Abstract
Atrial fibrillation, the most common cardiac arrhythmia, results in substantial morbidity and mortality related to its increased risks of stroke, heart failure, and impaired cognitive function. The incidence and prevalence of atrial fibrillation in the general population is rising, making atrial fibrillation treatment and management of its risk factors highly relevant clinical targets. One well-studied risk factor for the development of atrial fibrillation is diabetes mellitus. Inhibitors of sodium-glucose cotransporter 2 (SGLT2), common medications used to treat diabetes mellitus, have been observed to decrease the incidence of atrial fibrillation. This review discusses the SGLT2 and its role in glucose homeostasis, molecules inhibiting the transporter, possible physiological mechanisms responsible for the decreased incident atrial fibrillation in patients treated with SGLT2 inhibitors and proposes mechanistic studies to further our understanding of the biological processes involved.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) and atrial flutter (AFL) are the most common cardiac arrhythmias afflicting an estimated 59.7 million individuals worldwide in 2019 [1]. The Framingham Heart Study data indicates that AF-induced risk for stroke increases 4 to 5-fold and is associated with a doubling of mortality in both sexes [2]. The disease condition is also associated with a 3-fold risk of heart failure [3, 4] and a 2-fold risk of dementia and impaired cognitive function [5]. Despite concerted efforts to manage risk factors for AF [6,7,8,9], the prevalence and incidence of AF in the general population continue to rise [1].
Current pharmacotherapies targeting ion channels to treat AF have their own arrhythmogenic potentials [10, 11] and invasive interventional procedures (while highly effective), are not scalable given the vast numbers of AF patients needing treatment. Thus, a clear need exists for novel therapies or approaches addressing atrial fibrillation. A meta-analysis of published studies reaffirmed that patients with diabetes have an approximately 40% greater risk of AF compared to unaffected patients [12]. In this context, it is notable that dapagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor approved for the treatment of patients with type 2 diabetes, decreased the incidence of AF/AFL in those patients [13].
In this review, we provide a brief background on SGLT2, its role in glucose homeostasis, molecules inhibiting the transporter, potential mechanistic basis for the apparent protective effects on AF by the inhibitors, and prospective studies to further elucidate responsible mechanisms.
SGLT2 inhibition
The original finding that the glucose filtered and reabsorbed in human kidneys is sensitive to competitive inhibition by a naturally occurring compound called phlorizin [14] prompted research into the role of the kidneys in glucose homeostasis and diabetes. Preclinical studies in rats [15] validated the critical role of the kidney in glucose regulation and led to the identification of a low-affinity Na+-coupled glucose transporter in S1 segments and a high-affinity Na+-coupled glucose transporter located in S3 segments [16]. Kanai et al. [17] characterized the low-affinity, high-capacity Na+/glucose cotransporter that mediated saturable Na+-dependent and phlorizin-sensitive transport of D-glucose and labeled it SGLT2 to distinguish it from the high-affinity isoform SGLT1. SGLT2 expressed in the S1 segment of the nephron is responsible for approximately 90% of glucose reabsorption with the remainder done by SGLT1 in the S3 segment [18, 19]. The SGLTs are now known to comprise a family of active glucose transporter members [20] of which SGLT1 and SGLT2 are the most studied isoforms to date given their prominent role in glucose homeostasis and as logical drug targets for the treatment of diabetes.
Phlorizin, the prototypical SGLT1/SGLT2 dual inhibitor, while effective in lowering glucose levels in animal and human studies, is not an antidiabetic drug candidate given its poor pharmacodynamic and pharmacokinetic properties [21, 22]. Nonetheless, it served as a molecular starting point for the discovery and development of selective inhibitors of SGLT2 for the treatment of diabetes. This led to the synthesis of novel, relatively selective and efficacious SGLT2 inhibitors such as dapagliflozin, canagliflozin, empagliflozin, ertugliflozin, etc. [22, 23]. Canagliflozin was the first SGLT2 inhibitor approved by the US Food and Drug Administration (FDA) as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [24,25,26,27]. This was followed by FDA approvals of dapagliflozin, empagliflozin, and ertugliflozin for the treatment of type 2 diabetes based on data from randomized clinical trials.
The FDA’s regulatory guidance of 2008 recommended outcome trials to rule out increased cardiovascular risk for all glucose-lowering therapies undergoing evaluation. Surprisingly, in the EMPA-REG OUTCOME trial, patients with type 2 diabetes at high risk for cardiovascular events who received empagliflozin, as compared to placebo, had a lower rate of the primary composite cardiovascular outcome and of death from any cause when the study drug was added to standard care [28]. This unexpected finding was supported by a meta-analysis of data extracted from six regulatory submissions and 57 published trials for seven SGLT2 inhibitors suggesting net protection by the SGLT2 inhibitor class of drugs against cardiovascular outcomes and death [29]. A multitude of randomized clinical trials [28, 30,31,32,33,34,35,36,37,38,39,40] has extended the range of disease conditions including heart failure and chronic kidney disease treatable with individual SGLT2 inhibitors. Furthermore, the SGLT2 inhibitor dapagliflozin was found to reduce the incidence of AF/AFL in patients with diabetes [13] or heart failure [41]. That the protective effects extended beyond dapagliflozin to include the broad class of SGLT2 inhibitors were indicated by most meta-analyses [42,43,44,45,46,47] with exceptions [48, 49]. Analysis of a large pharmacologic database also indicated a consistent and convincing reduction in reported AF with SGLT2 inhibition [50]. These results merit mechanistic studies and prospective, randomized clinical trials. Collectively, they may offer a better understanding of the pathophysiology of diabetes and reveal novel targets for exploration while adding to the therapeutic options for patients with AF. With this perspective, this review discusses plausible mechanisms that individually or in combination confer protection against AF by SGLT2 inhibitors.
Mechanistic insights
SGLT2 is reportedly located almost exclusively in the epithelium of the renal proximal tubular segment with no detectable expression in the human heart [51,52,53]. Khemais-Benkhiat et al. [54] reported that SGLT2 expression is upregulated in cultured senescent endothelial cells of the porcine coronary artery when exposed to high glucose despite absence of expression under normal conditions, implying that the expression of the transporter could be induced under pathologic conditions in tissues with low basal expression. However, no expression of SGLT2 at both gene and protein level was found in tissue biopsies of healthy, ischemic, or hypertrophic human hearts [53]. Given these observations, the purported beneficial effect of SGLT2 on AF is apparently mediated by indirect metabolic or hemodynamic mechanisms triggered by its renal actions rather than by direct, ‘on-target’ effects on the cardiomyocyte. However, the possibility of ‘off-target’ actions of the different SGLT2 inhibitors directly on the atrial cardiomyocyte as a ‘class effect’ cannot be ruled out entirely given that they are structurally related as derivatives of phlorizin.
