Abstract
The newly proposed term “metabolic dysfunction-associated fatty liver disease” (MAFLD) is replacing the old term “non-alcoholic fatty liver disease” (NAFLD) in many global regions, because it better reflects the pathophysiology and cardiometabolic implications of this common liver disease. The proposed change in terminology from NAFLD to MAFLD is not simply a single-letter change in an acronym, since MAFLD is defined by a set of specific and positive diagnostic criteria. In particular, the MAFLD definition specifically incorporates within the classification recognized cardiovascular risk factors. Although convincing evidence supports a significant association between both NAFLD and MAFLD, with increased risk of CVD morbidity and mortality, neither NAFLD nor MAFLD have received sufficient attention from the Cardiology community. In fact, there is a paucity of scientific guidelines focusing on this common and burdensome liver disease from cardiovascular professional societies. This Perspective article discusses the rationale and clinical relevance for Cardiologists of the newly proposed MAFLD definition.
Similar content being viewed by others
Introduction
Many Cardiologists are not aware of the increased risk of cardiovascular disease (CVD) among patients with non-alcoholic fatty liver disease (NAFLD) [1]. Whilst Cardiologists pay close attention to traditional CVD risk factors, there is currently little awareness that fatty liver per se may contribute to CVD risk. To date, however, it remains debatable whether screening for fatty liver disease should be given the same priority as other established cardiometabolic risk factors. Although NAFLD is associated with increased CVD risk, routine screening is not recommended in current cardiovascular guidelines. It is reasonable to assume that the lack of clear recommendations for NAFLD screening likely relates to the lack of any effective pharmacotherapies other than lifestyle modification. The lack of awareness of the existing link between NAFLD and increased CVD risk further exacerbates clinical inertia amongst Cardiologists, Primary-care practitioners and non-liver clinician specialists [2, 3].
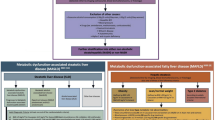
In 2020, metabolic dysfunction-associated fatty liver disease (MAFLD) was proposed as a more appropriate term than NAFLD, because this nomenclature better defines the pathophysiology of this liver disease and its associated metabolic abnormalities [4, 5]. The proposed change is more than a name change because it affects how clinicians perceive the association of this common liver disease with CVD and metabolic risk. NAFLD is defined as a group of heterogeneous conditions in which there is liver fat accumulation in the absence of secondary causes of hepatic steatosis, such as excessive alcohol consumption, viral hepatitis and other known causes of hepatic steatosis [6]. These “negative” (by exclusion) diagnostic criteria are not appropriate, meaning that NAFLD is only present when all other causes of fatty liver are excluded. In addition, fatty liver disease may coexist with viral hepatitis, excessive alcohol intake or other liver diseases. This renders it difficult for clinicians to make a definitive diagnosis of NAFLD in the face of other potential causes of hepatic steatosis. The term “non-alcoholic” may also confuse patients in terms of the real cause of their disease, which is not conducive to a good therapeutic relationship. Significantly different from NAFLD, MAFLD is defined as a condition characterized by liver fat accumulation in the presence of at least one of the following three metabolic conditions: overweight/obesity, T2DM, or at least two of seven metabolic risk abnormalities in those subjects who do not have T2DM and are lean by ethnic-specific body mass index (BMI) criteria (Fig. 1) [7]. The “positive” diagnostic criteria for MAFLD are based on the coexistence of hepatic steatosis and metabolic dysfunction and hence MAFLD may also coexist with other liver diseases. This is not possible when using the NAFLD definition, which requires the exclusion of all other causes of hepatic steatosis as a prerequisite for diagnosis. To date, the newly proposed definition of MAFLD has been accepted by many experts in the field, and by some pan-national societies; although debate is ongoing and there is not uniform agreement [8, 9]. For some experts the change in terminology/definition from NAFLD to MAFLD seems premature and they suggest that such a change could also lead to confusion [10, 11]. In addition, there is not consensus on what constitutes “metabolic health”. That said, taken together, the MAFLD definition better emphasizes the pathogenic role of metabolic dysregulation in the development and progression of this common and burdensome liver disease. Additionally, the inclusion of recognized cardiovascular risk factors within the definition, highlights the need for treatment of these specific coexisting cardiometabolic risk factors.
Comparison of diagnostic criteria between NAFLD and MAFLD definitions. Hepatic steatosis is detected either by imaging techniques, blood biomarkers and scores or by liver histology. The definition of NAFLD is based on the evidence of hepatic steatosis in the absence of excessive alcohol consumption, chronic viral hepatitis, or other competing causes of hepatic steatosis. The definition of MAFLD is based on the evidence of hepatic steatosis in the presence of at least one of the following three metabolic conditions, overweight/obesity, type 2 diabetes, or the presence of at least two of the following metabolic abnormalities: (1) waist circumference ≥ 102/88 cm in Caucasian men and women (or ≥ 90/80 cm in Asian men and women); (2) blood pressure ≥ 130/85 mmHg or specific drug treatment; (3) plasma triglycerides ≥ 150 mg/dl (≥ 1.70 mmol/L) or specific drug treatment; (4) plasma high-density lipoprotein (HDL)-cholesterol < 40 mg/dl (< 1.0 mmol/L) for men and < 50 mg/dl (< 1.3 mmol/L) for women or specific drug treatment; (5) prediabetes (i.e., fasting glucose levels 100 to 125 mg/dl [5.6 to 6.9 mmol/L], or 2-h post-load glucose levels 140 to 199 mg/dl [7.8 to 11.0 mmol] or HbA1c 5.7% to 6.4% [39 to 47 mmol/mol]); (6) Homeostasis model assessment (HOMA) of insulin resistance score ≥ 2.5; and (7) plasma high-sensitivity C-reactive protein (CRP) level > 2 mg/L. Thus, MAFLD diagnosis does not require exclusion of other liver diseases but as a prerequisite it must have evidence of metabolic dysregulation. NAFLD non-alcoholic fatty liver disease, MAFLD metabolic dysfunction-associated fatty liver disease
To date, there are few consensus statements about NAFLD or MAFLD published by national or international cardiovascular societies (Fig. 2). The first position paper was published by the Indian College of Cardiology in 2015 and raised questions as to whether NAFLD itself may predispose to CVD risk, independent of other common CVD risk factors [12]. In 2022, the American Heart Association (AHA) issued the first scientific statement on NAFLD and CVD risk [13]. This AHA statement highlighted the strong and independent association between NAFLD and increased risk of CVD and sounded the alarm to increase awareness among clinicians, particularly Cardiologists. We are now at the stage where it is germane to consider and understand the emerging relationship between MAFLD and CVD risk from a Cardiologist’s perspective (Table 1). This Perspectives article discusses issues related to NAFLD and MAFLD that are of concern for Cardiologists, divided into the following five sections: is the estimated risk of CVD similar when using the NAFLD or MAFLD definitions? Why is MAFLD associated with an increased risk of CVD? What is the role of MAFLD in CVD; is it a bystander or a mediator of CVD? Is routine screening for MAFLD necessary for CVD risk assessment? What is the effect of treatment interventions for MAFLD on the risk of CVD?
Is the estimated risk of CVD similar when using the NAFLD or MAFLD definitions?
