Abstract
Objective
Admission hyperglycemia is associated with poor prognosis in patients with acute myocardial infarction (AMI), but the effects of baseline diabetes status on this association remain elusive. We aim to investigate the impact of admission hyperglycemia on short and long-term outcomes in diabetic and non-diabetic AMI patients.
Methods
In this retrospective cohort study, 3330 patients with regard to first-time AMI between July 2012 and July 2020 were identified. Participants were divided into two groups according to diabetes status (1060 diabetic patients and 2270 non-diabetic patients). Thereafter, they were divided into four groups according to diabetes status-specific cutoff values of fasting blood glucose (FBG) identified by restricted cubic spline. Short-term outcomes included in-hospital death and cardiac complications. Long-term outcomes were all-cause mortality and major adverse cardiovascular events (MACE). Inverse probability of treatment weighting (IPTW) was conducted to adjust for baseline differences among the groups, followed by a weighted Cox proportional hazards regression analysis to calculate hazard ratios and 95% confidence intervals for all-cause mortality associated with each FBG category. Subgroup analysis and sensitivity analysis were performed to test the robustness of our findings.
Results
During a median follow-up of 3.2 years, 837 patients died. There was a significant interaction between diabetes status and FBG levels for all-cause mortality during long-term follow-up (p-interaction < 0.001). Moreover, restricted cubic spline curves for the association between FBG and all-cause mortality followed a J shape in patients with diabetes and a non-linear in patients without diabetes. Kaplan–Meier analysis demonstrated greater survival in non-hyperglycemia patients compared to hyperglycemia patients for both diabetic and non-diabetic patients groups. Survival of hyperglycemia patients without diabetes greater than in hyperglycemia patients with diabetes. In the weighted Multivariable cox analysis, admission hyperglycemia predicted higher short and long-term mortality. Subgroup analysis and sensitivity analysis showed the robustness of the results.
Conclusions
The inflection points of FBG level for poor prognosis were 5.60 mmol/L for patients without diabetes and 10.60 mmol/L for patients with diabetes. Admission hyperglycemia was identified as an independent predictor of worse short and long-term outcomes in AMI patients, with or without diabetes. These findings should be explored further.
Similar content being viewed by others
Introduction
Hyperglycemia during hospital admission is common in patients with AMI and independently associated with worse prognosis [1,2,3,4], although the association may be nonlinear [5], and data conflict as to whether this association varies by diabetes status [4, 6, 7]. Admission hyperglycemia occurs in 25–50% of patients, depending on the definition of admission hyperglycemia [8]. There is still no consensus on what blood glucose level defines admission hyperglycemia. It is well known that diabetes is a common comorbidity in patients with cardiovascular diseases [9]. Patients with AMI and diabetes show a more than two-fold higher risk for short and long-term mortality than patients without diabetes [10, 11]. Previous studies showed a stronger association between a diagnosis of clinical diabetes and incident mortality in hyperglycemia patients than non-hyperglycemia patients without diabetes when using the same prognostic cutoff value for both diabetic and non-diabetic patients [1]. Contributors to such diabetes status-based differences are not clear, although disparities in the prevalence of uncontrolled blood glucose and mortality are a possibility. Data are also lacking on diabetes status differences in the prognostic relevance of the blood glucose levels for defining admission hyperglycemia.
Although less is known about the association between admission hyperglycemia and mortality by diabetes status, recent studies demonstrated that admission hyperglycemia was an independent predictor of mortality in AMI patients without diabetes when used the same or different cutoff values for diabetic and non-diabetic patients [4, 7]. Data are lacking on diabetes status differences in absolute measures of mortality risk associated with admission hyperglycemia. Therefore, there is a critical need to take patients’ diabetes status into account to avoid incorrect estimation of the real prevalence of admission hyperglycemia.
Studies evaluating diabetes status-based differences in mortality risk associated with admission hyperglycemia have not used the optimal cutoff values of FBG separately for diabetic and non-diabetic patients or demonstrated whether differences in mortality risk persist across varying levels of FBG. Thus, we investigated the severity of hyperglycemia by diabetes status subgroups, differences in the risk of incident mortality across increasing levels of FBG between diabetes status subgroups, and evaluated FBG cutoff values in patients with and without diabetes to establish their value in predicting the short and long-term prognosis of patients with AMI.