Body weight reduction
The association of AF with obesity was recognized in the long-term prospective Framingham Heart Study [55]. A 4.7% linear increase in the risk of AF with each kg/m2 increase in body mass index (BMI) was evident in the Women’s Health Study [56]. Interestingly, women who were obese at baseline but then attained a BMI less than 30 kg/m2 by year 5 no longer had a significantly increased risk of subsequent AF in adjusted analyses in that study. This is substantiated by the observation in the LEGACY study that progressive weight loss has a dose-dependent effect on long-term freedom from AF in obese individuals with symptomatic AF [57]. In this context, it is interesting that therapy with SGLT2 inhibitors consistently results in a 1–3% body-weight loss in patients with type 2 diabetes [58] and that the effect is associated with a lower risk of new-onset AF [59]. The loss of about 1–3 kg body weight with treatment is characterized initially by a combination of early and rapid loss of water following osmotic diuresis associated with increased glucose excretion (calorie loss) and fat loss, and then by a maintained fat loss [58]. Dual-energy x-ray absorptiometry was used by Bolinder et al. [60] to demonstrate that the eventual weight loss in type 2 diabetes patients treated with dapagliflozin is the result of changes primarily in total-body fat mass, visceral adipose tissue, and subcutaneous adipose tissue. A study using bioimpedance spectroscopy confirmed the significant decrease in adipose tissue mass and fat tissue index without any observable change in lean tissue parameters in type 2 diabetes patients treated with dapagliflozin or empagliflozin [61].
The mechanistic basis for the association of obesity with AF is unclear. Obesity increases epicardial adipose tissue (EAT) mass overlying the posterior left atrium, atrioventricular groove, and infiltrating the atria [62,63,64]. Physiologically, the metabolically active fat depot is believed to release fatty acids through lipolysis for energy use by the heart under normal conditions–a process facilitated by a shared microcirculation given the lack of a muscle fascial plane between the fat depot and the myocardium [65, 66]. Under pathological conditions, dysfunctional EAT can release proinflammatory adipokines such as tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), IL-1β, plasminogen activator inhibitor-1 (PAI-1), resistin, etc. [66,67,68,69], potentially contributing to fibrosis and structural and electrical remodeling of the impacted atrial myocardium [63, 70,71,72,73]. Interestingly, extracellular vesicles derived from epicardial fat specimens collected from patients with AF showed proinflammatory, profibrotic, and proarrhythmic signature suggesting that the vesicles may promote atrial fibrosis, the arrhythmogenic substrate for AF [74]. The Framingham Heart Study reported that pericardial fat (defined in the report as total adipose tissue within the pericardial sac; epicardial fat) but not other fat deposits was associated with prevalent AF even after adjustment for AF risk factors including body mass index [75]. The higher fat volumes were associated with approximately 40% higher odds of prevalent AF. Epicardial adipose tissue accumulation is associated with atrial pathophysiology predisposing to AF [76,77,78,79,80]. Dapagliflozin, the SGLT2 inhibitor reported to reduce the incidence of AF/AFL in patients with diabetes [13], also caused a substantial and rapid reduction in epicardial fat thickness [81]. Furthermore, treatment of patients with type 2 diabetes and coronary artery disease with dapagliflozin significantly decreased epicardial adipose tissue volume and P-wave indices such as P-wave dispersion and P-wave variation [82, 83]. A similar reduction in EAT volume was noted with empagliflozin following a sub-analysis of the EMPA-TROPISM trial in nondiabetic patients with heart failure with reduced ejection fraction [84]. The mechanism for the reduction in volume is unclear but is postulated to result from the glycosuric effects of the drugs, inducing a negative caloric balance and altering the ratio of glucagon and insulin to trigger enhanced lipolysis and reduction of epicardial adipose tissue [85]. Thus, SGLT2 inhibitor-mediated reduction in the volume of epicardial adipose tissue and potential detrimental remodeling effects on the atrial myocardium apparently contribute to the reported reduction in the incidence of AF, warranting prospective studies.
Left ventricular function
Atrial fibrillation and congestive heart failure (HF) often coexist, with each condition predisposing to the other [86]. They share common risk factors including hypertension, diabetes, ischemic heart disease, obesity, and age. Atrial fibrillation occurs in more than half of individuals with HF and HF occurs in more than one third of individuals with AF at some point in time [87]. Amelioration of one often has favorable effects on the other. In the CASTLE-AF trial, AF ablation in heart failure patients led to a significant reduction of AF burden, an increase in left ventricular ejection fraction, and to a reduction in heart failure-related hospitalization and mortality [88]. Similar beneficial effects of AF-related ablation in patients with heart failure were noted in multiple studies [89,90,91,92,93,94]. However, the benefits of the procedure did not extend fully to patients with severely advanced heart failure [95]. Conversely, inhibitors of the renin-angiotensin-aldosterone system approved for the treatment of heart failure inhibit atrial remodeling and fibrosis and decrease incidence or recurrence of AF [96,97,98,99]. Thus, the observed benefits of SGLT2 inhibitors in reducing AF is seemingly secondary to the benefits of the drugs in cardiac function.
Empagliflozin treatment of individuals with diabetes and established cardiovascular disease produced a rapid and significant improvement in diastolic function [100]. A subsequent 3-month mechanistic study with the drug in that patient population revealed that the rapid, significant, and sustained improvement in left ventricular diastolic function as assessed by early mitral inflow velocity relative to early diastolic left ventricular relaxation (E/e’) was not accompanied by any change in systemic vascular resistance, cardiac index, stroke volume index, or pulse rate [101]. Similar improvements in left ventricular diastolic function were also observed within 3 months of treatment of patients with type 2 diabetes with canagliflozin [102], indicating that the improvement in diastolic function is a class effect of SGLT2 inhibitors. The apparent rapid improvement in left ventricular filling pressure implies that it is evoked by hemodynamic or metabolic alterations and not by structural changes in the tissue.
