Abstract
Currently, the differentiation between type 1 diabetes (T1D) and type 2 diabetes (T2D) is not straightforward, and the features of both types of diabetes coexist in one subject. This situation triggered the need to discriminate so-called double diabetes (DD), hybrid diabetes or type 1.5 diabetes, which is generally described as the presence of the insulin resistance characteristic of metabolic syndrome in individuals diagnosed with T1D. DD not only raises the question of proper classification of diabetes but is also associated with a significantly greater risk of developing micro- and macroangiopathic complications, which was independent of glycaemic control. When considering the global obesity pandemic and increasing incidence of T1D, the prevalence of DD may also presumably increase. Therefore, it is of the highest priority to discover the mechanisms underlying the development of DD and to identify appropriate methods to prevent or treat DD. In this article, we describe how the definition of double diabetes has changed over the years and how it is currently defined. We discuss the accuracy of including metabolic syndrome in the DD definition. We also present possible hypotheses connecting insulin resistance with T1D and propose possible methods to identify individuals with double diabetes based on indirect insulin resistance markers, which are easily assessed in everyday clinical practice. Moreover, we discuss adjuvant therapy which may be considered in double diabetic patients.
Similar content being viewed by others
Introduction
According to the World Health Organisation (WHO), diabetes comprises a group of metabolic diseases characterised by chronic hyperglycaemia that may lead to damage in various organs (especially the eyes, kidneys, nerves, heart and blood vessels), which eventually results in dysfunction [1]. Two main types of diabetes (among others) have been distinguished: type 1 diabetes (T1D) and type 2 diabetes (T2D). This classification system is based on various factors that differ between patients with T1D and T2D, such as age at disease onset, excessive weight, degree of insulin resistance (IR), presence of metabolic syndrome (MS), degree of loss of pancreatic β-cell function, presence of specific autoantibodies associated with β-cell destruction, presence of a systematic subclinical inflammatory state, concentration of C-peptide in the blood and requirement for treatment with insulin to survive [2]. A patient suffering from T1D is typically represented as being a young lean person who has lost 90–100% of his/her β-cell function and needs insulin treatment from the time that the disease started because of a direct lack of insulin production and secretion. However, an individual with T2D is represented as being an older, overweight or obese person who develops the state known as insulin resistance, which causes the functional failure of β-cells and, consequently, insulin deficiency. Patients usually suffer from other diseases, especially cardiovascular diseases, and can be treated with oral antidiabetic drugs at the beginning of the course of the disease, whereas insulin treatment is needed at a later point in time.
However, at the current time, distinguishing between these two major types of diabetes is not straightforward, and the features of both T1D and T2D may coexist within one subject [3]. This state is known as double diabetes (DD), hybrid diabetes or type 1.5 diabetes and is generally described as the presence of the IR characteristic of metabolic syndrome in individuals diagnosed with type 1 diabetes [3, 4]. Merger et al. showed that one in four patients suffering from T1D meets the criteria of MS according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and can be identified as an individual with DD [5]. This is associated with a significantly increased risk for the development of micro- and macroangiopathic complications (independent of glycaemic control) [5]. Moreover, the prevalence of cardiovascular comorbidities and diabetic nephropathy in individuals with DD is more similar to that in patients suffering from T2D than to that in patients with T1D [5]. This phenomenon creates new diagnostic and therapeutic challenges.
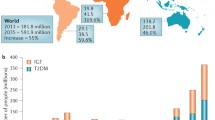
We do not currently possess global data on the prevalence of metabolic syndrome, and the estimates vary according to the criteria that are used to establish MS. However, given that MS is three times more common than diabetes, it can be estimated that metabolic syndrome affects approximately a quarter of the population worldwide [6]. A 2015 study highlighted the fact that 604 million adults and 108 million children in 195 countries were obese and that the prevalence of obesity doubled in more than 70 countries in individuals older than 25 years from 1980 to 2015 [7]. A trend towards an increasing incidence of excessive body weight has also been observed among patients suffering from T1D, most of whom are overweight or obese and gain weight faster than the general population [7, 8]. Of course, obesity is not always synonymous with the presence of MS but is usually one of its components and is easily diagnosed in most cases in everyday practice; thus, it should be particularly highlighted. When considering the global obesity pandemic and increasing incidence of type 1 diabetes, it is of the highest priority to establish the exact definition of double diabetes, as well as to discover the mechanisms underlying the development of DD and to identify appropriate methods to prevent or treat DD [7, 9].
In this article, we describe how the definition of double diabetes has changed over the years and how it is currently defined. We focused on the problems that could arise from the inclusion of the metabolic syndrome definition in the DD definition. We also present possible hypotheses connecting insulin resistance with type 1 diabetes. Moreover, we discuss the possible methods based on indirect insulin resistance markers for identifying individuals with double diabetes.
Double diabetes—efforts to establish the classification criteria
The first mention of double diabetes was made in 1991. Teupe et al. showed that, compared with individuals without a family history of T2D, some patients with T1D who had relatives suffering from T2D have increased bodyweights and require higher doses of insulin to maintain normoglycaemia [3]. The authors proposed the classification of DD patients as type 1 diabetic individuals with at least one type 2 diabetic relative [3].
Moreover, paediatricians have reported an increased occurrence of T2D in children, which is unusual, as well as problems in differentiating between T1D and T2D in this population [4, 10,11,12]. Gilliam et al. defined DD patients as individuals who were atypical or who had clinical features characteristic of T1D and T2D at presentation, which raised doubts about the proper diagnosis [13]. Pozzilli et al. claimed that DD occurs in individuals with clinical features of T2D and T1D, with a metabolic component outweighing an autoimmune component [14]. Cleland also described DD as the presence of T1D and features characteristic of T2D [15]. Kietsiriroje et al. defined double diabetes as type 1 diabetes in individuals with a family history of T2D, being overweight, with features of MS or insulin resistance, thus highlighting the significance of an indirect marker of IR known as the estimated glucose disposal rate (eGDR) [16]. The double diabetes phenomenon may raise doubts as to whether there are more type 1 diabetes with coexisting features of type 2 diabetes or more type 2 diabetes with a present autoimmune component. It seems that DD represents a grey zone between T1D and T2D and is a continuum of diabetes [17]. A summary of DD definitions that have been created over the years is presented in Table 1.