Methods
Study design and population
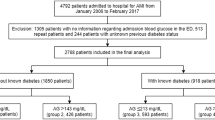
This retrospective, population-based cohort study included patients who were admitted to the chest pain center in the First People’s Hospital of Kashi Prefecture for their first AMI between July 2012 and July 2020. AMI was defined according to the current European guidelines [12], as non-ST-elevation myocardial infarction (NSTEMI) or ST-elevation myocardial infarction (STEMI). Exclusion criteria encompassed patients with previous myocardial infarction, missing crucial laboratory data (FBG on admission or glycated hemoglobin [HbA1c]), severe valvular heart diseases, severe renal failure, or tumors. This study was approved by the local institutional review board of First People’s Hospital of Kashi Prefecture (Approval No. 2021-03). All participants provided written informed consent.
Data collection and definitions
Demographic and clinical data were collected at baseline through electrical medical records review. Demographic data included age, sex, body mass index (BMI), ethnicity. smoking and drinking habits, and family history of cardiovascular disease. Clinical data consisted of diagnosis, medical history, laboratory test results, medications at discharge, and clinical therapies. The FBG levels were assessed within 24 h of admission. Pre-existing diabetes mellitus was defined as a known reported history of diabetes at admission, either treated with diet and lifestyle measures alone or with the additional use of oral glucose-lowering medications and insulin. Newly diagnosed diabetes was defined based on the oral glucose tolerance test, fasting glucose test or glycated hemoglobin ≥ 6.5% during hospitalization. Patients without a history of diabetes and with HbA1c < 6.5% mmol/mol were considered non-diabetic. Pre-existing diabetes mellitus with admission glucose level > 20 mmol/L received insulin–glucose infusion to decrease blood glucose between 7 and 15 mmol/L for the first 24 h, followed by subcutaneous insulin injections. Newly diagnosed diabetes and non-diabetic patients were not received glucose-lowering treatment for the first 24 h. Hyperglycemia was defined as FBG levels ≥ 5.6 mmol/L for non-diabetic patients or ≥ 10.6 mmol/L for diabetic patients.
Follow-up and outcomes
Patients’ follow-up data, including survival data and clinical event data, were obtained by review of all available medical records, personal communication with the patient’s physician, and a telephone interview with the patient or a patient's close relative conducted by trained personnel. Short-term outcomes included in-hospital death and cardiac complications (ventricular fibrillation, cardiogenic shock, atrial fibrillation, heart failure, and arrhythmia). Long-term outcomes were all-cause mortality and MACE [cardiovascular mortality, re-hospitalization for AMI, target vessel revascularization (TVR), heart failure, and stroke].
Statistical analyses
Baseline characteristics of the patients by categorical FBG level were summarized using descriptive statistics (frequencies with proportions or means with SDs as appropriate). Demographic and clinical characteristics were compared using the chi-square test for categorical variables and the Kruskal–Wallis test for continuous variables. Missing values at baseline were imputed by using the Multivariate Imputation by Chained Equations (MICE) package in python using random forest imputations. The median follow-up time was estimated by using the reverse Kaplan Meier method.
Cox proportional hazard models were used to assess the association between FBG and mortality, both with FBG as a categorical variable (to account for the glycemic threshold) and then as a restricted cubic spline, to explore a potential nonlinear relationship between FBG and mortality.
FBG as a restricted cubic spline
For FBG as a restricted cubic spline, we performed Cox proportional hazards regression with mortality as the outcome. The relative hazards of mortality (with FBG of 5.6 mmol/L for non-diabetic and 10.6 mmol/L for diabetic patients as reference) were graphed, stratified by diabetes status. Splines were modeled by restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles.