Because the overlap between the NAFLD and MAFLD definitions in the general population is reported to be around 70–90%, it is expected that patients with MAFLD have essentially similar CVD risk to those with NAFLD [14,15,16]. However, emerging evidence suggests a greater risk of CVD events in patients with MAFLD than in those with NAFLD. Using the National Health and Nutrition Examination Survey (NHANES 1999–2016) database, Zhang et al. [17] reported that patients with MAFLD had a significantly higher 10-year CVD risk (as assessed by the Framingham risk score) compared to those with NAFLD. These data provided the first hint that the CVD risk burden may be greater for MAFLD. Kim et al. [18] analyzed data in 2144 subjects without a prior history of CVD and showed that individuals with MAFLD had a remarkably higher risk of intermediate to high 10-year CVD risk compared to those with NAFLD only (defined as presence of NAFLD but not MAFLD), with an odds ratio (OR) of 8.17 (95% CI 2.40–36.1) in adjusted regression analyses. It is known that the Suita score is a CVD risk prediction tool that may improve CVD risk prediction relative to the Framingham risk score in Japanese individuals [19]. Tsutsumi et al. [20] reported that MAFLD better identified patients at high CVD risk (as estimated by Suita and Framingham risk scores) compared with NAFLD. In a community-based cohort of 6232 participants followed for a median of 4.3 years, Liu et al. [21] reported that MAFLD was associated with a greater risk of developing subclinical atherosclerosis, defined as increased carotid intima-media thickness and plaque, elevated brachial ankle pulse wave velocity, or microalbuminuria. Liu H et al. [22] reported that MAFLD was associated with an increased CVD risk in a cohort of 3306 patients with chronic coronary syndrome. Finally, in a prospective study of nearly 500 hospitalized patients with acute coronary syndromes (ACS) and hepatic steatosis, Noda et al. [23] showed that the coexistence of MAFLD and impaired physical function tests independently predicted the risk of adverse CVD outcomes. Collectively, therefore, accumulating evidence indicates that MAFLD may increase the risk of developing adverse CVD outcomes.
A recent large meta-analysis of 17 observational studies (including more than 12 million individuals) also reported that MAFLD is significantly associated with higher risk of overall mortality (hazard ratio (HR) 1.24, 95% confidence interval [CI] 1.13–1.34), CVD mortality (HR 1.28, 95% CI 1.03–1.53), nonfatal CVD events (HR 1.49, 95% CI 1.34–1.64) and stroke (HR: 1.55, 95% CI 1.37–1.73) [24]. Moreover, a matched cohort study, using electronic primary healthcare databases from four European countries, reported that NAFLD appears not to be significantly associated with risk of acute myocardial infarction or stroke after adjustment for common CVD risk factors, (although it should be noted that in this large registry-based study it was not possible to prove that control subjects did not have NAFLD, giving rise to the potential for misclassification bias attenuating the strength of any association between NAFLD and CVD, towards the null) [25]. Additionally, although there are important limitations of Mendelian randomization studies, a recent study did not find evidence supporting the existence of causal associations of NAFLD itself with acute myocardial infarction and any stroke subtypes [26]. In contrast to the criteria necessary for diagnosing NAFLD, MAFLD by definition, is closely associated with T2DM, obesity and atherogenic dyslipidaemia, which are established risk factors for CVD [27].
Recent cohort studies that compared MAFLD-only and NAFLD-only patient populations suggest that the MAFLD-only status is more strongly associated with risk of overall mortality, CVD mortality and nonfatal CVD events, compared with the NAFLD-only status (Fig. 3) [14, 28,29,30,31]. In particular, as shown in Fig. 3A, the MAFLD-only status seems to be more closely associated with a higher risk of nonfatal CVD events. In a retrospective cohort study of 2985 participants followed for 7 years, Niriella et al. [28] reported that the NAFLD-only status was not associated with CVD events compared with control individuals (HR = 1.90, 95% CI = 0.25–14.8) (although it should be noted the CIs were wide and the study may be underpowered), whilst the MAFLD-only status was associated with a greater risk of CVD events compared with control individuals (HR = 7.2, 95% CI = 2.4–21.5). In another study of ~ 9.5 million South Korean subjects from a health screening population, Lee et al. [14] reported that individuals with MAFLD only were at higher risk of CVD events compared with those without MAFLD or NAFLD (HR = 1.43, 95% CI = 1.41–1.45), whereas the association between the NAFLD-only status and risk of CVD events was modest (HR = 1.09, 95% CI = 1.03–1.15). As shown in Fig. 3B, in the study by Lee et al. [14], patients with MAFLD were also at higher risk of CVD mortality compared with individuals without MAFLD or NAFLD (HR = 1.46, 95% CI = 1.41–1.52), whereas NAFLD patients were not (HR = 1.12, 95% CI = 0.96–1.30). For all-cause mortality (Fig. 3C), the difference in CVD risk associated with MAFLD or NAFLD was even more apparent. Kim et al. [30] analyzed data from 7,761 participants in the NHANES-III database and showed that MAFLD was associated with a higher risk of all-cause mortality compared to those without MAFLD or NAFLD (HR = 1.66, 95% CI = 1.19–2.32), whereas NAFLD was not (HR = 0.94, 95% CI = 0.60–1.46). Similarly, Nguyen et al. [29] reported that the MAFLD-only status identified a group of patients with higher all-cause mortality compared with individuals without MAFLD or NAFLD (HR = 2.4, 95% CI = 1.2–4.6), whereas there was no increased risk for all-cause mortality with the NAFLD-only status (HR = 1.5, 95% CI = 0.8–2.8).
Comparative effects of MAFLD-only and NAFLD-only on the risk of fatal and nonfatal CVD events and all-cause mortality. The figure shows the forest plots of the effects of the MAFLD-only or the NAFLD-only status on the risk of CVD mortality and events and all-cause mortality in cohort studies that simultaneously used the MAFLD and NAFLD definitions. The NAFLD-only status is defined as presence of NAFLD but not MAFLD; the MAFLD-only status is defined as presence of MAFLD but not NAFLD. The reference category in these statistical analyses is the absence of both NAFLD and MAFLD. Data are expressed as hazard ratios and 95% confidence intervals (in parenthesis). (A) CVD events [14, 28]: the MAFLD-only status was associated with a higher risk of CVD events than the NAFLD-only status; (B) CVD mortality [14, 29, 30]: either the MAFLD-only status or NAFLD-only status was not associated with CVD mortality; (C) all-cause mortality [29, 30]: the MAFLD-only status was associated with a higher risk of all-cause mortality than the NAFLD-only status. Abbreviations: CVD: cardiovascular disease; MAFLD: metabolic dysfunction-associated fatty liver disease; NAFLD: non-alcoholic fatty liver disease
We recently performed a meta-analysis of seven observational cohort studies (mostly from Asian countries) that examined the comparative effects of NAFLD and MAFLD definitions on risk of CVD events [32]. This meta-analysis showed that each of the two definitions were significantly associated with a higher risk of incident CVD events (pooled random-effects HR 1.50, 95% CI 1.30–1.72 for MAFLD vs. no-MAFLD; and pooled random-effects HR 1.27, 95% CI 1.12–1.45 for NAFLD vs. no-NAFLD, respectively). Although MAFLD identified a numerically greater number of CVD events than NAFLD, the risk for incident CVD events associated with either definition was not significantly different [32].