In patients with cardiac disease, tissue Doppler assessment of ventricular relaxation can be used to assess the effect of left ventricular relaxation in mitral E velocity, and the E/e’ ratio can be applied for the prediction of left ventricular filling pressures [103, 104] and atrial fibrillation [105]. As is well known from the work by Haissaguerre et al. [106], the cardiomyocytes in the pulmonary vein sleeves are a significant ectopic source of aberrant electrical signals triggering left atrial arrhythmogenesis. Increased left ventricular filling pressure exerts hemodynamic stress on the left atrium and pulmonary veins, especially during the ‘atrial kick,’ causing stretching of the resident cardiomyocytes. Stretch of the pulmonary veins triggers arrhythmogenesis in the left atrium [107, 108]. Significant dilation of both superior pulmonary veins and the left atrium reportedly occurs in patients with atrial fibrillation [109]. Accordingly, the incidence of AF is related to pulmonary vein volume index [110]. Thus, the structure and function of the left atrium are directly impacted by left ventricular filling pressure. Reduction of the hemodynamic stress on the left atrium and pulmonary veins by SGLT2 inhibitor-mediated improvement in left ventricular diastolic function may thus ameliorate the trigger mechanism and acutely inhibit AF.
The mechanism underlying the acute improvement in left ventricular function by SGLT2 inhibitors is unclear. The effect of empagliflozin is thought to result from osmotic diuresis with electrolyte-free water excretion leading to immediate cardiac preload reduction, suggested by reduced early mitral inflow velocity E [101]. A mathematical model-based investigation suggested that SGLT2 inhibitors achieve diuresis by a mechanism distinct from that of other diuretic classes [111]. SGLT2 inhibition apparently elicits greater fluid clearance from the interstitial fluid than from the circulation, potentially reducing elevated cardiac filling pressures without causing neurohumoral activation. Additionally, the increased availability of circulating ketone bodies from the glucagon-mediated ketogenesis [112], may lead to improvement in myocardial energetics [113]. Dapagliflozin reduced left ventricular mass in subjects with type 2 diabetes and left ventricular hypertrophy in a longer, 12-month study [114], indicating that it reverses pathologic cardiac remodeling to possibly improve diastolic function. A significant increase in hematocrit, thought to be from decreased plasma volume and resultant hemoconcentration, was also observed in that study–suggesting presence of an additional mechanism for enhancing oxygen supply to the tissue. There may be other mechanisms contributing to this as well. There is a large body of literature investigating the mechanism(s) potentially responsible for the beneficial effects of SGLT2 inhibitors in patients with heart failure with or without diabetes as summarized in several review articles [115,116,117,118,119,120] that could relate a reduction in AF via improvements in ventricular function. Finally, the AF-reducing effect is multifactorial with the early effects being triggered by acute improvement in diastolic function as described above and sustained later by reversal of adverse structural and electrical remodeling of the left ventricle, left atrium, and pulmonary veins.
Antihyperglycemic effect and blood pressure lowering
The relationship between type 2 diabetes and new-onset AF is well documented [121]. A meta-analysis comprising 7 prospective studies and 4 case-control studies involving 1.7 million subjects indicated an approximately 40% higher risk of developing AF in patients with diabetes compared to unaffected patients. The increased risk remained significant at about 25% after adjustment for confounding multiple risk factors and publication bias [12]. The antihyperglycemic effects by the SGLT2 inhibitors were relatively modest [28, 31, 33, 34], suggesting that they are unlikely to account for the observed benefits of AF considering that intensive glycemic control per se does not affect the rate of new-onset AF [122].
Similarly, on a population level, elevated blood pressure is a risk factor for incident AF. A 20-mmHg increase in systolic blood pressure is associated with 21% higher risk of AF [123]. Intensive blood pressure lowering in patients with hypertension is associated with a 26% lower risk of developing incident AF [124]. A meta-analysis characterizing the blood pressure-lowering effects of SGLT2 inhibitors in 27 randomized clinical trials including canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and remogliflozin [125], revealed moderate reductions in both systolic blood pressure (approximately 5 mmHg) and diastolic blood pressure (approximately 2 mmHg) attributable to osmotic diuresis and natriuresis [126]. The magnitude of the reductions was essentially similar across the entire drug class. The dapagliflozin treatment-related reduced risk of AF was not modified by systolic blood pressure [13], suggesting that the marginally lowered blood pressure is unlikely to fully account for the beneficial effect.
Possible ‘off-target’ actions on cardiomyocytes
As discussed earlier, SGLT2 is located almost exclusively in the epithelium of the renal proximal tubular segment with no detectable expression in the human heart [51,52,53]. However, studies using human, or animal cardiomyocytes have shown that empagliflozin inhibits the sodium-hydrogen exchanger (NHE) activity [127, 128] similar in magnitude to that produced by cariporide [127], a selective inhibitor of the exchanger [129]. Molecular binding studies have demonstrated high binding affinities of SGLT2 inhibitors possibly with the extracellular Na+-binding site of the NHE [130], suggesting that an ‘off-target’ effect of the inhibitors on cardiomyocyte ionic homeostasis may contribute to the beneficial effects. The NHE is a major regulator of intracellular pH under normal physiological conditions as well as during pathological conditions such as cardiac ischemia or cardiac hypertrophy/remodeling [131, 132]. It mediates extrusion of protons from the cell with a concomitant increase in intracellular Na+ concentration. This is expected to activate the reverse mode of the Na+/Ca++ exchanger to increase intracellular Ca++, cause Ca++ overload, induce atrial electrical remodeling, and predispose to AF. Inhibition of the NHE in such a situation would be expected to interrupt this sequence of events and inhibit AF [133]. However, results from experimental studies examining the effects of NHE inhibitors in animal models of AF have been mixed. Jayachandran et al. [134] reported that blockade of the cardiac NHE by HOE648 (cariporide) blocked the shortening of the atrial effective refractory period in dogs subjected to rapid atrial pacing, suggesting a beneficial effect of the agent against AF. In contrast, Shinagawa et al. [135] observed no benefit by cariporide against atrial remodeling in dogs exposed to seven days of rapid atrial pacing. Similarly, in a goat model of AF, the NHE inhibitors EMD87580 or EMD125021 did not prevent or revert AF-induced atrial remodeling, leading the investigators to conclude that blockers of the exchanger are unlikely to be of benefit in the prevention or treatment of AF [136]. Thus, the role of SGLT2-inhibitor-mediated block of the NHE in preventing AF is currently unclear, warranting further investigation.
Shao et al. [137] have postulated that empagliflozin is potentially useful in the prevention of diabetes-related AF as it can improve mitochondrial function and biogenesis, and prevent atrial structural and electrical remodeling in a high-fat diet/streptozotocin rat model of insulin resistance, obesity, and diabetes. There was expectedly a significant reduction in body weight, and blood glucose in the treated rats relative to the control diabetic animals in that study. Mitochondria in the diabetic human atrium do show impaired capacity to oxidize fatty acids and glutamate and indicate persistent oxidative stress [138]. Whether the improved mitochondrial function and biogenesis observed in the study by Shao et al. [137] are secondary to the beneficial metabolic changes produced by the known pharmacological actions of the drug or to direct effects on mitochondrial dynamics are yet to be ascertained.