FBG as a categorical variable
For FBG as a categorical variable, the 4 categories of FBG were chosen a priori based on the optimal cutoff values determined by our restricted cubic spline analysis: non-hyperglycemia patients without diabetes (< 5.6 mmol/L), hyperglycemia patients without diabetes (≥ 5.6 mmol/), non-hyperglycemia patients with diabetes (< 10.6 mmol/L), and hyperglycemia patients with diabetes (≥ 10.6 mmol/L). Kaplan–Meier survival curves were used for survival analysis. We applied IPTW to the Cox models for all-cause mortality to adjust for baseline differences. The treatment probabilities were calculated from a logistic regression using a set of covariates deemed to have affected baseline differences, including patient characteristics, medical history, baseline assessments Systolic, laboratory values, revascularization, and medications. Hazard ratios (HRs) or odds ratios (ORs) with 95% confidence intervals (CIs) for the outcomes according to FBG categories were calculated using Cox proportional hazards models, all of which were inverse probability of treatment weighted. Model 1 included FBG categories only. Model 2 included FBG categories, age (≤ 55 and > 55 years), gender, ethnic, hypertension, diagnosis (NSTEMI and STEMI), Killip class (< II and ≥ II), and EF (< 40 and ≥ 40). Model 3 included FBG categories, age, gender, Killip class, ethnic, drinking, hypertension, COPD, liver disease, lung disease, diagnosis, EF, PCI, CABG, ACE inhibitor/ARB, and beta-blocker. These variables were the multiple Cox proportional hazard model, derived from the LASSO method and stepwise backward selection procedures. We performed tests for linear trends by entering the median value of each category of FBG level as a continuous variable in the models.
In addition, to examine the presence or absence of covariates differences in outcomes, we conducted subgroup analyses by age (≤ 55 and > 55 years), gender (male and female), ethnic (Uyghur and Han), hypertension (no and yes), diagnosis (NSTEMI and STEMI), Killip class (< II and ≥ II), and EF (< 40 and ≥ 40). Tests of interaction were used to assess the differences in FBG levels across these subgroups. To assess the robustness of the study findings, we conducted a series of sensitivity analyses. First, nonlinear associations between FBG and all-cause mortality were further examined using restricted cubic splines with multivariable-adjusted Cox proportional hazards models. Second, the first sensitivity analysis was repeated with the time to MACE instead of time to mortality. Third, we performed the restricted cubic spline analysis after excluding the participants who died during hospitalization (n = 221). Fourth, we conducted Cox proportional hazard models without applying IPTW. Models with significant interaction terms were visualized using the visreg package. All reported p-values were 2-tailed, and a p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS statistics (version 25), R (version 4.1.2), and Python (version 3.10.0).
Results
FBG level categorization
There was a significant interaction between diabetes status and increasing FBG levels on all-cause mortality (p < 0.001), with higher FBG levels being associated with greater all-cause mortality among non-diabetic than among diabetic patients (Additional file 1: Figure S2). Therefore, these analyses were stratified according to diabetes status and defined diabetes status-specific FBG cutoff values. Restricted cubic spline analysis was performed to evaluate cutoff values for FBG levels associated with all-cause mortality in patients with and without diabetes (Fig. 1). The risk of all-cause mortality was relatively flat and the hazard ratio was < 1 until around 5.60 mmol/L (100 mg/dL) of predicted FBG level and then started to increase rapidly afterwards (p for overall < 0.001 and p for non-linearity < 0.238) in patients without diabetes. Above 5.6 mmol/L, the hazard ratio per standard deviation higher predicted all-cause mortality was 1.19 (1.09–1.31). We observed a J shaped association between FBG and all-cause mortality in patients with diabetes, the plot showed a substantial reduction of the risk within the lower range of predicted FBG level, which reached the lowest risk around 10.6 mmol/L (190 mg/dL) and then increased thereafter (p for overall < 0.001 and p for non-linearity < 0.001). Above 10.6 mmol/L, the hazard ratio per standard deviation higher predicted all-cause mortality was 1.27 (1.10–1.46). Accordingly, we chose FBG of 5.60 mmol/L for patients without diabetes and 10.60 mmol/L for patients with diabetes as convenient cutoff values to classify patients into four groups.
Hazard Ratios of All-Cause Mortality According to FBG levels in AMI patients. A non-diabetic patients and all-cause mortality. B diabetic patients and all-cause mortality. Solid red lines are hazard ratios and dashed lines show 95% confidence intervals based on restricted cubic spline regressions. Reference line for no association (hazard ratio: 1.0) is indicated by dashed grey line while areas of purple show fraction of population at different FBG concentrations. The red points for hazard ratio = 1
Baseline characteristics
A total of 3330 patients with first-time AMI were enrolled in our study. The mean age of participants was 56.3 (SD 12.3) years, 78% of participants were male, 87% of participants were Uyghur, and 32% of participants were diabetes. Among the patients, 1091 (33%) were classified as non-hyperglycemia without diabetes, 1179 (35%) as hyperglycemia without diabetes, 635 (19%) as non-hyperglycemia with diabetes, and 425 (13%) as hyperglycemia with diabetes (Additional file 1: Fig. S1).