Almost all of these authors focused on subjects with established T1D with features characteristic of T2D. The diagnostic criteria show one similarity involving elements of MS. Therefore, a type 1 diabetic individual with a diagnosis of metabolic syndrome may be classified as being a double diabetic individual. However, more precisely, insulin resistance underlies the pathogenesis of double diabetes, as explained further in the article. Moreover, the inclusion of MS in the criteria necessary to recognise DD may have some complications, thus making the diagnosis of double diabetes incoherent. Therefore, clinicians should first focus on assessing the presence of insulin resistance.
In the double diabetes definition, does metabolic syndrome truly make the diagnosis easier?
As the thorough assessment of the degree of insulin resistance in type 1 diabetic patients in clinical practice is difficult, the presence of metabolic syndrome features may be helpful for identifying individuals at risk for double diabetes. The definition of metabolic syndrome has changed over the years. The most commonly used classification criteria are those proposed by the WHO, NCEP ATP III and the International Diabetes Federation (IDF), and modified later by (among others) IDF and American Heart Association (AHA) [18,19,20,21,22,23]. These data are presented in Table 2.
The MS criteria are based on uncomplicated measurements and basic blood tests; however, all of them involve hyperglycaemia or diagnosed diabetes, which are intended to be related to IR and T2D. However, type 1 diabetic individuals will always meet these criteria, but not necessarily because of the development of insulin resistance. Moreover, patients may also be diagnosed with MS according to the WHO definition when they only possess microalbuminura, even if they have a normal body weight and lipid profile. For this reason, the definitions of metabolic syndrome should be used carefully in these patients so that they are not overdiagnosed with MS. This also raises the question of whether this glycaemic criterion could be supported by or replaced with other markers indicating the presence of insulin resistance in individuals with type 1 diabetes. This problem was addressed by Lecumberri et al. They showed that the prevalence of MS among type 1 diabetic patients is lower when using standard IDF criteria and when excluding hyperglycaemia as a criterion [24]. Moreover, they proposed the modification of the MS definition in T1D, including the assessment of IR by using indirect insulin resistance markers [24]. Another disadvantage of using MS definitions is the lack of cohesion among them. These conditions are not the same, and some discrepancies in the epidemiology of metabolic syndrome may occur, as the prevalence of MS may vary depending on the criteria that are used while performing research [25,26,27,28,29]. Hence, special attention should be given when using MS definitions among individuals with type 1 diabetes.
Due to the fact that double diabetes is associated with a significantly greater risk of developing micro- and macroangiopathic complications (similar to T2D rather than T1D), the quick, appropriate and efficient identification of the population at risk for DD development is crucial [5]. The aforementioned issues indicate the need for more precise tools to diagnose double diabetes that focus on the presence of insulin resistance and not on the presence of metabolic syndrome itself, which may be unreliable and insufficient in double diabetes diagnosis.
Insulin resistance and type 1 diabetes—possible explanations for this association
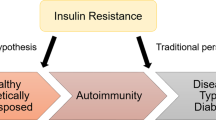
The pathophysiology of the coexistence of type 1 diabetes and insulin resistance is likely multifactorial and has not been fully established. First, type 1 diabetic subjects may also have a genetic predisposition to IR and T2D development, especially when they have a relative with diagnosed T2D [16]. This claim is supported by the fact that people with a family history of T2D tend to have a higher BMI and body fat percentage and exhibit decreased insulin sensitivity [3, 30]. It has been suggested that some type 1 diabetic individuals with a T2D family history develop T2D at some point in life if they have not developed T1D that is initially triggered by an independent pathological process [3]. This hypothesis points to an autoimmune process as a phenomenon that occurs accidentally and independently from insulin resistance and obesity in predisposed individuals.
In contrast, the accelerator hypothesis focuses on obesity-driven insulin resistance in at-risk individuals, which leads to glucotoxicity and accelerates the apoptosis of pancreatic β-cells [31, 32]. It increases immunogenicity and consequently leads to T1D occurrence [31, 32]. To a certain extent, the overload hypothesis is similar. In individuals with triggered autoimmune processes, excessive body weight leads to insulin resistance, which causes the overload of β-cells and the acceleration of apoptosis in these cells [33]. Both of these hypotheses are supported by research, in which insulin resistance and excessive body mass were proven to have an impact on T1D development by accelerating its onset and increasing its risk [34,35,36].
It is possible that insulin resistance develops later after T1D diagnosis due to treatment. Intensive insulin therapy reduces the risk of long-term diabetic complications but is also associated with weight gain [37,38,39]. Obesity is a well-known factor associated with IR that causes chronic inflammation [40, 41]. As previously mentioned, type 1 diabetic subjects gain weight faster than does the general population [8]. This is caused not only by intensive insulin treatment but also by cultural, social and lifestyle changes that favour unhealthy diets and sedentary lifestyles. More flexible insulin treatment adjusted to patients’ actual meals allows for a less cautious dietary pattern, such as an increased frequency of snack consumption. Additionally, repetitive episodes of hypoglycaemia induced by insulin therapy or the presence of fear of hypoglycaemia may result in maladaptive eating habits that promote weight gain [42, 43]. Moreover, obesity itself does not seem to be the only factor responsible for IR. Donga et al. demonstrated that insulin resistance is also present in lean individuals suffering from type 1 diabetes [44]. These findings suggest that the pathogenesis of IR in T1D patients cannot be solely explained by excessive body weight.