Future directions
The post hoc analysis of the DECLARE-TIMI 58 trial indicating decreased incidence of AF in high-risk patients with type 2 diabetes treated with dapagliflozin [13] is hypothesis-generating since it was not the prespecified primary outcome in the trial. Adequately powered, prospective randomized clinical trials with AF (new onset, progression, or reversal) as the primary outcome are needed to extend the findings. Determination of the timeline of new-onset AF in asymptomatic subjects even with established clinical risk factors is challenging without continuous rhythm monitoring. The value of this parameter is questionable as well, since it is often dependent on an initiating trigger which may occur by chance (indicating that AF burden instead may be a better approach) [139]. Examining the effect of SGLT2 inhibitors on the time-to-first recurrence of AF after ablation or cardioversion may be a more pragmatic approach given the increased likelihood of AF recurrence in this patient population.
SGLT2 inhibitors elicit pleotropic effects. It is critical to identify the principal driver of the drug-mediated reduction in incident AF. Delineation of the beneficial structural and functional changes in various organ systems relevant to AF pathogenesis as highlighted in this review will facilitate subsequent investigations of the molecular basis for the beneficial effects of the drugs on AF.
Comparative proteomic analysis of EAT samples biopsied before and after drug treatment may yield insights to specific effects on pathways related to inflammation, fibrosis, apoptosis, arrhythmogenesis, etc. Inflammatory cytokines such as TNF-α, IL-1, IL-6 directly modulate the function of cardiac ion channels to promote arrhythmia [140]. For instance, TNF induces atrial structural remodeling and downregulation of connexin40 to promote arrhythmia in a transgenic mouse model with cardiac targeted overexpression of TNF [141]. Valuable information may be obtained by investigating the relationship between changes in left ventricular relaxation (E/e’ ratio) and left atrial strain in diabetic patients with or without AF upon treatment with SGLT2 inhibitors. Left atrial strain measurements may provide early detection of functional changes before any changes in left atrial dilation can be detected [142]. Furthermore, left atrial strain or strain rate can predict postoperative AF, AF recurrence after ablation, and facilitate grading of diastolic dysfunction [143].
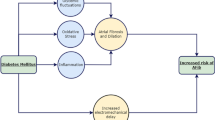
Stretch of the pulmonary veins triggers arrhythmia as discussed earlier. Elevated left atrial pressure resulting from left ventricular diastolic dysfunction is transmitted to the pulmonary veins given the anatomical continuity between the two. This leads to increased pulmonary vein volume and dilated vein orifices [109] and is predictive of AF [144]. It is thus important to investigate if amelioration of diastolic dysfunction by SGLT2 inhibitors has a direct upstream effect on pulmonary vein dimensions using computed tomography (CT) in longitudinal studies. A visual representation of potential mechanisms mediating reduction in AF by SGLT2 inhibitors is shown in Fig. 1.
Mechanistic studies with SGLT2 inhibitors may be supported by analysis of circulating biomarkers or fibrosis including type I procollagen C-terminal propeptide (a marker of collagen type 1 synthesis), type III collagen N-terminal propeptide (a marker of collagen type 3 synthesis), [145, 146] and on inflammation including high-sensitivity C-reactive protein, interleukin-6, and tumor necrosis factor alpha [147]. Thus, an expanded analysis involving functional, structural, and biochemical assessments will likely yield valuable clues regarding the clinical significance of the potential beneficial effects of SGLT2 inhibitors and their biological bases.
Conclusions
The post hoc analysis of the DECLARE-TIMI 58 trial demonstrated a reduction in AF incidence in diabetes mellitus patients treated with SGLT2 inhibitors. The mechanism for this reduction in AF is unknown. Possible mechanisms for the SGLT2 inhibitor-induced reduction in incident AF include reduction in epicardial fat inhibiting pathological atrial remodeling and rapid improvement in left ventricular diastolic function ameliorating hemodynamic stress on the atrium and pulmonary veins. Off-target interactions by the drugs potentially mitigating cardiomyocyte ionic imbalance and ameliorating oxidative stress-induced mitochondrial dysfunction are ancillary mechanisms worth exploring as well. Given the modest reductions in hemoglobin A1c and blood pressure in patients treated with SGLT2 inhibitors, these are unlikely to be predominant mechanisms by which SGLT2 inhibitors reduce AF. Biochemical, functional, and structural studies as highlighted in this review will further help elucidate the mechanistic bases for the observed beneficial effects of SGLT2 inhibitors on incident AF.
Availability of data and materials
Not applicable.
Abbreviations
- AF:
-
Atrial fibrillation
- AFL:
-
Atrial flutter
- SGLT2:
-
Sodium-glucose cotransporter 2
- EAT:
-
Epicardial adipose tissue
- E/e’:
-
Early mitral inflow velocity relative to early diastolic left ventricular relaxation
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. 10.1016/j.jacc.2020.11.010. Erratum in: J Am Coll Cardiol. 2021;77(15):1958–1959.
Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N–9N. https://doi.org/10.1016/s0002-9149(98)00583-9.
Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98(5):476–84. https://doi.org/10.1016/S0002-9343(99)80348-9.
Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–64. https://doi.org/10.1016/s0002-9343(02)01236-6.
Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28(2):316–21. https://doi.org/10.1161/01.str.28.2.316.
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-267. 10.1161/CIR.0000000000000041. Epub 2014 Mar 28. Erratum in: Circulation. 2014;130(23):e272-4.
Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–4.
Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–6. https://doi.org/10.1164/rccm.200509-1442OC. Epub 2006 Jan 19.
Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92(1):17–40. https://doi.org/10.1016/j.mcna.2007.09.002.
Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2007;(4):CD005049. https://doi.org/10.1002/14651858.CD005049.pub2. Update in: Cochrane Database Syst Rev. 2012;5:CD005049.
Freemantle N, Lafuente-Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace. 2011;13(3):329–45. https://doi.org/10.1093/europace/euq450. Epub 2011 Jan 11.
Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108(1):56–62. https://doi.org/10.1016/j.amjcard.2011.03.004. Epub 2011 Apr 27.
Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: Insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141(15):1227–1234. https://doi.org/10.1161/CIRCULATIONAHA.119.044183. Epub 2020 Jan 27.
Chasis H, Jolliffe N, Smith HW. The action of phlorizin on the excretion of glucose, xylose, sucrose, creatinine and urea by man. J Clin Invest. 1933;12(6):1083–90. https://doi.org/10.1172/JCI100559.
Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79(5):1510–5. https://doi.org/10.1172/JCI112981.
Barfuss DW, Schafer JA. Differences in active and passive glucose transport along the proximal nephron. Am J Physiol. 1981;241(3):F322-32. https://doi.org/10.1152/ajprenal.1981.241.3.F322.
Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93(1):397–404. https://doi.org/10.1172/JCI116972.
Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22(1):104–12. https://doi.org/10.1681/ASN.2010030246. Epub 2010 Jul 8.
Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–94. https://doi.org/10.1152/physrev.00055.2009.
Gyimesi G, Pujol-Giménez J, Kanai Y, Hediger MA. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflugers Arch. 2020;472(9):1177–1206. https://doi.org/10.1007/s00424-020-02433-x. Epub 2020 Aug 7.
Choi CI. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors from Natural Products: Discovery of Next-Generation Antihyperglycemic Agents. Molecules. 2016;21(9):1136. https://doi.org/10.3390/molecules21091136.
Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61(10):2079–2086. https://doi.org/10.1007/s00125-018-4654-7. Epub 2018 Aug 22.
Manoj A, Das S, Ramachandran AK, Alex AT, Joseph A. SGLT2 inhibitors, an accomplished development in field of medicinal chemistry: an extensive review. Future Med Chem. 2020;12(21):1961–90. https://doi.org/10.4155/fmc-2020-0154. Epub 2020 Oct 30.
Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–50. https://doi.org/10.1016/S0140-6736(13)60683-2. Epub 2013 Jul 12.
Davis SN. Canagliflozin versus glimepiride treatment in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU trial). Expert Rev Clin Pharmacol. 2014;7(1):21–3. https://doi.org/10.1586/17512433.2014.864950. Epub 2013 Dec 2.
Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16(5):467–77. https://doi.org/10.1111/dom.12273 Epub 2014 Mar 12.
Wilding JPH, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67(12):1267–82. https://doi.org/10.1111/ijcp.12322. Epub 2013 Oct 13.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720. Epub 2015 Sep 17.
Wu JHY, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4(5):411-9. https://doi.org/10.1016/S2213-8587(16)00052-8. Epub 2016 Mar 18. Erratum in: Lancet Diabetes Endocrinol. 2016 Sep;4(9):e9.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M et al. EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34. https://doi.org/10.1056/NEJMoa1515920. Epub 2016 Jun 14.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. https://doi.org/10.1056/NEJMoa1611925. Epub 2017 Jun 12.
Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140(9):739–50. https://doi.org/10.1161/CIRCULATIONAHA.119.042007 Epub 2019 Jul 11.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. https://doi.org/10.1056/NEJMoa1811744. Epub 2019 Apr 14.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. https://doi.org/10.1056/NEJMoa1812389. Epub 2018 Nov 10.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303. Epub 2019 Sep 19.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24. https://doi.org/10.1056/NEJMoa2022190. Epub 2020 Aug 28.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. https://doi.org/10.1056/NEJMoa2024816. Epub 2020 Sep 24.
Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA. 2020;323(14):1353–68. https://doi.org/10.1001/jama.2020.1906. Erratum in: JAMA. 2021 Apr 6;325(13):1335.
Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: Results of DAPA-HF. Circulation. 2021;143(4):298–309. https://doi.org/10.1161/CIRCULATIONAHA.120.050391. Epub 2020 Oct 12.
Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(1):22–31. https://doi.org/10.1016/S2213-8587(20)30369-7.
Zhou L, Yang Y, Han W. Sodium-glucose cotransporter-2 inhibitors protect against atrial fibrillation in patients with heart failure. Ann Palliat Med. 2021;10(10):10887-95. https://doi.org/10.21037/apm-21-2694.
Li HL, Lip GYH, Feng Q, Fei Y, Tse YK, Wu MZ, et al. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. 2021;20(1):100. https://doi.org/10.1186/s12933-021-01293-8. Erratum in: Cardiovasc Diabetol. 2021 Sep 4;20(1):177.
Li D, Liu Y, Hidru TH, Yang X, Wang Y, Chen C, et al. Protective effects of sodium-glucose transporter 2 inhibitors on atrial fibrillation and atrial flutter: A systematic review and meta-analysis of randomized placebo-controlled trials. Front Endocrinol (Lausanne). 2021;12:619586. https://doi.org/10.3389/fendo.2021.619586.
Zhou Z, Jardine MJ, Li Q, Neuen BL, Cannon CP, de Zeeuw D, et al. CREDENCE Trial Investigators*. Effect of sglt2 inhibitors on stroke and atrial fibrillation in diabetic kidney disease: Results from the CREDENCE trial and meta-analysis. Stroke. 2021;52(5):1545–56. https://doi.org/10.1161/STROKEAHA.120.031623. Epub 2021 Apr 20.
Zheng RJ, Wang Y, Tang JN, Duan JY, Yuan MY, Zhang JY. Association of SGLT2 inhibitors with risk of atrial fibrillation and stroke in patients with and without type 2 diabetes: A systemic review and meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol. 2022;79(2):e145–52. https://doi.org/10.1097/FJC.0000000000001183.
Yin DG, Qiu M, Duan XY. Association between SGLT2is and cardiovascular and respiratory diseases: A meta-analysis of large trials. Front Pharmacol. 2021;12:724405. https://doi.org/10.3389/fphar.2021.724405.
Li CX, Liang S, Gao L, Liu H. Cardiovascular outcomes associated with SGLT-2 inhibitors versus other glucose-lowering drugs in patients with type 2 diabetes: A real-world systematic review and meta-analysis. PLoS One. 2021;16(2):e0244689. https://doi.org/10.1371/journal.pone.0244689.
Li WJ, Chen XQ, Xu LL, Li YQ, Luo BH. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol. 2020;19(1):130. https://doi.org/10.1186/s12933-020-01105-5.
Usman MS, Siddiqi TJ, Memon MM, Khan MS, Rawasia WF, Ayub MT, et al. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25(5):495–502. https://doi.org/10.1177/2047487318755531.
Bonora BM, Raschi E, Avogaro A, Fadini GP. SGLT-2 inhibitors and atrial fibrillation in the Food and Drug Administration adverse event reporting system. Cardiovasc Diabetol. 2021;20(1):39. https://doi.org/10.1186/s12933-021-01243-4.
Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010;1(2):57–92. https://doi.org/10.1007/s13300-010-0006-4. Epub 2010 Dec 17.