The patient characteristics were compared between groups categorized by FBG level (Table 1). Among both diabetic and non-diabetic patients, hyperglycemia patients tended to have higher heart rate, Killip class, procalcitonin, c-reactive protein, triglyceride, HDL cholesterol, LDL cholesterol, apolipoprotein A, and peak hs Troponin I. Among the non-diabetic patients, total cholesterol was lower in hyperglycemia patients, while hyperglycemia patients had higher in diabetic patients. In the non-diabetic patients, higher uric acid and a greater prevalence of STEMI were more often observed in hyperglycemia patients, while no statistically significant differences were detected in the diabetic patients. When comparing the two hyperglycemia subgroups, hyperglycemia patients without diabetes tended to have more frequent of younger, men, former smokers, and STEMI; were more likely to have less frequent of hyperglycemia, liver disease, and lung disease; exhibited lower Killip class, heart rate, procalcitonin, c-reactive protein, and triglyceride; had greater Left ventricular ejection fraction value, haemoglobin, glomerular filtration rate, HDL cholesterol, apolipoprotein A, uric acid, and peak hs Troponin I.
FBG and clinical outcomes
During hospitalization, 221 deaths occurred. Among both diabetic and non-diabetic patients, in-hospital death and cardiogenic shock occurred more frequently among hyperglycemia patients (p < 0.01). In the non-diabetic patients, hyperglycemia patients exhibited a greater arrhythmogenic burden (atrial fibrillation and ventricular arrhythmias) during hospitalization when compared to non-hyperglycemia patients (p < 0.01). In-hospital death, heart failure, and cardiogenic shock occurred more frequently among hyperglycemia patients with diabetes compared to hyperglycemia patients without diabetes (p < 0.01, Fig. 2).
During a median follow-up period of 3.2 years, 837 deaths (756 from cardiovascular causes) and 1600 MACE were recorded. Kaplan–Meier survival analysis showed a significant difference in the incidence of all-cause mortality and MACE among the four groups(p < 0.001). In non-diabetic patients, all-cause mortality occurred more frequently among hyperglycemia patients (p = 0.003). In both non-diabetic and diabetic patients, MACE occurred more frequently among hyperglycemia patients (p = 0.053 and p = 0.018). When comparing the two hyperglycemic subgroups, all-cause mortality and MACE were more often observed in hyperglycemia patients with diabetes (p < 0.001, Fig. 2).
IPTW Cox models were performed to calculate the HRs of mortality across FBG categories. In a weighted univariable Cox model, hyperglycemia (non-diabetic patients: HR 2.26, 95% CI 1.35–3.79; diabetic patients: HR 1.70, 95% CI 1.16–2.49) was a significant predictor of short-term mortality (in-hospital death). As for the long-term outcome, hyperglycemia (non-diabetic patients: 1.23; 95% CI 1.01–1.49; diabetic patients: HR 1.23, 95% CI 1.00–1.58) was identified as an independent predictor of long-term mortality (all-cause mortality). Additional adjustment for age, gender, ethnic, hypertension, diagnosis, Killip class, and EF (model 2) or the clinical variables from the multiple Cox proportional hazard model (c-indices of the model was 0.81) (model 3) did not alter the significance of the results. Moreover, there was a consistent dose–response relationship between the FBG and short and long-term mortality (p for trend < 0.001 for all) (Fig. 3).
Risk for short and long-term mortality according to FBG levels. All models were inverse probability of treatment weighted (IPTW). IPTW included all the clinical variables listed in Table 1. Model 1 included FBG categories only. Model 2 included FBG categories, age, gender, ethnic, hypertension, diagnosis, Killip class, and EF. Model 3 included FBG categories, age, gender, Killip class, ethnic, drinking, hypertension, COPD, liver disease, lung disease, diagnosis, EF, PCI, CABG, ACE inhibitor/ARB, and beta-blocker. CI confidence interval; HR hazard ratio. Test for trend based on variable containing median value for each quintile
Subgroup and sensitivity analysis
Subgroup analyses of risk for all-cause mortality based on demographics and clinical characteristics and FBG categories showed consistent results across different subgroups (p for interaction > 0.05 for all, Additional file 1: Fig. S3).