Moreover, treatment for T1D is not completely physiological. Normally, insulin is secreted from the pancreas into the portal vein and subsequently reaches the liver, where it is mostly metabolised. Conversely, during T1D treatment, insulin is absorbed from the subcutaneous tissue to the peripheral circulation, thus resulting in peripheral hyperinsulinaemia and hepatic hypoinsulinaemia. It has been suggested that adaptation to this condition may result in decreased peripheral glucose uptake mediated by insulin and increased glucose production by the liver [45]. Chronically elevated levels of insulin in peripheral tissues may modify the expression and activity of insulin receptors, thus influencing insulin signalling pathways and worsening the degree of insulin sensitivity in peripheral tissues [46]. Hyperinsulinaemia interferes with mitochondrial function and enhances oxidative stress in T1D mice, thus increasing whole-body and hepatic insulin resistance [47]. Aditionally, chronic hyperglycaemia increases IR by inducing glucotoxicity, which participates in reducing peripheral glucose uptake; moreover, intensive insulin therapy in T1D patients was shown to increase the degree of insulin sensitivity [48,49,50]. One of the available methods for treating T1D is continuous intraperitoneal insulin infusion, which has been shown to mimic the physiological route of insulin, as insulin reaches the liver first after diffusion (mainly via the portal vein) [51]. This results in the restoration of the natural gradient of insulin concentrations between the liver circulation and the peripheral circulation [52]. In contrast, a decrease in peripheral insulin concentration due to continuous intraperitoneal insulin infusion was not associated with an improvement in insulin resistance, as assessed by using the hyperinsulinaemic-euglycaemic clamp technique (HEC) [53]. This fact indicates that the presence of insulin resistance cannot be solely explained by the nonphysiological nature of type 1 diabetes treatment.
In 1986, Yki-Jarvinen et al. showed that most individuals with T1D of long duration are characterised by varying degrees of IR [54]. This was shown in both poorly controlled and adequately controlled type 1 diabetic patients [55, 56]. These findings were further confirmed in a meta-analysis performed by Donga et al. [57]. Therefore, the identification of tools for assessing the exact degree of insulin sensitivity in patients with type 1 diabetes and distinguishing between individuals with a “normal” level of insulin resistance in type 1 diabetic patients and those with a pathologically high level of insulin resistance are highly important. The pathophysiology of double diabetes is presented in Fig. 1.
Recognition of double diabetes—the role of indirect insulin resistance markers
The gold standard for measuring insulin-mediated glucose uptake is still the hyperinsulinaemic-euglycaemic clamp technique, in which the glucose disposal rate is obtained (GDR) [58]. However, this method is relatively invasive, expensive, time-consuming and technically complicated; as a result, it is not used in everyday clinical practice. The homeostatic model assessment for insulin resistance (HOMA-IR) is typically used for routine assessment of insulin resistance [59]. It has been acknowledged to be a useful tool in epidemiology studies for assessing insulin sensitivity [60, 61]. However, the HOMA-IR cannot be used in patients suffering from type 1 diabetes, because the function of pancreatic β-cells is not known. Additional indirect insulin resistance indices, such as the quantitative insulin sensitivity check index (QUICKI) or the Matsuda index, have been established; however, most of these indices cannot be applied in type 1 diabetic patients [62, 63]. The assessment of insulin sensitivity by these aforementioned indices among individuals who are completely dependent on exogenous insulin is problematic, as fasting glycaemia and insulinaemia do not reflect insulin and glucose metabolism but rather the treatment itself. Therefore, further research must be performed to establish helpful markers indicating the presence of insulin resistance in patients with T1D and to consequently diagnose double diabetes properly.
An alternative method for assessing the presence of IR is to use indirect markers of insulin resistance based on widely available clinical parameters, such as medical history, anthropometrical parameters and basic laboratory tests. The first such marker was the estimated glucose disposal rate (eGDR) [64, 65]. As previously mentioned, a value of eGDR less than 8 was used in the classification criteria for double diabetes by Kietsiriroje et al. [16]. Other surrogate measures of insulin resistance include the lipid accumulation product (LAP), the triglyceride-glucose index (TyG-index) and the visceral adiposity index (VAI) [66,67,68,69]. In addition, other measures include parameters related to the TyG index, such as the triglyceride-glucose-body mass index (TyG-BMI) and the triglyceride-glucose-waist circumference (TyG-WC) [70]. Moreover, other parameters include the estimated insulin sensitivity (eIS), the natural logarithm of the glucose disposal rate (lnGDR), the metabolic score for IR (METS-IR), the triglyceride-glucose-waist-to-height ratio (TyG-WHtR) and the triglyceride-glucose-waist-to-hip ratio (TyG-WHpR) [71,72,73,74,75]. Furthermore, the insulin dosage that is needed for obtaining normoglycaemia per kg, waist-to-height ratio (WHtR), waist circumference (WC) and the triglyceride/HDL cholesterol ratio (TG/HDL-C) may also be used in the screening of metabolic syndrome among adult patients suffering from type 1 diabetes [76,77,78,79]. The aforementioned indirect insulin resistance markers are fast, inexpensive and noninvasive methods and may be useful tools for identifying IR in people for whom the HOMA-IR, QUICKI or Matsuda index cannot be applied. A summary of these data is presented in Additional file 1.
Most of these indirect markers of insulin resistance were compared with the gold standard (HEC) in different populations, which showed efficacy in assessing the presence of insulin resistance; these markers included eGDR, VAI, eIS, lnGDR, METS-IR, the TG-HDL-C ratio, the TyG index, the LAP and the TyG-BMI [64, 69, 71,72,73, 79, 94, 120, 121]. Most likely, the TyG index and related parameters (TyG-BMI, TyG-WC, TyG-WHpR, and TyG-WHtR) are not suitable for routine use in type 1 diabetic patients because they contain fasting plasma glucose in their formulas, which is strictly dependent on intensive insulin treatment; however, this hypothesis has not yet been confirmed. Perhaps a modification of their formulas and replacement of fasting glycaemia with other clinical values would be sufficient for use them in T1D patients.
The results of studies comparing indirect insulin resistance markers are incoherent and it is impossible to indicate at the moment which marker is better than others [71, 81, 122]. Moreover, the specific cut-points of different insulin resistance markers for IR and/or MS detection may vary depending on the ethnicity and the statistical methods used in studies. The existing differences were presented in Additional file 1, highlighting the need to be cautious while comparing studies.
Treatment in double diabetes, can insulin succeed alone?