Collectively, since the MAFLD definition better captures underlying metabolic dysfunction, it is perhaps not surprising that MAFLD definition might also increase CVD risk more strongly than NAFLD definition. However, further cohort studies from different countries are certainly needed to elucidate whether MAFLD may better predict the risk of developing incident CVD events than NAFLD.
Why is MAFLD associated with an increased risk of CVD?
There are at least two possible explanations for the increased CVD risk observed in individuals with MAFLD. First, the MAFLD definition has as an obligate requirement for the presence of overweight/obesity, T2DM or other features of the metabolic syndrome, all of which are associated with increased CVD risk. In MAFLD, the presence of T2DM marks the most severe form of metabolic dysfunction and hence has the worst prognosis [33]. Indeed, recent studies have shown that MAFLD patients with T2DM have a worse clinical outcome than their counterparts without T2DM (i.e. MAFLD patients with overweight/obesity, or nondiabetic MAFLD patients with other metabolic risk abnormalities) [34]. Several pathophysiological pathways may link MAFLD and T2DM to an increased CVD risk, including a proatherogenic lipid phenotype, as well as an increase in prothrombotic factors, insulin resistance, low-grade inflammation, and intestinal dysbiosis [35].
Second, the impact of MAFLD on CVD risk may also be affected by other coexisting liver diseases, such as viral hepatitis or moderate alcohol consumption. Whereas it is necessary to always exclude these coexisting liver diseases to establish a diagnosis of NAFLD, this is not necessary for a diagnosis of MAFLD. Indeed, some studies showed that patients with MAFLD and concomitant viral hepatitis or moderate alcohol consumption have a higher 10-year calculated CVD risk compared to those with MAFLD only [29, 36, 37].
That said, MAFLD itself may increase risk of CVD possibly via multiple pathophysiological mechanisms associated with metabolic dysfunction; these include increased oxidative stress, systemic/hepatic insulin resistance, low-grade inflammation and endothelial dysfunction (Fig. 4) [38,39,40,41]. Patients with MAFLD exhibit excessive reactive oxygen species (ROS), and ROS overproduction leads to hepatic inflammation and fibrosis, mostly through activation of hepatic stellate cells [42]. ROS overproduction also leads to low-density lipoprotein (LDL)-cholesterol oxidation, which may promote transformation of macrophages into foam cells, which is a key step in the formation of atherosclerotic lesions and atherosclerosis progression. The latter occurs through a variety of pathways, including endothelial cell dysfunction and vascular smooth muscle cell proliferation [43]. Insulin resistance is considered one of the core pathophysiological changes in MAFLD [44]. Insulin resistance promotes hepatic de novo lipogenesis and may affect microvascular and macrovascular homeostasis in a variety of ways to promote atherosclerosis [44]. In addition, previous studies confirmed that chronic hyperglycemia damages vascular endothelial cells, stimulates proliferation of smooth muscle cells, improves platelet activity, and induces ROS overproduction, thus promoting accelerated atherogenesis [45]. Low-grade inflammation also aggravates endothelial dysfunction, changes vascular tone, and promotes vascular plaque formation [46]. All these mechanisms promote the development and progression of CVD including vascular inflammation, lipid deposition, vascular remodeling, endothelial injury and hypercoagulability. Given that MAFLD is defined by the presence of hepatic steatosis plus at least one of its diagnostic cardiometabolic criteria [4], it is reasonable to hypothesize that there will be a strong mechanistic association between MAFLD and adverse CVD outcomes [18, 20, 47, 48].
Putative shared pathophysiological mechanisms in MAFLD and CVD. MAFLD is closely associated with metabolic dysfunction and typical features of the metabolic syndrome. These metabolic risk abnormalities include visceral adipose tissue deposition, systemic low-grade inflammation, increased activity of RAAS systems, enhanced oxidative stress and insulin resistance. These metabolic risk abnormalities induce progression of coronary atherosclerosis, including vascular inflammation, lipids deposition, vascular remodeling, endothelial injury, as well as hypercoagulability, thereby contributing to increased risk of CVD. CVD cardiovascular disease, MAFLD metabolic dysfunction-associated fatty liver disease, RAAS renin–angiotensin–aldosterone system
What is the role of MAFLD in CVD? Is it a simple bystander or a mediator?
To date, most Cardiologists are not aware that NAFLD (or MAFLD) is a CVD risk factor [30, 49, 50]. In 2015, a position paper published by the Indian College of Cardiology [51] identified the increased CVD risk in patients with NAFLD, but raised some doubts as to whether NAFLD per se may predispose to CVD development. However, since the publication of that position paper, further new data has provided yet more evidence that MAFLD is a CVD risk factor [14, 52]. Interestingly, there is a discrepancy for the risk of CVD outcomes in MAFLD and NAFLD after adjustment for coexisting cardiometabolic risk factors (Fig. 5). As shown in Fig. 5A, in a cohort study of ~ 6.8 million Japanese individuals, Yoneda et al. [52] reported that the risk of CVD events was almost the same (adjusted HR 1.02, 95% CI 0.92–1.14) in the NAFLD and non-NAFLD groups after adjusting for cardiometabolic risk factors. In contrast, after adjusting for the same cardiometabolic risk factors, the risk of CVD was higher in the MAFLD group compared with the non-MAFLD group (adjusted HR 1.89, 95% CI 1.78–2.01). However, there are conflicting data (Fig. 5B) [38, 47, 53,54,55]. In a smaller prospective study, Kim et al. [30] reported a significant association between MAFLD and CVD mortality (HR 2.14, 95% CI 1.71–2.70), but this risk was attenuated after adjusting for cardiometabolic risk factors. Similarly, these authors did not find any association between NAFLD and CVD mortality in adjusted regression analyses. Using the NHANES III database, Huang et al. [47] reported that MAFLD was associated with a greater risk of CVD mortality compared with NAFLD (HR 2.01, 95% CI 1.66–2.44 vs. HR 1.53, 95% CI 1.26–1.86, respectively). However, the increased risk of CVD mortality was attenuated after adjustment for cardiometabolic risk factors. Previous meta-analyses reported that NAFLD was associated with a higher risk of nonfatal CVD events but not CVD mortality [55,56,57]. However, the largest updated meta-analysis to date by Mantovani et al. [39] has clearly shown that NAFLD was associated with a higher risk of both nonfatal CVD events (pooled random-effects HR1.40, 95% CI 1.20–1.64) and CVD mortality (pooled random-effects HR 1.30, 95% CI 1.08–1.56), and that this risk was further increased with the severity of NAFLD (especially with higher fibrosis stage). A nationwide Swedish cohort study by Simon et al. [58] provided further evidence of a strong association between the presence and severity of biopsy-proven NAFLD and the risk of CVD mortality.