Multivariable-adjusted restricted cubic splines showed similar trends for continuous levels of FBG and risk of all-cause mortality and MACE among patients with and without diabetes. Reimplementing Multivariable-adjusted restricted cubic spline analysis after excluding participants who died during hospitalization resulted in a similar shaped association between FBG and all-cause mortality among patients with and without diabetes (Additional file 1: Fig. S4). Multivariable analysis without IPTW also yielded consistent results with those of the main analyses (Additional file 1: Fig. S5).
Discussion
We investigated the diabetes status-specific risks of mortality across increasing levels of FBG, a key glycemic maker in identifying a group of “high-risk” patients who could probably benefit from a proper secondary prevention medical therapy. When treating FBG as a restricted cubic spline term, our findings demonstrated that the association between FBG and all-cause mortality followed a J shape in patients with diabetes and a non-linear in patients without diabetes. The HRs of mortality significantly increased when FBG levels ≥ 5.60 mmol/L for patients without diabetes and ≥ 10.60 mmol/L for patients with diabetes. Based on FBG categories, our findings demonstrated that admission hyperglycemia was independently associated with short and long-term outcomes in AMI patients, regardless of diabetes status.
In this study, we observed that elevated fasting blood glucose in AMI has a strong association with worse outcomes. Previous studies have primarily focused on the prognosis value of admission hyperglycemia in both patients with and without diabetes [1,2,3,4]. Some studies showed that persisting hyperglycaemia was a more accurate and stronger independent predictor of risk than admission blood glucose. Hyperglycaemia (blood glucose ≥ 8.9 mmol/L), persists from admission to at least 24 h after symptom onset, is associated both with reduced myocardial perfusion despite patency of the infarct-related artery and with pre-discharge left ventricular impairment [13]. In another study, persistent hyperglycemia in myocardial infarction has a stronger relation with 30-day MACE than elevated glucose at admission [14]. Fasting glucose was superior to admission glucose with regard to 30-day mortality in previous study [15]. The superiority of FBG over random glucose levels in predicting outcome probably results from factors such as differences in the amount of caloric intake and time since the last meal. In a recent study, random blood glucose and FBG were positively correlated with the Gensini score in AMI patients, and FBG was an independent risk factor for the Gensini score in AMI patients [16]. These findings demonstrate that acute and continuing elevation of blood glucose, rather than an underlying “diabetic state”, may promote microvascular dysfunction, contributing to poorer outcomes. In 1219 non-diabetic patients after AMI, higher admission glucose concentrations predicted greater mortality, while larger reductions in glucose over 24 h predicted lower 30-day mortality. Baseline glucose and the 24-h change in glucose remained significant predictors of 180-day death [17]. Therefore, we considered that FBG levels at admission may better represent the continuing elevation of blood glucose and a greater potential predictor of clinical outcomes in AMI patients.
There are no accurate cutoff values of blood glucose in AMI patients to predict adverse events or accurate definition for admission hyperglycemia with AMI. Prior studies elevated that Stress Hyperglycaemia Ratio (SHR), hemoglobin a1c (HbA1c), and glucose-HbA1c-ratio (GHR) were reported as predictors of clinical outcomes in AMI and other pathological processes. Still, their optimal values were not clarified as thresholds have been arbitrarily selected [4, 18, 19]. In this study, we found a significant interaction between diabetes status and increasing FBG levels on all-cause mortality. This finding is similar to that of a previous study and found a significant interaction between diabetes status and increasing blood glucose levels on mortality. We also identified the nonlinear association between the FBG and all-cause mortality in patients with or without diabetes. The optimal cutoff values for FBG levels were 10.6 mmol/L for diabetic patients and 5.6 mmol/L for non-diabetic patients, above which there was a statistically significant increase in mortality. Our study corroborates these findings that there are different cutoff values for blood glucose to predict outcomes depending on the diabetes status [2, 7].
This study demonstrated that patients with higher FBG levels (≥ 10.6 mmol/L for diabetic patients and ≥ 5.6 mmol/L for non-diabetic patients) had higher heart rate, Killip class, procalcitonin, c-reactive protein, triglyceride, HDL cholesterol, LDL cholesterol, apolipoprotein A, and a greater prevalence of in-hospital death and cardiogenic shock than those with lower FBG levels. The finding indices are paralleled by a higher GRACE score observed in hyperglycemic subjects, both diabetic and non-diabetic groups. Our findings are consistent with those of previous studies, which whether patients are diagnosed with diabetes or not, elevated blood glucose concentrations at the time of hospital admission are independently associated with a higher risk of in-hospital mortality and in-hospital complications, such as cardiogenic shock, and arrhythmia in hyperglycemia patients compared with non-hyperglycemia patients without diabetes [1, 20,21,22]. Several explanations have been proposed for the association between hyperglycemia and adverse events, including hyperglycemic status, or diabetes, affects the pro-inflammatory/oxidative properties and the pro-thrombotic properties in the arterial plaque lesion [23, 24].