The only treatment for type 1 diabetes is insulin treatment (preferably as intensive insulin injections) and must not be changed into other antihyperglycaemic drugs. But intensive insulin treatment is associated with weight gain which escalates insulin resistance and leads to higher doses of insulin needed to achieve glycaemic targets [123]. Because of that, glucose-lowering drugs commonly used in therapy of type 2 diabetes (especially metformin, sodium-glucose co-transporter type 2 inhibitors and glucagon-like peptide-1 receptor agonists) can be promising possibilities in double diabetes. These medications are not officially indicated to be used among patients with T1D, but they may improve insulin sensitivity in these patients. Moreover, they can influence the components of metabolic syndrome, such as blood pressure or lipid profile.
Metformin decreases hepatic gluconeogenesis and increases peripheral glucose uptake stimulated by insulin through different, still not fully understood pathways [124]. A 2015 meta-analysis showed a significant reduction in weight and total daily dose of insulin when metformin was applied in T1D patients, but these positive effects vanished in the long-term observation [125, 126]. The REMOVAL trial suggested that metformin may have a wider role in cardiovascular risk management in patients with T1D [127]. Metformin addition to insulin therapy can also increase insulin sensitivity in type 1 diabetic individuals [128, 129]. Oza et al. suggested that metformin addition may prevent form deteriorating insulin sensitivity in type 1 diabetic adolescents and, consequently, prevent from the development of double diabetes [130]. The positive benefits of metformin were not associated with the improvement of glycaemic control, nor with the increased risk of hypoglycaemia or diabetic ketoacidosis (DKA) [125,126,127]. Metformin seems to be a reasonable choice in double diabetic individuals because of its ability to reduce insulin resistance, beneficial cardiovascular influence and its safety.
Sodium-glucose co-transporter type 2 (SGLT-2) inhibitors reduce reabsorption of filtered glucose in renal proximal tubules which causes glycosuria and lowers glycaemia. It is a group of drugs which attracts attention of many different specialists by its additional functions. Beneficial influence of SGLT-2 inhibitors on cardiovascular and kidney outcomes in patients with type 2 diabetes and in non-diabetic patients triggers the need to assess whether they could be used in T1D.The EASE trial, the DEPICT-1 and DEPICT-2 study investigated the use of empagliflozin and dapagliflozin, respectively, in type 1 diabetic patients [131, 132]. Both of the substances showed the reduction in HbA1c, body mass and daily dose of insulin but increased the risk of diabetic ketoacidosis [131, 132]. SGLT-2 inhibitors seem to be one of the possible therapies in double diabetes, especially in individuals with an excessive body weight and/or present cardiovascular risk factors, but the patients should be aware of the risk of diabetic ketoacidosis and should be able to recognise the symptoms of diabetic ketoacidosis and react properly by themselves.
Glucagon-like peptide-1 receptor agonists (GLP1-RAs) are incretin drugs which stimulate glucose-dependent insulin secretion, inhibit glucagon secretion from pancreatic α cells during hyperglycaemia, slow down the emptying of stomach and influence the appetite leading to the reduction in food intake [133]. The ADJUNCT trials showed that liraglutide administration reduced HbA1c, daily dose of insulin and body weight in type 1 diabetic patients, but increased the risk of hypoglycaemia and ketosis [134, 135]. GLP1-RAs should be considered when a patient has excessive body weight.
Lifestyle changes are also necessary to prevent and treat insulin sensitivity disorders in type 1 diabetic patients. Diet is an important modifiable risk factor of the development of insulin resistance in T1D, and diet with high protein, low fat and optimum carbohydrate intake with increased intake of dietary fiber may improve insulin sensitivity [136]. Dietary modifications, especially an isocaloric low-fat diet, may improve insulin sensitivity, which is not associated with weight loss or improvement in glycaemic control [137]. Regular physical activity can also be beneficial, as it reduces insulin resistance and daily dose of insulin without influencing HbA1c [138,139,140]. These lifestyle modifications should not be underestimated as an adjunctive treatment in double diabetes.
Summary
Double diabetes, which is a situation in which two main types of diabetes exist in one patient, raises doubts about whether the current classification of diabetes is still in use. It is a serious clinical problem, as it is associated with a significantly increased risk of developing complications (especially macrovascular complications), independent of glycaemic control. Moreover, it usually requires additional interventions, such as lifestyle modification or the addition of metformin, SGLT-2 inhibitors or GLP-1RAs to insulin therapy. Validation of a proper definition of DD is necessary to recognise individuals who are at risk for or who have already developed this condition. The use of the definition of metabolic syndrome may be helpful in double diabetes diagnosis; however, its role should not be overestimated, as the lack of unified criteria and glycaemic criteria that are consistently present in type 1 diabetic individuals may cause some discrepancies and may be misleading. The assessment of the degree of insulin resistance should eliminate these problems arising from using the metabolic syndrome definition in DD diagnosis, especially as IR underlies the pathophysiology of double diabetes. This approach will likely make the diagnosis of DD more reliable and objective. Indirect insulin resistance markers deserve special attention because they can be helpful in assessing the severity of insulin sensitivity disorders, and they are based on easy, inexpensive, widely available clinical parameters, such as medical history, anthropometrical parameters and basic laboratory tests. Furthermore, they are easy to incorporate in everyday clinical practice and could be used in the future to both recognise double diabetes without doubt (after further studies are performed on the parameters to validate them) and to monitor responses to particular interventions.
Availability of data and materials
Not applicable.
