specifically, in the multivariate stepwise linear regression, metformin and acarbose use were significantly associated with lower LAD-PCAT attenuation, indicating targeted medications could play a key role in effective T2DM management to reduce coronary inflammation. Metformin, a first-line medication for T2DM, offers benefits that go beyond GC. It also provides additional cardiovascular protection for T2DM patients. Several studies have shown that its potential to reduce inflammation within blood vessels and improve endothelial function, which can contribute to a lower risk of cardiovascular events. This anti-inflammatory effect may be related to metformin’s impact on certain inflammatory mediators, such as C-reactive protein and interleukin-6, which are known risk factors for cardiovascular diseases [30]. These markers are known risk factors for cardiovascular diseases. Additionally, metformin has been reported to inhibit proinflammatory responses and the activation of Nuclear Factor-kappa B (NF-κB) in human vascular wall cells, which further supports its role in cardiovascular protection [31]. Furthermore, a meta-analysis on the effects of metformin treatment indicated that early therapy with metformin might ameliorate chronic inflammation, as evidenced by reductions in serum levels of CRP [32]. Interestingly, research by Sardu et al. [33] also supports metformin’s potential for alleviating coronary inflammation - non-metformin users had more adipose inflammation, higher leptin/adiponectin ratios, and more cardiovascular events than metformin users, which further supported our results. Our study suggested that metformin use was significantly associated with lower LAD-PCAT attenuation.
Similarly, acarbose (a carbohydrate absorption inhibitor) reduces postprandial hyperglycemia and glucose fluctuations in T2DM patients, thereby mitigating inflammatory responses linked to glycemic variability [28, 34, 35]. These inferences still need to be further verified in the future.
Furthermore, diabetes, a persistent chronic inflammatory illness, is intricately tied to alterations in cardiac structure and function, like diminished left ventricular performance and cardiac enlargement [36]. Elevated blood glucose spurs excessive protein accumulation in the myocardium and rising glycation end products (AGEs) [37]. These stimulate inflammatory signaling and apoptosis pathways in endothelial cells, heightening oxidative stress and inflammation. This exacerbates myocardial cell damage, impacting cardiac structure and function. However, the linkage between heart function and coronary artery inflammation remains sparsely explored, underscoring the need for more extensive investigation in this domain.
This study had several limitations that may affect the interpretation of our results and conclusions. First, the cross-sectional design used in this study means that we cannot determine the causal relationship between diabetes management and coronary artery inflammation, and we also cannot completely rule out the possibility of selection bias. In addition, the study data came from only two tertiary hospitals’ patient populations, which may limit the applicability of our findings to the broader population of patients with type 2 diabetes. Therefore, future research needs to be conducted across multiple centers and cover larger sample sizes to enhance the generalizability and reliability of the conclusions. Second, although we used propensity score matching to adjust for known confounding factors and sample size analysis to ensure the reliability and statistical power of the study, we were unable to consider all potential confounding variables such as dietary habits, physical activity levels, and complications. These unmeasured factors may affect the relationship between diabetes management and coronary artery inflammation. Therefore, future research should include these potential confounding factors and use more comprehensive data collection to reduce their interference with the study results. Third, this study failed to provide detailed information on the duration and dosage of GLDIS, limiting our ability to understand how these factors affect coronary artery inflammation. This highlights the necessity for future research to collect and analyze detailed information such as patients’ medication adherence, specific dosages, and treatment durations to assess the effects of GLDIS more accurately on coronary artery health. In summary, we recognize that selection bias, unmeasured confounding factors, and the lack of detailed GLDIS dosage and usage time information may affect our study results. Future research should adopt multi-center, prospective longitudinal cohort study designs and include more potential confounding factors such as dietary habits, physical activity levels, and complications. Through more rigorous study designs and comprehensive data collection methods, we can more accurately assess the correlation between cardiovascular events and PCAT attenuation and GLDIS, further validating the efficacy and reliability of the study. This can provide more convincing evidence and a deeper understanding of the connections between diabetes management, coronary artery inflammation, and cardiovascular events.