In terms of long-term prognosis, hyperglycemia patients in both diabetic and non-diabetic groups exhibited a higher rate of all-cause and MACE. Similarly, hyperglycemia patients with diabetes had significantly higher rates of heart failure [25] and macrovascular complications (stroke and re-AMI). These results might confirm recent studies which demonstrated that Over-inflammation, chronic or acute hyperglycemia, insulin resistance, hyperinsulinemia, and dyslipidemia in T2DM patients, together with decreased levels of high density lipoprotein (HDL) and increased levels of low density lipoprotein (LDL), represent the triggering causes of endothelial disfunction (i.e., impaired balance between the vasoconstriction and vasodilatory properties of endothelium) [26, 27]. The management of hyperglycemic patients during AMI is unclear. An earlier study demonstrated a positive effect of tight glycaemic control, associated to the achievement of glucose target, compared to standard therapy on myocardial injury in hyperglycaemic patients addressed to pPCI [28]. A recent study reported that thrombus aspiration during the primary percutaneous intervention (pPCI) for STEMI reduced clinical outcomes in hyperglycaemic patients, whereas it did not in normoglycaemic ones [24].
We found that hyperglycemia was an independent predictor of all-cause mortality during long-term follow-up which corroborates the key finding from a recent report based on the optimal values (140 mg/dL) for glucose in patients with and without diabetes [4]. Similar findings have also been reported admission hyperglycemia is an independent predictor of in-hospital mortality (admission hyperglycemia defined as ≥ 200 mg/dL) and long-term prognosis (the optimal FBG cutoff values were 14.80 and 6.77 mmol/L for patients with and without diabetes, respectively) in non-diabetic patients after AMI. However, those studies had conflicting results in patients with diabetes, no difference was observed between the hyperglycemic and non-hyperglycemic groups, which might be due to the small sample size or low cutoff value [4, 7].
Study strengths and limitations
A major strength of our study is the report of both relative estimates of the association between elevated FBG and mortality in diabetic status subgroups as well as absolute mortality risks according to diabetes status and specific FBG levels. To our knowledge, this is the first study verifying the J-shaped or nonlinear association between the FBG and clinical outcomes in AMI patients with and without diabetes. Participants were classified into 4 groups based on the optimal cutoff values of FBG. In addition, we were able to adjust for a wide range of baseline covariates using the IPTW method. Last, short and long-term outcomes were analyzed simultaneously, all of which yielded consistent results. Subgroup analysis, as well as sensitivity analysis, further supported the robustness of our findings. Our study has several limitations. First, due to the long enrollment period, guidelines and recommendations on diagnosis and treatment of AMI have changed over the years which might also influence results of the outcomes. Second, the FBG levels were measured after the PCI procedure in a very small subset of AMI patients. Third, admission hyperglycemia levels may have been influenced by multiple factors such as last meal composition and timing and day versus night measurements.
Conclusions
According to diabetes status and specific fasting blood glucose levels, our findings demonstrated admission hyperglycemia is an important predictor of short and long-term outcomes in AMI patients, regardless of diabetes status. Subgroup-specific measurements of the relative increase in mortality risk are critical to understanding potential diabetes status differences in the relative contribution of disordered glucose metabolism to mortality risk after AMI. Further research is need to identify how such differences might be incorporated into clinical guidelines the use of diabetes status-specific treatment recommendations across the spectrum of FBG.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMI:
-
Acute myocardial infarction
- FBG:
-
Fasting blood glucose
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- IPTW:
-
Inverse probability of treatment weighted
- LVEF:
-
Left ventricular ejection fraction
- STEMI:
-
ST-segment elevation myocardial infarction
- CVD:
-
Cardiovascular disease
- COPD:
-
Chronic obstructive pulmonary disease
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- LAD:
-
Left anterior descending coronary
- LCX:
-
Left circumflex artery
- RCA:
-
Right coronary artery
- PCI:
-
Percutaneous coronary intervention
- CABG:
-
Coronary artery bypass grafting
- ACEI:
-
Angiotensin-converting enzyme inhibitor
- ARB:
-
Angiotensin-receptor blocker
References
Paolisso P, Foà A, Bergamaschi L, Angeli F, Fabrizio M, Donati F, et al. Impact of admission hyperglycemia on short and long-term prognosis in acute myocardial infarction: MINOCA versus MIOCA. Cardiovasc Diabetol. 2021;20:1–10. https://doi.org/10.1186/s12933-021-01384-6.