Abbreviations
- ACR:
-
Albumin-to-creatinine ratio
- AHA:
-
American Heart Association
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CV:
-
Cardiovascular
- DD:
-
Double diabetes
- DDI:
-
Daily dose of insulin
- eGDR:
-
The estimated glucose disposal rate
- eIS:
-
The estimated insulin sensitivity
- FPG:
-
Fasting plasma glucose
- GDR:
-
Glucose disposal rate
- GLP-1RAs:
-
Glucagon-like peptide-1 receptor agonists
- HbA1c/HbA1:
-
Glycated haemoglobin
- HDL-C:
-
High-density lipoprotein cholesterol
- HEC:
-
Hyperinsulinaemic-euglycaemic clamp
- HOMA-IR:
-
The homeostatic model assessment for insulin resistance
- HT:
-
Hypertension
- IDF:
-
International Diabetes Federation
- IR:
-
Insulin resistance
- LAP:
-
The lipid accumulation product
- lnGDR:
-
The natural logarithm of the glucose disposal rate
- METS-IR:
-
The metabolic score for insulin resistance
- MS:
-
Metabolic syndrome
- NCEP ATP III:
-
National Cholesterol Education Program Adult Treatment Panel III
- QUICKI:
-
The quantitative insulin sensitivity check index
- SGLT-2:
-
Sodium-glucose co-transporter type 2
- ST1RE:
-
The Steno type 1 risk engine
- T1D:
-
Type 1 diabetes
- T2D:
-
Type 2 diabetes
- TG:
-
Triglyceride
- TG/HDL-C ratio:
-
The triglyceride/high-density lipoprotein cholesterol ratio
- TyG index:
-
The triglyceride-glucose index
- TyG-BMI:
-
Triglyceride-glucose-body mass index
- TyG-WC:
-
Triglyceride-glucose-waist circumference
- TyG-WHpR:
-
Triglyceride-glucose-waist-to-hip ratio
- TyG-WHtR:
-
Triglyceride-glucose-waist-to-height ratio
- U:
-
Unit
- VAI:
-
The visceral adiposity index
- WC:
-
Waist circumference
- WHO:
-
World Health Organisation
- WHR = WHpR:
-
Waist-to-hip ratio
- WHtR:
-
Waist-to-height ratio
References
WHO. Classification of diabetes mellitus 2019. Geneva: WHO; 2019.
Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia. 2016;59(1):13–20.
Teupe B, Bergis K. Epidemiological evidence for ‘double diabetes.’ Lancet. 1991;337(8737):361–2.
Libman IM, Becker DJ. Coexistence of type 1 and type 2 diabetes mellitus: ‘double’ diabetes? Pediatr Diabetes. 2003;4(2):110–3.
Merger SR, Kerner W, Stadler M, Zeyfang A, Jehle P, Müller-Korbsch M, et al. Prevalence and comorbidities of double diabetes. Diabetes Res Clin Pract. 2016;1(119):48–56.
Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12.
Afshin A, Forouzanfar M, Reitsma M, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Szadkowska A, Madej A, Ziółkowska K, Szymańska M, Jeziorny K, Mianowska B, et al. Gender and age—dependent effect of type 1 diabetes on obesity and altered body composition in young adults. Ann Agric Environ Med. 2015;22(1):124–8.
Gale EAM. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51(12):3353–61.
Fagot-Campagna A, Pettitt DJ, Engelgau MM, Ríos Burrows N, Geiss LS, Valdez R, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136(5):664–72.
Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5 Pt 1):608–15.
Singh R, Shaw J, Zimmet P. Epidemiology of childhood type 2 diabetes in the developing world. Pediatr Diabetes. 2004;5(3):154–68.
Gilliam LK, Brooks-Worrell BM, Palmer JP, Greenbaum CJ, Pihoker C. Autoimmunity and clinical course in children with type 1, type 2, and type 1.5 diabetes. J Autoimmun. 2005;25(3):244–50.
Pozzilli P, Buzzetti R. A new expression of diabetes: double diabetes. Trends Endocrinol Metab. 2007;18(2):52–7.
Cleland SJ. Cardiovascular risk in double diabetes mellitus–when two worlds collide. Nat Rev Endocrinol. 2012;8(8):476–85.
Kietsiriroje N, Pearson S, Campbell M, Ariëns RAS, Ajjan RA. Double diabetes: a distinct high-risk group? Diabetes Obes Metab. 2019;21(12):2609–18.
Pozzilli P, Guglielmi C, Caprio S, Buzzetti R. Obesity, autoimmunity, and double diabetes in youth. Diabetes Care. 2011;34(Suppl 2):S166–70.
Alberti K, Zimmet P. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation—PubMed. Diabet Med. 1998;15(7):539–53.
World Health Organisation. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. Geneva: World Health Organisation; 1999.
Cleeman JI. Executive summary of the Third report of the National Cholesterol Education Program (NCEP) expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52.
Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–80.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5.
Lecumberri E, Nattero-Chávez L, Quiñones Silva J, Alonso Díaz S, Fernández-Durán E, Dorado Avendaño B, et al. Impact of excluding hyperglycemia from international diabetes federation metabolic syndrome diagnostic criteria on prevalence of the syndrome and its association with microvascular complications, in adult patients with type 1 diabetes. Endocrine. 2022;76(3):601–11.
de Melo DA, Dos Santos AM, da Silveira VNC, Silva MB, da Diniz AS. Prevalence of metabolic syndrome in adolescents based on three diagnostic definitions: a cross-sectional study. Arch Endocrinol Metab. 2023;67(5):e000634.
Haverinen E, Paalanen L, Palmieri L, Padron-Monedero A, Noguer-Zambrano I, Sarmiento Suárez R, et al. Comparison of metabolic syndrome prevalence using four different definitions—a population-based study in Finland. Arch Public Health. 2021;79(1):231.
Huang Y, Chen Z, Wang X, Zheng C, Shao L, Tian Y, et al. Comparison of the three most commonly used metabolic syndrome definitions in the Chinese population: a prospective study. Metabolites. 2022;13(1):12.
Nwankwo M, Okamkpa CJ, Danborno B. Comparison of diagnostic criteria and prevalence of metabolic syndrome using WHO, NCEP-ATP III, IDF and harmonized criteria: a case study from urban southeast Nigeria. Diabetes Metab Syndr. 2022;16(12): 102665.
Pokharel DR, Khadka D, Sigdel M, Yadav NK, Acharya S, Kafle RC, et al. Prevalence of metabolic syndrome in Nepalese type 2 diabetic patients according to WHO, NCEP ATP III, IDF and Harmonized criteria. J Diabetes Metab Disord. 2014;13(1):104.