Comparative effects of MAFLD and NAFLD on the risk of fatal and nonfatal CVD and all-cause mortality independently of cardiometabolic risk factors. The figure shows the forest plots of the effects of MAFLD and NAFLD on the risk of CVD mortality and events and all-cause mortality after adjustment for coexisting cardiometabolic risk factors, in cohort studies that simultaneously used the MAFLD and NAFLD definitions. Data are expressed as hazard ratios and 95% confidence intervals (in parenthesis). A CVD events [14, 50, 52]: MAFLD is associated with a greater risk of CVD events than NAFLD B CVD mortality [30, 47]: the risk for CVD mortality is attenuated after adjustment for cardiometabolic risk factors in MAFLD or NAFLD; C all-cause mortality [30, 47, 50]: MAFLD is associated with a higher risk of all-cause mortality but this association is diminished after adjustment for cardiometabolic risk factors. CVD cardiovascular disease, MAFLD metabolic dysfunction-associated fatty liver disease, NAFLD non-alcoholic fatty liver disease
MAFLD is associated with a higher risk of all-cause mortality but this association is attenuated after adjustment for cardiometabolic risk factors (Fig. 5C) [30, 47, 50]. For example, Kim et al. [30] reported that the association with higher all-cause mortality in MAFLD became non-significant, after adjustment for cardiometabolic risk factors. Huang et al. [47] showed that MAFLD was associated with higher all-cause mortality compared with NAFLD and control subjects, but the associations lost significance after adjustment for cardiometabolic risk factors, in both MAFLD and NAFLD. On the other hand, in a community-based cohort study of 8919 subjects Moon et al. [50] reported that MAFLD significantly predicted the risk of all-cause mortality even after adjustment for cardiometabolic risk factors (HR 1.36, 95% CI 1.08–1.73), whereas NAFLD did not (HR 1.20, 95% CI 0.94–1.53).
Although the recent AHA scientific statement identified NAFLD as an independent risk factor for CVD, the question as to whether MAFLD is a simple bystander or an active mediator in the pathogenesis of CVD remains [59]. Based on the available evidence [13], the shared cardiometabolic risk factors play an important role but likely do not account for the entire relationship between MAFLD and the risk of CVD events. Apart from shared cardiometabolic risk factors, the precise mechanism(s) underlying the association between MAFLD and CVD risk is (are) not clear, but some potential mechanisms (such as, for example, activation of the renin–angiotensin–aldosterone system, some NAFLD-related genetic polymorphisms and intestinal dysbiosis) may also play a role in both MAFLD and CVD [1], but further research is needed.
Is routine screening for MAFLD necessary for CVD risk assessment?
Based on current evidence, whether a diagnosis of MAFLD improves CVD risk prediction remains uncertain [46, 60]. Currently, in high-risk patient populations with obesity, T2DM or MetS, screening for MAFLD has been recommended by many scientific guidelines [61,62,63]. Conversely, routine screening for MAFLD has not been recommended by scientific guidelines from cardiovascular societies [2, 13, 64]. Before MAFLD screening can be recommended, it is necessary to demonstrate that routine screening may improve both liver-related and cardiovascular outcomes in a cost-effective manner [65]. Wong et al. [66] performed a study of 612 patients referred for coronary angiography with 3679 patient-years of follow-up to test the utility of MAFLD for CVD risk prediction. These authors found that whilst the presence of MAFLD was associated with significant coronary artery disease and need for coronary revascularization procedures, the rates of mortality and CVD events were the same among the MAFLD and non-MAFLD patient cohorts.
In fatty liver disease, it is often overlooked that the severity of liver fibrosis is strongly associated with an increased risk of fatal and nonfatal CVD events [67]. Non-invasive tests for diagnosing liver fibrosis may reduce the number for unnecessary liver biopsies and identify patients at higher risk of CVD. As proof, in a population-based cohort study of 3512 individuals, Tamaki et al. [68] examined the associations between non-invasive biomarkers of liver fibrosis [including Fibrosis-4 (FIB-4) index, non-alcoholic fatty liver disease fibrosis score (NFS), and Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA+-M2BP)] and risk of CVD events. The authors showed that advanced fibrosis (defined as FIB-4 ≥ 2.67, NFS ≥ 0.675, or WFA+-M2BP ≥ 1.0) was associated with higher CVD risk (using the Framingham risk score), independent of traditional CVD risk factors. In another prospective study of nearly 900 outpatients with metabolic syndrome followed for a median period of 41 months, Baratta et al. [54] showed that subjects with NAFLD and FIB-4 ≥ 2.67 had a fourfold increase in fatal and nonfatal CVD events (HR 4.02, 95% CI 1.06–5.74). Although further prospective studies are needed, these findings are proof of concept for the use of non-invasive tools for a better CVD risk stratification in MAFLD.
What is the effect of treatment interventions for MAFLD on the risk of CVD?
Safe, effective and acceptable pharmacotherapies for MAFLD must halt or delay the progression from simple steatosis to cirrhosis, end-stage liver disease and/or hepatocellular carcinoma. The efficacy and safety of potential treatments for MAFLD that reduce the risk of CVD are summarized in Fig. 6.
Assessment of lifestyle interventions and pharmacotherapies on CVD risk and liver histology features. ACC Acetyl-CoA carboxylase, ACEi angiotensin converting enzyme inhibitor, ARBs angiotensin II receptor blockers, CVD cardiovascular disease, GLP-1RA glucagon-like peptide 1 receptor agonist, NASH non-alcoholic steatohepatitis, PCSK9 proprotein convertase subtilisin/kexin type 9, SGLT-2i sodium-glucose cotransporter 2 inhibitor
Interventions with benefit in both CVD and MAFLD
Lifestyle intervention continues to play a key role in the primary and secondary prevention of CVD, as also recommended in several guidelines for management of MAFLD [63, 69, 70]. Adopting a diet rich in vegetables, fruits, legumes, nuts, whole grains and fish is recommended in order to reduce CVD risk and to improve hepatic steatosis and inflammation [64]. A Mediterranean-type diet may reduce hepatic steatosis, improve insulin resistance [71, 72], and is also effective in primary and secondary prevention of CVD [64, 73].
Weight loss is an essential treatment component for reducing CVD risk. Weight loss of 5% to 10% has been shown to be an achievable goal in most lifestyle interventions and results in significant improvements of hepatic histology features (steatosis, inflammation and fibrosis) and CVD risk reduction [63, 74]. With regard to physical activity, at least 150 min per week of accumulated moderate-intensity aerobic physical activity or 75 min per week of vigorous-intensity aerobic physical activity, can improve hepatic steatosis and reduce CVD risk [75, 76]. There may be no lower limit to the amount of moderate to vigorous physical activity at which the benefits of CVD risk reduction begins [77]. Therefore, for adults who cannot meet the minimum level of physical activity, engaging in some moderate or vigorous physical activity may help to reduce risk of CVD [76, 77].
Sleep is an emerging risk factor for cardiometabolic disease with strong relationships between obstructive sleep apnea and fatty liver disease, possibly mediated (at least in part) by recurrent nocturnal hypoxemia [78]. Importantly, in light of the increasing prevalence of inadequate sleep worldwide, sleep deprivation has been causally implicated in increased visceral fat deposition even in young and healthy subjects [79]. Indeed, the AHA recently included sleep in its list of “Life’s Essential 8”, as a behavioral strategy for improving cardiovascular and metabolic population health (see https://www.heart.org/en/healthy-living/healthy-lifestyle/lifes-essential-8).