Sia CH, Chan MHH, Zheng H, Ko J, Ho AFW, Chong J, et al. Optimal glucose, HbA1c, glucose-HbA1c ratio and stress-hyperglycaemia ratio cut-off values for predicting 1-year mortality in diabetic and non-diabetic acute myocardial infarction patients. Cardiovasc Diabetol. 2021;20:1–14. https://doi.org/10.1186/s12933-021-01395-3.
Ferreira JA, Baptista RM, Monteiro SR, Gonçalves FM, Monteiro PF, Gonçalves LM. Admission hyperglycemia and all-cause mortality in diabetic and non-diabetic patients with acute myocardial infarction: a tertiary center analysis. Intern Emerg Med. 2021;16:2109–19. https://doi.org/10.1007/s11739-021-02693-0.
Kim EJ, Jeong MH, Kim JH, Ahn TH, Seung KB, Oh DJ, et al. Clinical impact of admission hyperglycemia on in-hospital mortality in acute myocardial infarction patients. Int J Cardiol. 2017;236:9–15. https://doi.org/10.1016/j.ijcard.2017.01.095.
Cid-Alvarez B, Gude F, Cadarso-Suarez C, Gonzalez-Babarro E, Rodriguez-Alvarez MX, Garcia-Acuna JM, et al. Admission and fasting plasma glucose for estimating risk of death of diabetic and nondiabetic patients with acute coronary syndrome: nonlinearity of hazard ratios and time-dependent comparison. Am Heart J. 2009;158:989–97. https://doi.org/10.1016/j.ahj.2009.10.004.
Marenzi G, Cosentino N, Milazzo V, De MM, Cecere M, Mosca S, et al. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diabetes Care. 2018;41:847–53.
Yan CC, Gang ZM, Chao CL, Ye T, Mei ZY, Zhu F, et al. Admission hyperglycemia as an independent predictor of long-term prognosis in acute myocardial infarction patients without diabetes: a retrospective study. J Diabetes Investig. 2021;12:1244–51.
Chakrabarti AK, Singh P, Gopalakrishnan L, Kumar V, Elizabeth Doherty M, Abueg C, et al. Admission hyperglycemia and acute myocardial infarction: outcomes and potential therapies for diabetics and nondiabetics. Cardiol Res Pract. 2012;1:1–6.
Grant PJ, Cosentino F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;40:3215–7.
Arnold SV, Spertus JA, Jones PG, McGuire DK, Lipska KJ, Xu Y, et al. Predicting adverse outcomes after myocardial infarction among patients with diabetes mellitus. Circ Cardiovasc Qual Outcomes. 2016;9:372–9.
Nauta ST, Akkerhuis KM, Deckers JW, Van Domburg RT. Short- and long-term mortality after myocardial infarction in patients with and without diabetes: changes from 1985 to 2008. Diabetes Care. 2012;35:2043–7.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98.
Kosuge M, Kimura K, Ishikawa T, Shimizi T, Hibi K, Toda N, et al. Persistent hyperglycemia is associated with left ventricular dysfunction in patients with acute myocardial infarction. Circ J. 2005;69:23–8.
van der Horst ICC, Nijsten MWN, Vogelzang M, Zijlstra F. Persistent hyperglycemia is an independent predictor of outcome in acute myocardial infarction. Cardiovasc Diabetol. 2007;6:2–9.
Suleiman M, Hammerman H, Boulos M, Kapeliovich MR, Suleiman A, Agmon Y, et al. Fasting glucose is an important independent risk factor for 30-day mortality in patients with acute myocardial infarction: a prospective study. Circulation. 2005;111:754–60.