Arslanian SA, Bacha F, Saad R, Gungor N. Family history of type 2 diabetes is associated with decreased insulin sensitivity and an impaired balance between insulin sensitivity and insulin secretion in white youth. Diabetes Care. 2005;28(1):115–9.
Wilkin TJ. The accelerator hypothesis: a review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int J Obes. 2009;33(7):716–26.
Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia. 2001;44(7):914–22.
Dahlquist G. Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia. 2006;49(1):20–4.
Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia. 2004;47(10):1661–7.
Betts P, Mulligan J, Ward P, Smith B, Wilkin T. Increasing body weight predicts the earlier onset of insulin-dependant diabetes in childhood: testing the ‘accelerator hypothesis’ (2). Diabet Med. 2005;22(2):144–51.
Xu P, Cuthbertson D, Greenbaum C, Palmer JP, Krischer JP. Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care. 2007;30(9):2314–20.
Nathan D, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial JAMA. 1998;280(2):140–6.
Nathan D, Cleary P, Backlund J, Genuth S, Lachin J, Orchard T, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53.
Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30.
Martyn-Nemeth P, Quinn L, Penckofer S, Park C, Hofer V, Burke L. Fear of hypoglycemia: influence on glycemic variability and self-management behavior in young adults with type 1 diabetes. J Diabetes Complications. 2017;31(4):735–41.
Przezak A, Bielka W, Molęda P. Fear of hypoglycemia—an underestimated problem. Brain Behav. 2022;12(7): e2633.
Donga E, van Dijk M, Hoogma RPLM, Corssmit EPM, Romijn JA. Insulin resistance in multiple tissues in patients with type 1 diabetes mellitus on long-term continuous subcutaneous insulin infusion therapy. Diabetes Metab Res Rev. 2013;29(1):33–8.
Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia. 2013;56(7):1462–70.
Catalano KJ, Maddux BA, Szary J, Youngren JF, Goldfine ID, Schaufele F. Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS ONE. 2014;9(9): e108693.
Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem. 2009;284(40):27090–100.
Yki-jarvinen H. Glucose toxicity. Endocr Rev. 1992;13(3):415–31.
Fasching P, Ratheiser K, Damjancic P, Schneider B, Nowotny P, Vierhapper H, et al. Both acute and chronic near-normoglycaemia are required to improve insulin resistance in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36(4):346–51.
Yki-Järvinen H, Koivisto VA. Continuous subcutaneous insulin infusion therapy decreases insulin resistance in type 1 diabetes. J Clin Endocrinol Metab. 1984;58(4):659–66.
Dandona P, Fonseca V, Fernando O, Menon RK, Weerakoon J, Kurtz A, et al. Control of diabetes through a subcutaneous peritoneal access device (SPAD) in patients with resistance to subcutaneously injected insulin—PubMed. Diabetes Res. 1987;5(1):47–9.
Giacca A, Caumo A, Galimberti G, Petrella G, Librenti MC, Scavini M, et al. Peritoneal and subcutaneous absorption of insulin in type I diabetic subjects. J Clin Endocrinol Metab. 1993;77(3):738–42.
Beylot M, Khalfallah Y, Laville M, Sautot G, Dechaud H, Serusclat P, et al. Insulin-mediated glucose disposal in type 1 (insulin-dependent) diabetic subjects treated by continuous subcutaneous or intraperitoneal insulin fusion—PubMed. Diabete Metab. 1987;13(4):450–6.
Yki-Järvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med. 1986;315(4):224–30.
Kacerovsky M, Brehm A, Chmelik M, Schmid AI, Szendroedi J, Kacerovsky-Bielesz G, et al. Impaired insulin stimulation of muscular ATP production in patients with type 1 diabetes. J Intern Med. 2011;269(2):189–99.
Simonson DC, Tamborlane WV, Sherwin RS, Smith JD, DeFronzo RA. Improved insulin sensitivity in patients with type I diabetes mellitus after CSII. Diabetes. 1985;34(Suppl 3):80–6.
Donga E, Dekkers OM, Corssmit EPM, Romijn JA. Insulin resistance in patients with type 1 diabetes assessed by glucose clamp studies: systematic review and meta-analysis. Eur J Endocrinol. 2015;173(1):101–9.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997;20(7):1087–92.
Anderson RL, Hamman RF, Savage PJ, Saad MF, Laws A, Kades WW, et al. Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests. The Insulin Resistance Atherosclerosis Study. Am J Epidemiol. 1995;142(7):724–32.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70.
Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49(4):626–32.
Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care. 2005;28(8):2019–24.
Kahn HS. The ‘lipid accumulation product’ performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;8(5):26.
Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29(1):151–3.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11(3): e0149731.
Duca LM, Maahs DM, Schauer IE, Bergman BC, Nadeau KJ, Bjornstad P, et al. Development and validation of a method to estimate insulin sensitivity in patients with and without type 1 diabetes. J Clin Endocrinol Metab. 2016;101(2):686–95.
Zheng X, Huang B, Luo S, Yang D, Bao W, Li J, et al. A new model to estimate insulin resistance via clinical parameters in adults with type 1 diabetes. Diabetes Metab Res Rev. 2017. https://doi.org/10.1002/dmrr.2880.
Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–44.
Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS ONE. 2019;14(3): e0212963.
Raimi TH, Dele-Ojo BF, Dada SA, Fadare JO, Ajayi DD, Ajayi EA, et al. Triglyceride-Glucose index and related parameters predicted metabolic syndrome in Nigerians. Metab Syndr Relat Disord. 2021;19(2):76–82.
Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19(7):527–34.
Ovalle F. Clinical approach to the patient with diabetes mellitus and very high insulin requirements. Diabetes Res Clin Pract. 2010;90(3):231–42.
Ferreira-Hermosillo A, Ramírez-Rentería C, Mendoza-Zubieta V, Molina-Ayala MA. Utility of the waist-to-height ratio, waist circumference and body mass index in the screening of metabolic syndrome in adult patients with type 1 diabetes mellitus. Diabetol Metab Syndr. 2014;6(1):32.
Uruska A, Zozulinska-Ziolkiewicz D, Niedzwiecki P, Pietrzak M, Wierusz-Wysocka B. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J Clin Lipidol. 2018;12(3):734–40.