Vrhovac I, Eror BD, Klessen D, Burger C, Breljak D, Kraus O, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467(9):1881–98. https://doi.org/10.1007/s00424-014-1619-7. Epub 2014 Oct 11.
Di Franco A, Cantini G, Tani A, Coppini R, Zecchi-Orlandini S, Raimondi L, et al. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: A new potential pharmacological target. Int J Cardiol. 2017;243:86–90. https://doi.org/10.1016/j.ijcard.2017.05.032. Epub 2017 May 9.
Khemais-Benkhiat S, Belcastro E, Idris-Khodja N, Park SH, Amoura L, Abbas M, et al. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J Cell Mol Med. 2020;24(3):2109–22. https://doi.org/10.1111/jcmm.14233. Epub 2019 Mar 30.
Wang TJ, Parise H, Levy D, D’Agostino RB Sr, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–7. https://doi.org/10.1001/jama.292.20.2471.
Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. 2010;55(21):2319–27. https://doi.org/10.1016/j.jacc.2010.02.029.
Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. 2015;65(20):2159–69. https://doi.org/10.1016/j.jacc.2015.03.002.
Lee PC, Ganguly S, Goh SY. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes Rev. 2018;19(12):1630–41. https://doi.org/10.1111/obr.12755. Epub 2018 Sep 25.
Chan YH, Chen SW, Chao TF, Kao YW, Huang CY, Chu PH. The impact of weight loss related to risk of new-onset atrial fibrillation in patients with type 2 diabetes mellitus treated with sodium-glucose cotransporter 2 inhibitor. Cardiovasc Diabetol. 2021;20(1):93. https://doi.org/10.1186/s12933-021-01285-8.
Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–31. https://doi.org/10.1210/jc.2011-2260. Epub 2012 Jan 11.
Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18(1):46. https://doi.org/10.1186/s12933-019-0852-y.
Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res. 2014;102(2):205–13. https://doi.org/10.1093/cvr/cvu045. Epub 2014 Mar 18.
Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, et al. Electroanatomical remodeling of the atria in obesity: Impact of adjacent epicardial fat. JACC Clin Electrophysiol. 2018;4(12):1529–40. https://doi.org/10.1016/j.jacep.2018.08.014. Epub 2018 Nov 1.
Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JPM, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66(1):1–11. https://doi.org/10.1016/j.jacc.2015.04.058.
Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2(10):536–43. https://doi.org/10.1038/ncpcardio0319.
Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22(11):450–7. https://doi.org/10.1016/j.tem.2011.07.003. Epub 2011 Aug 16.
Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11(6):363–71. https://doi.org/10.1038/nrendo.2015.58. Epub 2015 Apr 7.
Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. https://doi.org/10.1161/01.CIR.0000099542.57313.C5. Epub 2003 Oct 27.
Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H, et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation. 2012;126(19):2324–34. https://doi.org/10.1161/CIRCULATIONAHA.111.039586. Epub 2012 Oct 12.
Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36(13):795-805a. https://doi.org/10.1093/eurheartj/eht099. Epub 2013 Mar 22.
Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J. 2017;38(1):53–61. https://doi.org/10.1093/eurheartj/ehv625. Epub 2015 Nov 26.
Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71(20):2360–72. https://doi.org/10.1016/j.jacc.2018.03.509.
Wang Q, Xi W, Yin L, Wang J, Shen H, Gao Y, et al. Human epicardial adipose tissue cTGF expression is an independent risk factor for atrial fibrillation and highly associated with atrial fibrosis. Sci Rep. 2018;8(1):3585. https://doi.org/10.1038/s41598-018-21911-y.
Shaihov-Teper O, Ram E, Ballan N, Brzezinski RY, Naftali-Shani N, Masoud R, et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation. 2021;143(25):2475–93. https://doi.org/10.1161/CIRCULATIONAHA.120.052009. Epub 2021 Apr 1.
Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3(4):345–50. https://doi.org/10.1161/CIRCEP.109.912055. Epub 2010 Jun 17.
Oba K, Maeda M, Maimaituxun G, Yamaguchi S, Arasaki O, Fukuda D, et al. Effect of the epicardial adipose tissue volume on the prevalence of paroxysmal and persistent atrial fibrillation. Circ J. 2018;82(7):1778–87. https://doi.org/10.1253/circj.CJ-18-0021. Epub 2018 May 25.
van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. 2018;20(11):1559–66. https://doi.org/10.1002/ejhf.1283. Epub 2018 Aug 1.
Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol. 2020;76(10):1197–1211. https://doi.org/10.1016/j.jacc.2020.07.017.
Couselo-Seijas M, Rodríguez-Mañero M, González-Juanatey JR, Eiras S. Updates on epicardial adipose tissue mechanisms on atrial fibrillation. Obes Rev. 2021;22(9):e13277. https://doi.org/10.1111/obr.13277. Epub 2021 May 17.
Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2017;38(17):1294–1302. https://doi.org/10.1093/eurheartj/ehw045.
Iacobellis G, Gra-Menendez S. Effects of dapagliflozin on epicardial fat thickness in patients with type 2 diabetes and obesity. Obesity (Silver Spring). 2020;28(6):1068–74. https://doi.org/10.1002/oby.22798. Epub 2020 Apr 30.
Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17(1):6. https://doi.org/10.1186/s12933-017-0658-8.
Sato T, Aizawa Y, Yuasa S, Fujita S, Ikeda Y, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume and p-wave indices: An ad-hoc analysis of the previous randomized clinical trial. J Atheroscler Thromb. 2020;27(12):1348–58. https://doi.org/10.5551/jat.48009. Epub 2020 Feb 28.
Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: From the EMPA-TROPISM study. JACC Heart Fail. 2021;9(8):578 – 89. https://doi.org/10.1016/j.jchf.2021.04.014.
Mullens W, Martens P. Empagliflozin-induced changes in epicardial fat: The centerpiece for myocardial protection? JACC Heart Fail. 2021;9(8):590–93. https://doi.org/10.1016/j.jchf.2021.05.006.
Paolillo S, Scardovi AB, Campodonico J. Role of comorbidities in heart failure prognosis Part I: Anaemia, iron deficiency, diabetes, atrial fibrillation. Eur J Prev Cardiol. 2020;27(2_suppl):27–34. https://doi.org/10.1177/2047487320960288.
Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa: Temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484–92. https://doi.org/10.1161/CIRCULATIONAHA.115.018614. Epub 2016 Jan 8.
Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417–27. https://doi.org/10.1056/NEJMoa1707855.
Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, et al. PABA-CHF Investigators. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778–85. https://doi.org/10.1056/NEJMoa0708234.
Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61(18):1894–903. https://doi.org/10.1016/j.jacc.2013.01.069.
Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: Results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637–44. https://doi.org/10.1161/CIRCULATIONAHA.115.019406. Epub 2016 Mar 30.
Pott A, Jäck S, Schweizer C, Baumhardt M, Stephan T, Rattka M, et al. Atrial fibrillation ablation in heart failure patients: improved systolic function after cryoballoon pulmonary vein isolation. ESC Heart Fail. 2020;7(5):2258–67. https://doi.org/10.1002/ehf2.12735. Epub 2020 Jun 24.
Rattka M, Kühberger A, Pott A, Stephan T, Weinmann K, Baumhardt M, et al. Catheter ablation for atrial fibrillation in HFpEF patients-A propensity-score-matched analysis. J Cardiovasc Electrophysiol. 2021;32(9):2357–67. https://doi.org/10.1111/jce.15200. Epub 2021 Aug 18.
Hohendanner F, Heinzel FR, Blaschke F, Pieske BM, Haverkamp W, Boldt HL, et al. Pathophysiological and therapeutic implications in patients with atrial fibrillation and heart failure. Heart Fail Rev. 2018;23(1):27–36. https://doi.org/10.1007/s10741-017-9657-9.
Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schlüter M, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: The randomized AMICA trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. https://doi.org/10.1161/CIRCEP.119.007731. Epub 2019 Nov 25.
Madrid AH, Bueno MG, Rebollo JMG, Marín I, Peña G, Bernal E, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;106(3):331–6. https://doi.org/10.1161/01.cir.0000022665.18619.83.
Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003;107(23):2926–31. https://doi.org/10.1161/01.CIR.0000072793.81076.D4. Epub 2003 May 27.
Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–9. https://doi.org/10.1016/j.jacc.2007.09.064.
Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999;100(4):376–80. https://doi.org/10.1161/01.cir.100.4.376.
Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: An important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39(12):e212-e213. https://doi.org/10.2337/dc16-1312. Epub 2016 Sep 27.
Rau M, Thiele K, Hartmann NK, Schuh A, Altiok E, Möllmann J, et al. Empagliflozin does not change cardiac index nor systemic vascular resistance but rapidly improves left ventricular filling pressure in patients with type 2 diabetes: a randomized controlled study. Cardiovasc Diabetol. 2021;20(1):6. https://doi.org/10.1186/s12933-020-01175-5.
Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):73. https://doi.org/10.1186/s12933-018-0717-9.
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33. https://doi.org/10.1016/j.echo.2008.11.023.
Nagueh SF. Non-invasive assessment of left ventricular filling pressure. Eur J Heart Fail. 2018;20(1):38–48. https://doi.org/10.1002/ejhf.971. Epub 2017 Oct 8.
Costabel JP, Galve E, Terricabras M, Ametrano C, Ronderos R, Baranchuk A, et al. E/e’ ratio and left atrial area are predictors of atrial fibrillation in patients with hypertrophic cardiomyopathy. Echocardiography. 2018;35(7):935–40. https://doi.org/10.1111/echo.13857 Epub 2018Mar 5.
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–66. https://doi.org/10.1056/NEJM199809033391003.
Chang SL, Chen YC, Chen YJ, Wangcharoen W, Lee SH, Lin CI, et al. Mechanoelectrical feedback regulates the arrhythmogenic activity of pulmonary veins. Heart. 2007;93(1):82–8. https://doi.org/10.1136/hrt.2006.089359. Epub 2006 Aug 11.
Chan CS, Lin YK, Chen YC, Lu YY, Chen SA, Chen YJ. Heart failure differentially modulates natural (sinoatrial node) and ectopic (pulmonary veins) pacemakers: Mechanism and therapeutic implication for atrial fibrillation. Int J Mol Sci. 2019;20(13):3224. https://doi.org/10.3390/ijms20133224.
Tsao HM, Yu WC, Cheng HC, Wu MH, Tai CT, Lin WS, et al. Pulmonary vein dilation in patients with atrial fibrillation: detection by magnetic resonance imaging. J Cardiovasc Electrophysiol. 2001;12(7):809–13. https://doi.org/10.1046/j.1540-8167.2001.00809.x.
Kim S, Kim YH, Lee SH, Kim JS. Pulmonary vein enlargement as an independent predictor for new-onset atrial fibrillation. J Clin Med. 2020;9(2):401. https://doi.org/10.3390/jcm9020401. PMID: 32024250; PMCID: PMC7074413.
Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20(3):479–487. https://doi.org/10.1111/dom.13126. Epub 2017 Nov 15. PMID: 29024278.
Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190–5. https://doi.org/10.2337/db15-1356. Epub 2016 Feb 9.
Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931–44. https://doi.org/10.1016/j.jacc.2019.01.056.
Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J. 2020;41(36):3421–32. https://doi.org/10.1093/eurheartj/ehaa419.
Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(4):422–434. https://doi.org/10.1016/j.jacc.2019.11.031. Erratum in: J Am Coll Cardiol. 2020 Sep 22;76(12):1505.
Perry RJ, Shulman GI. Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem. 2020;295(42):14379–90. https://doi.org/10.1074/jbc.REV120.008387. Epub 2020 Aug 12.
Brown E, Wilding JPH, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2021;53(1):2072–89. https://doi.org/10.1080/07853890.2020.1841281.
Rasalam R, Atherton JJ, Deed G, Molloy-Bland M, Cohen N, Sindone A. Sodium-glucose cotransporter 2 inhibitor effects on heart failure hospitalization and cardiac function: systematic review. ESC Heart Fail. 2021;8(5):4093–4118. https://doi.org/10.1002/ehf2.13483. Epub 2021 Jul 5.
Pabel S, Hamdani N, Singh J, Sossalla S. Potential mechanisms of SGLT2 inhibitors for the treatment of heart failure with preserved ejection fraction. Front Physiol. 2021;12:752370. https://doi.org/10.3389/fphys.2021.752370.
Wu VC, Li YR, Wang CY. Impact of sodium-glucose co-transporter 2 inhibitors on cardiac protection. Int J Mol Sci. 2021;22(13):7170. https://doi.org/10.3390/ijms22137170.
Bell DSH, Goncalves E. Atrial fibrillation and type 2 diabetes: Prevalence, etiology, pathophysiology and effect of anti-diabetic therapies. Diabetes Obes Metab. 2019;21(2):210–17. https://doi.org/10.1111/dom.13512. Epub 2018 Sep 25.
Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol. 2014;114(8):1217–22. https://doi.org/10.1016/j.amjcard.2014.07.045. Epub 2014 Jul 30.