Conclusions
Our research indicated that RCA-PCAT attenuation was significantly higher in poor GC-T2DM compared with non-T2DM. Additionally, PCAT attenuation was significantly lower in GLDIS T2DM patients versus non-GLDIS T2DM patients. Our research reveals a preliminary signal that inadequate diabetes management, including poor GC and lack of GLDIS, may be associated with increased coronary artery inflammation in T2DM patients, as indicated by PCAT attenuation on CCTA. These insights could assist healthcare providers in identifying T2DM patients at increased cardiovascular risk and developing improved protocols for cardiovascular management, with the potential to reduce subsequent cardiovascular-related mortality.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- GLDIS:
-
Glucose-lowering drug interventions
- GC:
-
Glycemic control
- CAD:
-
Coronary artery disease
- CTTA:
-
Coronary Computed tomography angiography
- PCAT:
-
Pericoronary adipose tissue
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- RCA:
-
Right coronary artery
- CVD:
-
Cardiovascular disease
- BMI:
-
Body mass index
- HbA1c:
-
Glycated hemoglobin
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- HU:
-
Hounsfield units
- DS:
-
Diameter stenosis
- PSM:
-
Propensity score matching
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
References
Guo Y, Xie C, Li X, Yang J, Yu T, Zhang R, Zhang T, Saxena D, Snyder M, Wu Y et al. Succinate and its G-protein-coupled receptor stimulates osteoclastogenesis. Nat Commun 2017;815621.
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22.
Zhao C, Yang C, Wai STC, Zhang Y, M PP, Paoli P, Wu Y, San Cheang W, Liu B, Carpéné C, et al. Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit Rev Food Sci Nutr. 2019;59(6):830–47.
Collaborators GD. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of Disease Study 2021. Lancet. 2023;402(10397):203–34.
Group IHS. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7(5):385–96.
Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a therapeutic target in atherosclerosis. J Clin Med 2019;8(8).
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97.
Gimbrone MA Jr., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–36.
Si N, Shi K, Li N, Dong X, Zhu C, Guo Y, Hu J, Cui J, Yang F, Zhang T. Identification of patients with acute myocardial infarction based on coronary CT angiography: the value of pericoronary adipose tissue radiomics. Eur Radiol. 2022;32(10):6868–77.
Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17(2):115–30.
Milasan A, Smaani A, Martel C. Early rescue of lymphatic function limits atherosclerosis progression in Ldlr(-/-) mice. Atherosclerosis 2019;283106–19.
Taher M, Nakao S, Zandi S, Melhorn MI, Hayes KC, Hafezi-Moghadam A. Phenotypic transformation of intimal and adventitial lymphatics in atherosclerosis: a regulatory role for soluble VEGF receptor 2. Faseb j. 2016;30(7):2490–9.
Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol. 2014;34(8):1631–6.
Antoniades C, Shirodaria C. Detecting coronary inflammation with Perivascular Fat Attenuation Imaging: making sense from Perivascular Attenuation maps. JACC Cardiovasc Imaging. 2019;12(10):2011–14.
Antoniades C, Kotanidis CP, Berman DS. State-of-the-art review article. Atherosclerosis affecting fat: what can we learn by imaging perivascular adipose tissue? J Cardiovasc Comput Tomogr. 2019;13(5):288–96.
Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218(1):7–29.
Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, Margaritis M, Shirodaria C, Kampoli AM, Akoumianakis I, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9:398.
Yu Y, Ding X, Yu L, Dai X, Wang Y, Zhang J. Increased coronary pericoronary adipose tissue attenuation in diabetic patients compared to non-diabetic controls: a propensity score matching analysis. J Cardiovasc Comput Tomogr. 2022;16(4):327–35.
Malavazos AE, Meregalli C, Sorrentino F, Vignati A, Dubini C, Scravaglieri V, Basilico S, Boniardi F, Spagnolo P, Malagoli P, et al. Semaglutide therapy decreases epicardial fat inflammation and improves psoriasis severity in patients affected by abdominal obesity and type-2 diabetes. Endocrinol Diabetes Metab Case Rep. 2023;2023:3.
Ichikawa K, Miyoshi T, Osawa K, Nakashima M, Miki T, Nishihara T, Toda H, Yoshida M, Ito H. High pericoronary adipose tissue attenuation on computed tomography angiography predicts cardiovascular events in patients with type 2 diabetes mellitus: post-hoc analysis from a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):44.
Elnabawi YA, Oikonomou EK, Dey AK, Mancio J, Rodante JA, Aksentijevich M, Choi H, Keel A, Erb-Alvarez J, Teague HL, et al. Association of Biologic Therapy with Coronary Inflammation in patients with psoriasis as assessed by Perivascular Fat Attenuation Index. JAMA Cardiol. 2019;4(9):885–91.
Classification and Diagnosis of Diabetes. Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–31.
Aronson R, Orzech N, Ye C, Goldenberg R, Brown V. Specialist-led diabetes registries and predictors of poor glycemic control in type 2 diabetes: insights into the functionally refractory patient from the LMC Diabetes Registry database. J Diabetes. 2016;8(1):76–85.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
Ren J, Gao Z, Zhao R, Lu S, Zhao G, Li D, Jun J. Guidelines for the Prevention and Treatment of Dyslipidemia in Chinese adults (revised Edition 2016). Chin Circulation J 2016;31(10).