Chillarón JJ, Goday A, Flores-Le-Roux JA, Benaiges D, Carrera MJ, Puig J, et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab. 2009;94(9):3530–4.
Ferreira-Hermosillo A, Ibarra-Salce R, Rodríguez-Malacara J, Molina-Ayala MA. Comparison of indirect markers of insulin resistance in adult patients with double diabetes. BMC Endocr Disord. 2020;20(1):87. https://doi.org/10.1186/s12902-020-00570-z.
Šimonienė D, Platūkiene A, Prakapienė E, Radzevičienė L, Veličkiene D. Insulin resistance in type 1 diabetes mellitus and its association with patient’s micro- and macrovascular complications, sex hormones, and other clinical data. Diabetes Ther. 2020;11(1):161–74.
Vladu M, Clenciu D, Efrem IC, Forţofoiu MC, Amzolini A, Micu ST, et al. Insulin resistance and chronic kidney disease in patients with type 1 diabetes mellitus. J Nutr Metab. 2017;2017:6425359.
Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(3):556–63.
Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: ‘double diabetes’ in the diabetes control and complications trial. Diabetes Care. 2007;30(3):707–12.
Cano A, Llauradó G, Albert L, Mazarico I, Astiarraga B, González-Sastre M, et al. Utility of insulin resistance in estimating cardiovascular risk in subjects with type 1 diabetes according to the scores of the steno type 1 risk engine. J Clin Med. 2020;9(7):1–12.
Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care. 2013;36(8):2280–5.
Marques CL, Beretta MV, Prates RE, de Almeida JC, Rodrigues T da C. Body adiposity markers and insulin resistance in patients with type 1 diabetes. Arch Endocrinol Metab. 2023. 67(3):401–7.
Karatas S, Beysel S. Visceral adiposity index, triglyceride/high-density lipoprotein ratio, and lipid accumulation product index to discriminate metabolic syndrome among adult type 1 diabetes patients. Metab Syndr Relat Disord. 2021;19(9):507–12.
Taverna MJ, Martínez-Larrad MT, Frechtel GD, Serrano-Ríos M. Lipid accumulation product: a powerful marker of metabolic syndrome in healthy population. Eur J Endocrinol. 2011;164(4):559–67.
Ahn N, Baumeister SE, Amann U, Rathmann W, Peters A, Huth C, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep. 2019;9(1):9693.
Wakabayashi I, Daimon T. A strong association between lipid accumulation product and diabetes mellitus in japanese women and men. J Atheroscler Thromb. 2014;21(3):282–8.
Lee JW, Lim NK, Park HY. The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle-aged Koreans. BMC Endocr Disord. 2018;18(1):33.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2011;95(7):3347–51.
Moon S, Park JS, Ahn Y. The cut-off values of triglycerides and glucose index for metabolic syndrome in American and Korean adolescents. J Korean Med Sci. 2017;32(3):427–33.
Vasques ACJ, Novaes FS, de Oliveira MS, Matos Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98-100.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;5(10):74.
Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE. 2014;9(2): e90430.
Yu X, Wang L, Zhang W, Ming J, Jia A, Xu S, et al. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: a nationwide study. J Diabetes Investig. 2019;10(4):1050–8.
Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. 2014;62(2):345–9.
Guarnotta V, Pillitteri G, Gambino G, Radellini S, Vigneri E, Pizzolanti G, et al. Levothyroxine and insulin requirement in autoimmune polyglandular type 3 syndrome: a real-life study. J Endocrinol Invest. 2021;44(7):1387–94.
Chen C, Xu Y, Guo ZR, Yang J, Wu M, Hu XS. The application of visceral adiposity index in identifying type 2 diabetes risks based on a prospective cohort in China. Lipids Health Dis. 2014;8(13):108.
Du T, Sun X, Huo R, Yu X. Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: the China Health and Nutrition Survey 2009. Int J Obes (Lond). 2014;38(6):840–7.
Zheng S, Shi S, Ren X, Han T, Li Y, Chen Y, et al. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: cross-sectional and prospective cohort study. J Transl Med. 2016;14(1):260.
Yang Y, Feng Y, Ma X, Chen K, Wu N, Wang D, et al. Visceral adiposity index and insulin secretion and action in first-degree relatives of subjects with type 2 diabetes. Diabetes Metab Res Rev. 2015;31(3):315–21.
Kuang M, Yang R, Huang X, Wang C, Sheng G, Xie G, et al. Assessing temporal differences in the predictive power of baseline TyG-related parameters for future diabetes: an analysis using time-dependent receiver operating characteristics. J Transl Med. 2023;21(1):299.
Bjornstad P, Maahs DM, Duca LM, Pyle L, Rewers M, Johnson RJ, et al. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the coronary artery calcification in type 1 diabetes study. J Diabetes Complications. 2016;30(4):586–90.
Cheng H, Yu X, Li YT, Jia Z, Wang JJ, Xie YJ, et al. Association between METS-IR and prediabetes or type 2 diabetes mellitus among elderly subjects in China: a large-scale population-based study. Int J Environ Res Public Health. 2023;20(2):1053.
Xuan W, Liu D, Zhong J, Luo H, Zhang X. Impacts of triglyceride glucose-waist to height ratio on diabetes incidence: a secondary analysis of a population-based longitudinal data. Front Endocrinol. 2022;22(13): 949831.
Malek M, Khamseh ME, Chehrehgosha H, Nobarani S, Alaei-Shahmiri F. Triglyceride glucose-waist to height ratio: a novel and effective marker for identifying hepatic steatosis in individuals with type 2 diabetes mellitus. Endocrine. 2021;74(3):538–45.
Li WC, Chen IC, Chang YC, Loke SS, Wang SH, Hsiao KY. Waist-to-height ratio, waist circumference, and body mass index as indices of cardiometabolic risk among 36,642 Taiwanese adults. Eur J Nutr. 2013;52(1):57–65.
Ashwell M, Gibson S. Waist-to-height ratio as an indicator of ‘early health risk’: simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open. 2016;6(3): e010159.