T2DM is often present in MAFLD and diabetic cardiomyopathy is a risk factor for CVD [80]. Recent data also suggests that some newer glucose-lowering agents may not only improve the histological features of NAFLD, but also significantly reduce CVD outcomes because these agents induce weight loss and improve glycemic control [81,82,83]. Glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT-2i) are two newer classes of glucose-lowering agents that are highly effective for both T2DM treatment and risk reduction of CVD and kidney outcomes [81, 84]. A meta-analysis of phase-2 randomized controlled trials demonstrated that treatment with GLP-1RAs (especially subcutaneous liraglutide and semaglutide) significantly reduce body weight and improve liver histology in NAFLD [85]. Tirzepatide, a novel, dual GLP-1RAs and glucose-dependent insulinotropic polypeptide (GIP) may also exert beneficial effects on liver fat content and the volume of visceral and abdominal subcutaneous adipose tissues. Importantly, tirzepatide did not increase the risk of major CVD events in patients with T2DM [86, 87]. Some phase-2 randomized controlled trials have reported that SGLT2i treatment may also improve hepatic fat content and fibrosis [88, 89]. Nevertheless, the beneficial effects of these newer glucose-lowering agents on hepatic fibrosis beyond weight loss require further study. Pioglitazone, a peroxisome proliferator-activated receptor (PPAR)-gamma agonist, is another glucose-lowering drug that also improves hepatic histology features in patients with biopsy-proven non-alcoholic steatohepatitis, irrespective of the coexistence of T2DM [82, 90]. The benefits of pioglitazone on CVD outcomes in patients with and without T2DM are also well-known [91, 92]. However, safety concerns and moderate weight gain have severely impacted the long-term use of this drug in clinical practice [93, 94].
Therapies with benefit in MAFLD but with cardiovascular safety concerns
Acetyl-CoA carboxylase (ACC) is a key enzyme in fatty acid synthesis that has been explored as a therapeutic target for metabolic steatohepatitis [95]. ACC inhibitors may improve hepatic steatosis, inflammation and fibrosis [96]. Unfortunately, in a randomized controlled trial, ACC inhibitors reduced liver fat content but increased plasma triglyceride levels, raising concerns about their CVD safety [96]. To date, Mendelian randomization studies have not provided sufficient evidence to support the conclusion that hepatic fat accumulation is causally associated with CVD [97]. Conversely, some studies reported that MAFLD susceptibility genotypes (e.g., genetic variants in patatin-like phospholipase domain containing 3 (PNPLA3) and trans-membrane 6 superfamily member 2 (TM6SF2)) are associated with higher risk of fatty liver and steatohepatitis, but with a less atherogenic lipid profile and lower risk of CVD [98, 99].
Farnesoid X receptor (FXR) agonists have therapeutic potential for MAFLD by correcting abnormalities in intermediary metabolism and lipid accumulation, inhibiting p53 activation induced by metabolic stress, inhibiting the progression of fibrosis, and reducing hepatic inflammation [100, 101]. However, obeticholic acid as the first FXR agonist to be submitted for approval for treatment of nonalcoholic steatohepatitis was rejected by the U.S. Food and Drug Administration in 2020 citing uncertainty over the expected benefits based on alternative histopathological endpoints and after consideration that the treatment benefits did not outweigh the potential risks of increasing plasma LDL-C concentrations.
Saroglitazar, a peroxisome proliferator-activated receptor (PPAR) α/γ dual agonist is the first drug to be approved for non-cirrhotic non-alcoholic steatohepatitis (NASH). A randomized, double-blind, placebo-controlled trial demonstrated that high dose saroglitazar (4 mg daily) for 16 weeks reduced liver fat content and improved insulin resistance, serum triglyceride, and transaminase levels in obese patients with NAFLD or NASH [102]. Saroglitazar was approved in India in 2020, but regulatory approval outside of India has not occurred.
Lanifibranor is a pan-PPAR agonist that activates PPAR, α, γ and δ receptors. In the phase 2B placebo-controlled NATIVE trial [103], the histological SAF-A (activity of liver steatosis, activity, and fibrosis) score was reduced in obese patients with biopsy-confirmed nonalcoholic steatohepatitis. Additionally, multiple secondary endpoints were achieved with satisfactory resolution of steatohepatitis without worsening of fibrosis, and improvement in fibrosis stage of at least one stage without worsening of NASH. However, there is little evidence of its impact on CVD risk.
Vitamin E effectively improves hepatic histology in adult patients with biopsy-proven NASH [104]. Combined low-dose spironolactone plus vitamin E also decreased NAFLD liver fat score [105]. However, studies evaluating vitamin E for histological benefit have generally been negative or have produced inconsistent results in small groups of patients [106,107,108]. The results of some randomized placebo-controlled clinical trials also indicate that vitamin E supplementation not only failed to prevent major CVD events, but in fact may increase the risk of developing heart failure [109].
Therapies with benefit in CVD but unknown or uncertain effects in MAFLD
Statins are the first-line treatment to prevent atherosclerotic CVD in patients with hypercholesterolemia [110]. Statins reduce the risk of CVD in MAFLD patients with dyslipidemia, even without any beneficial effect on liver histology [63, 111]. Statins are known to be safe in NAFLD and statin use is not associated with abnormal serum liver enzyme levels, even in patients with hepatic steatosis [112,113,114]. An unexpected concern is that statin treatment might be suboptimal for subjects with MAFLD [114], however further research is needed to test this further. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors represent an alternative pharmacological approach to reducing plasma LDL-C concentrations. While some studies reported a possible beneficial effect on hepatic pathology, it is premature to recommend this agent for specifically treating MAFLD.[115,116,117] Daily aspirin use has been associated with fewer severe histologic features of MAFLD and a lower risk of progressing to advanced fibrosis in a recent observational study [118]. Angiotensin converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARBs) are also thought to exert a moderate anti-fibrotic effect on the liver in experimental and clinical studies [119]. Given the current evidence, more and larger controlled clinical trials are needed before a recommendation for use of these anti-hypertensive agents can be recommended for specifically treating MAFLD.
Conclusions
The AHA statement in 2022 [13] identifies liver fat accumulation (NAFLD) as an independent risk factor for CVD. However, routine screening for MAFLD in patients with pre-existing CVD is not currently recommended. There is increasing scientific and clinical interest in the link between MAFLD and CVD risk, not least because newer glucose-lowering drugs, such as GLP-1RAs and SGLT2i may exert benefit on both hepatic fat content and CVD outcomes. That said, when safe and effective pharmacological treatments for MAFLD are licensed, management will involve close liaison between Cardiologists and other physicians treating this multisystem disease.
Availability of data and materials
Not applicable.