Yu L, Chen X, Ling R, Yu Y, Yang W, Sun J, Zhang J. Radiomics features of pericoronary adipose tissue improve CT-FFR performance in predicting hemodynamically significant coronary artery stenosis. Eur Radiol. 2023;33(3):2004–14.
Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, Thomas S, Herdman L, Kotanidis CP, Thomas KE et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet (London England) 2018;392929–39.
Valentina A, VeronikaA M, Cristina VM, Maurizio R, Paola S, Ilaria M, Nicola C, Donato M, Vincenza V, Michele C et al. The role of Glycemic Variability in Cardiovascular disorders. Int J Mol Sci 2021;22(16).
Janssen AWM, van Heck JIP, Stienstra R, Aarntzen EHJG, van Diepen JA, Riksen NP, Tack CJ. Arterial wall inflammation assessed by 18F-FDG-PET/CT is higher in individuals with type 1 diabetes and associated with circulating inflammatory proteins. Cardiovascular Res. 2023;119(10):1942–51.
Bai B, Chen H. Metformin: a Novel Weapon against inflammation. Front Pharmacol 2021;12622262.
Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schönbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-kappab in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26(3):611–7.
Wang J, Zhu L, Hu K, Tang Y, Zeng X, Liu J, Xu J. Effects of metformin treatment on serum levels of C-reactive protein and interleukin-6 in women with polycystic ovary syndrome: a meta-analysis: a PRISMA-compliant article. Med (Baltim). 2017;96(39):e8183.
Sardu C, D’Onofrio N, Torella M, Portoghese M, Loreni F, Mureddu S, Signoriello G, Scisciola L, Barbieri M, Rizzo MR, et al. Pericoronary fat inflammation and major adverse cardiac events (MACE) in prediabetic patients with acute myocardial infarction: effects of metformin. Cardiovasc Diabetol. 2019;18(1):126.
Mori Y, Shiozaki M, Matsuura K, Tanaka T, Yokoyama J, Utsunomiya K. Evaluation of efficacy of acarbose on glucose fluctuation and postprandial glucose using continuous glucose monitoring in type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13(4):467–70.
Li FF, Xu XH, Fu LY, Su XF, Wu JD, Lu CF, Ye L, Ma JH. Influence of Acarbose on plasma glucose fluctuations in insulin-treated patients with type 2 diabetes: a pilot study. Int J Endocrinol 2015;2015903524.
Nguyen JP, Ramirez-Sanchez I, Garate-Carrillo A, Navarrete-Yañez V, Carballo-Castañeda RA, Ceballos G, Moreno-Ulloa A, Villarreal F. Effects of aging and type 2 diabetes on cardiac structure and function: underlying mechanisms. Exp Gerontol 2023;173112108.
Askin L, Tanrıverdi O, Tibilli H, Turkmen S. New Method improves the evaluation of subclinical left ventricular dysfunction in type 2 diabetes Mellitus. Arq Bras Cardiol. 2019;113(2):216–17.
Acknowledgements
None.
Funding
The work was supported by National Key R&D Program of China(2019YFE0196800).
Author information
Authors and Affiliations
Contributions
Dr. Y-KL contributed to image analysis, statistical analysis, data acquisition and manuscript drafting, including preparing all figures and tables. Dr. L-SD contributed to study conception and statistical analysis. Dr. DY contributed to manuscript drafting and data collection. Dr. CM contributed to manuscript drafting. Dr. P-PC contributed to study conception research design. C-PJ and Dr. YL contributed to data acquisition. Dr. X-YX and Dr. XW contributed to revise the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Review Committee of Wuhan Central Hospital and the Ethics Review Committee of Liyuan Hospital (Wuhan Central Hospital: WHZXKYL2023-168; Liyuan Hospital: [2023] IEC RYJ010) and conducted by the principles outlined in the Declaration of Helsinki. However, patient informed consent was waived as this was a retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1
. Table S1. Comparison of clinical characteristics and CT parameters in diabetic patients according to GC status. Table S2. Comparison of clinical characteristics and CT parameters in GLDIS T2DM patients and non-GLDIS T2DM patients. Table S3. Comparison of clinical characteristics and CT parameters in GLDIS T2DM patients and non-T2DM patients. Table S4. Comparison of clinical characteristics and CT parameters in non-GLDIS T2DM patients and non-T2DM patients. Figure S1. PCAT attenuation in three main coronary arteries stratified by GLDIS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Dai, L., Dong, Y. et al. Coronary inflammation based on pericoronary adipose tissue attenuation in type 2 diabetic mellitus: effect of diabetes management. Cardiovasc Diabetol 23, 108 (2024). https://doi.org/10.1186/s12933-024-02199-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02199-x