Gibson S, Ashwell M. A simple cut-off for waist-to-height ratio (0·5) can act as an indicator for cardiometabolic risk: recent data from adults in the Health Survey for England. Br J Nutr. 2020;123(6):681–90.
Parente EB, Mutter S, Harjutsalo V, Ahola AJ, Forsblom C, Groop PH. Waist-height ratio and waist are the best estimators of visceral fat in type 1 diabetes. Sci Rep. 2020;10(1):18575.
McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96(3):399–404.
Boizel R, Benhamou PY, Lardy B, Laporte F, Foulon T, Halimi S. Ratio of triglycerides to HDL cholesterol is an indicator of LDL particle size in patients with type 2 diabetes and normal HDL cholesterol levels. Diabetes Care. 2000;23(11):1679–85.
Wilson DP, Fesmire JD, Endres RK, Blackett PR. Increased levels of HDL-cholesterol and apolipoprotein A-I after intensified insulin therapy for diabetes. South Med J. 1985;78(6):636–8.
Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Leiva Sisnieguez CE, March CE, et al. Identifying cardiovascular disease risk and outcome: use of the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio versus metabolic syndrome criteria. J Intern Med. 2013;273(6):595–601.
Salazar MR, Carbajal HA, Espeche WG, Leiva Sisnieguez CE, March CE, Balbín E, et al. Comparison of the abilities of the plasma triglyceride/high-density lipoprotein cholesterol ratio and the metabolic syndrome to identify insulin resistance. Diabetes Vasc Dis Res. 2013;10(4):346–52.
Fiorentino TV, Marini MA, Succurro E, Andreozzi F, Sesti G. Relationships of surrogate indexes of insulin resistance with insulin sensitivity assessed by euglycemic hyperinsulinemic clamp and subclinical vascular damage. BMJ Open Diabetes Res care. 2019;7(1): e000911.
Almeda-Valdés P, Bello-Chavolla OY, Caballeros-Barragán CR, Gómez-Velasco DV, Viveros-Ruiz T, Vargas-Vázquez A, et al. Índices para la evaluación de la resistencia a la insulina en individuos mexicanos sin diabetes. Gac Med Mex. 2018;154(Supp 2):S50–5.
Oza C, Khadilkar A, Karguppikar M, Gondhalekar K, Khadilkar V. Comparison of insulin sensitivity indices for detection of double diabetes in Indian adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2022;35(8):1010–9.
Purnell JQ, John EH, Cleary PA, Nathan DM, Lachin JM, Zinman B, et al. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127(2):180–7.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85.
Liu C, Wu D, Zheng X, Li P, Li L. Efficacy and safety of metformin for patients with type 1 diabetes mellitus: a meta-analysis. Diabetes Technol Ther. 2015;17(2):142–8.
Staels F, Moyson C, Mathieu C. Metformin as add-on to intensive insulin therapy in type 1 diabetes mellitus. Diabetes Obes Metab. 2017;19(10):1463–7.
Petrie JR, Chaturvedi N, Ford I, Brouwers MCGJ, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Diabetes Endocrinol. 2017;5(8):597–609.
Särnblad S, Kroon M, Åman J. Metformin as additional therapy in adolescents with poorly controlled type 1 diabetes: randomised placebo-controlled trial with aspects on insulin sensitivity. Eur J Endocrinol. 2003;149(4):323–9.
Moon RJ, Bascombe LA, Holt RIG. The addition of metformin in type 1 diabetes improves insulin sensitivity, diabetic control, body composition and patient well-being. Diabetes Obes Metab. 2007;9(1):143–5.
Oza C, Mondkar S, Shah N, More C, Khadilkar V, Khadilkar A. A pilot study to assess effect of metformin therapy on prevention of double diabetes in Indian adolescents with type-1 diabetes. Indian J Endocrinol Metab. 2023;27(3):201–7.
Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. 2018;41(12):2560–9.
Phillip M, Mathieu C, Lind M, Araki E, di Bartolo P, Bergenstal R, et al. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: pooled 52-week outcomes from the DEPICT-1 and -2 studies. Diabetes Obes Metab. 2021;23(2):549–60.
Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–56.
Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, et al. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care. 2016;39(10):1702–10.
Ahren B, Hirsch IB, Pieber TR, Mathieu C, Gomez-Peralta F, Hansen TK, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care. 2016;39(10):1693–701.
Khadilkar A, Oza C, Mondkar SA. Insulin resistance in adolescents and youth with type 1 diabetes: a review of problems and solutions. Clin Med Insights Endocrinol Diabetes. 2023;1:16.
Rosenfalck AM, Almdal T, Viggers L, Madsbad S, Hilsted J. A low-fat diet improves peripheral insulin sensitivity in patients with Type 1 diabetes. Diabet Med. 2006;23(4):384–92.
Yki-Jarvinen H, DeFronzo RA, Koivisto VA. Normalization of insulin sensitivity in type I diabetic subjects by physical training during insulin pump therapy. Diabetes Care. 1984;7(6):520–7.
Wallberg-Henriksson H, Gunnarsson R, Henriksson J, DeFronzo R, Felig P, Ostman J, et al. Increased peripheral insulin sensitivity and muscle mitochondrial enzymes but unchanged blood glucose control in type I diabetics after physical training. Diabetes. 1982;31(12):1044–50.
Landt KW, Campaigne BN, James FW, Sperling MA. Effects of exercise training on insulin sensitivity in adolescents with type I diabetes. Diabetes Care. 1985;8(5):461–5.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
WB, AP and PM contributed to the conception and design of the work. WB and AP drafted the manuscript. PM, EPS and BM critically revised the manuscript. All authors reviewed the manuscript. All authors gave final approval and agreed to be accountable for all aspects of this work and to ensure integrity and accuracy.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Summary of indirect insulin resistance markers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bielka, W., Przezak, A., Molęda, P. et al. Double diabetes—when type 1 diabetes meets type 2 diabetes: definition, pathogenesis and recognition. Cardiovasc Diabetol 23, 62 (2024). https://doi.org/10.1186/s12933-024-02145-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02145-x