Qin Y, Yan G, Qiao Y, Ma C, Liu J, Tang C. Relationship between random blood glucose, fasting blood glucose, and gensini score in patients with acute myocardial infarction. Biomed Res Int. 2019. https://doi.org/10.1155/2019/9707513.
Goyal A, Mahaffey KW, Garg J, Nicolau JC, Hochman JS, Weaver WD, et al. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. 2006;27:1289–97.
Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association diabetes committee of the council on nutrition, physical activity, and metabolism. Circulation. 2008;117:1610–9.
Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015;100:4490–7.
Ishihara M, Kagawa E, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, et al. Impact of Admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. 2007;99:1674–9.
Ding XS, Wu SS, Chen H, Zhao XQ, Li HW. High admission glucose levels predict worse short-term clinical outcome in non-diabetic patients with acute myocardial infraction: a retrospective observational study. BMC Cardiovasc Disord. 2019;19:1–9.
Tran HV, Gore JM, Darling CE, Ash AS, Kiefe CI, Goldberg RJ. Hyperglycemia and risk of ventricular tachycardia among patients hospitalized with acute myocardial infarction 11 Medical and Health Sciences 1103 Clinical Sciences. Cardiovasc Diabetol. 2018;17:1–9. https://doi.org/10.1186/s12933-018-0779-8.
Paolisso P, Foà A, Bergamaschi L, Donati F, Fabrizio M, Chiti C, et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol. 2021;20:1–11. https://doi.org/10.1186/s12933-021-01222-9.
Sardu C, Barbieri M, Balestrieri ML, Siniscalchi M, Paolisso P, Calabrò P, et al. Thrombus aspiration in hyperglycemic ST-elevation myocardial infarction (STEMI) patients: clinical outcomes at 1-year follow-up. Cardiovasc Diabetol. 2018;17:1–16. https://doi.org/10.1186/s12933-018-0795-8.
Jomaa W, El Mhamdi S, Ben Ali I, Azaiez MA, El Hraiech A, Ben Hamda K, et al. Prognostic value of hyperglycemia on-admission in diabetic versus non-diabetic patients presenting with ST-elevation myocardial infarction in Tunisia. Indian Heart J. 2018;70:772–6. https://doi.org/10.1016/j.ihj.2018.01.005.
Kristiansen SB, Pælestik KB, Johnsen J, Jespersen NR, Pryds K, Hjortbak MV, et al. Impact of hyperglycemia on myocardial ischemia-reperfusion susceptibility and ischemic preconditioning in hearts from rats with type 2 diabetes. Cardiovasc Diabetol. 2019;18:1–10. https://doi.org/10.1186/s12933-019-0872-7.
Paolisso P, Bergamaschi L, Rambaldi P, Gatta G, Foà A, Angeli F, et al. Impact of admission hyperglycemia on heart failure events and mortality in patients with Takotsubo syndrome at long-term follow-up: data from HIGH-GLUCOTAKO Investigators. Diabetes Care. 2021;44:2158–61.
Marfella R, Sasso FC, Siniscalchi M, Paolisso P, Rizzo MR, Ferraro F, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction. J Clin Endocrinol Metab. 2012;97:2862–71.
Acknowledgements
Authors would thank the physicians and patients of involved Institutions.
Statement of guarantor
M.H. is the guarantor of the research.
Permissions information
The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
Funding
This study was supported by Guangdong Provincial Key Laboratory of Construction Foundation (2020B1212060034).
Author information
Authors and Affiliations
Contributions
HU wrote the manuscript and researched data, J-LL researched data, X-GZ and Y-YH provided critical feedback and suggestions on the draft versions of the manuscript, H-YY, AA, AA and MA acquired the data, and MH designed and carried out the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local institutional review board of First People’s Hospital of Kashi Prefecture (Approval No. 2021-03). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Flow chart of the study. Figure S2. Significant interactions between diabetes status and FBG levels for all-cause mortality. Figure S3. Subgroup Analyses of the Risk for All-Cause Mortality. Figure S4. Association between FBG and outcomes using restricted cubic splines with multivariable-adjusted Cox proportional hazards models. Figure S5. Risk for short and long-term mortality according to FBG levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Upur, H., Li, JL., Zou, XG. et al. Short and long-term prognosis of admission hyperglycemia in patients with and without diabetes after acute myocardial infarction: a retrospective cohort study. Cardiovasc Diabetol 21, 114 (2022). https://doi.org/10.1186/s12933-022-01550-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01550-4