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- ACEi:
-
Angiotensin converting enzyme inhibitor
- ACS:
-
Acute coronary syndrome
- AHA:
-
American heart association
- ARBs:
-
Angiotensin II receptor blockers
- CVD:
-
Cardiovascular disease
- FDA:
-
Food and drug administration
- FIB-4:
-
Fibrosis-4 index
- FXR:
-
Farnesoid X receptor
- GLP-1RA:
-
Glucagon-like peptide 1 receptor agonist
- LDL-C:
-
Low-density lipoprotein cholesterol
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- MetS:
-
Metabolic syndrome
- NAFLD:
-
Non-alcoholic fatty liver disease
- NFS:
-
Non-alcoholic fatty liver disease fibrosis score
- NASH:
-
Non-alcoholic steatohepatitis
- NHANES III:
-
Third National Health and Nutrition Examination Survey
- OR:
-
Odds ratio
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- SGLT-2i:
-
Sodium-glucose cotransporter 2 inhibitor
- PPAR:
-
Peroxisome proliferator-activated receptor
- ROS:
-
Reactive oxygen species
- T2DM:
-
Type 2 diabetes mellitus
- WFA+-M2BP:
-
Wisteria floribunda agglutinin-positive Mac-2 binding protein
References
Cai J, Zhang X, Ji Y, Zhang P, She Z, Li H. Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. Circ Res. 2020;126(5):679–704.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey R, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81.
Eslam M, Newsome P, Sarin S, Anstee Q, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour J, Schattenberg J, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–9.
Zhang XL, Fan JG, Wei L, Shi JP, Zheng MH. Promoting the term MAFLD: China in action. Lancet Gastroenterol Hepatol. 2022;7(7):598.
Chalasani N, Younossi Z, Lavine J, Charlton M, Cusi K, Rinella M, Harrison S, Brunt E, Sanyal A. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–57.
Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999-2014.e1991.
Fouad Y, Elwakil R, Elsahhar M, Said E, Bazeed S, Ali Gomaa A, Hashim A, Kamal E, Mehrez M, Attia D. The NAFLD-MAFLD debate: Eminence vs evidence. Liver International. 2021;41(2):255–60.
Wang T, George J, Zheng M. Metabolic (dysfunction) associated fatty liver disease: more evidence and a bright future. Hepatobiliary Surg Nutr. 2021;10(6):849–52.
van Kleef L, de Knegt R. The transition from NAFLD to MAFLD: one size still does not fit all—time for a tailored approach? Hepatology. 2022. https://doi.org/10.1002/hep.32552.
Younossi Z, Rinella M, Sanyal A, Harrison S, Brunt E, Goodman Z, Cohen D, Loomba R. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2021;73(3):1194–8.
Non-alcoholic fatty liver disease in adults 2021: A clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO).[J] .Dig Liver Dis, 2022,;54:170–182. PMID: 34924319.
Duell P, Welty F, Miller M, Chait A, Hammond G, Ahmad Z, Cohen D, Horton J, Pressman G, Toth P. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the american heart association. Arter Thromb Vasc Biol. 2022. https://doi.org/10.1161/ATV0000000000000153.
Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19(10):2138-2147.e2110.
Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, Targher G, Byrne CD, Yuan WJ, Zheng MH. MAFLD and risk of CKD. Metabolism. 2021. https://doi.org/10.1016/j.metabol.2020.154433.
Zheng KI, Sun DQ, Jin Y, Zhu PW, Zheng MH. Clinical utility of the MAFLD definition. J Hepatol. 2021;74(4):989–91.
Zhang H, Wang Y, Chen C, Lu Y, Wang N. Cardiovascular and renal burdens of metabolic associated fatty liver disease from serial US national surveys, 1999–2016. Chin Med J. 2021;134(13):1593–601.
Kim H, Lee C, Ahn S, Lee K, Lee B, Baik S, Kim S, Lee J. MAFLD predicts the risk of cardiovascular disease better than NAFLD in asymptomatic subjects with health check-ups. Dig Dis Sci. 2022. https://doi.org/10.1007/s10620-022-07508-6.
Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A, Miyamoto Y. Predicting coronary heart disease using risk factor categories for a japanese urban population, and comparison with the Framingham risk score: the suita study. J Atheroscler Thromb. 2016;23(9):1138–9.
Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, Koseki M, Yoshinaga S, Takahashi H, Anzai K, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: generalized estimating equation approach. Hepatol Res. 2021;51(11):1115–28.
Liu S, Wang J, Wu S, Niu J, Zheng R, Bie L, Xin Z, Wang S, Lin H, Zhao Z, et al. The progression and regression of metabolic dysfunction-associated fatty liver disease are associated with the development of subclinical atherosclerosis: a prospective analysis. Metabolism. 2021. https://doi.org/10.1016/j.metabol.2021.154779.
Liu H, Cao Y, Jin J, Guo Y, Zhu C, Wu N, Gao Y, Xu R, Dong Q, Zheng M, et al. Metabolic-associated fatty liver disease and major adverse cardiac events in patients with chronic coronary syndrome: a matched case-control study. Hep Int. 2021;15(6):1337–46.
Noda T, Kamiya K, Hamazaki N, Nozaki K, Ichikawa T, Yamashita M, Uchida S, Maekawa E, Terada T, Reed J, et al. The prevalence of metabolic dysfunction-associated fatty liver disease and its association with physical function and prognosis in patients with acute coronary syndrome. J Clin Med. 2022. https://doi.org/10.3390/jcm11071847.
Quek J, Ng CH, Tang ASP, Chew N, Chan M, Khoo CM, Wei CP, Chin YH, Tay P, Lim G, et al. Metabolic associated fatty liver disease increases the risk of systemic complications and mortality a meta-analysis and systematic review of 12 620 736 individuals. Endocr Pract. 2022;28(7):667–72.
Alexander M, Loomis A, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367: l5367.
Wu M, Zha M, Lv Q, Xie Y, Yuan K, Zhang X, Liu X. Non-alcoholic fatty liver disease and stroke: a Mendelian randomization study. Eur J Neurol. 2022;29(5):1534–7.
George J, Gish RG, Geier A. MAFLD and cardiovascular events: what does the evidence show? Clin Gastroenterol Hepatol. 2021;19(10):2025–8.
Niriella M, Ediriweera D, Kasturiratne A, De Silva S, Dassanayaka A, De Silva A, Kato N, Pathmeswaran A, Wickramasinghe A, de Silva H. Outcomes of NAFLD and MAFLD: results from a community-based, prospective cohort study. PLoS ONE. 2021;16(2): e0245762.
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19(10):2172-2181.e2176.
Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75(6):1284–91.
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–9.
Mantovani A, Csermely A, Tilg H, Byrne C, Targher G. Comparative effects of non-alcoholic fatty liver disease and metabolic dysfunction-associated fatty liver disease on risk of incident cardiovascular events: a meta-analysis of about 13 million individuals. Gut. 2022. https://doi.org/10.1136/gutjnl-2022-328224.
Davis TME. Diabetes and metabolic dysfunction-associated fatty liver disease. Metabolism. 2021. https://doi.org/10.1016/j.metabol.2021.154868.
Zhang Y, Lyu Z, Ma B, Li L, Wang W, Sheng C, Dai H, Huang Y, Song F, Song F, et al. A new risk stratification strategy for fatty liver disease by incorporating MAFLD and fibrosis score in a large US population. Hep Intl. 2022;16(4):835–45.
Caussy C, Aubin A, Loomba R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr DiabRep. 2021;21(5):15.
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–9.
Lee KK, Stelzle D, Bing R, Anwar M, Strachan F, Bashir S, Newby DE, Shah JS, Chung MH, Bloomfield GS, et al. Global burden of atherosclerotic cardiovascular disease in people with hepatitis C virus infection: a systematic review, meta-analysis, and modelling study. Lancet Gastroenterol Hepatol. 2019;4(10):794–804.
Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–53.
Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(11):903–13.
Han E, Lee Y, Kim Y, Kim B, Park J, Kim D, Ahn S, Lee B, Kang E, Cha B, et al. Nonalcoholic fatty liver disease and sarcopenia are independently associated with cardiovascular risk. Am J Gastroenterol. 2020;115(4):584–95.
Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–10.
Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radical Biol Med. 2020;152:116–41.
Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19.
Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22084156.
Pasterkamp G. Methods of accelerated atherosclerosis in diabetic patients. Heart. 2013;99(10):743–9.
Stahl E, Dhindsa D, Lee S, Sandesara P, Chalasani N, Sperling L. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art review. J Am Coll Cardiol. 2019;73(8):948–63.
Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: which has closer association with all-cause and cause-specific mortality?-Results from NHANES III. Front Med. 2021;8: 693507.
Ampuero J, Aller R, Gallego-Durán R, Banales J, Crespo J, García-Monzón C, Pareja M, Vilar-Gómez E, Caballería J, Escudero-García D, et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48:1260–70.
Koo BK, Allison MA, Criqui MH, Denenberg JO, Wright CM. The association between liver fat and systemic calcified atherosclerosis. J Vasc Surg. 2020;71(1):204-211.e204.
Moon J, Kim W, Koo B, Cho N. Metabolic dysfunction-associated fatty liver disease predicts long-term mortality and cardiovascular disease. Gut and liver. 2022;16(3):433–42.
Duseja A, Singh SP, Saraswat VA, Acharya SK, Chawla YK, Chowdhury S, Dhiman RK, Jayakumar RV, Madan K, Misra SP, et al. Non-alcoholic fatty liver disease and metabolic syndrome-position paper of the indian national association for the study of the liver, endocrine society of india, indian college of cardiology and indian society of gastroenterology. J Clin Exp Hepatol. 2015;5(1):51–68.
Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, Kobayashi T, Nogami A, Higurashi T, Kato S, et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: a retrospective nationwide claims database study in Japan. J Gastroenterol. 2021;56(11):1022–32.
Zeb I, Li D, Budoff M, Katz R, Lloyd-Jones D, Agatston A, Blumenthal R, Blaha M, Blankstein R, Carr J, et al. Nonalcoholic fatty liver disease and incident cardiac events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2016;67(16):1965–6.
Baratta F, Pastori D, Angelico F, Balla A, Paganini AM, Cocomello N, Ferro D, Violi F, Sanyal AJ, Del Ben M. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2020;18(10):2324-2331.e2324.
Liu Y, Zhong GC, Tan HY, Hao FB, Hu JJ. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis. Sci Rep. 2019;9(1):11124.
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600.
Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep. 2016;6:33386.
Simon T, Roelstraete B, Khalili H, Hagström H, Ludvigsson J. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70(7):1375–82.
Xun Z, Zhao H. Letter to the editor: Is NAFLD a bystander or contributor to coronary artery disease? Hepatology. 2022. https://doi.org/10.1002/hep.32606.
Glen J, Floros L, Day C, Pryke R. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ. 2016;354: i4428.
Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the american association for the study of liver diseases (AASLD). Endocr Pract. 2022;28(5):528–62.
Younossi Z, Corey K, Lim J. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021;160(3):912–8.
Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, et al. The Asian Pacific Association for the study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hep Intl. 2020;14(6):889–919.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–646.
Corey KE, Klebanoff MJ, Tramontano AC, Chung RT, Hur C. Screening for nonalcoholic steatohepatitis in individuals with type 2 diabetes: a cost-effectiveness analysis. Dig Dis Sci. 2016;61(7):2108–17.
Wong VW, Wong GL, Yeung JC, Fung CY, Chan JK, Chang ZH, Kwan CT, Lam HW, Limquiaco J, Chim AM, et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: A prospective cohort study. Hepatology. 2016;63(3):754–63.
Mantovani A, Csermely A, Petracca G, Beatrice G, Corey K, Simon T, Byrne C, Targher G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(11):903–13.
Tamaki N, Kurosaki M, Takahashi Y, Itakura Y, Inada K, Kirino S, Yamashita K, Sekiguchi S, Hayakawa Y, Osawa L, et al. Liver fibrosis and fatty liver as independent risk factors for cardiovascular disease. J Gastroenterol Hepatol. 2021;36(10):2960–6.
Petroni ML, Brodosi L, Bugianesi E, Marchesini G. Management of non-alcoholic fatty liver disease. BMJ. 2021;372: m4747.
Targher G, Byrne C, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–705.
Gepner Y, Shelef I, Komy O, Cohen N, Schwarzfuchs D, Bril N, Rein M, Serfaty D, Kenigsbuch S, Zelicha H, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71(2):379–88.
Yaskolka Meir A, Rinott E, Tsaban G, Zelicha H, Kaplan A, Rosen P, Shelef I, Youngster I, Shalev A, Blüher M, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. 2021;70(11):2085–95.
Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, Quintana-Navarro GM, Fuentes F, Garcia-Rios A, Ortiz-Morales AM, Gonzalez-Requero AI, Perez-Caballero AI, Yubero-Serrano EM, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399(10338):1876–85.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78.
Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based Interventions for nonalcoholic fatty liver disease: a meta-analysis and meta-regression. Clin Gastroenterol Hepatol. 2016;14(10):1398–411.
Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, Kaur A, Friedemann Smith C, Wilkins E, Rayner M, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc. 2016. https://doi.org/10.1161/JAHA.115.002495.
Sattelmair J, Pertman J, Ding EL, Kohl HW 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–95.
Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. 2019;199(7):830–41.
Covassin N, Singh P, McCrady-Spitzer SK, St Louis EK, Calvin AD, Levine JA, Somers VK. Effects of experimental sleep restriction on energy intake, energy expenditure, and visceral obesity. J Am Coll Cardiol. 2022;79(13):1254–65.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Moon J, Hong J, Jung Y, Ferrannini E, Nauck M, Lim S. SGLT-2 inhibitors and GLP-1 receptor agonists in metabolic dysfunction-associated fatty liver disease. Trends Endocrinol Metab. 2022;33(6):424–42.
Mantovani A, Byrne CD, Targher G. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol. 2022;7(4):367–78.
Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G. Glucagon-Like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta-analysis of randomized controlled trials. Metabolites. 2021. https://doi.org/10.3390/metabo11020073.
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90.
Tang W, Xu Q, Hong T, Tong G, Feng W, Shen S, Bi Y, Zhu D. Comparative efficacy of anti-diabetic agents on nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized and non-randomized studies. Diabetes Metab Res Rev. 2016;32(2):200–16.
Targher G. Tirzepatide adds hepatoprotection to its armoury. Lancet Diabetes Endocrinol. 2022;10(6):374–5.
Sattar N, McGuire D, Pavo I, Weerakkody G, Nishiyama H, Wiese R, Zoungas S. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28(3):591–8.
Mantovani A, Petracca G, Csermely A, Beatrice G, Targher G. Sodium-Glucose cotransporter-2 inhibitors for treatment of nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Metabolites. 2020. https://doi.org/10.3390/metabo11010022.
Lai LL, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci. 2020;65(2):623–31.
Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307.