Emdin CA, Anderson SG, Salimi-Khorshidi G, Woodward M, MacMahon S, Dwyer T, et al. Usual blood pressure, atrial fibrillation and vascular risk: evidence from 4.3 million adults. Int J Epidemiol. 2017;46(1):162–172. https://doi.org/10.1093/ije/dyw053.
Soliman EZ, Rahman AF, Zhang ZM, Rodriguez CJ, Chang TI, Bates JT, et al. Effect of Intensive Blood Pressure Lowering on the Risk of Atrial Fibrillation. Hypertension. 2020;75(6):1491–96. https://doi.org/10.1161/HYPERTENSIONAHA.120.14766. Epub 2020 May 4.
Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):262-75.e9. https://doi.org/10.1016/j.jash.2014.01.007 Epub 2014 Jan 26.
Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Miyata M, Kawasoe M, et al. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol. 2017;18(1):23. https://doi.org/10.1186/s40360-017-0125-x.
Trum M, Riechel J, Lebek S, Pabel S, Sossalla ST, Hirt S, et al. Empagliflozin inhibits Na+ /H+ exchanger activity in human atrial cardiomyocytes. ESC Heart Fail. 2020;7(6):4429–37. https://doi.org/10.1002/ehf2.13024.
Uthman L, Baartscheer A, Schumacher CA, Fiolet JWT, Kuschma MC, Hollmann MW, et al. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol. 2018;9:1575. https://doi.org/10.3389/fphys.2018.01575.
Scholz W, Albus U, Counillon L, Gögelein H, Lang HJ, Linz W, et al. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res. 1995;29(2):260–8.
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na + and vasodilation. Diabetologia. 2018;61(3):722–26. https://doi.org/10.1007/s00125-017-4509-7. Epub 2017 Dec 2.
Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K. The myocardial Na(+)-H(+) exchange: structure, regulation, and its role in heart disease. Circ Res. 1999;85(9):777–86. https://doi.org/10.1161/01.res.85.9.777.
Madonna R, De Caterina R. Sodium-hydrogen exchangers (NHE) in human cardiovascular diseases: interfering strategies and their therapeutic applications. Vascul Pharmacol. 2013;59(5–6):127–30. https://doi.org/10.1016/j.vph.2013.10.001. Epub 2013 Oct 15.
Peng X, Li L, Zhang M, et al. Sodium-Glucose Cotransporter 2 Inhibitors Potentially Prevent Atrial Fibrillation by Ameliorating Ion Handling and Mitochondrial Dysfunction. Front Physiol. 2020;11:912. https://doi.org/10.3389/fphys.2020.00912. eCollection 2020.
Jayachandran JV, Zipes DP, Weksler J, Olgin, JE. Role of the Na+/H+ exchanger in short-term atrial electrophysiological remodeling. Circulation. 2000;101(15):1861–6. https://doi.org/10.1161/01.cir.101.15.1861.
Shinagawa K, Mitamura H, Ogawa S, Nattel S. Effects of inhibiting Na+/H+-exchange or angiotensin converting enzyme on atrial tachycardia-induced remodeling. Cardiovasc Res. 2002;54(2):438–46. https://doi.org/10.1016/s0008-6363(01)00515-6.
Blaauw Y, Beier N, van der Voort P, van Hunnik A, Schotten U, Allessie MA. Inhibitors of the Na+/H+ exchanger cannot prevent atrial electrical remodeling in the goat. J Cardiovasc Electrophysiol. 2004;15(4):440–6. https://doi.org/10.1046/j.1540-8167.2004.03498.x.
Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z et al. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18(1):165. https://doi.org/10.1186/s12933-019-0964-4.
Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol 2009;54(20):1891–8. https://doi.org/10.1016/j.jacc.2009.07.031.
Linz D, Elliott AD, Marwick TH, Sanders P. Biomarkers and new-onset atrial fibrillation to assess atrial cardiomyopathy. Int J Cardiol. 2017;248:208–10. https://doi.org/10.1016/j.ijcard.2017.08.031.
Lazzerini PE, Laghi-Pasini F, Boutjdir M, Capecchi PL. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19:63–64. https://doi.org/10.1038/s41577-018-0098-z.
Sawaya SE, Rajawat YS, Rami TG, Szalai G, Price RL, Sivasubramanian N, et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am. J. Physiol. Heart Circ. Physiol 2007; 292(3), H1561–7. https://doi.org/10.1152/ajpheart.00285.2006. Epub 2006 Nov 22.
Sun BJ, Park JH. Echocardiographic measurement of left atrial strain-A key requirement in clinical practice. Circ J. 2021;86(1):6–13. https://doi.org/10.1253/circj.CJ-21-0373.
Peters DC, Lamy J, Sinusas AJ, Baldassarre LA. Left atrial evaluation by cardiovascular magnetic resonance: sensitive and unique biomarkers. Eur Heart J Cardiovasc Imaging. 2021;23(1):14–30. https://doi.org/10.1093/ehjci/jeab221.
Kurata M, Asano T, Mori H, Mase H, Nagumo S, Wakatsuki D, et al. Can an increase in the pulmonary vein volume measured by three dimensional computed tomography predict the presence of atrial fibrillation? J Arrhythm. 2019;35(2):230–37. https://doi.org/10.1002/joa3.12158.
López B, González A, Ravassa S, Beaumont J, Moreno MU, San José G et al. Circulating biomarkers of myocardial fibrosis: The need for a reappraisal. J Am Coll Cardiol. 2015;65(22):2449–56. https://doi.org/10.1016/j.jacc.2015.04.026.
Duprez DA, Gross MD, Kizer JR, Ix JH, Hundley WG, Jacobs DR Jr. Predictive value of collagen biomarkers for heart failure with and without preserved ejection fraction: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(5):e007885. https://doi.org/10.1161/JAHA.117.007885.
Wu N, Xu B, Xiang Y, Wu L, Zhang Y, Ma X, et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol. 2013;169(1):62–72. 10.1016/j.ijcard.2013.08.078. Epub 2013 Sep 8.
Acknowledgements
Special acknowledgement to Dr. John Fisher for his feedback and proofreading of this review.
Funding
None.
Author information
Authors and Affiliations
Contributions
SS performed the literature review and was a major contributor in manuscript writing. AK provided guidance regarding the supporting bodies of the manuscript and assisted with manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shetty, S.S., Krumerman, A. Putative protective effects of sodium-glucose cotransporter 2 inhibitors on atrial fibrillation through risk factor modulation and off-target actions: potential mechanisms and future directions. Cardiovasc Diabetol 21, 119 (2022). https://doi.org/10.1186/s12933-022-01552-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01552-2