Young LH, Viscoli CM, Curtis JP, Inzucchi SE, Schwartz GG, Lovejoy AM, Furie KL, Gorman MJ, Conwit R, Abbott JD, et al. Cardiac outcomes after ischemic stroke or transient ischemic attack: effects of pioglitazone in patients with insulin resistance without diabetes mellitus. Circulation. 2017;135(20):1882–93.
Young LH, Viscoli CM, Schwartz GG, Inzucchi SE, Curtis JP, Gorman MJ, Furie KL, Conwit R, Spatz ES, Lovejoy A, et al. Heart Failure after ischemic stroke or transient ischemic attack in insulin-resistant patients without diabetes mellitus treated with pioglitazone. Circulation. 2018;138(12):1210–20.
Mehtälä J, Khanfir H, Bennett D, Ye Y, Korhonen P, Hoti F. Pioglitazone use and risk of bladder cancer: a systematic literature review and meta-analysis of observational studies. Diabetol Int. 2019;10(1):24–36.
Portillo-Sanchez P, Bril F, Lomonaco R, Barb D, Orsak B, Bruder J, Cusi K. Effect of pioglitazone on bone mineral density in patients with nonalcoholic steatohepatitis: a 36-month clinical trial. J Diabetes. 2019;11(3):223–31.
Zhang X, Ji Y, Cheng X, Cheng Y, Yang H, Wang J, Zhao L, Huang Y, Sun D, Xiang H, et al. A small molecule targeting ALOX12-ACC1 ameliorates nonalcoholic steatohepatitis in mice and macaques. Sci Transl Med. 2021;13(624):8116.
Zhang X, She Z, Wang J, Sun D, Shen L, Xiang H, Cheng X, Ji Y, Huang Y, Li P, et al. Multiple omics study identifies an interspecies conserved driver for nonalcoholic steatohepatitis. Sci Transl Med. 2021;13(624):8117.
Eslam M, George J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat Rev Gastroenterol Hepatol. 2020;17(1):40–52.
Simons N, Isaacs A, Koek GH, Kuč S, Schaper NC, Brouwers M. PNPLA3, TM6SF2, and MBOAT7 genotypes and coronary artery disease. Gastroenterology. 2017;152(4):912–3.
Rüschenbaum S, Schwarzkopf K, Friedrich-Rust M, Seeger F, Schoelzel F, Martinez Y, Zeuzem S, Bojunga J, Lange CM. Patatin-like phospholipase domain containing 3 variants differentially impact metabolic traits in individuals at high risk for cardiovascular events. Hepatol Commun. 2018;2(7):798–806.
Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021;18(12):809–23.
Francque S, Szabo G, Abdelmalek M, Byrne C, Cusi K, Dufour J, Roden M, Sacks F, Tacke F. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2021;18(1):24–39.
Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–33.
Francque S, Bedossa P, Ratziu V, Anstee Q, Bugianesi E, Sanyal A, Loomba R, Harrison S, Balabanska R, Mateva L, et al. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385(17):1547–58.
Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, Nakade Y, Ito K, Fukuzawa Y, Yoneda M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31(7–8):923–30.
Polyzos SA, Kountouras J, Mantzoros CS, Polymerou V, Katsinelos P. Effects of combined low-dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: a randomized controlled trial. Diabetes Obes Metab. 2017;19(12):1805–9.
Sarkhy AA, Al-Hussaini AA, Nobili V. Does vitamin E improve the outcomes of pediatric nonalcoholic fatty liver disease? A systematic review and meta-analysis. Saudi J Gastroenterol. 2014;20(3):143–53.
Bril F, Biernacki D, Kalavalapalli S, Lomonaco R, Subbarayan S, Lai J, Tio F, Suman A, Orsak B, Hecht J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42(8):1481–8.
Amanullah I, Khan Y, Anwar I, Gulzar A, Mallhi T, Raja A. Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomised controlled trials. Postgrad Med J. 2019;95(1129):601–11.
Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–47.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–143.
Du J, Ma YY, Yu CH, Li YM. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20(2):569–77.
Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376(9756):1916–22.
Fatima K, Moeed A, Waqar E, Atif A, Kamran A, Rizvi H, Suri N, Haider H, Shuja S, Khalid M, et al. Efficacy of statins in treatment and development of non-alcoholic fatty liver disease and steatohepatitis: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2021;46(4): 101816.
Khoo S, Wong V, Goh G, Fan J, Chan W, Seto W, Chow W. Suboptimal treatment of dyslipidemia in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35(2):320–5.
Kothari S, Dhami-Shah H, Shah SR. Antidiabetic drugs and statins in nonalcoholic fatty liver disease. J Clin Exp Hepatol. 2019;9(6):723–30.
Theocharidou E, Papademetriou M, Reklou A, Sachinidis A, Boutari C, Giouleme O. The role of PCSK9 in the pathogenesis of non-alcoholic fatty liver disease and the effect of PCSK9 inhibitors. Curr Pharm Des. 2018;24(31):3654–7.
Rimbert A, Smati S, Dijk W, Le May C, Cariou B. Genetic inhibition of PCSK9 and liver function. JAMA Cardiol. 2021;6(3):353–4.
Severo M. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18(2):523.
Mantovani A, Dalbeni A. Treatments for NAFLD: state of art. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22052350.
Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, Yang C, Zong G, Wu J, Jia W. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 4.6-year cohort study in China. J Clin Endocrinol Metab. 2022;107(1):88–97.
Jeong S, Oh Y, Choi S, Chang J, Kim S, Son J, Lee G, Kim W, Park S. Metabolic dysfunction-associated fatty liver disease better predicts incident cardiovascular disease. Gut and liver. 2021. https://doi.org/10.5009/gnl210256.
Matsubayashi Y, Fujihara K, Yamada-Harada M, Mitsuma Y, Sato T, Yaguchi Y, Osawa T, Yamamoto M, Kitazawa M, Yamada T, et al. Impact of metabolic syndrome and metabolic dysfunction-associated fatty liver disease on cardiovascular risk by the presence or absence of type 2 diabetes and according to sex. Cardiovasc Diabetol. 2022;21(1):90.
Acknowledgements
GT is supported in part by grants from the School of Medicine, University of Verona, Verona, Italy. CDB is supported in part by the Southampton National Institute for Health and Care (NIHR) Biomedical Research Centre (IS-BRC-20004), UK. VKS is supported by NIH HL65176 and HL160619. MHZ is supported by National Natural Science Foundation of China (82070588), High Level Creative Talents from Department of Public Health in Zhejiang Province (S2032102600032). JG is supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney; a National Health and Medical Research Council of Australia (NHMRC) Program Grant (APP1053206) and Project, ideas grants and investigator grants (APP2001692, APP1107178, APP1108422, APP1196492).
Author information
Authors and Affiliations
Consortia
Contributions
X-DZ and M-HZ designed the study. X-DZ and JC draft the manuscript. X-DZ were responsible for information retrieval. X-DZ and L–LC prepared the figures. GT, CDB, MDS, K-CS, VKS, C. AAC, JG and YZ contributed to writing and proof reading the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, XD., Cai, J., Targher, G. et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc Diabetol 21, 270 (2022). https://doi.org/10.1186/s12933-022-01697-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01697-0